ABSTRACT
Exposure of cancer cells to particular chemotherapeutic agents or γ-irradiation induces a form of cell death that stimulates an immune response in mice. This “immunogenic cell death” requires calreticulin (CRT) translocation to the plasma membrane, which has been shown to promote cancer cell phagocytosis. However, it remains unclear whether the effect of CRT on cancer cell phagocytosis is alone sufficient to affect tumor immunity. Acute myeloid leukemia (AML) cells expressing cell-surface CRT were generated in order to characterize the mechanism(s) through which CRT activates tumor immune responses. Potent immune-mediated control or rejection of AML was observed in mice with CRT-expressing leukemia. The “CRT effect” was ultimately T-cell dependent, but dendritic cells (DCs), and CD8α+ DCs in particular, were also necessary, indicating that CRT might act directly on these DCs. CRT-expressing AML cells were slightly more susceptible to phagocytosis by DCs in vivo, but this effect was unlikely to explain the potent immunity observed. CRT did not affect classical DC maturation markers, but induced expression of type I interferon (IFN), which was critical for its positive effect on survival. In conclusion, CRT functions as a “danger signal” that promotes a host type I IFN response associated with the induction of potent leukemia-specific T-cell immunity.
KEYWORDS: Acute myeloid leukemia, calreticulin, immunity, type I interferon
Introduction
Exposure of cancer cells to particular chemotherapeutic drugs or γ-irradiation induces a form of apoptosis that stimulates an immune response in mice.1,2 This “immunogenic cell death” requires translocation of a chaperone protein called calreticulin (CRT) from the endoplasmic reticulum (ER), where it normally resides, to the plasma membrane.3 Under homeostatic conditions, calreticulin is involved in major histocompatibility complex (MHC) class I assembly, and in the maintenance of ER calcium homeostasis.4 Cell surface CRT translocation requires activation of pancreatic ER kinase (PERK), which phosphorylates eukaryotic translation initiation factor (eIF2α). eIF2α-mediated triggering of ER stress pathways culminates in anterograde transport of CRT through the Golgi to the cell surface.3 Following translocation, CRT functions as an “eat me” signal to promote efferocytosis by APCs through a receptor called low-density lipoprotein-related protein (LRP; CD91).5,6 CRT translocation also occurs in viable malignant cells,7 suggesting that apoptosis may not be required for CRT translocation, and that activation of “ER stress” programs can be sufficient to promote its cell surface expression.
A major unanswered question regarding CRT is whether its effect on tumor immunity depends exclusively on its ability to enhance cancer cell engulfment. By inducing phagocytosis of apoptotic tumor cells by APCs, CRT might promote cross-presentation of tumor antigens to T cells. However, antigen cross-presentation by immature dendritic cells (DCs) results in T-cell tolerance, rather than activation.8 We therefore hypothesized that CRT might also directly stimulate APCs, leading to increased cross-priming of tumor-specific T cells. In support of this hypothesis, soluble CRT was recently shown to stimulate production of NF-kB-related cytokines by peritoneal macrophages in a CD91-dependent manner.9
Increased cell-surface CRT exposure on malignant cells has been reported in a number of human cancers,7,10-12 including acute myeloid leukemia.7,13 In a recent study, the degree of cell-surface CRT expression on human acute myeloid leukemia (AML) cells was found to correlate with enhanced T-cell immunity to AML-associated antigens and improved clinical outcomes.14 In addition, our laboratory is interested in characterizing pathways that promote innate immune sensing of AML.15 With these notions in mind, we sought to investigate mechanisms through which CRT translocation on AML cells might promote anti-leukemia immunity. Thus, AML cells with stable cell-surface CRT expression were generated in order to investigate how CRT, as an isolated variable (i.e., without the requirement for chemotherapy or radiation exposure), affects anti-leukemia immunity. In a systemic disease model known to induce a potent T-cell tolerant state,16 CRT expression on AML cells was associated with prolonged survival in wild-type, but not in T-cell-deficient mice. Moreover, leukemia-specific CD8+ T-cell responses were significantly augmented in mice harboring CRT-expressing AML, indicating that T cells were required to mediate the CRT effect on anti-leukemia immunity. CD8α+ DCs were also necessary for the “CRT effect,” which suggested that CRT acts upstream of adaptive immunity. A modest increase in engulfment of CRT-expressing AML cells by DCs was observed, but did not appear to explain its potent effect on immunity. Rather, CRT-induced expression of type I interferon (IFN) in leukemia-bearing mice, and host type I IFN signaling, was required for the effect of CRT on AML survival. In conclusion, CRT functions as a “danger signal” to promote innate immune sensing of cancer through type I IFN.
Results
Generation of CRT-expressing C1498 AML cells
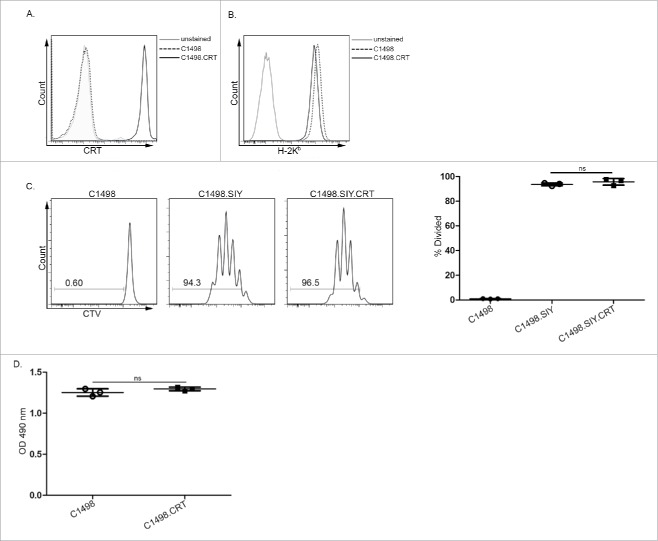
To investigate how CRT, as an isolated variable, regulates antitumor immune responses, its cell surface expression was engineered on C1498 and C1498.SIY AML cells through a GPI anchor (Fig. 1A). GPI-mediated protein anchoring to the plasma membrane is sensitive to enzymatic cleavage by phospholipase C.17 Treatment of CRT-expressing C1498 cells with phospholipase C resulted in significantly decreased cell surface CRT (Fig. S1A and B), clearly demonstrating its GPI-dependent anchoring to the plasma membrane. Cell surface CRT expression on engineered C1498 AML cells was only slightly higher than that on control C1498 cells undergoing doxorubicin-induced apoptosis (Fig. S1C). Thus, we have generated an AML cell line that expresses cell surface CRT at a similar level to that observed following treatment with a chemotherapy agent known to induce CRT translocation.2
Figure 1.
Generation of CRT-expressing AML cells. Representative FACS plots depicting expression of (A) CRT and (B) H-2Kb on control C1498 and C1498.CRT cells. (C) CTV-labeled 2C T cells were cultured in vitro for 3 d with parental C1498 cells (left), C1498.SIY cells (center) or C1498.SIY.CRT cells (right). Representative FACS plots demonstrating CTV dilution of 2C T cells are shown after gating on live CD8+1B2+ cells. Numbers indicate the percentage of divided 2C T cells. Quantified data are shown to the right. (D) MTS assay of in vitro cultured C1498 and C1498.CRT cells. n.s.: not significant. (C, D) Data are representative of 2–4 experiments, each performed in triplicate.
CRT is a member of a multi-protein peptide-loading complex (PLC), which is involved in MHC class I folding and peptide loading.4 Thus, engineered CRT expression could conceivably affect the MHC class I presentation pathway in C1498 cells. However, equivalent cell surface Kb levels were observed on CRT-expressing and control C1498 cells (Fig. 1B). Also, SIY-specific CD8+ 2C T cells proliferated similarly when cultured with C1498.SIY or C1498.SIY.CRT cells, indicating that MHC class I presentation of the SIY peptide antigen was not influenced by induced CRT expression (Fig. 1C). Last, the in vitro growth of C1498 and C1498.CRT cells was identical, demonstrating that engineered CRT expression did not affect AML cell viability or proliferation (Fig. 1D).
CRT expression on AML cells is associated with impaired tumor development
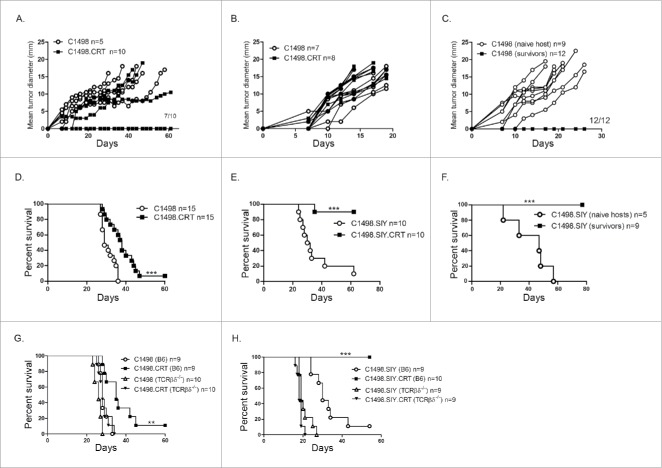
To determine whether CRT expression on C1498 cells affected their capacity to develop and progress as localized tumors, C1498 or C1498.CRT cells were inoculated subcutaneously (SC) into recipient hosts. Control C1498 tumors progressed rapidly in C57BL/6 mice. However, following SC C1498.CRT inoculation, 70% of mice remained tumor-free (Fig. 2A). Conversely, both control and C1498.CRT tumors progressed similarly in Rag2−/− hosts (Fig. 2B), which indicated that the adaptive immune system was necessary to prevent localized growth of CRT-expressing C1498 tumors. Further, wild-type mice that rejected a SC C1498.CRT challenge were resistant to re-challenge with parental C1498 cells, suggesting that CRT expression on AML cells was sufficient to promote immunological memory against native leukemia antigens (Fig. 2C). This result also indicates that CRT itself is not a direct antigenic target of adaptive immune cells in mice harboring CRT-expressing tumors. Collectively, these results demonstrate that CRT expression on cancer cells negatively impacts localized tumor progression through a mechanism which requires adaptive immunity.
Figure 2.
CRT expression impairs tumor development and delays progression of systemic AML. C1498 or C1498.CRT cells were inoculated SC into groups of C57BL/6 (A) or Rag2−/− (B) mice, and tumor growth was assessed. Data represent tumor growth in individual mice. (C) Mice from (A) that remained tumor-free for 60 d after a SC C1498.CRT challenge received a secondary challenge with parental C1498 cells in the opposite flank. Tumor growth was compared to mice receiving a primary C1498 challenge. (D, E) Survival of C67BL/6 mice challenged with C1498 versus C1498.CRT IV (D) or with C1498.SIY versus C1498.SIY.CRT IV (E). (F) Mice from (E) that remained alive for 60 d after an IV C1498.SIY.CRT challenge received a secondary challenge with C1498.SIY cells IV. Survival was compared to mice receiving a primary IV C1498.SIY challenge; ***p <0.001. (G and H) Survival of C57BL/6 versus TCRβ/δ−/− mice challenged with C1498 versus C1498.CRT IV (G) or with C1498.SIY versus C1498.SIY.CRT IV (H). **p <0.001 for comparison of survival between C57BL/6 and TCRβ/δ−/− challenged with C1498.CRT cells. ***p <0.0001 for comparison of survival between C57BL/6 and TCRβ/δ−/− challenged with C1498.SIY.CRT cells. (A–H) Data are pooled from two to three independent experiments, each with 2–5 mice/group.
CRT promotes enhanced survival in animals with systemic AML
It was next of interest to determine whether a similar result would occur in a systemic AML setting known to induce a T-cell tolerant state, and which more accurately recapitulates human AML progression.16 To that end, survival of C57BL/6 mice was assessed following an intravenous (IV) challenge with C1498 or C1498.CRT cells. As shown in Fig. 2D, survival of mice harboring disseminated C1498.CRT AML was significantly prolonged compared to those with control C1498 leukemia. The effect on survival was much more striking when CRT-expressing C1498.SIY cells were introduced IV, where 90% of mice survived long-term (Fig. 2E), and were consistently able to reject a secondary C1498.SIY cell challenge (Fig. 2F). Thus, CRT expression on AML cells is sufficient to generate effective immunological memory responses, even in a disease setting associated with a profound T-cell tolerant state.
Adaptive immunity was also required to promote CRT-mediated survival in mice with systemic AML, as the survival benefit associated with CRT expression on leukemia cells was abrogated in Rag2−/− mice (Fig. S2A), in TCRβ/δ−/− hosts (Fig. 2G and H), and also in C57BL/6 mice depleted of CD4+ and CD8+ T cells (Fig. S2B). In conclusion, the enhanced survival of mice with systemic CRT-expressing AML requires the adaptive immune system, and T cells in particular.
Augmented leukemia-specific T-cell responses in mice with CRT-expressing AML
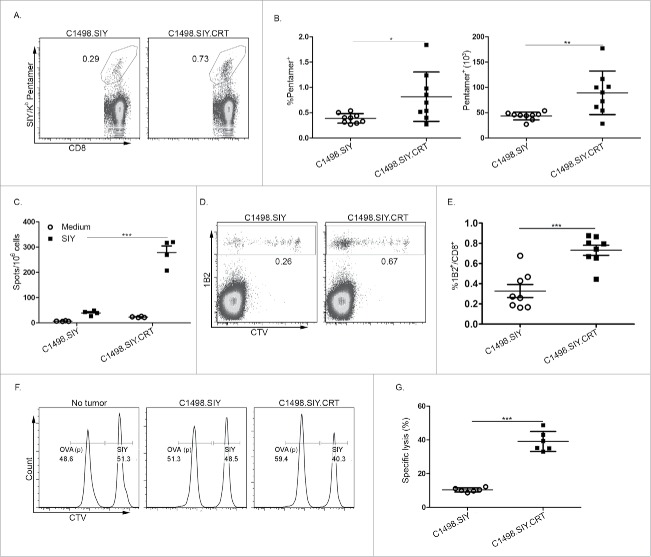
Because T cells were found to be necessary for the CRT-mediated effect on systemic leukemia progression, their ability to promote leukemia-specific T-cell responses was investigated. Six days following AML induction, SIY/Kb pentamer staining revealed a significantly higher frequency (Fig. 3A and B) and absolute number (Fig. 3B) of SIY antigen-specific CD8+ T cells in the spleens of mice with C1498.SIY.CRT versus control C1498.SIY AML. More impressive was the capacity for SIY-specific CD8+ T cells from C1498.SIY.CRT-challenged mice to produce IFNγ upon in vitro re-stimulation, which was approximately 6-fold higher compared to mice challenged with control C1498.SIY (Fig. 3C).
Figure 3.
CRT promotes enhanced leukemia antigen-specific T-cell responses. (A) Representative FACS plots depicting the frequency of SIY/Kb pentamer-reactive CD8+ T cells in the spleen of C57BL/6 mice challenged 6 d earlier with C1498.SIY (left) or C1498.SIY.CRT (right) IV. Numbers indicate the frequency of SIY+CD8+ cells among the entire CD8+ T-cell population. (B) Quantification of the frequency (left) and absolute number (right) of SIY/Kb pentamer-reactive splenic CD8+ T cells following IV challenge with C1498.SIY versus C1498.SIY.CRT cells; *p <0.05; **p <0.01. (C) Spleen cells from individual mice challenged IV with C1498.SIY versus C1498.SIY.CRT 6 d earlier were re-stimulated overnight in the presence of media or SIY peptide in an IFNγ ELISPOT assay; ***p <0.001. (D) Representative FACS plots demonstrating CTV dilution of 2C T cells 7 d following adoptive transfer into mice challenged IV with C1498.SIY (left) versus C1498.SIY.CRT (right). Numbers indicate 2C T-cell frequency among the entire CD8+ T-cell population. (E) Quantification of data in (D). ***p <0.001. (F, G) In vivo cytolysis of SIY or OVA peptide-pulsed, CTV-labeled B6.SJL splenocytes (CD45.1/.1) 6 h after IV inoculation into C57BL/6 mice (CD45.2/.2) that previously received adoptive 2C T-cell transfer and IV challenge with C1498.SIY or C1498.SIY.CRT cells. Representative plots are shown in (F) after gating on CD45.1+ donor cells. Numbers indicate the relative frequency of CTV-labeled CD45.1+ cells pulsed either with SIY or OVA peptide. Data are quantified in (G); ***p <0.0001. (B, E, G) Data are pooled from two independent experiments each with 3–4 mice/group. (C) Data are representative of three independent experiments each with 3–4 mice/group.
To track the proliferation and accumulation of leukemia antigen-specific CD8+ T cells in leukemia-bearing mice, a T-cell adoptive transfer model was employed. CTV-labeled, SIY antigen-specific, T-cell receptor (TCR) transgenic 2C T cells were transferred, and 1 d later, animals received C1498.SIY or C1498.SIY.CRT cells IV. 2C T cells accumulated poorly in mice with C1498.SIY leukemia as we have previously demonstrated (Fig. 3D and E).16 However, 2C T-cell accumulation was enhanced 2-fold in C1498.SIY.CRT-bearing mice (Fig. 3D and E; Fig. S3A and B). CRT expression on AML cells also led to an improved capacity of 2C T cells to produce IFNγ (Fig. S3C), and to lyse SIY antigen-pulsed targets in vivo (Fig. 3F and G). Taken together, these data reveal that CRT-expression on leukemia cells significantly enhances the priming of highly functional antigen-specific CD8+ T cells with the capacity to control, and even eradicate leukemia.
CRT modestly enhances the uptake of AML cells by splenic DCs in vivo
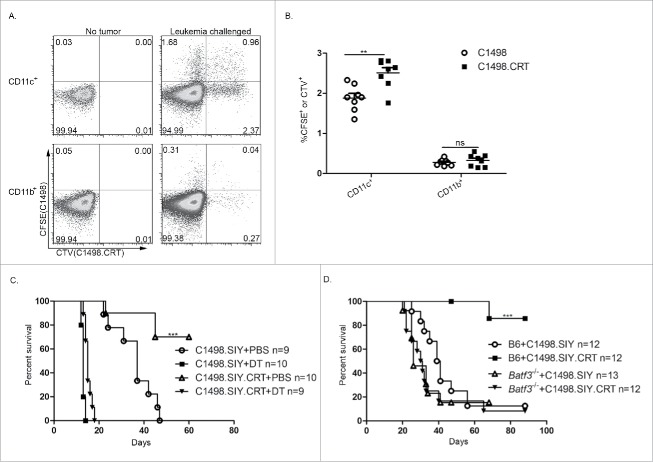
To determine whether cell surface CRT would enhance leukemia cell engulfment in vivo, C1498 and C1498.CRT cells were labeled with unique fluorescent dyes (CFSE and CTV, respectively) and were co-injected IV into mice in equal numbers. Three hours later, spleens of recipient animals were analyzed by flow cytometry for the frequency of fluorescence-containing CD11b+CD11c− and CD11c+ cells as a readout for phagocytosis. Very few CD11b+ cells from mice challenged with either C1498 or C1498.CRT cells contained a CFSE or CTV signal, suggesting that macrophages were not adept at AML cell uptake (Fig. 4A and B). However, a small population of CD11c+ cells was also CTV+ or CFSE+, indicating that splenic DCs engulf AML cells in vivo (Fig. 4A and B). Moreover, the frequency of CD11c+ cells that were CTV+ was significantly higher than for CFSE (Fig. 4A and B), demonstrating in a competitive setting that CRT modestly enhances DC-mediated phagocytosis of AML cells in vivo. To control for the possibility that the difference in observed uptake of cellular material from control and CRT-expressing AML cells was dependent on the particular fluorescent dye utilized, control C1498 cells were labeled either with CFSE or with CTV and a similar experiment was performed. Here, identical frequencies of CD11c+ cells contained CTV versus CFSE fluorescence (Fig. S4A and B), indicating that the enhanced uptake of C1498.CRT cells by CD11c+ cells was not an experimental artifact.
Figure 4.
DCs are required to mediate the CRT effect in vivo. (A, B) C1498 or C1984.CRT cells were labeled with CFSE or CTV, respectively, were mixed at a 1:1 ratio, and were co-inoculated IV into recipient C57BL/6 mice. Three hours later, spleens were harvested and CD11b+ or CD11c+ cells were analyzed by flow cytometry for CTV and CFSE fluorescence. (A) Representative flow plots are shown after gating on CD11c+ (top row) and CD11b+ cells (bottom row) from spleens of naive (left column) and leukemia-challenged (right column) mice. Numbers indicate the frequency of CD11c+ or CD11b+ cells that contained CFSE, CTV (or both) fluorescence. (B) Summary of data from (A); **p <0.001. (C) CD11cDTR/GFP bone marrow chimeric (BMC) mice were treated with DT or PBS as indicated, and received an IV challenge with C1498.SIY or C1498.SIY.CRT. Survival is shown. ***p <0.0001 for survival comparison of C1498.SIY.CRT challenged mice treated with DT versus PBS. (D) C1498.SIY or C1498.SIY.CRT cells were inoculated IV into groups of C57BL/6 or Batf3−/− mice, and survival was monitored. ***p <0.0001 for survival comparison of C57BL/6 and Batf3−/− mice challenged with C1498.SIY.CRT. (B, C) Data are pooled from two independent experiments each with 4–5 mice/group. (D) Data are pooled from three independent experiments each with 3–5 mice/group.
DCs are required for the CRT effect on survival in leukemia-bearing hosts
The role of DCs in regulating the effect CRT expression on AML survival was next examined. To ablate DCs from leukemia-bearing hosts, CD11cDTR/GFP BMC mice were generated. DT-mediated elimination of CD11c-expressing cells was associated with a rapid demise of mice harboring both control and CRT-expressing C1498.SIY leukemia (Fig. 4C), indicating that DCs are critical to control the progression of disseminated AML in general. The extended survival observed in mice with CRT-expressing leukemia was lost following CD11c+ cell depletion (Fig. 4C), revealing that the CRT-mediated effect on anti-leukemia immunity requires DCs.
Our laboratory has recently identified that splenic CD8α+ DCs, uniquely capable of engulfing and cross-presenting leukemia-derived antigens to CD8+ T cells in vivo, mediate a form of deletional T-cell tolerance in mice with AML (D. Kline, unpublished observation). To investigate the role of CD8α+ DCs in mediating the CRT effect, Batf3−/− mice, which lack CD8α+ DCs,18 were utilized. As shown in Fig. 4D, the survival benefit associated with a CRT-expressing AML cell challenge was completely abolished in the absence of CD8α+ DCs. Collectively, these data suggest that, although T cells are ultimately essential as effectors in leukemia cell killing, CRT likely acts upstream of adaptive immunity—possibly through CD8α+ DCs.
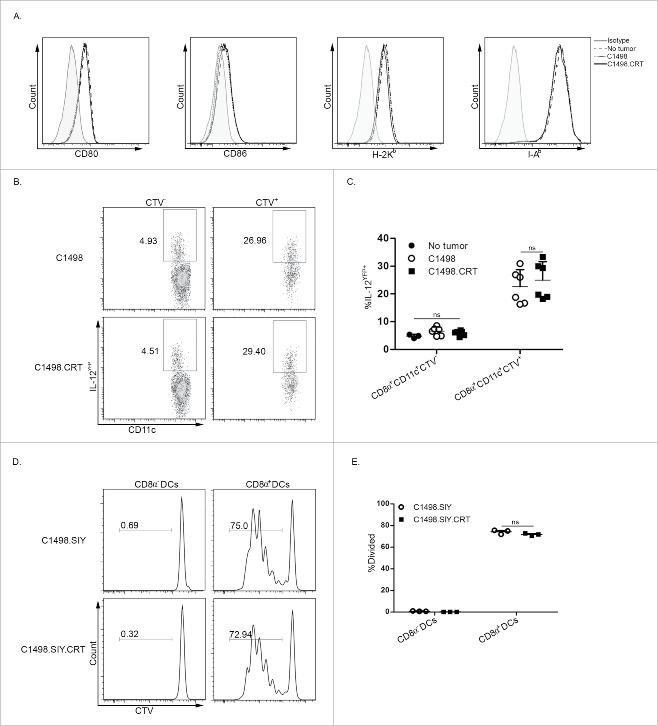
CRT does not induce classical measures of DC activation in vivo
The requirement for DCs, and specifically for CD8α+ DCs, to mediate the powerful CRT effect in vivo suggested that its expression on leukemia cells might directly activate DCs, enhancing their ability to cross-prime leukemia-specific CD8+ T cells. First, the phenotypes of DCs from leukemia-bearing mice were analyzed. Surprisingly, expression of co-stimulatory B7 and MHC class I and II molecules was identical among CD11c+ DCs that engulfed CRT-expressing or control C1498 AML cells in vivo (Fig. 5A). IL-12 production by CD8α+CD11c+ cells that had acquired AML cells in vivo was higher than those that had not (Fig. 5B and C), suggesting either that phagocytosis of AML cells induced IL-12 production by DCs, or that a subpopulation of IL-12-producing DCs was more efficient at AML cell uptake. Regardless, there was no difference between the frequencies of IL-12-producing CD8α+CD11c+ cells that had acquired C1498 versus C1498.CRT cells in vivo (Fig. 5B and C). Furthermore, purified CD8α+ DCs from C1498.SIY or C1498.SIY.CRT-bearing animals equivalently induced the proliferation of 2C T cells directly ex vivo (Fig. 5D and E), demonstrating that CRT expression on AML cells did not appear to enhance cross-presentation of leukemia cell-derived antigens by DCs to T cells. Taken together, these results indicate, at least at the time points chosen for analysis, that CRT does not appear to activate classical pathways associated with DC maturation.
Figure 5.
CRT does not affect expression of DC activation molecules or enhance ex vivo antigen cross-presentation. (A) Expression of CD80, CD86, H-2Kb and I-Ab on live splenic CD11c+ cells from naive C57BL/6 mice or those challenged 12 h earlier with C1498 or C1498.CRT cells IV. (B) C1498 or C1498.CRT cells were labeled with CTV and inoculated IV into IL-12YFP reporter mice. Six hours later, YFP expression was analyzed by flow cytometry in splenic CTV+ or CTV− CD8α+CD11c+ cells. Data are summarized in (C). (D, E) 4×106 C1498.SIY or C1498.SIY.CRT cells were inoculated IV into C57BL/6 mice, and 3 h later, splenic CD8α− or CD8α+ DCs were isolated by FACS and cultured with CTV-labeled CD8+ 2C T cells for 65–72 h. Subsequently, 2C T-cell division was monitored by flow cytometry. (D) Representative FACS plots depicting CTV dilution of 2C T cells after gating on CD8+1B2+ cells. (E) Summary of data from (D). (C) Data are pooled from two independent experiments each with 3 mice/group. (E) Data are representative of three independent experiments each with 3 mice/group.
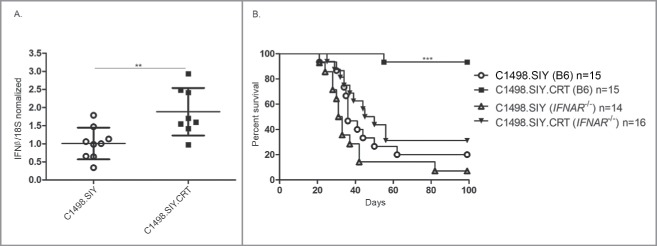
CRT activates the host type I IFN pathway
Type I IFNs are essential for bridging innate and adaptive immune responses against solid tumors.19,20 However, we recently observed that disseminated AML fails to induce a host type I IFN response,15 which may help to explain the dense T-cell tolerant state that exists in leukemia-bearing hosts. To determine whether CRT expression on AML cells would induce type I IFN transcription, levels of ifnb mRNA were measured in spleen cells from mice challenged with C1498.CRT or control C1498 AML. Interestingly, a 2-fold increase in ifnb mRNA expression was detected in spleens of mice as early as 24 h following IV challenge with C1498.CRT cells (Fig. 6A). Experiments were performed to identify the specific cell type(s) in which ifnb mRNA expression was induced in the spleen following exposure to CRT-expressing AML cells. Here, CD11b+ cells, but not CD11c+ or CD11b−CD11c− cells, were found to express increased ifnb mRNA following an IV C1498.CRT cell versus control C1498 cell challenge (data not shown). To test whether enhanced ifnb expression in mice harboring C1498.CRT AML cells correlated with improved outcome, wild-type and Ifnar−/− mice (defective in type I IFN signaling) were challenged with C1498.SIY or C1498.SIY.CRT cells, and survival was assessed. As previously demonstrated,15 the survival of wild-type and Ifnar−/− mice with control C1498.SIY cells was similar (Fig. 6B). Nearly all wild-type mice challenged with C1498.SIY.CRT cells survived long-term, as expected (Fig. 6B). Strikingly, the enhanced survival associated with CRT-expressing AML cells was nearly completely abrogated in Ifnar−/− mice (Fig. 6B). Collectively, these results reveal that CRT-expressing AML cells stimulate a type I IFN response in vivo, and that type I IFN signaling in host cells is essential for the CRT-mediated effect on survival. Thus, the type I IFN pathway is an important mechanism through which CRT promotes anti-leukemia immunity.
Figure 6.
Type I IFN signaling mediates prolonged survival in mice with CRT-expressing AML. (A) C1498.SIY or C1498.SIY.CRT cells were inoculated IV into C57BL/6 mice. Twenty-four hours later, ifnβ expression was analyzed by qRT-PCR in bulk spleen cells. Ifnβ expression levels were normalized to those measured in spleen cells of naive C57BL/6 mice. 18S was used as an internal control; **p <0.01. Data are pooled from three independent experiments each with 2–3 mice/group. (B) C1498.SIY or C1498.SIY.CRT cells were inoculated IV into groups of C57BL/6 or Ifnar−/− mice, and survival was monitored. Data are pooled from three experiments each with 4–6 mice/group. ***p <0.01 for comparison of C1498.SIY.CRT challenged C57BL/6 versus Ifnar−/− mice.
Discussion
“Danger signals” released by dying tumor cells, including tumor-derived nucleic acids and their byproducts, facilitate innate immune sensing of cancer.21 CRT translocation is also an important mechanism through which stressed or dying cancer cells place the immune system on alert,2 possibly through its ability to promote malignant cell phagocytosis.2,5 The role of CRT in activating antitumor immunity has been demonstrated in multiple murine tumor models,1,2 and in human studies including acute myeloid leukemia, non-Hodgkin lymphoma, bladder cancer, ovarian cancer and non-small cell lung cancer, where CRT exposure correlates with favorable clinical outcomes.7,10,11,14 Within tumor microenvironment, hyperploid cancer cells undergoing constitutive ER stress were shown to trigger the CRT translocation to promote early cancer immunoserveillance.22 Long non-coding RNA expressed from tumor suppressor retinoblastoma bidirectional promoter were reported as one mechanism that regulated CRT cell surface translocation in response to chemotherapy.23 However, the precise mechanism through which CRT stimulates antitumor immunity remains unknown, and the impact of CRT translocation on tumor immune responses has been described only after exposure of cancer cells to chemotherapy or radiation,1,2 which could result in CRT-independent effects. To circumvent this problem, AML cells exhibiting stable cell surface CRT expression were generated to directly examine its effect on anti-leukemia immunity.
Our results reveal that CRT expression on leukemia cells leads to potent immune-mediated control, and in some cases, eradication of disseminated AML. This observation is particularly interesting in light of our previous studies, which have revealed that a dense T-cell tolerant state is present in mice with AML.16 Furthermore, the finding that CRT expression on AML cells enhanced functional leukemia-specific CD8+ T-cell responses, and generated effective immunological memory, indicated that adaptive immunity was crucial for its effect. However, the observation that DCs were also required to mediate enhanced survival in mice with CRT-expressing AML argued against a direct effect of CRT on T cells. The data presented in Fig. 3 reveal that a major effect of CRT is to promote the activation of leukemia-specific CD8+ T cells. Experiments in which CD8+ or CD4+ T cells are separately depleted could also be performed to examine whether one or both populations are required to mediate anti-leukemia immunity downstream of CRT.
It has been demonstrated that CRT translocation on malignant cells promotes their phagocytosis by macrophages and DCs.2,5 This observation has led to the hypothesis that CRT-induced efferocytosis, particularly by DCs, augments cross-presentation of tumor-derived antigens to T cells, which ultimately drives the protective immunity that ensues. This model relies on the theory that tumor antigen cross-presentation by DCs is a limiting factor in generating functional antitumor T-cell responses. However, the activation status of DCs is also critical in their ability to either prime or tolerize antigen-specific T cells. For example, targeting antigen for cross-presentation by DCs leads to deletional T-cell tolerance, unless an activating signal is also provided.8 Thus, in addition to promoting engulfment of cancer cells, we hypothesized that CRT might also directly activate DCs, licensing them to stimulate superior antitumor T-cell responses.
In agreement with published studies, CRT expression on AML cells did result in their enhanced uptake by splenic DCs in vivo, although the effect was not striking (Fig. 4A and B). In our opinion, this finding did not likely explain the marked differences in functional CD8+ T-cell responses generated in mice with CRT-expressing versus control AML (Fig. 3A, F and G). We therefore examined the phenotypes of DCs that had been exposed or not to CRT-expressing leukemia cells in vivo, and found no differences in expression of classical maturation markers or production of IL-12. On the surface, these results seemed to argue against a hypothesis supporting direct activation of antigen-presenting cells by CRT, until it was observed that splenocytes (and CD11b+ cells specifically) from mice with CRT-expressing AML expressed significantly higher levels of ifnb mRNA, which is interesting in lieu of our recent finding that disseminated AML fails to induce a host type I IFN response.15
A clear role for type I IFN in mediating the CRT effect was demonstrated in an experiment in which CRT-expressing or control AML cells were inoculated into wild-type or Ifnar−/− mice. As previously demonstrated,15 host type I IFN signaling played no role in regulating survival of mice with control AML. Strikingly, however, the survival benefit associated with CRT expression on AML cells was almost completely abolished in Ifnar−/− hosts. Together, these observations support a model in which CRT stimulates a host type I IFN response that may act through CD8α+ DCs to promote their ability to cross-prime leukemia-specific CD8+ T-cell responses. Although speculative, our result demonstrating that the CRT effect on survival was completely eliminated in Batf3−/− mice strongly supports the conclusion that this DC subset is the target of type I IFN in vivo. Others have shown that type I IFN produced in solid tumor-bearing hosts acts directly on CD8α+ DCs, licensing them to prime antitumor CD8+ T-cell responses.19
Immunotherapeutic approaches aimed at enhancing CRT translocation with chemotherapeutic agents or radiation have been difficult to develop because these modalities can concomitantly induce apoptosis of the immune cells necessary to mediate the CRT effect in vivo. However, the observation that CRT stimulates type I IFN suggests that activating this pathway, for example, with stimulator of interferon genes (STING) agonists,15,24 may be an efficacious alternative immunotherapeutic strategy. Regardless, the identification of type I IFN as a mechanism through which CRT translocation stimulates host antitumor immunity represents an important step forward in our understanding of how cancers are sensed by the host immune system.
Material and methods
Mice
Mice were maintained in a specific pathogen-free environment. Animal experimentation was carried out under a protocol approved by an Institutional Animal Use and Care Committee. C57BL/6 mice (H-2b; CD45.2/.2) were purchased from Taconic. Rag2−/− and 2C TCR transgenic mice were bred in our facility. TCRβ/δ−/− mice were provided by M. Alegre (University of Chicago). CD11cDTR/GFP B6.FVB-Tg (Itgax-DTR/EGFP) mice and Ifnar−/− mice were provided by X.Y. Fu (University of Texas Southwestern). YET40 B6.129-Il12btm1Lky/J mice, B6.SJL-Ptprca Pepcb/BoyJ mice (CD45.1/.1) and Batf3−/− mice were purchased from Jackson Labs and bred in our facility.
Cell lines
The C1498 AML cell line (H-2b)25 was purchased from ATCC. C1498 cells expressing the Kb-restricted model SIY (SIYRYYGL) peptide antigen were previously generated to facilitate monitoring of endogenous antigen-specific CD8+ T-cell responses in leukemia-bearing animals.26 SIY is also the cognate antigen for the 2C transgenic TCR. C1498 and C1498.SIY cells were transduced by the retroviral pRetroX-IRES-DsRed Express vector containing full-length murine CRT cDNA in-frame with the cDNA of the 5′ end of decay accelerating factor (DAF), which encodes a signal sequence for attachment of a glycophosphatidylinositol (GPI) anchor to the C-terminus of the resulting CRT-DAF fusion protein to facilitate CRT anchoring in the plasma membrane (C1498.CRT and C1498.SIY.CRT). The CRT-GPI construct contains an IRES-DSRED; an empty IRES-DSRED construct was also transduced to generate control C1498 and C1498.SIY cells. All C1498 derivative cell lines were cultured in DMEM (Invitrogen) supplemented with 10% FBS, 2-mercaptoethanol, essential amino acids, penicillin and streptomycin.
C1498 AML model
To induce systemic AML, 106 C1498 cells were injected IV through the lateral tail vein. For in vivo CD4+ and CD8+ T-cell depletion, anti-CD4 (L3T4) and anti-CD8α (2.43) antibodies (BioXCell) were administered intra-peritoneal (IP) at a dose of 200 μg on day −1, and 100 μg on day 0, and every 3 d thereafter until day 14. To induce localized tumor growth, 106 C1498 cells were inoculated subcutaneously (SC) in the lower lateral abdominal wall. Tumor growth was monitored 2–3 times per week with calipers, and mean tumor diameter was recorded.
Flow cytometry
Following red blood cell lysis and blockade of Fc receptors with anti-CD16/32 antibodies, spleen cells were stained with the following directly conjugated antibodies: CD11b (M1/70), CD11c (HL3), CD86 (GL1), I-A/I-E (M5/114.15.2) and H-2Kb (AF6-88.5) (BD Biosciences); CD8α (53-6.7), CD80 (16-10A) and CD19 (eBio1D3) (eBiosciences); CD45.2 (104) CD45.1 (A20), and CD3ε (145-2C11) (BioLegend). Rabbit anti-calreticulin monoclonal antibody and fluorescence-labeled goat-anti-rabbit IgG polyclonal secondary antibody were purchased from Abcam. Fixable viability dyes (Invitrogen) were used to exclude dead cells. Flow cytometry was performed on LSRII or LSRFortessa cytometers (BD Biosciences). Analysis was performed using FlowJo software (Treestar). Fluorescence-activated cell sorting (FACS) was performed using a FACSAria (BD Biosciences).
For DC isolation, spleens were injected with 1 mg/mL collagenase IV (Sigma), 20 µg/mL DNAse I (Roche) were incubated at 37 °C for 15–20 min, and passed through a 70-µm filter to generate single cell suspensions. Cells were stained with CD3ε (145-2C11) and CD19 (eBio1D3) biotinylated antibodies, followed by secondary streptavidin staining to eliminate T and B cells.
IFNγ ELISPOT
At the indicated time points, 106 spleen cells from individual leukemia-bearing animals were re-stimulated with media alone or with SIY peptide (100 nM) in triplicate overnight in 96-well, flat-bottom plates using the mouse IFNγ ELISPOT kit (BD Biosciences).16 ELISPOT plates were read with an ImmunoSpot Series 3 Analyzer. Data were analyzed with ImmunoSpot software (Cellular Technology, Ltd.).
Adoptive transfer of 2C T cells into leukemia-bearing mice
2C CD8+ T cells were isolated with a mouse CD8 microbead kit (Miltenyi), labeled with CellTrace Violet (CTV) (Invitrogen), and 106 were injected IV. Twenty-four hours later, mice were challenged IV with 106 C1498.SIY or C1498.SIY.CRT cells. Six days later, spleen cells of recipient mice were stained with anti-CD8α and anti-1B2 antibodies (the 1B2 antibody specifically binds the 2C TCR). Flow cytometry was performed to assess the frequency and dilution of the CTV signal in 2C T cells (as a read-out of in vivo 2C T-cell proliferation).
In vivo cytolysis assay
4×106 2C T cells were transferred into C57BL/6 mice (CD45.2/.2) on day −1. On day 0, half of the mice were challenged with C1498.SIY or C1498.SIY.CRT cells IV. Six days later, spleen cells isolated from naive B6.SJL (CD45.1/.1) mice were separately labeled with different concentrations of CTV (2.5 µM, 50 nM), and were pulsed with SIYRYYGL or SIINFEKL (irrelevant control) peptides, respectively. CTV-labeled, peptide-pulsed cells were mixed 1:1, and a total of 8×106 were injected IV into leukemia-bearing C57BL/6 mice. Six hours later, spleen cells were stained with an anti-CD45.1 antibody, and analyzed by flow cytometry. After gating on CD45.1+ cells, the ratio of CTVhi to CTVlo cells present was calculated using the following equations:
In vivo phagocytosis and cross-presentation assays
C1498 or C1498.CRT cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) or CTV, respectively, were mixed at a 1:1 ratio, and 8×106 cells were injected IV into C57BL/6 mice. Three hours later, spleen cells were stained with antibodies against CD11c, CD11b, CD8α, CD3ε and CD19. The frequency of CD11c+ and CD11b+CD11c− cells that contained either a CFSE or CTV signal was analyzed by flow cytometry. For antigen cross-presentation assays, C1498.SIY or C1498.SIY.CRT cells (4×106) were inoculated IV into C57BL/6 mice. Splenic CD8α+ and CD8α− DCs from these animals were isolated by FACS. Sorted DC populations were cultured 1:1 with purified CTV-labeled 2C T cells for 65–72 h, followed by analysis of CTV dilution by 2C T cells.
Generation of bone marrow chimeric (BMC) mice
C57BL/6 mice were lethally irradiated (900 rads) and reconstituted 1 d later with 2.5×106 bone marrow cells from CD11cDTR/GFP mice. Eight weeks later, mice were utilized experimentally. To deplete CD11c+ cells from leukemia-bearing CD11cDTR/GFP BMC mice, diphtheria toxin (DT) (500 ng) was administered IP 1 d prior to C1498 cell inoculation and continued every 48 h until day 11.
Quantitative real-time PCR analysis
C57BL/6 mice were challenged with 5×106 C1498.SIY or C1498.SIY.CRT cells IV. Twenty-four hours later, spleen cells from recipient mice were re-suspended in Trizol (Life Technologies). Total RNA was isolated via chloroform extraction. cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Ifnb expression was measured by real-time quantitative PCR (RT-qPCR) using specific primers/probes, as previously reported.24
Statistical analysis
Grouped data were analyzed via two-way ANOVA with Bonferroni post-tests. Survival differences were analyzed with the Log-rank test. Statistics were performed using GraphPrism software. Data are presented as mean ± SD unless otherwise indicated. A p value of < 0.05 was considered statistically significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by R01 CA166770 (J.K.) and Wendy Will Case Foundation (J.K.). D.E.K. is funded by the immunology training grant at the University of Chicago (T32 AI007090).
Author contributions
X.C. designed and executed experiments, analyzed data and drafted and reviewed the manuscript. D.F. designed and executed experiments, analyzed data and reviewed the manuscript. D.E.K. designed experiments and reviewed the manuscript. J.K. designed experiments, analyzed data, drafted and reviewed the manuscript.
References
- 1.Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, Zitvogel L, Kroemer G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ 2007; 14:1848-50; PMID:17657249; http://dx.doi.org/ 10.1038/sj.cdd.4402201 [DOI] [PubMed] [Google Scholar]
- 2.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N et al.. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13:54-61; PMID:17187072; http://dx.doi.org/ 10.1038/nm1523 [DOI] [PubMed] [Google Scholar]
- 3.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res 2010; 16:3100-4; PMID:20421432; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-2891 [DOI] [PubMed] [Google Scholar]
- 4.Gao B, Adhikari R, Howarth M, Nakamura K, Gold MC, Hill AB, Knee R, Michalak M, Elliott T. Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity 2002; 16:99-109; PMID:11825569; http://dx.doi.org/ 10.1016/S1074-7613(01)00260-6 [DOI] [PubMed] [Google Scholar]
- 5.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005; 123:321-34; PMID:16239148; http://dx.doi.org/ 10.1016/j.cell.2005.08.032 [DOI] [PubMed] [Google Scholar]
- 6.Orr AW, Pedraza CE, Pallero MA, Elzie CA, Goicoechea S, Strickland DK, Murphy-Ullrich JE. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J Cell Biol 2003; 161:1179-89; PMID:12821648; http://dx.doi.org/ 10.1083/jcb.200302069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Volkmer J, Weiskopf K, Willingham SB, Raveh T et al.. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med 2010; 2:63ra94; http://dx.doi.org/ 10.1126/scitranslmed.3001375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med 2004; 199:815-24; PMID:15024047; http://dx.doi.org/ 10.1084/jem.20032220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawaria S, Binder RJ. CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat Commun 2011; 2:521; PMID:22045000; http://dx.doi.org/ 10.1038/ncomms1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoll G, Iribarren K, Michels J, Leary A, Zitvogel L, Cremer I, Kroemer G. Calreticulin expression: interaction with the immune infiltrate and impact on survival in patients with ovarian and non-small cell lung cancer. Oncoimmunology 2016; 5:e1177692; PMID:27622029; http://dx.doi.org/ 10.1080/2162402X.2016.1177692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fucikova J, Becht E, Iribarren K, Goc J, Remark R, Damotte D, Alifano M, Devi P, Biton J, Germain C et al.. Calreticulin expression in human non-small cell lung cancers correlates with increased accumulation of antitumor immune cells and favorable prognosis. Cancer Res 2016; 76:1746-56; PMID:26842877; http://dx.doi.org/ 10.1158/0008-5472.CAN-15-1142 [DOI] [PubMed] [Google Scholar]
- 12.Matsukuma S, Yoshimura K, Ueno T, Oga A, Inoue M, Watanabe Y, Kuramasu A, Fuse M, Tsunedomi R, Nagaoka S et al.. Calreticulin is highly expressed in pancreatic cancer stem-like cells. Cancer Sci 2016; 107:1599-609; PMID:27561105; http://dx.doi.org/ 10.1111/cas.13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wemeau M, Kepp O, Tesniere A, Panaretakis T, Flament C, De Botton S, Zitvogel L, Kroemer G, Chaput N. Calreticulin exposure on malignant blasts predicts a cellular anticancer immune response in patients with acute myeloid leukemia. Cell Death Dis 2010; 1:e104; PMID:21368877; http://dx.doi.org/ 10.1038/cddis.2010.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fucikova J, Truxova I, Hensler M, Becht E, Kasikova L, Moserova I et al.. Calreticulin exposure by malignant blasts correlates with robust anticancer immunity and improved clinical outcome in AML patients. Blood 2016; 128:3113-24; PMID:27802968; http://dx.doi.org/ 10.1182/blood-2016-08-731737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran E, Chen X, Corrales L, Kline DE, Dubensky TW Jr., Duttagupta P, Kortylewski M, Kline J. STING pathway activation stimulates potent immunity against acute myeloid leukemia. Cell Rep 2016; 15:2357-66; PMID:27264175; http://dx.doi.org/ 10.1016/j.celrep.2016.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Chen X, Liu X, Kline DE, Teague RM, Gajewski TF, Kline J. CD40 ligation reverses T cell tolerance in acute myeloid leukemia. J Clin Invest 2013; 123:1999-2010; PMID:23619361; http://dx.doi.org/ 10.1172/JCI63980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Low MG, Saltiel AR. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science 1988; 239:268-75; PMID:3276003; http://dx.doi.org/ 10.1126/science.3276003 [DOI] [PubMed] [Google Scholar]
- 18.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS et al.. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 2008; 322:1097-100; PMID:19008445; http://dx.doi.org/ 10.1126/science.1164206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med 2011; 208:2005-16; PMID:21930765; http://dx.doi.org/ 10.1084/jem.20101159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U et al.. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med 2011; 208:1989-2003; PMID:21930769; http://dx.doi.org/ 10.1084/jem.20101158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA et al.. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014; 41:830-42; PMID:25517615; http://dx.doi.org/ 10.1016/j.immuni.2014.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, Galluzzi L, Adjemian S, Kepp O, Niso-Santano M et al.. An immunosurveillance mechanism controls cancer cell ploidy. Science 2012; 337:1678-84; PMID:23019653; http://dx.doi.org/ 10.1126/science.1224922 [DOI] [PubMed] [Google Scholar]
- 23.Musahl AS, Huang X, Rusakiewicz S, Ntini E, Marsico A, Kroemer G, Kepp O, Ørom UA. A long non-coding RNA links calreticulin-mediated immunogenic cell removal to RB1 transcription. Oncogene 2015; 34:5046-54; PMID:25579178; http://dx.doi.org/ 10.1038/onc.2014.424 [DOI] [PubMed] [Google Scholar]
- 24.Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ et al.. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep 2015; 11:1018-30; PMID:25959818; http://dx.doi.org/ 10.1016/j.celrep.2015.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldie H, Butler CH, Anderson MM, Maxwell MC, Hahn PF. Growth characteristics of free C1498 granulocytic leukemia tumor cells in the peritoneal fluid and the blood of C57 mice. Cancer Res 1953; 13:125-9; PMID:13042796 [PubMed] [Google Scholar]
- 26.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 2009; 114:1545-52; PMID:19417208; http://dx.doi.org/ 10.1182/blood-2009-03-206672 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.