ABSTRACT
The survival of patients with metastatic or relapsed Ewing sarcoma (ES) remains dismal despite intensification of combination chemotherapy and radiotherapy, precipitating the need for novel alternative therapies with minimal side effects. Natural killer (NK) cells are promising additions to the field of cellular immunotherapy. Adoptive NK cell therapy has shown encouraging results in hematological malignancies. Despite these initial promising successes, however, NK cell therapy for solid tumors remains to be investigated using in vivo tumor models. The purpose of this study is to evaluate the efficacy of ex vivo expanded human NK cells in controlling primary and metastatic ES tumor growth in vitro and in vivo. Using membrane-bound IL-21 containing K562 (K562-mbIL-21) expansion platform, we were able to obtain sufficient numbers of expanded NK (eNK) cells that display favorable activation phenotypes and inflammatory cytokine secretion, along with a strong in vitro cytotoxic effect against ES. Furthermore, eNK therapy significantly decreased lung metastasis without any significant therapeutic effect in limiting primary tumor growth in an in vivo xenograft model. Our data demonstrate that eNK may be effective against pulmonary metastatic ES, but challenges remain to direct proper trafficking and augmenting the cytotoxic function of eNK to target primary tumor sites.
KEYWORDS: Ewing sarcoma, lung metastasis, natural killer cells, NK expansion
Abbreviations
- aAPCs
artificial antigen-presenting cells
- ES
Ewing sarcoma
- eNK cells
expanded natural killer cells
- mbIL-21
membrane-bound interleukin 21
- MHC
major histocompatibility complex
Introduction
Ewing sarcoma (ES) is the second most common malignant sarcoma in childhood and the adolescent and young adult (AYA) population. Appearing as a small round blue cell tumor, ES often harbors the characteristic translocation t(11; 22) with diffuse CD99 expression on immunohistology.1 Despite the overall improved outcome of localized ES with aggressive combination of chemotherapy, radiotherapy and surgery that results in a 5 y event-free survival of 75%, outcomes for metastatic and relapsed ES remain a major challenge, with a dismal 5 y event-free survival rate between 20–30%.2,3 Unfortunately, systemic chemotherapy trials have not been able to improve outcomes for patients with metastatic ES, with lung being the most frequent site of metastasis. For these patients, novel therapeutic approaches are urgently needed.
In recent years, antibody-mediated and cell-based immunotherapy approaches have enjoyed great successes in childhood cancers. Among those therapies, NK cells have gained considerable interests. NK cells are known for their natural ability to lyse tumor cells in vitro without the need for prior sensitization.4 They are the major cytotoxic effector cells of the innate immune system, constituting 5–15% of mature lymphocytes. NK cells are defined phenotypically by the presence of CD16 or CD56 and the absence of CD3 and CD19. The cytolytic activity of NK cells is determined by the balance of signals from both inhibitory and activating receptors expressed on their surface.5 The inhibitory receptors include killer immunoglobulin receptor (KIR), which recognizes HLA-A, B and C, and NKG2A/CD94, which recognizes HLA-E. Activating receptors include CD16, NKG2D (which recognizes MICA/B and ULBPs) and DNAM-1 (which recognizes CD155).6,7 Many tumor cells have been shown to upregulate activating receptor ligands, making them potential targets for NK cells.8-10 Furthermore, tumor cells with low MHC class I expression are potentially even more sensitive to NK-mediated cytotoxic killing due to a lack of inhibitory signals through KIR or NKG2A.11,12 In this regard, ES has been shown to be exquisitely sensitive to NK cell killing in vitro.13-15
NK cells have demonstrated anticancer effect in ongoing clinical trials. However, their widespread use is hampered by the ability to generate large numbers of these cells ex vivo.16,17 Moreover, the limited life span of NK cells in vivo is another crucial obstacle. In order to overcome those challenges, several methods have been examined to effectively expand NK cells. Using genetically modified K562 artificial antigen-presenting cells (aAPCs) as feeder cells, Denman and coworkers established an ex vivo method to expand NK cells with increased cytotoxicity and decreased senescence.18,19 Combining NK cell therapy with IL-2 infusion can possibly circumvent the limitation of limited in vivo life span. Genetically engineered aAPCs expressing membrane-bound IL-15 (mbIL-15) have been used to propagate clinical-grade NK cells for adoptive immunotherapy clinical trials, but ex vivo proliferation has been limited by telomere shortening. In contrast to mbIL-15, membrane-bound IL-21 (mbIL-21) expressing aAPCs promoted log-phase NK expansion without evidence of senescence for up to 6 weeks of culture.19-21 Here, we demonstrate that human NK cells expanded using genetically modified mbIL-21 K562 aAPCs can potently inhibit ES pulmonary metastasis in NSG mice.
Results
TC106 does not express HLA-ABC and expresses NKG2D and DNAM-1 ligands
To investigate the effectiveness of ex vivo expanded NK cells (eNK) against ES, we chose TC106 cell line, which was derived from an 19 y old patient with pelvic ES that harbored the t(11; 22) translocation and spontaneously metastasizes to the lungs upon subcutaneous inoculation.22 We first sought to determine whether TC106 expresses MHC class I and/or NK cell recognition ligands, as the presence of these molecules is important for sensitizing NK cell killing. At baseline, TC106 does not express appreciable HLA-ABC and does not upregulate HLA-ABC after 24 h exposure to IFNγ (Fig. 1A). In addition, TC106 expresses high levels of ULBP-1 and CD155, the ligands for the activating NK receptors NKG2D and DNAM-1, respectively (Fig. 1B). The lack of HLA-ABC and presence of NK activating ligands indicate that TC106 is a suitable target for NK cell therapy.
Figure 1.

Lack of HLA and expression of activating NK receptor ligands on Ewing sarcoma cell line. (A) TC106 cells were incubated in the presence or absence of 100 ng/mL IFNγ for 24 h, then surface MHC-I expression was analyzed by flow cytometry using an antibody against human HLA-A,B,C. No significant difference in expression after IFNγ treatment was seen. (B) TC106 cells were stained for ULBP-1 (NKG2D ligand) and CD155 (DNAM-1 ligand) and analyzed by flow cytometry.
Human NK cell purification and expansion
Consistent with previous reports, we were able to successfully expand peripheral blood-derived NK cells ex vivo using the K562-mbIL21 aAPC system generously provided by Dean Lee (Nationwide Children's Hospital, Columbus, Ohio).19 First, NK cells were isolated from PBMCs using an NK enrichment kit with CD3 depletion. Irradiated feeder K562-mbIL-21 cells were then co-cultured with the NK cells in the presence of human IL-2 (50 U/mL). Every week for a total of 3 weeks, NK cells were re-stimulated with irradiated K562-mbIL-21 and fresh hIL-2. Using this method, we were able to exponentially expand NK cells 25,000 to 90,000-fold from different donors (Fig. 2), which is within the range of expansion as previously reported.18
Figure 2.

Ex vivo expansion of NK cell using the K562-mbIL-21 system. At days 7, 14 and 21 after expansion, total number of NK cells from four healthy donors were enumerated and graphed above as cumulative fold expansion on day 21 relative to day 1.
Phenotypic characterization of expanded human NK cells
We assessed surface expression of the major NK receptors as well as intracellular cytokine content in the eNK cells. Flow cytometric analysis of the eNK cell population revealed that these cells maintain important surface markers of functional NK cells after 21 d (Fig. 3A). There was no CD3 contamination, confirming efficient CD3 depletion on day 0. NK activating receptors NKG2D and DNAM-1 (CD226) were upregulated by day 7 and maintained at high levels throughout the subsequent 2 week expansion period (Fig. 3B). We did not observe any significant expression of CD244 or PD-1 on eNK cells at any time during expansion, consistent with previous reports.18 As for homing receptors, CXCR3 was upregulated at day 7 and continued to increase with time, reaching maximal expression at day 21 (Fig. 3C). Consistent with acquisition of an activated phenotype, CD62L expression was downregulated at day 14 through day 21, while the lymph node homing receptor CCR7 was absent on eNKs (Fig. 3C). In addition, modest increases in CXCR6, CCR5 and CCR6 expression were observed over time, while CXCR1, CXCR4, CCR4, CCR9 and CX3CR1 remained low at day 21 (Fig. S1), consistent with previously reported profiles for freshly isolated CD16+ NK cells.23 Intracellular staining for perforin, granzyme B, IFNγ and TNFα revealed that these cytotoxic molecules as well as surface FasL were all maintained throughout expansion (Fig. 3D).
Figure 3.

Phenotype of ex vivo expanded NK cells. Flow cytometric characterization of NK cell markers (A), activating receptors (B), homing receptors (C) and cytotoxic molecules (D) at day 0, 7, 14 and 21 of expansion from a representative donor.
Expanded NK cells are cytotoxic against TC106 in vitro
In order to test eNK cytotoxic function, we used an in vitro Pantoxilux assay with TC106 as the target cells. Using eNKs from different donors, we found that both day 14 and day 21 eNKs are able to efficiently kill TC106 at varying effector-to-target ratios over the course of 2 h (Fig. 4A and B). While day 21 eNKs appeared to be slightly more cytotoxic than day 14 eNKs, there was no statistical difference between day 14 and day 21 eNK cytotoxicity, consistent with the similar NK activating receptor profile and cytotoxic molecular expressions as observed by flow cytometry (Fig. 3).
Figure 4.

TC106 Ewing sarcoma cells are sensitive to eNK cell killing in vitro. eNK cells from donor 1 (A) and donor 2 (B) were used in the Pantoxilux flow cytometric assay to quantitate early cytotoxicity as measured by granzyme B and upstream caspase cleavage at 2 h of co-incubation.
Expanded NK cells decrease the number of pulmonary metastatic nodules in vivo
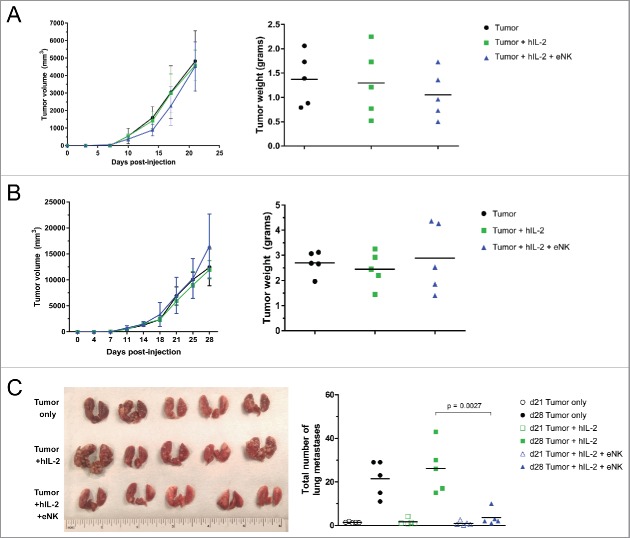
In order to test the differential potential of eNK cells to control various ES sites in vivo, we inoculated NSG mice subcutaneously with TC106, which grows progressively and readily metastasizes to the lungs over a 2–3 week window. As opposed to metastatic models involving direct intravenous inoculation of tumor, our approach allowed us to interrogate tumor control by eNK cells at both the primary and metastatic tumor sites. Shortly after tumor inoculation, we began weekly treatment of mice with 30 × 106 eNK cells given i.v. supplemented with hIL-2, and measured primary tumor progression until a 2 to 3 week time point (Fig. 5A and B). Throughout the whole treatment regimen, mice steadily gained weight and generally appeared healthy, indicating that this high dose of eNK therapy is safe (Fig. S2).
Figure 5.
eNK therapy inhibits pulmonary metastases but not primary tumor growth. NSG mice were inoculated with 50,000 TC106 cells s.c. and given hIL-2 and/or eNK therapy, followed out to 21 d (A) and 28 d (B) with primary tumor measurements. In (C) day 28 mice lungs were dissected for gross examination (left) and both day 21 and day 28 metastases were quantified following sectioning (right). Unpaired t test was used to calculate significant differences in tumor size.
We found that repeated weekly administration of eNKs was unable to influence the rate of primary tumor growth, as the tumor size and weights showed no difference between treated and control groups at the end of therapy period. This is likely a result of the inability of eNK to home to the primary tumor site, as assessed by two-photon imaging of explanted tumor tissues (Fig. S3A). Interestingly, however, weekly i.v. administration of eNKs was able to significantly reduce the number of ES lung metastases at the end of 3 weeks of therapy, with clear gross and microscopic reduction in tumor burden (Fig. 5C). As eNKs largely home to the lung after i.v. administration (Fig. S3B), efficient tumor control in this important metastatic site may benefit from this trafficking tropism of eNK. In addition, we see that eNKs also home to spleen and liver after adoptive transfer, which may help protect these organs from metastases (Fig. S3C and D). Based on these studies, we believe that eNK therapy may be beneficial in humans with lung invasive ES.
Discussion
Intensification of salvage chemotherapy has not made any significant impact on long-term survival in patients with metastatic or relapsed ES, even when combined as multimodal therapy or stem cell transplantation.24,25 Ongoing excitement in the development of novel immunotherapeutic strategies holds promise in improving the treatment outcome of advanced stage ES.26 Here, using a spontaneous metastatic xenograft model of ES, we show that ex vivo-expanded NK cells were able to inhibit ES lung metastasis in NSG mice, although they were not effective in reducing primary ES tumor burden in peripheral sites.
NK cells are the major cytotoxic effector cells of the innate immune system.27 NK cells harvested from donor blood leukapheresis have limited viability and life span in vitro. Several methods have been investigated in order to effectively expand, maintain and activate NK cells to facilitate treatment in vivo, but these efforts have been limited by senescence of eNK cells resulting from telomere shortening. To circumvent this challenge, we used genetically modified K562 aAPCs expressing mbIL-21 to expand human NK cells ex vivo, which has been developed by Denman and colleagues to enable sustained ex vivo expansion of NK cells with increased telomere lengths and greatly reduced senescence.19 Using this method, we were able to expand NK cells successfully with up to tens of thousands fold expansion, consistent with the results reported by Denman et al. We observed some variability in overall yield from donor to donor, but the mean fold-expansion remains superior to other expansion platforms.
Our rationale behind using eNK cells to treat ES comes from a handful of published reports indicating a preferentially high NK cytotoxicity against ES.28-30 In a more recent report, it has been shown that among pediatric solid tumors, ES cells are exquisitely sensitive in vitro to the cytotoxicity exerted by activated eNK cells.13 The authors demonstrated that eNK cells could kill nearly all ES cells within 4 h at 1:1 effector-to-target ratio. Verhoeven and coworkers have confirmed these results, concluding that cytokine-activated NK cells rather than resting NK cells might be instrumental in optimizing NK cell reactivity to ES in immunotherapy settings.15
NK cell recognition of targets cells is a complex interplay between both activating and inhibitory receptors and their corresponding ligands. The balance between activating and inhibitory signals will, for the most part, determine NK cell killing and activation.31 The interaction between the activating receptors, NKG2D and DNAM-1, on the surface of NK cells with their corresponding ligands on ES cells (ULBP1-6, MICA/B and CD155, CD112, respectively) are critical for NK cytotoxicity against ES, as interference with these molecules reduced cell killing considerably.13,15 On the other hand, the presence of the major histocompatibility complex (MHC), also known as human leukocyte antigen class I (HLA-I), on target cells serves as an inhibitory ligand for MHC class I-specific receptors (KIRs) on NK cells upon binding. Consequently, those tumors that do not express HLA-I and are therefore thought to be capable of evading a cytotoxic T-cell-mediated attack may become susceptible instead to a NK cell-mediated immune response. Complete or partial absence of HLA-I expression was observed in 79% of ES tumors among six ES cell lines, with lung metastasis consistently lacked HLA-I expression.11,12 We observed in our ES cell line (TC106) an absence of MHC class I expression even after IFNγ stimulation, which would theoretically decrease inhibitory signals to NK cells. Moreover, we also observed the expression of the NKG2D ligand ULBP-1 and the DNAM-1 ligand CD155 on TC106, which may again potentially increase activation signals to NK cells. Taken together, both the increased activation and decreased inhibition of NK cells by ES suggest a favorable scenario for NK cell immunotherapy against ES.
Our eNK cells were phenotypically consistent with those expanded by Denman and colleagues. Throughout the 3 week expansion process, we showed upregulation of the activating receptors NKG2D and DNAM-1 along with high expression of intracellular granzyme B and perforin in addition to IFNγ and TNF-α. In addition, we also detected expression of the homing CXCR3 with downregulation of CD62L and absent CCR7 over time. Therefore, we expect eNK cells to have high cytotoxicity and cytokine secretion against tumor targets, as long as they are able to home efficiently to the tumor site expressing ligands to CXCR3, CXCL9/CXCL10. Day 21 eNK were somewhat more cytotoxic in vitro compared to day 14 eNK cells. The reason for this is not clear but could be due to upregulation of the homing receptor CXCR3 during the last week of expansion, as previous reports have shown improved migration capacity of NK cells with higher CXCR3 expression toward solid tumors, which was dependent on tumor-derived CXCL10.32
Clinically, lung is the most common site of metastasis in ES and the majority, if not all, of advanced stage patients show pulmonary metastases. Therapies that can control lung metastasis would significantly impact the survival of this subset of patients who face otherwise dismal prognosis. Few groups have investigated the effect of NK cells on lung metastasis in tumor types other than ES. Guma and colleagues showed that NK cell therapy and aerosol IL-2 has some efficacy against osteosarcoma lung metastasis.8 Hong and coworkers demonstrated that NK cells inhibited lung metastasis of hepatocellular carcinoma but did not inhibit tumor growth in the liver of nude mice.9 IL-2 is routinely used to maintain and sustain the activation and viability of injected NK cells in vivo. In our NSG xenograft model, we evaluated the activity of eNK cells to control primary and metastatic tumor. We show a significant decrease in both micro- and macro-metastasis in the NK-treated group. These data were consistent with mice weights, as none of the NK-treated mice lost weight over time, while a number of the control mice began to lose weight in the last week of study. However, these very encouraging results against metastatic disease were not associated with any significant differences in primary tumor size between the control and the NK groups. There could be many reasons for this observation: first, there is likely inadequate homing of i.v. injected eNK cells to primary tumor. Second, even if eNK cells homed successfully, they may become dysfunctional due to an immunosuppressive tumor microenvironment. These challenges may be addressed in future studies investigating approaches such as intratumoral NK injections, modification of tumor microenvironment, and modulation of eNK homing receptors. An interesting observation from our study is that hIL-2 control group lungs were qualitatively worse compared to the tumor only group. We hypothesize this could be a side effect from hIL-2 in the lung tissue, which may cause leaky blood vessels and promote metastasis through hematogenous and local spread.
One limitation of our current study is lack of a detailed dissection on the regulation of NK homing and trafficking receptors over the course of ex vivo expansion. As we show downregulation of CD62L and disappearance of CCR7 with sequential expansion, it can be argued that day 7 eNK cells might be better NK cell source to use for hematological malignancies, while NK cells from longer expansions may be more suitable for solid tumors in peripheral sites. In addition, the mechanism underlying NK-mediated inhibition of ES lung metastasis is unclear. Previous studies have demonstrated that NK cell receptors, including NKG2D and DNAM-1 and their corresponding ligands, may play an important role in NK killing of ES.
In conclusion, our data show that eNK cell therapy inhibits ES lung metastasis, although eNK cells do not inhibit primary tumor growth. This therapy may therefore be a new therapeutic approach for patients with metastatic or relapsed ES who otherwise carry dismal prognosis. In order to increase NK cytotoxic capacity in vivo, functional enhancers such as IL-15 superagonists and TGF-β antagonists may improve eNK lifespan and efficacy.33-35 Further investigations are needed to identify mechanisms that enable NK cells to inhibit cancer metastasis and clarify strategies to enforce efficient NK homing to primary tumor sites for therapies against solid tumors including ES.
Materials and methods
Ewing sarcoma cell line and culture
TC106 is a ES cell line harboring the classical t(11; 22) translocation that was derived from a 19 y old male with ES which arose in the pelvis and demonstrated widespread metastasis to bone, bone marrow and lungs.22 The line was established from a scalp mass from this male patient in 1982. TC106 cells were cultured in RPMI 1640 medium (Corning) supplemented with 10% fetal bovine serum (Gemcell), 2 mmol/L L-glutamine (Corning), 1 mmol/L sodium pyruvate (Corning), 1 mmol/L nonessential amino acids (Corning), and 1× penicillin/streptomycin (Corning). TC106-GFP cells were created by stably transfecting TC106 cells with eGFP under puromycin selection.
Human NK cells isolation, purification, ex vivo expansion and culture
Peripheral blood was obtained from healthy adult volunteer donors at the Case Comprehensive Cancer Center following informed consent. Human NK cells were harvested from buffy coat fractions using Ficoll-Paque (GE Healthcare) and the Human NK cell Isolation Kit (Miltenyi Biotec Cat. 130-092-657) by negative selection. Post-selection purity by flow cytometry staining for NK cell markers was routinely over 80–90%. NK cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 1 mmol/L nonessential amino acids, 1 × antibiotic-antimycotic (Corning), and 50 IU/mL recombinant human IL-2 (rhIL2, PeproTech). Genetically engineered K562 cells with mbIL-21 were used as aAPCs after 100-Gy irradiation for in vitro expansion of isolated human NK cells. aAPCs were a generous gift from Dr. Dean Lee at Nationwide Children's Hospital. As previously described,18 NK cell cultures were refreshed with complete media and hIL-2 every 3 d. Every 7 d the cultures were restimulated with aAPCs at 1:1 NK to aAPC ratio. The expansion process continued for 3 weeks. At the end of 21 d, eNK cells were counted and either used for in vitro and in vivo studies or cryopreserved in liquid nitrogen to be used for future experiments.
Flow cytometry
The phenotype of eNK cells was analyzed weekly using flow cytometry. The following mouse anti-human antibodies were used for staining: CD16 (3G8), CD56 (NCAM 16.2), NKG2D (1D11), DNAM-1 (DX11), CD3 (HIT3a) from BD PharMingen; CXCR1 (8F1), CXCR3 (CEW33D), CXCR4 (L276F12), CXCR6 (K041E5), CCR4 (L291H4), CCR5 (J418F1), CCR6 (G034E3), CCR9 (L053E8), CX3CR1 (2A9-1), Fas-L (NOK-1), IFNγ (B27) from BioLegend; CD62L (DREG-56), intracellular Perforin (dG9), Granzyme B (GB11), and TNF-α (MAb11) from eBioscience. For surface staining, NK cells were resuspended in FACS buffer (0.5% FBS and 2 mM EDTA in PBS), blocked with 10% mouse serum for 30 min, then incubated with the indicated antibodies for 30 min at 4°C using concentrations recommended by the manufacturers. For subsequent intracellular staining, NK cells were stained using the BD Cytofix/Cytoperm Kit following manufacturer protocol. Data was acquired using a BD FACS Accuri C6 and analyzed using FlowJo v10.2 software. Isotype IgG antibodies were used as negative controls. Human NK cells were defined as CD16+, CD56+ and CD3−. Mouse anti-human HLA-ABC (W6/32), MICA/B (6D4), CD155 (2H7) and PD-L1 (MIH1) from eBioscience, ULBP-1 (170818) from R&D, and Fas (DX2) from BioLegend were used to determine MHC-I, NKG2D ligand and DNAM-1 ligand expression on TC106 with surface staining performed as above for NK cells.
In vitro cytotoxicity assay
PanToxiLux (OncoImmunin, Gaithersburg, MD), a fluorescence-based caspase-8 cleavage and granzyme B activity assay, was used to assess cytotoxic activity of day 14 and day 21 eNK cells. Day 14 and 21 eNK cells were incubated with the TC106 cells in 1:1, 4:1, 8:1 and 16:1 effector: target ratios for 2 h at 37°C. Following manufacturer protocol, TC106 incubated with target dye (fluorescing in FL-4) and/or PanToxiLux substrate (fluorescing in FL-1) served as target cell background staining control. In addition, eNK incubated with PanToxiLux substrate alone served as effector cell background staining control. Flow cytometry was used to quantify the percentage of TC106 cell death at the various effector: target ratios, indicated by double staining in FL-4 and FL-1 channels.
Animal experiments and quantification of lung metastases
Six-week-old male NOD SCID Gamma (NSG) mice were obtained from the in-house breeding facility. All mice were housed in standard cages at five mice per cage. NSG mice were split into three experimental groups: (1) TC106 only, (2) TC106 and hIL-2 and (3) TC106, hIL-2 and day 21 eNK cells. TC106 was injected subcutaneously in to the left thigh at a dose of 50,000 cells per mouse, and rhIL-2 was injected intraperitoneally at a dose of 25,000 IU per mouse daily for the first week then twice weekly after that. 30 × 106 day 21 eNK cells were injected intravenously via tail vein at the time of TC106 injection then weekly thereafter. Mice weights and primary tumor size were measured twice weekly by digital scale and calipers, with tumor volume calculated as π × long diameter × small diameter × small diameter.36 All mice were sacrificed at day 21 or day 28 post TC106 injection. Upon sacrifice, all mice were dissected and organs including lungs, tumor, liver, spleen were isolated and frozen in OCT following overnight 10% formalin fixation. Samples were cryosectioned into 10 µm sections spanning the entire organ on a Leica Cryostat, followed by hematoxylin/eosin (IHC World) staining. Metastases in lungs sections were manually counted by microscopy (10–20 representative sections of lung/mouse), with the number of metastases normalized to the total number of sections counted. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at Case Western Reserve University.
Multi-photon explant imaging of eNK organ homing
NSG mice were inoculated with 50,000 TC106-GFP cells s.c. in the flank. At day 13–14 post inoculation, ∼30 × 106 eNK cells were labeled with 5 µm SNARF-1 (Molecular Probes) then i.v. injected 24 h prior to explant multi-photon imaging of the primary tumor, lungs, spleen and liver using a Leica SP5 on a DM6000 stage fitted with a 16W IR laser (Chameleon; Coherent) tuned to 880 nm. Imaging data were subsequently processed in Imaris (BitPlane).
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors acknowledge Dr Dean Lee at Nationwide Children's Hospital for his generous gift of K562-mbIL-21, and Dr. John Letterio at CWRU for provision of TC106.
Funding
This work was supported by NIH R01CA154656 (A.H.), NIH R21CA181875 (A.H.), NIH P30CA043703 (A.H.), F31CA192874 (F.A.), the Marc Joseph Fund (H.H.), the Fellowship Research Award in Pediatrics (H.H.), the Samuel Szabo Foundation (A.T.), Alex's Lemonade Stand Foundation (D.K. and A.H.), the St. Baldrick's Foundation (A.H.), the Angie Fowler AYA Cancer Research Initiative at the Case Comprehensive Cancer Center (A.H.), The Keira Kilbane Foundation (A.H.), and the Theresia G. & Stuart F. Kline Family Foundation in Pediatric Oncology (A.H.).
References
- 1.Delattre O, Zucman J, Melot T, Garau XS, Zucker JM, Lenoir GM, Ambros PF, Sheer D, Turc-Carel C, Triche TJ et al.. The Ewing family of tumors–a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med 1994; 331:294-9; PMID:8022439; http://dx.doi.org/ 10.1056/NEJM199408043310503 [DOI] [PubMed] [Google Scholar]
- 2.Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, Marcus K, Sailer S, Healey JH, Dormans JP et al.. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol 2012; 30:4148-54; PMID:23091096; http://dx.doi.org/ 10.1200/JCO.2011.41.5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA et al.. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003; 348:694-701; PMID:12594313; http://dx.doi.org/ 10.1056/NEJMoa020890 [DOI] [PubMed] [Google Scholar]
- 4.Bryceson YT, Ljunggren HG. Natural killer cells: biology, physiology and medicine–part 1. J Innate Immun 2011; 3:213-5; PMID:21454963; http://dx.doi.org/ 10.1159/000325332 [DOI] [PubMed] [Google Scholar]
- 5.Moretta L, Montaldo E, Vacca P, Del Zotto G, Moretta F, Merli P, Locatelli F, Mingari MC. Human natural killer cells: origin, receptors, function, and clinical applications. Int Arch Allergy Immunol 2014; 164:253-64; PMID:25323661; http://dx.doi.org/ 10.1159/000365632 [DOI] [PubMed] [Google Scholar]
- 6.Sutlu T, Alici E. Natural killer cell-based immunotherapy in cancer: current insights and future prospects. J Intern Med 2009; 266:154-81; PMID:19614820; http://dx.doi.org/ 10.1111/j.1365-2796.2009.02121.x [DOI] [PubMed] [Google Scholar]
- 7.Leung W. Infusions of allogeneic natural killer cells as cancer therapy. Clin Cancer Res 2014; 20:3390-400; PMID:24987108; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-1766 [DOI] [PubMed] [Google Scholar]
- 8.Guma SR, Lee DA, Yu L, Gordon N, Hughes D, Stewart J, Wang WL, Kleinerman ES. Natural killer cell therapy and aerosol interleukin-2 for the treatment of osteosarcoma lung metastasis. Pediatr Blood Cancer 2014; 61:618-26; PMID:24136885; http://dx.doi.org/ 10.1002/pbc.24801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong ZF, Zhao WX, Yin ZY, Xie CR, Xu YP, Chi XQ, Zhang S, Wang XM. Natural killer cells inhibit pulmonary metastasis of hepatocellular carcinoma in nude mice. Oncol Lett 2016; 11:2019-26; PMID:26998115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández L, Valentín J, Zalacain M, Leung W, Patiño-García A, Pérez-Martínez A. Activated and expanded natural killer cells target osteosarcoma tumor initiating cells in an NKG2D-NKG2DL dependent manner. Cancer Lett 2015; 368:54-63; PMID:26276724; http://dx.doi.org/ 10.1016/j.canlet.2015.07.042 [DOI] [PubMed] [Google Scholar]
- 11.Berghuis D, de Hooge AS, Santos SJ, Horst D, Wiertz EJ, van Eggermond MC, van den Elsen PJ, Taminiau AH, Ottaviano L, Schaefer KL et al.. Reduced human leukocyte antigen expression in advanced-stage Ewing sarcoma: implications for immune recognition. J Pathol 2009; 218:222-31; PMID:19274709; http://dx.doi.org/ 10.1002/path.2537 [DOI] [PubMed] [Google Scholar]
- 12.Haworth KB, Leddon JL, Chen CY, Horwitz EM, Mackall CL, Cripe TP. Going back to class I: MHC and immunotherapies for childhood cancer. Pediatr Blood Cancer 2015; 62:571-6; PMID:25524394; http://dx.doi.org/ 10.1002/pbc.25359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho D, Shook DR, Shimasaki N, Chang YH, Fujisaki H, Campana D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin Cancer Res 2010; 16:3901-9; PMID:20542985; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn YO, Weigel B, Verneris MR. Killing the killer: natural killer cells to treat Ewing's sarcoma. Clin Cancer Res 2010; 16:3819-21; PMID:20554750; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verhoeven DH, de Hooge AS, Mooiman EC, Santos SJ, ten Dam MM, Gelderblom H, Melief CJ, Hogendoorn PC, Egeler RM, van Tol MJ et al.. NK cells recognize and lyse Ewing sarcoma cells through NKG2D and DNAM-1 receptor dependent pathways. Mol Immunol 2008; 45:3917-25; PMID:18657862; http://dx.doi.org/ 10.1016/j.molimm.2008.06.016 [DOI] [PubMed] [Google Scholar]
- 16.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 2013; 10:230-52; PMID:23604045; http://dx.doi.org/ 10.1038/cmi.2013.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klingemann H. Challenges of cancer therapy with natural killer cells. Cytotherapy 2015; 17:245-9; PMID:25533934; http://dx.doi.org/ 10.1016/j.jcyt.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 18.Somanchi SS, Senyukov VV, Denman CJ, Lee DA. Expansion, purification, and functional assessment of human peripheral blood NK cells. J Vis Exp 2011; 48(2540); PMID:21339714; http://dx.doi.org/22279576 10.3791/2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, Singh H, Hurton L, Maiti SN, Huls MH et al.. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PloS One 2012; 7:e30264; PMID:22279576; http://dx.doi.org/ 10.1371/journal.pone.0030264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imamura M, Shook D, Kamiya T, Shimasaki N, Chai SM, Coustan-Smith E, Imai C, Campana D. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood 2014; 124:1081-8; PMID:25006133; http://dx.doi.org/ 10.1182/blood-2014-02-556837 [DOI] [PubMed] [Google Scholar]
- 21.Cho D, Campana D. Expansion and activation of natural killer cells for cancer immunotherapy. Korean J Lab Med 2009; 29:89-96; PMID:19411773; http://dx.doi.org/ 10.3343/kjlm.2009.29.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whang-Peng J, Triche TJ, Knutsen T, Miser J, Kao-Shan S, Tsai S, Israel MA. Cytogenetic characterization of selected small round cell tumors of childhood. Cancer Genet Cytogenet 1986; 21:185-208; PMID:3004699; http://dx.doi.org/ 10.1016/0165-4608(86)90001-4 [DOI] [PubMed] [Google Scholar]
- 23.Berahovich RD, Lai NL, Wei Z, Lanier LL, Schall TJ. Evidence for NK cell subsets based on chemokine receptor expression. J Immunol 2006; 177:7833-40; PMID:17114454; http://dx.doi.org/ 10.4049/jimmunol.177.11.7833 [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Galindo C, Navid F, Liu T, Billups CA, Rao BN, Krasin MJ. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 2008; 19:814-20; PMID:17998282; http://dx.doi.org/ 10.1093/annonc/mdm521 [DOI] [PubMed] [Google Scholar]
- 25.Cotterill SJ, Ahrens S, Paulussen M, Jürgens HF, Voûte PA, Gadner H, Craft AW. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol 2000; 18:3108-14; PMID:10963639; http://dx.doi.org/ 10.1200/JCO.2000.18.17.3108 [DOI] [PubMed] [Google Scholar]
- 26.Rossig C. Cellular immunotherapy strategies for Ewing sarcoma. Immunotherapy 2014; 6:611-21; PMID:24896629; http://dx.doi.org/ 10.2217/imt.14.36 [DOI] [PubMed] [Google Scholar]
- 27.Chester C, Fritsch K, Kohrt HE. Natural Killer Cell Immunomodulation: Targeting Activating, Inhibitory, and Co-stimulatory Receptor Signaling for Cancer Immunotherapy. Front Immunol 2015; 6:601; PMID:26697006; http://dx.doi.org/ 10.3389/fimmu.2015.00601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atzpodien J, Gulati SC, Shimazaki C, Bührer C, Oz S, Kwon JH, Kolitz JE, Clarkson BD. Ewing's sarcoma: ex vivo sensitivity towards natural and lymphokine-activated killing. Oncology 1988; 45:437-43; PMID:3263598; http://dx.doi.org/ 10.1159/000226661 [DOI] [PubMed] [Google Scholar]
- 29.Staege MS, Hansen G, Baersch G, Burdach S. Functional and molecular characterization of interleukin-2 transgenic Ewing tumor cells for in vivo immunotherapy. Pediatr Blood Cancer 2004; 43:23-34; PMID:15170886; http://dx.doi.org/ 10.1002/pbc.20013 [DOI] [PubMed] [Google Scholar]
- 30.Chin T, Toy C, Vandeven C, Cairo MS. Lymphokine-activated killer cytotoxicity in neonatal mononuclear cells: in vitro responses to tumor cell lines from pediatric solid tumors. Pediatr Res 1989; 25:156-60; PMID:2537488; http://dx.doi.org/ 10.1203/00006450-198902000-00016 [DOI] [PubMed] [Google Scholar]
- 31.Konjević G, Vuletić A, Mirjačić Martinović K. Natural killer cell receptors: alterations and therapeutic targeting in malignancies. Immunol Res 2016; 64:25-35; PMID:26374324; http://dx.doi.org/ 10.1007/s12026-015-8695-4 [DOI] [PubMed] [Google Scholar]
- 32.Wennerberg E, Kremer V, Childs R, Lundqvist A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol Immunother 2015; 64:225-35; PMID:25344904; http://dx.doi.org/ 10.1007/s00262-014-1629-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao YM, French AR. Mechanistic model of natural killer cell proliferative response to IL-15 receptor stimulation. PLoS Comput Biol 2013; 9:e1003222; PMID:24068905; http://dx.doi.org/ 10.1371/journal.pcbi.1003222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viel S, Marçais A, Guimaraes FS, Loftus R, Rabilloud J, Grau M, Degouve S, Djebali S, Sanlaville A, Charrier E et al.. TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci Signal 2016; 9:ra19; PMID:26884601; http://dx.doi.org/ 10.1126/scisignal.aad1884 [DOI] [PubMed] [Google Scholar]
- 35.Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGF-β targeted cancer therapy. Int J Biol Sci 2012; 8:964-78; PMID:22811618; http://dx.doi.org/ 10.7150/ijbs.4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merchant MS, Melchionda F, Sinha M, Khanna C, Helman L, Mackall CL. Immune reconstitution prevents metastatic recurrence of murine osteosarcoma. Cancer Immunol Immunother 2007; 56:1037-46; PMID:17149595; http://dx.doi.org/ 10.1007/s00262-006-0257-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.