Abstract
Detection of circulating tumor DNAs (ctDNAs) in cancer patients is an important component of cancer precision medicine ctDNAs. Compared to the traditional physical and biochemical methods, blood-based ctDNA detection offers a non-invasive and easily accessible way for cancer diagnosis, prognostic determination, and guidance for treatment. While studies on this topic are currently underway, clinical translation of ctDNA detection in various types of cancers has been attracting much attention, due to the great potential of ctDNA as blood-based biomarkers for early diagnosis and treatment of cancers. ctDNAs are detected and tracked primarily based on tumor-related genetic and epigenetic alterations. In this article, we reviewed the available studies on ctDNA detection and described the representative methods. We also discussed the current understanding of ctDNAs in cancer patients and their availability as potential biomarkers for clinical purposes. Considering the progress made and challenges involved in accurate detection of specific cell-free nucleic acids, ctDNAs hold promise to serve as biomarkers for cancer patients, and further validation is needed prior to their broad clinical use.
Keywords: Precision medicine, Liquid biopsy, Circulating tumor DNA, Biomarker, Clinical diagnosis, Cell-free nucleic acids
Introduction
Over 8.2 million people die of cancer each year due to the inaccessibility of appropriate detection procedures and treatments [1]. Researchers have been exploring methods for the detection and cure of cancers via cancer screening, prognostic determination, and monitoring. However, up till now, there are no known diagnostic methods that do not hurt the physical health of patients during the process of cancer detection. For example, radiology is extensively used in cancer detection, but excessive ionizing radiation could pose potential health risk to the examined patient [2], [3]. On the other hand, non-radiation modalities, such as ultrasound scans and magnetic resonance imaging (MRI) scans, are thought to be inefficient for the detection of minimal residual disease [4], [5], [6]. Furthermore, the “solid biopsy” method of detection is invasive, and cannot accurately track dynamic changes in tumors due to tumor heterogeneity [7], [8], [9]. Thus, developing non-invasive and precise methods for the early diagnoses of cancers is an increasingly urgent requirement in the era of precision medicine (PM).
Liquid biopsy is a type of technique for sampling and analyzing of non-solid biological tissues, mainly used in disease diagnosis [10]. Circulating tumor DNAs (ctDNAs), being a popular class of liquid biopsy biomarkers, are believed to be easily detected in the plasma of cancer patients even in the early stages of their disease [11], [12], [13]. ctDNAs display considerable variations in DNA sequences. Moreover, tumor-specific DNA methylations can also be consistently measured and reflected within ctDNAs, showing the potential for wide application in clinical detection of cancers [14], [15], [16], [17], [18]. To provide an overview of current utilities of clinical therapy and potential biomarkers, we summarized the methods of detection that are frequently used nowadays, such as imaging-based methods [19], [20], [21] and solid biopsies [22], [23], [24], [25] in Table 1. Biomarkers that are currently in use or under investigation in liquid biopsies are also shown in the table, including proteins [26], [27], [28], circulating tumor cells (CTCs) [7], [11], [29], ctDNAs [10], [30], [31], circulating cell-free RNAs [32], [33], [34], and exosomes [35], [36], [37]. In this article, we mainly discuss several new methods for using ctDNAs and ctDNA methylations in the early detection of cancers. The challenges and potential applications of ctDNA detection are also discussed in this review.
Table 1.
Comparison of different cancer detection methods for their clinical utilities
| Detection method | Strengths | Limitations | Refs. | |
|---|---|---|---|---|
| Imaging-based methods (CT, MRI, PET, etc.) | Rapid; easy to use; displaying solid tumor visually | Unable to detect minimal residual disease; exposing patients to additional ionizing radiation | [19], [20], [21] | |
| Solid biopsy | Reflecting certain histological issues; short operating time | Unable to represent the entre tumor due to the intra- and inter-tumor heterogeneity; serial biopsy often impractical; discomfort suffered by the patient; not accessible for some tumors | [22], [23], [24], [25] | |
| Liquid biopsy |
Protein (CA-125, CEA, PSA, etc.) | Non-invasive; easy to obtain | Low specificity; Unable to be detected in vast majority of patients with advanced cancers | [26], [27], [28] |
| CTCs | Non-invasive; high specificity; demonstrating colocalization of signals; evaluating protein expression; potentially addressing tumor heterogeneity | Low signal-to-noise; affected by heterogeneity on selection methods | [7], [11], [29] | |
| ctDNA | Non-invasive; high specificity and sensitivity; providing personalized snapshot of disease; fully representing tumors | Low signal-to-noise; lack of colocalization, protein expression, and functional studies | [10], [30], [31] | |
| Circulating cfRNA | Non-invasive; stable; demonstrating distinct gene expression patterns from particular tumor | Lack of large-scale studies; lack of correlations between tumor behavior and findings | [32], [33], [34] | |
| Exosomes | Non-invasive; stable within exosomes; easy to isolate or enrich | Lack of large-scale studies; hard to define | [35], [36], [37] | |
Note: CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; CA-125, carcinoma antigen-125; CEA, carcinoembryonic antigen; PSA, prostate-specific antigen; CTC, circulating tumor cell; ctDNA, circulating tumor DNA; cfRNA, cell-free RNA.
Profiles of ctDNAs and CTCs
On January 9, 2016, the Chinese Academy of Sciences (CAS) announced its precision medicine initiative (CASPMI). This initiative aims to establish a new medical paradigm characterized by high-efficiency and low-cost disease diagnoses and treatments of individual patients, based on their genetic and epigenetic composition. In this program, some studies would focus on the risks of occurrence of cancers and other major chronic diseases for early warning signs and interventions. Performing liquid biopsies, specifically by capturing CTCs and ctDNAs in the plasma or serum of cancer patients, is an ideal strategy for clinical utility in the PM research programs [38], [39].
For around 1000 years, biopsies have been used clinically for the diagnoses, management, and planning the treatments of diseases [10]. Given the many obstacles in sequentially obtaining repeated biopsies, including the inconvenience to the patients, the potential surgical complications, and the clinical risks, clinical use of multi-site biopsies is often impractical [22], [23], [24], [25]. As an alternate, liquid biopsies are currently being used to address the temporal and spatial heterogeneity in solid tumors. Liquid biopsies could even be used in cancer detection, thus facilitating early diagnoses and treatments [10], [40], [41], [42].
CTCs are shed into the bloodstream by primary tumors during early tumorigenesis [43]. They can be purified from blood, and separated from normal blood cells by the differences in their physicochemical characteristics [43]. Ashworth demonstrated the presence of CTCs in 1869 [44], but their value was overlooked until the 1990s [29]. The CTCs have immense potential for cancer detection and management of advanced disease, as reported in cases of breast cancers, prostate cancers, and colorectal cancers [45], [46], [47]. However, it is difficult to identify and isolate CTCs since they are present in circulation at the rate of only one CTC per 1 × 109 normal blood cells in patients with metastatic cancers [48]. Many new approaches have lately been designed to select and capture CTCs due to the recent technological advances. The semi-automated CellSearch (Veridex) system is the most commonly used selection technique for CTC detection. It enriches cells that express epithelial-cell adhesion molecule (EpCAM) but lack expression of the leukocyte-specific molecule, cluster of differentiation 45 (CD45) [49]. Several microfluidic devices have been developed for CTC capture, including the CTC-chip (based on microfluidic and chip technology), micro-Hall detector, and CTC-iChip (an inertial focusing-enhanced microfluidic CTC capture platform) [50], [51], [52].
The CTC-based liquid biopsy assays have high specificities. In addition, they also display low signal-to-noise ratios, particularly in the detection of early-stage disease. Compared to CTC detection, the ctDNA assay can provide personalized disease detection, and is disease- and treatment-specific for individual patients. CtDNA can provide a personalized snapshot of the patient’s disease status. Additionally, ctDNA is likely to exhibit increased sensitivity for early detection of cancers. Unlike CTC capture, ctDNA enrichment does not depend on the use of special equipment [7].
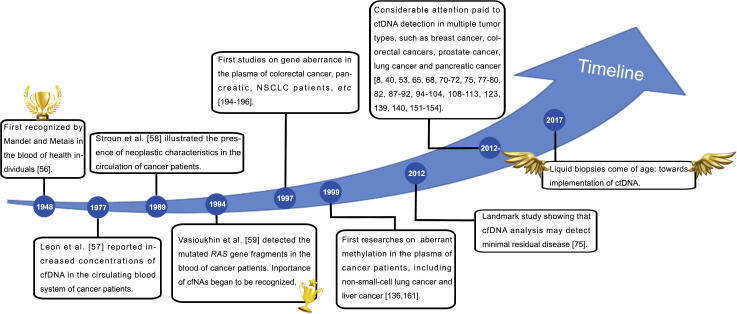
In solid tumors, the detection of ctDNAs is non-invasive and reproducible [30], [43], [53], [54], [55]. Plasma ctDNA was first recognized more than 60 years ago [56], representing the first step toward the development of liquid biopsies. In 1977, Leon et al. [57] detected increased concentrations of cell-free DNAs (cfDNAs) in the circulating blood of patients with lymphomas and tumors of the lungs, ovaries, uterus, and cervix using radioimmunoassay. In 1989, Stroun et al. [58] demonstrated that one-third of patients with various malignancies displayed an abundance of cfDNAs, whereas no cfDNAs could be detected in normal controls. Furthermore, it took another five years for the importance of cell-free nuclei acid (cfNA) to be recognized. Vasioukhin et al. [59] detected mutated RAS gene fragments in the blood of patients with myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML). In subsequent years, epigenetic aberrations in cfDNAs were identified. In 2005, Fujiwara et al. [60] demonstrated the presence of aberrant methylations on the promoters of five tumor-suppressor genes in the serum DNAs of patients with lung cancers. Within the past decade, large cohorts of studies have focused on the detection of ctDNAs in multiple types of tumors, such as cancers of the breast, colorectal region, prostate, lungs, and pancreas [61], [62], [63] (Figure 1). The details of the progress achieved by these studies would be discussed further in a later section.
Figure 1.
Landmarks in the detection of ctDNAs in patients with different cancers
This timeline shows the development of ctDNA detection of genetic and epigenetic alterations. Since the first validation of ctDNAs in 1948 [56], increasing interest has been attracted due to its ability for detection and broad clinical applicability. In 1977, Leon et al. [57] found increased concentrations of cfDNAs circulating in cancer patients. Ten years later, Stroun et al. [58] illustrated the presence of neoplastic characteristics in the circulation of cancer patients. The importance of cfNAs began to be recognized around the year 1994 [59]. At the time, the first studies on aberrant genetic alterations [194], [195], [196] and methylations [136], [161] were of high interest to the public. In 2012, a landmark study by Shaw et al. [75] showed that analyses of cfDNAs may help to detect minimal residual disease. cfDNA, cell-free DNA; cfNA, cell-free nucleic acid; ctDNA, circulating tumor DNA; NSCLC, non-small-cell lung cancer.
In cancer patients, ctDNAs represent a variable fraction of cfDNAs (ranging from 0.01% to more than 50%) [64]. Several studies have hypothesized that ctDNAs are produced via the release of nucleic acids during the apoptosis or necrosis of cancer cells or from tumor-derived exosomes [30]. In 2015, Sun et al. found that most cfDNAs in healthy people originate from the bone marrow [65]. The average length of cfDNAs in the blood of healthy people is 70–200 bp with concentrations ranging 0–100 ng/ml. However, ctDNAs from patients with malignant tumors have lengths ranging from 200 to more than 1 kb [30], [66]. The half-lives of ctDNAs range from 15 min to a few hours, and ctDNAs are removed by the liver and kidney [67].
Novel, non-invasive applications of liquid biopsies are transforming cancer research by enabling the accurate and reliable detections of ctDNAs in plasma and urine [12]. The percentage of detectable ctDNAs depends on distinct stages (49%–78% in localized tumors and 86–100% in metastatic tumors) [68]. The sensitivity of ctDNAs can enable detections of quantitative mutations in blood plasma [30], [68]. These ctDNAs are important emerging biomarkers in cancer diagnostics, and provide non-invasive diagnostic tools for identifying cancer relapses by detecting the dynamic qualitative and quantitative changes in ctDNAs in different stages of cancer.
Plasma ctDNA in the clinic
Monitoring of DNA mutations and epigenetic alterations presents two avenues to the detection and tracking of ctDNAs. Specific genetic variations in cancer cells can reflect the physical conditions and treatment responses of patients. Detecting DNAs with tumor-specific mutations in the peripheral blood of patients with malignancies may help to identify dynamic changes in cancer cells [69]. The ctDNA content varies in different tumor types and stages, and the mutation profiles for individual tumors may vary between patients [30], [70], [71], [72]. Unlike genetic alterations, methylation of ctDNAs is very consistent in cancer patients [14]. The aberrant methylations of ctDNAs have been described and investigated for clinical applications in most cancer types. The compositions of ctDNAs can be distinguished by analyzing the previously established methylation patterns. Under dissimilar conditions, the comparison of clinical sensitivities of detection across different studies presents enormous challenges. These conditions include the variabilities in methods of detection, number and types of targeted molecular alterations, tumor types and stages, and preselections of patients [73]. The applications of cfDNA assessments for the early diagnoses of cancers have been described extensively in previous reviews [10], [11], [12], [30]. We have illustrated previous literature on this topic published up to 2016, with emphasis on reports published between 2011 and 2016.
Breast cancer
Breast cancer is the leading cause of cancer-related deaths in women worldwide [74]. Over the past few years, an increasing number of researchers have attempted to utilize the blood-borne biomarkers of breast cancers for the early diagnoses and precise staging of tumors, and the monitoring of treatments in patients.
In the earlier half of 2016, Shaw et al. [75] directly compared the mutational profiles of CTCs and cfDNAs from the same patients with metastatic breast cancers (MBCs). From among 112 patients, they identified five patients with more than 100 CTCs and compared these CTCs with matched cfDNAs. Unlike the levels of carbohydrate antinegen 15-3 (CA15-3) and alkaline phosphatase (ALP), total cfDNA levels and cell counts were both significantly associated with overall survivals, suggesting that cfDNAs might reflect the persisting EpCAM-positive CTCs in patients with high CTC counts. This was not the first study that quantitatively compared ctDNAs and CTCs in the circulation of individual patients. In a larger scale study comprising 640 patients that was published in 2014, Bettegowda et al. [68] demonstrated that ctDNAs were detectable in more than 75% of patients with advanced diseases, including breast cancers, and in 50% of patients with localized tumors. However, there were no cases wherein ctDNAs were absent but CTCs were detected. In contrast, in many cases wherein ctDNAs were detected (13 of 16 cases; 81.25%), no CTCs were detectable with the identical assay. Interestingly, five of these cases were of patients with breast cancers, indicating that ctDNA detection is likely to be more sensitive than CTC detection in breast cancer.
Traditional detection methods, such as immunohistochemistry and fluorescence in situ hybridization (FISH), have limited capability for assessments of breast cancer especially the HER2 status [76]. Therefore, more effective methods for evaluating cancer status need to be devised. In 2012, Higgins et al. [77] screened for PIK3CA mutations in serum samples by beads, emulsification, amplification and magnetics (BEAMing) and reported sensitivities of 100% (14 of 14 patients). In 2013, Dawson et al. [53] described that ctDNAs are informative, inherently specific, and highly sensitive biomarkers for MBCs. Using microfluidic digital PCR and direct plasma sequencing, they were able to detect ctDNAs and CTCs in 29 (97%) and 26 (87%) of 30 women, respectively. Moreover, there existed stronger correlations between levels of ctDNAs and changes in tumor burdens than CTCs. In the meantime, Gevensleben et al. [78] detected the amplification of HER2 in ctDNAs of patients with MBCs by using digital PCRs. Seven of 11 (64%) patients with HER2-amplified cancers were classified as plasma digital PCR HER2-positive. In 2014, Beaver et al. [79] demonstrated that pre-surgical ctDNAs with PIK3CA mutations from breast cancer patients showed sensitivities of 93.3% and specificities of 100% by droplet digital PCRs (ddPCRs). Also using ddPCR method, Chu et al. showed that ctDNAs from patients with MBCs displayed high frequencies of ESR1 that encodes estrogen receptor 1. In 6 of 12 patients (50%), and seven mutations in the ctDNA of ESR1 were detected [80]. Several other studies also showed that the detection of ctDNAs is a surrogate procedure for the traditional biopsies for breast cancer detection [81], [82], [83], [84].
Aberrant ctDNA methylation offers a more consistent and broadly applicable marker of tumor DNA in serum in comparison to DNA mutations. It has been shown that methylated RARB2 was reduced in blood of patients following surgical removal of the tumor [85]. Additional studies also reported that methylated RASSF1 in cancer patient can serve as an indicator of response to tamoxifen treatment [86]. However, the aberrant methylation of ctDNAs (in clinical use) in patients with breast cancers remains to be further validated [87], [88], [89], [90], [91], [92].
Colorectal cancers
Colorectal cancers (CRCs) are a major health burden with a disease-specific mortality of about 33% [93]. Approximately 50% of the cases with CRCs are first diagnosed in late stages and about 50% of patients experience distant metastases. Thus, it is becoming increasingly urgent to develop new biomarkers for the early detection of CRCs. Some tumor-linked genetic alterations, such as in EGFR, BRAF, ALK, KIT, PDGFR, HER2, and KRAS [54], [94], [95], were only detected via ctDNA-based assays. Nonetheless, translating these laboratory findings into cancer therapy remains to be validated.
In 2012, Spindler et al. [96] demonstrated the expression levels of KRAS mutant alleles in the plasma of patients using CRCs. KRAS mutations were detected in 41 patients with primary or metastatic tumors and the levels of the plasma mutant KRAS (pmKRAS) were lower than 75%. Similar results were observed in another study by the same group [97] when examining 64 patients who were treated with temsirolimus, either alone or in combination with irinotecan. Additionally, the use of a biomarker panel consisting of three genes (KRAS, TP53, and APC) enabled the detection of at least one gene mutation from approximately 75% of CRC tissues [98]. In 2015, Tie et al. [99] reported that the patient-specific candidate mutations, such as KRAS G13D, in CRC tissues were detectable in the cfDNAs from 48 of 52 patients (concordance, 92.3%). In the same year, Kidess et al. [100] developed a novel assay named the sequence-specific synchronous coefficient of drag alteration (SCODA). Using the SCODA assay, they demonstrated that the detected mutations were concordant between tissues and plasma in 93% of metastatic patients (n = 38) and 54% of non-metastatic patients. Additionally, the analysis of circulating mutant DNAs has been useful in monitoring of patients receiving anti-EGFR therapies [94], [101], [102], [103], [104]. Several studies have also established that the mutations of beta-catenin DNA were specifically associated with CRC tissues [105], [106], [107], suggesting that the mutations of ctDNAs could serve as promising targets for the detection of CRCs.
Regarding the aberrant methylations of ctDNAs, the hypermethylation of SEPT9 encoding septin 9 is highly associated with the progression of CRCs. Powrozek et al. [108] showed that the test for SEPT9 methylation correctly identified lung cancers in 31 of 70 (44%) samples, whereas positive results were only detected in 4 of 100 controls. In addition, hypermethylated RASSF1A and E-CAD have been considered as new biomarkers for CRCs as well [109], [110].
Non-small-cell lung cancers
Tumor tissue genotyping is used routinely in cases of lung cancers to identify specific and targetable oncogenic alterations, including EGFR mutations and ALK rearrangements. In 2015, Alecensa (alectinib) and Tagrisso (osimertinib) were approved by the US Food and Drug Administration (USFDA) for personalized treatment of non-small-cell lung cancers (NSCLCs). In the past few years, studies have reported a series of achievements relevant to EGFR mutations [111], [112], [113]. The cobas® EGFR Mutation Test v2 from Roche was the first liquid biopsy test approved by the USFDA in June 2016 for the detection of EGFR exon 19 deletions or exon 21 (L858R) substitution mutations of NSCLC patients. More information can be found at http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm504540.htm. This represents great progress for the clinic utility of ctDNAs.
The sensitivities of detecting ctDNAs in NSCLC patients varied in different studies. For example, ctDNAs were detected in 7 of 8 (88%) patients with stage III disease [114], 1 of 1 (100%) with stage III, 4 of 4 (100%) with stage IV [115], and 4 of 5 (80%) with stage IV [68]. Therefore, studies on larger cohorts are needed for more reliable results.
In addition, the hypermethylation of 80 genes have been observed in lung cancers, and the methylation levels show spatial–temporal specificities [116].
Other types of tumors
A previous study showed that a high proportion of DNAs from cancerous tissues were found in the plasma of 29 patients with liver cancers. About 24% of the plasma DNAs were from the liver, whereas only 10.7% plasma DNAs were from the liver in the control group [65]. In addition, the sensitivities of detecting ctDNA mutations in primary pancreatic cancers are usually 30%–50%, and the specificities are usually higher (approximately 90%) [117]. More studies on other types of cancers, specifically prostate cancers [41], [68], [118], [119], [120], ovarian cancers [121], [122], and pancreatic carcinomas [123], were still underway. The deconvolution of methylations showed that the median percentage of cfDNA present in the plasma from patients with hepatocellular carcinomas (HCCs) and control subjects were 24.0% and 10.7%, respectively [65]. A summary of DNA methylation for cancer detection is shown in Table 2.
Table 2.
The DNA methylation for cancer detection
| Cancer type | Marker | Sensitivity | Specificity | Refs. |
|---|---|---|---|---|
| Colorectal cancer | MLH1 | 3/18 (17%) | N/A | [128] |
| CDKN2A (INK4A) | 14/52 (27%) | 44/44 (100%) | [129] | |
| ALX4 | 21/58 (36%) | N/A | [130] | |
| CDH4 | 25/30 (83%) | 36/52 (70%) | [131] | |
| NGFR | 32/46 (70%) | 17/17 (100%) | [127] | |
| RUNX3 | 68/133 (51%) | 150/179 (84%) | [132] | |
| SEPT9 | 11/17 (65%) | 10/10 (100%) | [133], [134] | |
| TMEFF2 | 87/133 (65%) | 123/179 (69%) | [132] | |
| Breast cancer | CDKN2A (INK4A) | 5/35 (14%) | N/A | [135] |
| Lung cancer | CDKN2A (INK4A) | 3/22 (14%) | N/A | [136] |
| DAPK1 | 4/22 (18%) | N/A | ||
| GSTP1 | 1/22 (5%) | N/A | ||
Note: Table was adapted from Jin et al. [126] with permission. MLH1, mutL homolog 1; CDKN2A (INK4A), cyclin dependent kinase inhibitor 2A; ALX4, ALX homeobox 4; CDH4, cadherin 4; NGFR, nerve growth factor receptor; RUNX3, Runt related transcription factor 3; SEPT9, septin 9; TMEFF2, transmembrane protein with EGF-like and two follistatin-like domains 2; DAPK1, death associated protein kinase 1; GSTP1, glutathione S-transferase Pi 1.
Our understanding of DNA methylations in cancers has been deepened during the last three decades [124], [125]. Circulating methylated DNAs have been considered as potential biomarkers for the detection of several cancers, including colorectal, lung, breast, and pancreatic cancers. The methylation of DNA that encodes tumor suppressors and metastases suppressors can be used to determine tumor proliferation and metastases in CTCs [15], [16], [17], [18]. Unlike DNA mutations, the aberrant methylation of specific promoter regions that typically occurs at multiple sites may be a consistent characteristic of cancers [17], [126]. Such consistency makes methylation of ctDNAs a great choice when designing broadly-applicable clinical assays [14], [65]. Small differentially-methylated regions (DMRs) such as CpG island shore methylations and large blocks (hypomethylated blocks), appear at early stages. They can potentially be used as biomarkers for early cancer detection [127].
Methods for detecting ctDNAs
Mutations of ctDNAs
The quantity of detectable ctDNAs depends on the tumor burden and type, as well as other potential biological mechanisms, such as the activities of plasma nucleases [137], [138]. It is necessary to understand the technical aspects of ctDNA detection, in order to evaluate the clinical value of current studies on ctDNAs. Due to recent technological advances, many methods are now available for detecting genetic mutations in cancers. Methods such as microarray-based comparative genome hybridization (CGH), single nucleotide polymorphism (SNP) analysis, and next-generation sequencing (NGS) have been adopted to tackle with the issue of sensitivity and accuracy for the detection of tumor genomes, and have yielded crucial insights into tumor biology [55]. In addition, array-based CGH and SNP arrays, both based on the hybridizations with the arrays of oligonucleotide probes immobilized on a slide, allow for the identification of genetic variations as well. The SNP arrays contain unique nucleotide sequences used as probes that hybridize with fragmented single-stranded DNAs (ssDNAs). Furthermore, genome-wide association studies (GWAS) facilitate the analyses of more than one million SNPs via the chip-based microarray technology, and open up the possibility of detecting tumor-specific DNAs for the development of blood-based diagnostic tests for cancers [139], [140]. There is an urgent need to update related technologies for detecting low levels of tumor DNAs in the circulation of patients with cancers (Table 3).
Table 3.
Comparison of methods for ctDNA detection
| Method | Description | Detection limit (% ctDNA) | Strengths | Limitations | Refs. |
|---|---|---|---|---|---|
| Allele-specific PCR | Preferentially amplifying rare mutant DNA molecules | 0.10–1.00 | Ease to use; lowest cost | Lower sensitivity; only able to test small number of genomic positions in a sample | [141] |
| Digital PCR | Counting mutant molecules via partitioning of DNA molecules | 0.01 | High sensitivity | Only able to test small number of genomic positions in a sample | [142] |
| NGS amplicon based | Deep sequencing of PCR amplicons | 0.01–2.00 | High sensitivity (some methods); less expensive than other NGS methods | Less comprehensive than other NGS methods; unable to detect SCNAs; unable to detect rearrangements without assay customization | [55] |
| WGS | Deep sequencing of entire genome | 1.00 | Interrogating entire genome; broadly applicable without personalization | Expensive; low sensitivity; mostly limited to SCNA detection | [41] |
| WES | Deep sequencing of exome | 5.00 | Interrogating entire exome; broadly applicable without personalization | Expensive; low sensitivity | [41] |
| CAPP-Seq | Targeted hybrid capture | 0.01 | High sensitivity for SNVs, indels, rearrangement, and SCNAs detection; broadly applicable without personalization | Less comprehensive than WGS or WES | [143], [144] |
| iDES-enhanced CAPP-Seq | Targeted hybrid capture and integrated digital error suppression | 0.01 | High scalability, flexibility, and coverage uniformity; able to reliably evaluate all mutation classes in a single assay | Less comprehensive than WGS or WES | [145] |
Note: Table was adapted from Chaudhuri et al. [4] with permission. ctDNA, circulating tumor DNA; SCNA, somatic copy number alteration; SNV, single nucleotide variation; WES, whole-exome sequencing; WGS, whole-genome sequencing; CAPP-Seq, CAncer personalized profiling by deep sequencing; iDES, integrated digital error suppression.
More cutting-edge technologies have emerged, resulting in the incorporation of higher sensitivities and personalized therapies for cancers. For instance, targeted plasma re-sequencing (TAm-Seq) was first used in 2012 to detect de novo mutations [140]. TAm-Seq could identify TP53 mutations by tracking ctDNAs in ovarian cancer patients [140]. For personalized healthcare, the massively parallel sequencing (MPS) technique, including the personalized analysis of rearranged ends (PARE) and shotgun MPS, can track alterations in ctDNA levels in patients before and after surgery [114]. In 2013, using shotgun MPS, Chan et al. identified single nucleotide variants (SNVs) and copy number variations (CNVs) from the plasma of four HCC patients [146]. In addition, many other methods for detecting ctDNAs are also available, such as allele-specific PCR-based methods, digital PCR-based methods, whole-genome sequencing (WGS), and whole-exome sequencing (WES). A comparison of these methods is presented in Table 3 [4].
In 1999, Vogelstein et al. described an approach termed digital PCR, on the basis of the traditional PCR, and analyzed mutations using fluorescent probes [147]. Using digital PCRs, they can detect a small number of mutant cells among several normal cells with a lower signal-to-noise ratio. ddPCR in combination with digital PCR and rapid microfluidic analysis can significantly increase the sensitivities of ctDNA detection. Compared to real-time qPCRs (RT-qPCRs), ddPCRs exhibits higher precision and can absolutely quantify nucleic acids [142]. Several studies have reported ddPCR as a method for ctDNA detection in several cancers, including breast cancers [72], [80], melanomas [148], [149], HCCs [150], and colorectal cancers [151], [152]. This technique may serve as a potential surrogate for ctDNA detection, and has exhibited great potential for clinical applications.
In addition, Alizadeh and Diehn developed a novel method, termed cancer personalized profiling by deep sequencing (CAPP-Seq). It is a capture-based NGS method for the detection of ctDNAs [143] and ultrasensitive for the quantification of ctDNAs. Application of CAPP-Seq in NSCLC samples lead to the identification of mutations in more than 95% of tumors [143]. For 100% of stage II–IV and 50% of stage I patients with NSCLCs, ctDNAs showed a specificity of 96% for mutant allele fractions down to approximately 0.02%. This new strategy can examine large portions of the genome for early cancer detection [143], [144]. In March 2016, Newman et al. [145] developed a novel method called integrated digital error suppression-enhanced cancer personalized profiling by deep sequencing (iDES-enhanced CAPP-Seq). With a sensitivity of 92% and a specificity of more than 99.99% for the variant alleles, and concurrently, a sensitivity of 90% and a specificity of 96% in patients, iDES-enhanced CAPP-Seq enabled the biopsy-free profiling of mutations in the EGFR kinase domain [145] (Table 3). As mentioned above, these techniques enable ctDNA analysis to track the tumor burden without conducting sequentially repeated biopsies.
ctDNA methylation
Strategies used in DNA methylation analysis can be classified into two groups: site-specific and genome-wide methylation detections. Currently, detection of site-specific methylations of DNA has been conduced more often [14], [153], [154], [155]. Many methods originally used for site-specific detection of genomic DNA methylations are suitable for site-specific detection of ctDNA methylations as well. Following bisulfite conversion or methylated ctDNA enrichment, detection of methylated ctDNAs can be facilitated via different PCR amplification methods, including the conventional methylation-specific PCR (MSP) [156], [157], [158], quantitative multiplexed methylation-specific PCR (QM-PCR) [159], and methylation on beads (MOB) [160]. A method for the enrichment of methylated CpG sequences has also been developed for use in kits [156]. The enriched methylated DNA can be used for both sequencing and PCR amplifications. Conventional MSP can be used directly in the detection of ctDNA methylations [157], [158]. This method requires only 5 ml of peripheral blood, and can be applied in non-invasive measurements clinically [161]. Furthermore, fluorescence-based real-time MSP, an improved form of the aforementioned technique, combines the use of fluorescent probes and detection in real-time to facilitate the quantitative detection of DNA methylations [162]. Furthermore, quantum dots (QDs) have been used as fluorophores and fluorescence resonance energy transfer (FRET) donors for biological sensing and detection of biomolecular targets [163]. Another modified version of conventional PCR, namely MOB, integrates three processes – DNA extraction, bisulfite conversion, and PCR – into a single tube by using silica superparamagnetic beads as DNA carriers [160], [164]. In 2014, the cMethDNA assay, a new method based on the standard QM-MSP, was reported for the identification of novel methylated breast cancer genes in serum. Owing to its ability of enhancing methylation signals, cMethDNA assay would be suitable for detection of ctDNA methylations [165].
Although there are many bisulfite conversion- and enrichment-based methods for genomic DNA methylation analysis, few of these methods can be applied in ctDNA methylation analysis, owing to the characteristics of ctDNAs. For example, bisulfite conversion-based methods, including MethylC-Seq or BS-Seq, are not suitable for this purpose because whole-genome bisulfite sequencing requires relatively large samples of DNAs [166] to be subjected to the sodium bisulfite-mediated conversion of unmethylated cytosines, and obtaining the sequence information through computing conversion ratio [167]. A method called reduced representation bisulfite sequencing (RRBS) was developed with a focus on CpG islands and promoter regions, allowing the sequencing of methylated regions that are otherwise unable to be properly profiled using conventional bisulfite sequencing techniques [168]. In addition, studies employing enrichment-based methods, including methylated DNA binding domain-sequencing (MBD-Seq) [169] and methylated DNA immunoprecipitation sequencing (MeDIP-Seq) [170], have not been reported for use at low concentrations (<150 ng) of DNA [171]. Both MBD-Seq and MeDIP-Seq are based on DNA enrichment technologies, which utilize methyl-CpG binding domain protein 2b and 5-methyl cytosine antibodies, respectively, may exhibit sensitivities lower than single-base resolution [169], [170].
In recent decades, an increasing number of studies have focused on the potential use of ctDNA methylations as biomarkers for early detection of cancers, for cancer screening, and for monitoring the efficacies of anticancer therapies [172], [173]. Shot-gun massively parallel bisulfite sequencing, another bisulfite sequencing-based technique, was also developed for the detection of ctDNA methylations. This method detects ctDNAs with high sensitivity and specificity, even at a low sequence depth. Additionally, the volume of sample used in this method can be reduced to 4 ml of plasma [172].
In 2015, Wen et al. [173] explored a genome-wide methylated CpG tandem amplification and sequencing (MCTA-Seq) method to detect hypermethylated CpG islands in ctDNAs. Given ctDNA is fragmented and accounts for only a small portion of cfDNA, this sensitive method is suitable for detecting ctDNA methylation, since it only requires small amounts of ctDNA (as low as 7.5 pg) [173]. This is the first genome-wide technique developed for the detection of ctDNA methylations (Table 4).
Table 4.
Methods of detection of DNA methylation in circulating cells
| Detection type | Method | Description | Refs. |
|---|---|---|---|
| Site-specific detection | Conventional MSP | Requiring a sample spot (5 ml of peripheral blood); Able to be used in the detection of certain methylated genes in the plasma of serum; using specific PCR primers for methylated sequences | [156], [157], [158] |
| Fluorescence-based real-time MSP | Facilitating quantitative detection; sensitive; requiring prior knowledge of the methylated sequences | [174] | |
| QDs-FRET | Able to reduce the background for detecting targets at low concentration; greater sensitivity; limited FRET efficiency; impractical for challenging samples such as serum and plasma | [163] | |
| MOB | Easy to handle; increased detection throughput; providing efficient, sensitive methylation detection in diagnosis; able to be used in blood samples | [160], [164] | |
| cMethDNA | High sensitivity, specificity, reproducibility, dynamic range, and quantitative advantages; detecting methylated site at low levels in cell-free circulating serum DNA; promising new liquid biopsy tool | [165] | |
| Genome-scale detection | Conventional bisulfite conversion-based methods | Gold standard for the detection of DNA methylation; requiring a relatively large amount of sample; focused on CpG islands or promoter regions | [166], [167], [168] |
| Conventional enrichment-based methods | No conversion treatment; requiring a high concentration of DNA; likely ignoring other methylated sites when using antibody against 5 mC or 5 mCG | [169], [170], [171] | |
| Short-gun massively parallel bisulfite sequencing | Detecting with high sensitivity and specificity even at a low sequence depth with 10 million sequencing data; requiring 4 ml plasma only | [172] | |
| MCTA-seq | Working well with ctDNA samples as small as 7.5 pg; able to simultaneously detect thousands of hypermethylated CpG islands in cfDNA | [173] |
Note: MSP, methylation-specific PCR; QDs-FRET, quantum dots-fluorescence resonance energy transfer; MOB, methylation on beads; MCTA-seq, methylated CpG tandem amplification and sequencing; ctDNA, circulating tumor DNA; cfDNA, cell-free DNA.
Challenges in circulating DNA for early diagnosis
Although tumor-specific mutations and methylations in ctDNAs are potential targets for the non-invasive cancer detection, and for the diagnoses, prognostic management, and guidance for treatments of these cancers, there are still barriers in the accurate detection of specific cell-free nucleic acids [175]. Common biomarkers for all types of tumors have not been discovered yet.
Notably, there is no unified standard for detection. There is no consensus yet regarding the typical concentrations of cfDNAs present in the blood of healthy people. Since steps involving ctDNA extraction are not always described in detail in published studies, the concentrations of ctDNA detected tend to vary a lot [67], [120], [176], [177], [178], [179]. Based on current situation, a large blood sample is needed for the detection of ctDNAs due to their low blood concentrations [180], [181], [182], [183]. The rate of purification of these samples needs to be improved greatly. Tumor DNA fragments are diluted with normal DNA in circulation, which may hamper subsequent analysis. Furthermore, false-positives still occur in several approaches due to the inadequate sensitivities and specificities of these techniques [4]. Technological improvements and a better understanding of ctDNA biology are necessary to achieve better outcomes. Techniques using ctDNA are limited in the clinical setting because of their high costs, and the clinical use of this technique is still a subject of debate due to the needs to further improve the accuracy and sensitivity of ctDNA detection.
ctDNA has the advantage and the potential of serving as a relatively stable biomarker. To develop clinically valuable biomarkers, close collaborations are needed between the clinicians and scientists. In the context of strong public expectation and the increasingly-established biotechnology companies as “cancer detection from a drop of blood”, liquid biopsy is perhaps overestimated to some extent. Diagnostic kits that are reliable and exhibit both higher sensitivities and specificities are therefore overdue. However, before translating basic research into clinical utility for precise treatment, it is prerequisite to gain in-depth mechanistic understanding on how heterogeneous cancer cells in different cancers behave at various stages, rather than merely improving the methods of detection.
To achieve the goal of PM, we would need to select the optimal strategies or drugs accordingly to the characteristics of patients, based on the available early detection and screening for cancer patients before initiation of treatments. However, due to the lack of subsequent treatment strategies or drugs, we have not benefitted much from the head start provided by PM for early detection of cancers, even though we have screened for several risk factors related to the cancers. Therefore, we need to discover and identify new targets in order to develop more effective drugs.
In the context of precision medicine, the construction of a comprehensive, accurate, and multi-dimensional cancer genomic-epigenetic map remains a challenge. Additionally, lack of timely and efficient communication between researchers and clinicians poses further challenges. A better appreciation of the patients’ needs by basic scientists, and a clearer understanding of the research progress and the potential clinical applications of the basic research by clinicians would help bridge the gaps.
Opportunities on the path to clinical utility
Given the well-recognized difficulties in repeatedly obtaining tissue biopsies, liquid biopsy can be an effective method for cancer detection. Individual patients with either metastatic or primary tumors may vary in their genomic, epigenetic, and transcriptomic compositions [30], [184]. Liquid biopsies help to overcome the problems of inherent tumor heterogeneity of tissue biopsies for personalized therapy, and can be used for detecting cancers even before diagnosis by imaging studies [185]. Despite the challenges in the clinical application of ctDNAs, their detection can be conducted repetitively in real-time, conferring the advantages of being non-invasive, non-injurious, as well as highly sensitive and specific. Also, ctDNAs might hold more promise as biomarkers, compared with protein biomarkers, as the ctDNAs may be more informative, accurate, and specific [53], [68]. In addition, technological advances facilitate the detection of thousands of CpG sites and whole-genome sequences, which can be applied to bisulfite converted ctDNAs and used for cancer detection as well [186], [187]. As the identification of DMRs becomes easier, they are likely to be used as cancer biomarkers [14], [15], [72], [108]. Furthermore, the cost of sequencing was $100,000 per genome in 2009, but fell remarkably to below $1500 by late 2015 (http://www.genome.gov/sequencingcosts/).
Targeted re-sequencing of cfDNAs can be performed to elucidate mutations in serum samples. In-depth re-sequencing and digital PCR [188] analyses enables more sensitive detection and monitoring of specific mutations in minute amounts of ctDNA [189]. It is expected that NGS will lower the overall costs, speed up the turnaround times, increase the detection sensitivities, as well as enable the detections of rare gene mutations and individual epigenetic markers in the near future. Additionally, in the clinic, NGS may become applicable to CTCs and cfDNAs in plasma. Targeted NGS of ctDNAs has the potential clinical utility of enabling early diagnoses of tumors and implementation of targeted therapies. This would create more opportunities for the discovery of cancer biomarkers and improve the sensitivity and specificity of such detection. This new task also requires cooperation among the leading research groups and investment from pharmaceutical and biotechnology companies.
Currently, the USFDA and the China FDA (CFDA) have certificated some DNA methylation-based biomarkers, including genes encoding NDRG family member 4 (NDRG4) and bone morphogenetic protein 3 (BMP3; USFDA approval in 2014) [190], SEPT9 [191], and the gene encoding short stature homeobox 2 (SHOX2; CFDA approval in 2015) [192], which give a boost to ctDNA detection on larger scales. Additionally, in 2016, the EGFR Mutation Test v2 (cobas) [193] became the first US FDA-approved in-vitro diagnostic (IVD) medical device for liquid biopsy for detecting EGFR mutations in NSCLCs. Biocept, Guardant Health, Neo Genomics Laboratories, Qiagen, and many other US-based companies have provided ctDNA detection services to their consumers. Founded by Illumina, a new company, named Grail, aims at developing liquid biopsy-based tests that would cost less than $1000 per test, and is recruiting the best talents in the field of cancer detection. Their products are planned to reach consumers by the year 2019 (http://www.grailbio.com/). The biotechnology companies in China also have invested in this emerging field; these companies include Surexam, Annroad, BGI, etc. The development trend and the current progress in the field afford more opportunities than challenges for liquid biopsy detection. From 2015 to 2016, nearly thirty kinds of new anti-cancer drugs were approved by the USFDA, such as Tecentriq (atezolizumab, Genentech, US), Tarceva (erlotinib, Astellas Pharm, US), Alecensa (alectinib, Hoffmann-La Rocha, Swiss) for treatment of NSCLCs, and Lenovima (lenvatinib, Eisai, Japan) and Cabometyx (cabozantinib, Exelixis, US) for the treatment of renal cell carcinomas (RCCs). The highly-sensitive ctDNA detection could be harnessed by the newly-approved targeted therapies and precise treatments and ultimately benefit the patients.
Monitoring of cancers by measuring ctDNA dynamics in blood or serum is a new and developing area of research. Based on the current research progress and the growth of the medical industry, we believe that ctDNA assays may be used to personalize treatments real-time for cancer patients in the future, based on their individual ctDNAs or ctDNA methylation levels, for diagnoses, prognoses, and guidance for treatments. However, there is much room for improvement before this technology can be routinely applied in clinical settings.
Competing interests
The authors declare that they have no conflict of interests.
Acknowledgments
This work was supported by the Precision Medicine Research Program of the Chinese Academy of Sciences (Grant No. KJZD-EW-L14), the National Basic Research Program of China (973 Program; Grant Nos. 2012CB518302 and 2013CB911001), the National Natural Science Foundation of China (Grant Nos. 31540033 and 91019024), and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA01040407).
Handled by Cesar Wong
Footnotes
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences and Genetics Society of China.
References
- 1.McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr. 2016;7:418–419. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dainiak N. Inferences, risk modeling, and prediction of health effects of ionizing radiation. Health Phys. 2016;110:271–273. doi: 10.1097/HP.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 3.Zhou D.D., Hao J.L., Guo K.M., Lu C.W., Liu X.D. Sperm quality and DNA damage in men from Jilin Province, China, who are occupationally exposed to ionizing radiation. Genet Mol Res. 2016;15:gmr8078. doi: 10.4238/gmr.15018078. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri A.A., Binkley M.S., Osmundson E.C., Alizadeh A.A., Diehn M. Predicting radiotherapy responses and treatment outcomes through analysis of circulating tumor DNA. Semin Radiat Oncol. 2015;25:305–312. doi: 10.1016/j.semradonc.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin N.E., D'Amico A.V. Progress and controversies: radiation therapy for prostate cancer. CA Cancer J Clin. 2014;64:389–407. doi: 10.3322/caac.21250. [DOI] [PubMed] [Google Scholar]
- 6.Mancebo S.E., Wang S.Q. Skin cancer: role of ultraviolet radiation in carcinogenesis. Rev Environ Health. 2014;29:265–273. doi: 10.1515/reveh-2014-0041. [DOI] [PubMed] [Google Scholar]
- 7.Ignatiadis M., Lee M., Jeffrey S.S. Circulating tumor cells and circulating tumor DNA: challenges and opportunities on the path to clinical utility. Clin Cancer Res. 2015;21:4786–4800. doi: 10.1158/1078-0432.CCR-14-1190. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Waters J., Leung M.L., Unruh A., Roh W., Shi X. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature. 2014;512:155–160. doi: 10.1038/nature13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiley C., Bruin E.C.D., Mcgranahan N., Swanton C. Deciphering intratumor heterogeneity and temporal acquisition of driver events to refine precision medicine. Genome Biol. 2014;15:1–10. doi: 10.1186/s13059-014-0453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowley E., Di Nicolantonio F., Loupakis F., Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10:472–484. doi: 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- 11.Alix-Panabieres C., Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discovery. 2016;6:479–491. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- 12.Diaz L.A., Jr., Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung F., Kulasingam V., Diamandis E.P., Hoon D.S., Kinzler K., Pantel K. Circulating tumor DNA as a cancer biomarker: fact or fiction? Clin Chem. 2016;62:1054–1060. doi: 10.1373/clinchem.2016.260331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warton K., Mahon K.L., Samimi G. Methylated circulating tumor DNA in blood: power in cancer prognosis and response. Endocr Relat Cancer. 2016;23:R157–R171. doi: 10.1530/ERC-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shivapurkar N., Gazdar A. DNA methylation based biomarkers in non-invasive cancer screening. Curr Mol Med. 2010;10:123–132. doi: 10.2174/156652410790963303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cairns P. Gene methylation and early detection of genitourinary cancer: the road ahead. Nat Rev Cancer. 2007;7:531–543. doi: 10.1038/nrc2170. [DOI] [PubMed] [Google Scholar]
- 17.Chimonidou M., Strati A., Tzitzira A., Sotiropoulou G., Malamos N., Georgoulias V. DNA methylation of tumor suppressor and metastasis suppressor genes in circulating tumor cells. Clin Chem. 2011;57:1169–1177. doi: 10.1373/clinchem.2011.165902. [DOI] [PubMed] [Google Scholar]
- 18.Dulaimi E., Hillinck J., Ibanez de Caceres I., Al-Saleem T., Cairns P. Tumor suppressor gene promoter hypermethylation in serum of breast cancer patients. Clin Cancer Res. 2004;10:6189–6193. doi: 10.1158/1078-0432.CCR-04-0597. [DOI] [PubMed] [Google Scholar]
- 19.Hendee W.R., Edwards F.M. Health effects of exposure to low-level ionizing radiation. Acta Radiol. 1998;39:453–454. [PubMed] [Google Scholar]
- 20.Morgan W.F., Sowa M.B. Non-targeted effects induced by ionizing radiation: mechanisms and potential impact on radiation induced health effects. Cancer Lett. 2015;356:17–21. doi: 10.1016/j.canlet.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Cho S.H., Krishnan S. CRC Press; Boca Raton, FL: 2013. Cancer nanotechnology: principles and applications in radiation oncology. [Google Scholar]
- 22.Dechet C.B., Sebo T., Farrow G., Blute M.L., Engen D.E., Zincke H. Prospective analysis of intraoperative frozen needle biopsy of solid renal masses in adults. J Urol. 1999;162:1282–1284. [PubMed] [Google Scholar]
- 23.Al-Leswas D., O'Reilly D.A., Poston G.J. Biopsy of solid liver tumors: adverse consequences. Hepatobiliary Pancreat Dis Int. 2008;7:325–327. [PubMed] [Google Scholar]
- 24.Yang Y., Li L., Qu C., Liang S., Zeng B., Luo Z. Endoscopic ultrasound-guided fine needle core biopsy for the diagnosis of pancreatic malignant lesions: a systematic review and meta-analysis. Sci Rep. 2016;6:22978. doi: 10.1038/srep22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hompes D., Ruers T. Review: incidence and clinical significance of Bevacizumab-related non-surgical and surgical serious adverse events in metastatic colorectal cancer. Eur J Surg Oncol. 2011;37:737–746. doi: 10.1016/j.ejso.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Mazzucchelli R., Colanzi P., Pomante R., Muzzonigro G., Montironi R. Prostate tissue and serum markers. Adv Clin Pathol. 2000;4:111–120. [PubMed] [Google Scholar]
- 27.Ruibal Morell A. CEA serum levels in non-neoplastic disease. Int J Biol Markers. 1992;7:160–166. doi: 10.1177/172460089200700307. [DOI] [PubMed] [Google Scholar]
- 28.Sikaris K.A. CA125 – a test with a change of heart. Heart Lung Circ. 2011;20:634–640. doi: 10.1016/j.hlc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Heitzer E., Auer M., Ulz P., Geigl J.B., Speicher M.R. Circulating tumor cells and DNA as liquid biopsies. Genome Med. 2013;5:1–11. doi: 10.1186/gm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarzenbach H., Hoon D.S., Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 31.Heitzer E., Ulz P., Geigl J.B. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112–123. doi: 10.1373/clinchem.2014.222679. [DOI] [PubMed] [Google Scholar]
- 32.Vickers K.C., Palmisano B.T., Shoucri B.M., Shamburek R.D., Remaley A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giovannetti E., van der Velde A., Funel N., Vasile E., Perrone V., Leon L.G. High-throughput microRNA (miRNAs) arrays unravel the prognostic role of MiR-211 in pancreatic cancer. PLoS One. 2012;7:e49145. doi: 10.1371/journal.pone.0049145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Gu J., Roth J.A., Hildebrandt M.A., Lippman S.M., Ye Y. Pathway-based serum microRNA profiling and survival in patients with advanced stage non-small cell lung cancer. Cancer Res. 2013;73:4801–4809. doi: 10.1158/0008-5472.CAN-12-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bang C., Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44:2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Simpson R.J., Lim J.W., Moritz R.L., Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 37.Thakur B.K., Zhang H., Becker A., Matei I., Huang Y., Costa-Silva B. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dienstmann R., Rodon J., Tabernero J. Optimal design of trials to demonstrate the utility of genomically-guided therapy: putting precision cancer medicine to the test. Mol Oncol. 2015;9:940–950. doi: 10.1016/j.molonc.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnedos M., Vicier C., Loi S., Lefebvre C., Michiels S., Bonnefoi H. Precision medicine for metastatic breast cancer-limitations and solutions. Nat Rev Clin Oncol. 2015;12:693–704. doi: 10.1038/nrclinonc.2015.123. [DOI] [PubMed] [Google Scholar]
- 40.Baccelli I., Schneeweiss A., Riethdorf S., Stenzinger A., Schillert A., Vogel V. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 41.Heitzer E., Ulz P., Belic J., Gutschi S., Quehenberger F., Fischereder K. Tumor-associated copy number changes in the circulation of patients with prostate cancer identified through whole-genome sequencing. Genome Med. 2013;5:30. doi: 10.1186/gm434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodgkinson C.L., Morrow C.J., Li Y., Metcalf R.L., Rothwell D.G., Trapani F. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med. 2014;20:897–903. doi: 10.1038/nm.3600. [DOI] [PubMed] [Google Scholar]
- 43.Alix-Panabieres C., Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623–631. doi: 10.1038/nrc3820. [DOI] [PubMed] [Google Scholar]
- 44.Ashworth T.R. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aust Med J. 1869;14:146–147. [Google Scholar]
- 45.Krishnamurthy S. The emerging role of circulating tumor cells in breast cancer. Cancer Cytopathol. 2012;120:161–166. doi: 10.1002/cncy.20207. [DOI] [PubMed] [Google Scholar]
- 46.Hu B., Rochefort H., Goldkorn A. Circulating tumor cells in prostate cancer. Cancers (Basel) 2013;5:1676–1690. doi: 10.3390/cancers5041676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schölch S., Bork U., Rahbari N.N., García S., Swiersy A., Betzler A.M. Circulating tumor cells of colorectal cancer. Cancer Cell Microenviron. 2014;1:e323. [Google Scholar]
- 48.Pantel K., Brakenhoff R.H., Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 49.Riethdorf S., Fritsche H., Muller V., Rau T., Schindlbeck C., Rack B. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the cell search system. Clin Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 50.Nagrath S., Sequist L.V., Maheswaran S., Bell D.W., Irimia D., Ulkus L. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Issadore D., Chung J., Shao H., Liong M., Ghazani A.A., Castro C.M. Ultrasensitive clinical enumeration of rare cells ex vivo using a micro-hall detector. Sci Transl Med. 2012;4:141ra92. doi: 10.1126/scitranslmed.3003747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karabacak N.M., Spuhler P.S., Fachin F., Lim E.J., Pai V., Ozkumur E. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9:694–710. doi: 10.1038/nprot.2014.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dawson S.J., Tsui D.W., Murtaza M., Biggs H., Rueda O.M., Chin S.F. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–1209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 54.Siravegna G., Bardelli A. Blood circulating tumor DNA for non-invasive genotyping of colon cancer patients. Mol Oncol. 2016;10:475–480. doi: 10.1016/j.molonc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mabert K., Cojoc M., Peitzsch C., Kurth I., Souchelnytskyi S., Dubrovska A. Cancer biomarker discovery: current status and future perspectives. Int J Radiat Biol. 2014;90:659–677. doi: 10.3109/09553002.2014.892229. [DOI] [PubMed] [Google Scholar]
- 56.Mandel P., Metais P. Les acides nucleiques du plasma sanguin chez l'homme. CR Acad Sci Paris. 1948;142:241–243. [PubMed] [Google Scholar]
- 57.Leon S.A., Shapiro B., Sklaroff D.M., Yaros M.J. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646–650. [PubMed] [Google Scholar]
- 58.Stroun M., Anker P., Maurice P., Lyautey J., Lederrey C., Beljanski M. Neoplastic characteristics of the DNA found in the plasma of cancer patients. Oncology. 1989;46:318–322. doi: 10.1159/000226740. [DOI] [PubMed] [Google Scholar]
- 59.Vasioukhin V., Anker P., Maurice P., Lyautey J., Lederrey C., Stroun M. Point mutations of the N-ras gene in the blood plasma DNA of patients with myelodysplastic syndrome or acute myelogenous leukaemia. Br J Haematol. 1994;86:774–779. doi: 10.1111/j.1365-2141.1994.tb04828.x. [DOI] [PubMed] [Google Scholar]
- 60.Fujiwara K., Fujimoto N., Tabata M., Nishii K., Matsuo K., Hotta K. Identification of epigenetic aberrant promoter methylation in serum DNA is useful for early detection of lung cancer. Clin Cancer Res. 2005;11:1219–1225. [PubMed] [Google Scholar]
- 61.Hardy T., Zeybel M., Day C.P., Dipper C., Masson S., McPherson S. Plasma DNA methylation: a potential biomarker for stratification of liver fibrosis in non-alcoholic fatty liver disease. Gut. 2016 doi: 10.1136/gutjnl-2016-311526. http://www.idf.org/gutjnl-2016-311526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jovelet C., Ileana E., Le Deley M.C., Motte N., Rosellini S., Romero A. Circulating cell-free tumor DNA analysis of 50 genes by next-generation sequencing in the prospective MOSCATO trial. Clin Cancer Res. 2016;22:2960–2968. doi: 10.1158/1078-0432.CCR-15-2470. [DOI] [PubMed] [Google Scholar]
- 63.Cheng F., Su L., Qian C. Circulating tumor DNA: a promising biomarker in the liquid biopsy of cancer. Oncotarget. 2016;7:48832–48841. doi: 10.18632/oncotarget.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diehl F., Schmidt K., Choti M.A., Romans K., Goodman S., Li M. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun K., Jiang P., Chan K.C., Wong J., Cheng Y.K., Liang R.H. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc Natl Acad Sci U S A. 2015;112:E5503–E5512. doi: 10.1073/pnas.1508736112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jahr S., Hentze H., Englisch S., Hardt D., Fackelmayer F.O., Hesch R.D. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 67.Fleischhacker M., Schmidt B. Circulating nucleic acids (CNAs) and cancer – a survey. Biochim Biophys Acta. 2007;1775:181–232. doi: 10.1016/j.bbcan.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Bettegowda C., Sausen M., Leary R.J., Kinde I., Wang Y., Agrawal N. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gold B., Cankovic M., Furtado L.V., Meier F., Gocke C.D. Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility? J Mol Diagn. 2015;17:209–224. doi: 10.1016/j.jmoldx.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang P., Chan C.W., Chan K.C., Cheng S.H., Wong J., Wong V.W. Lengthening and shortening of plasma DNA in hepatocellular carcinoma patients. Proc Natl Acad Sci U S A. 2015;112:E1317–E1325. doi: 10.1073/pnas.1500076112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang Z., Hua D., Hu Y., Cheng Z., Zhou X., Xie Q. Quantitation of plasma circulating DNA using quantitative PCR for the detection of hepatocellular carcinoma. Pathol Oncol Res. 2012;18:271–276. doi: 10.1007/s12253-011-9438-z. [DOI] [PubMed] [Google Scholar]
- 72.Hoque M.O., Feng Q., Toure P., Dem A., Critchlow C.W., Hawes S.E. Detection of aberrant methylation of four genes in plasma DNA for the detection of breast cancer. J Clin Oncol. 2006;24:4262–4269. doi: 10.1200/JCO.2005.01.3516. [DOI] [PubMed] [Google Scholar]
- 73.Webb S. The cancer bloodhounds. Nat Biotechnol. 2016;34:1090–1094. doi: 10.1038/nbt.3717. [DOI] [PubMed] [Google Scholar]
- 74.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 75.Shaw J.A., Page K., Blighe K., Hava N., Guttery D., Ward B. Genomic analysis of circulating cell-free DNA infers breast cancer dormancy. Genome Res. 2012;22:220–231. doi: 10.1101/gr.123497.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah S.S., Ketterling R.P., Goetz M.P., Ingle J.N., Reynolds C.A., Perez E.A. Impact of American Society of Clinical Oncology/College of American Pathologists guideline recommendations on HER2 interpretation in breast cancer. Hum Pathol. 2010;41:103–106. doi: 10.1016/j.humpath.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Higgins M.J., Jelovac D., Barnathan E., Blair B., Slater S., Powers P. Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clin Cancer Res. 2012;18:3462–3469. doi: 10.1158/1078-0432.CCR-11-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gevensleben H., Garcia-Murillas I., Graeser M.K., Schiavon G., Osin P., Parton M. Noninvasive detection of HER2 amplification with plasma DNA digital PCR. Clin Cancer Res. 2013;19:3276–3284. doi: 10.1158/1078-0432.CCR-12-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beaver J.A., Jelovac D., Balukrishna S., Cochran R.L., Croessmann S., Zabransky D.J. Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clin Cancer Res. 2014;20:2643–2650. doi: 10.1158/1078-0432.CCR-13-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chu D., Paoletti C., Gersch C., VanDenBerg D.A., Zabransky D.J., Cochran R.L. ESR1 mutations in circulating plasma tumor DNA from metastatic breast cancer patients. Clin Cancer Res. 2016;22:993–999. doi: 10.1158/1078-0432.CCR-15-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Mattos-Arruda L., Cortes J., Santarpia L., Vivancos A., Tabernero J., Reis-Filho J.S. Circulating tumour cells and cell-free DNA as tools for managing breast cancer. Nat Rev Clin Oncol. 2013;10:377–389. doi: 10.1038/nrclinonc.2013.80. [DOI] [PubMed] [Google Scholar]
- 82.Olsson E., Winter C., George A., Chen Y., Howlin J., Tang M.H. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med. 2015;7:1034–1047. doi: 10.15252/emmm.201404913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kamel A.M., Teama S., Fawzy A., El Deftar M. Plasma DNA integrity index as a potential molecular diagnostic marker for breast cancer. Tumour Biol. 2016;37:7565–7572. doi: 10.1007/s13277-015-4624-3. [DOI] [PubMed] [Google Scholar]
- 84.Madic J., Kiialainen A., Bidard F.C., Birzele F., Ramey G., Leroy Q. Circulating tumor DNA and circulating tumor cells in metastatic triple negative breast cancer patients. Int J Cancer. 2015;136:2158–2165. doi: 10.1002/ijc.29265. [DOI] [PubMed] [Google Scholar]
- 85.Liggett T.E., Melnikov A.A., Marks J.R., Levenson V.V. Methylation patterns in cell-free plasma DNA reflect removal of the primary tumor and drug treatment of breast cancer patients. Int J Cancer. 2011;128:492–499. doi: 10.1002/ijc.25363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fiegl H., Millinger S., Mueller-Holzner E., Marth C., Ensinger C., Berger A. Circulating tumor-specific DNA: a marker for monitoring efficacy of adjuvant therapy in cancer patients. Cancer Res. 2005;65:1141–1145. doi: 10.1158/0008-5472.CAN-04-2438. [DOI] [PubMed] [Google Scholar]
- 87.Fu D., Ren C., Tan H., Wei J., Zhu Y., He C. Sox17 promoter methylation in plasma DNA is associated with poor survival and can be used as a prognostic factor in breast cancer. Medicine (Baltimore) 2015;94:e637. doi: 10.1097/MD.0000000000000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wittenberger T., Sleigh S., Reisel D., Zikan M., Wahl B., Alunni-Fabbroni M. DNA methylation markers for early detection of women's cancer: promise and challenges. Epigenomics. 2014;6:311–327. doi: 10.2217/epi.14.20. [DOI] [PubMed] [Google Scholar]
- 89.Avraham A., Uhlmann R., Shperber A., Birnbaum M., Sandbank J., Sella A. Serum DNA methylation for monitoring response to neoadjuvant chemotherapy in breast cancer patients. Int J Cancer. 2012;131:E1166–E1672. doi: 10.1002/ijc.27526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamamoto N., Nakayama T., Kajita M., Miyake T., Iwamoto T., Kim S.J. Detection of aberrant promoter methylation of GSTP1, RASSF1A, and RARbeta2 in serum DNA of patients with breast cancer by a newly established one-step methylation-specific PCR assay. Breast Cancer Res Treat. 2012;132:165–173. doi: 10.1007/s10549-011-1575-2. [DOI] [PubMed] [Google Scholar]
- 91.Mirza S., Sharma G., Parshad R., Srivastava A., Gupta S.D., Ralhan R. Clinical significance of promoter hypermethylation of ERbeta and RARbeta2 in tumor and serum DNA in Indian breast cancer patients. Ann Surg Oncol. 2012;19:3107–3115. doi: 10.1245/s10434-012-2323-5. [DOI] [PubMed] [Google Scholar]
- 92.Li Z., Guo X.W., Tang L.L., Peng L.M., Chen M., Luo X.P. Methylation analysis of plasma cell-free DNA for breast cancer early detection using bisulfite next-generation sequencing. Tumor Biol. 2016;37:13111–13119. doi: 10.1007/s13277-016-5190-z. [DOI] [PubMed] [Google Scholar]
- 93.Siegel R., Desantis C., Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 94.Diaz L.A., Jr., Williams R.T., Wu J., Kinde I., Hecht J.R., Berlin J. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–540. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Siravegna G., Mussolin B., Buscarino M., Corti G., Cassingena A., Crisafulli G. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:827. doi: 10.1038/nm0715-827b. [DOI] [PubMed] [Google Scholar]
- 96.Spindler K.L., Pallisgaard N., Vogelius I., Jakobsen A. Quantitative cell-free DNA, KRAS, and BRAF mutations in plasma from patients with metastatic colorectal cancer during treatment with cetuximab and irinotecan. Clin Cancer Res. 2012;18:1177–1185. doi: 10.1158/1078-0432.CCR-11-0564. [DOI] [PubMed] [Google Scholar]
- 97.Spindler K.L., Sorensen M.M., Pallisgaard N., Andersen R.F., Havelund B.M., Ploen J. Phase II trial of temsirolimus alone and in combination with irinotecan for KRAS mutant metastatic colorectal cancer: outcome and results of KRAS mutational analysis in plasma. Acta Oncol. 2013;52:963–970. doi: 10.3109/0284186X.2013.776175. [DOI] [PubMed] [Google Scholar]
- 98.Wang J.Y., Hsieh J.S., Chang M.Y., Huang T.J., Chen F.M., Cheng T.L. Molecular detection of APC, K-ras, and p53 mutations in the serum of colorectal cancer patients as circulating biomarkers. World J Surg. 2004;28:721–726. doi: 10.1007/s00268-004-7366-8. [DOI] [PubMed] [Google Scholar]
- 99.Tie J., Kinde I., Wang Y., Wong H.L., Roebert J., Christie M. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol. 2015;26:1715–1722. doi: 10.1093/annonc/mdv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kidess E., Heirich K., Wiggin M., Vysotskaia V., Visser B.C., Marziali A. Mutation profiling of tumor DNA from plasma and tumor tissue of colorectal cancer patients with a novel, high-sensitivity multiplexed mutation detection platform. Oncotarget. 2015;6:2549–2561. doi: 10.18632/oncotarget.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Misale S., Yaeger R., Hobor S., Scala E., Janakiraman M., Liska D. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–536. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bardelli A., Corso S., Bertotti A., Hobor S., Valtorta E., Siravegna G. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discovery. 2013;3:658–673. doi: 10.1158/2159-8290.CD-12-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Misale S., Arena S., Lamba S., Siravegna G., Lallo A., Hobor S. Blockade of EGFR and MEK intercepts heterogeneous mechanisms of acquired resistance to anti-EGFR therapies in colorectal cancer. Sci Transl Med. 2014;6:162ra54. doi: 10.1126/scitranslmed.3007947. [DOI] [PubMed] [Google Scholar]
- 104.Mohan S., Heitzer E., Ulz P., Lafer I., Lax S., Auer M. Changes in colorectal carcinoma genomes under anti-EGFR therapy identified by whole-genome plasma DNA sequencing. PLoS Genet. 2014;10:e1004271. doi: 10.1371/journal.pgen.1004271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnson V., Volikos E., Halford S.E., Eftekhar Sadat E.T., Popat S., Talbot I. Exon 3 beta-catenin mutations are specifically associated with colorectal carcinomas in hereditary non-polyposis colorectal cancer syndrome. Gut. 2005;54:264–267. doi: 10.1136/gut.2004.048132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ilyas M., Tomlinson I.P.M., Rowan A., Pignatelli M., Bodmer W.F. β-Catenin mutations in cell lines established from human colorectal cancers. Proc Natl Acad Sci U S A. 1997;94:10330–10334. doi: 10.1073/pnas.94.19.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guo J., Cagatay T., Zhou G., Chan C.C., Blythe S., Suyama K. Mutations in the human naked cuticle homolog NKD1 found in colorectal cancer alter Wnt/Dvl/beta-catenin signaling. PLoS One. 2009;4:1493–1494. doi: 10.1371/journal.pone.0007982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Powrozek T., Krawczyk P., Kucharczyk T., Milanowski J. Septin 9 promoter region methylation in free circulating DNA-potential role in noninvasive diagnosis of lung cancer: preliminary report. Med Oncol. 2014;31:917. doi: 10.1007/s12032-014-0917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pack S.C., Kim H.R., Lim S.W., Kim H.Y., Ko J.Y., Lee K.S. Usefulness of plasma epigenetic changes of five major genes involved in the pathogenesis of colorectal cancer. Int J Colorectal Dis. 2013;28:139–147. doi: 10.1007/s00384-012-1566-8. [DOI] [PubMed] [Google Scholar]
- 110.Grawenda A.M., O'Neill E. Clinical utility of RASSF1A methylation in human malignancies. Br J Cancer. 2015;113:372–381. doi: 10.1038/bjc.2015.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Z., Lee J.C., Lin L., Olivas V., Au V., LaFramboise T. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bai H., Wang Z., Chen K., Zhao J., Lee J.J., Wang S. Influence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:3077–3083. doi: 10.1200/JCO.2011.39.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Akca H., Demiray A., Yaren A., Bir F., Koseler A., Iwakawa R. Utility of serum DNA and pyrosequencing for the detection of EGFR mutations in non-small cell lung cancer. Cancer Genet. 2013;206:73–80. doi: 10.1016/j.cancergen.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 114.Leary R.J., Sausen M., Kinde I., Papadopoulos N., Carpten J.D., Craig D. Detection of chromosomal alterations in the circulation of cancer patients with whole-genome sequencing. Sci Transl Med. 2012;4:162ra54. doi: 10.1126/scitranslmed.3004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Narayan A., Carriero N.J., Gettinger S.N., Kluytenaar J., Kozak K.R., Yock T.I. Ultrasensitive measurement of hotspot mutations in tumor DNA in blood using error-suppressed multiplexed deep sequencing. Cancer Res. 2012;72:3492–3498. doi: 10.1158/0008-5472.CAN-11-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vinayanuwattikun C., Sriuranpong V., Tanasanvimon S., Chantranuwat P., Mutirangura A. Epithelial-specific methylation marker: a potential plasma biomarker in advanced non-small cell lung cancer. J Thorac Oncol. 2011;6:1818–1825. doi: 10.1097/JTO.0b013e318226b46f. [DOI] [PubMed] [Google Scholar]
- 117.Jiao L., Zhu J., Hassan M.M., Evans D.B., Abbruzzese J.L., Li D. K-ras mutation and p16 and preproenkephalin promoter hypermethylation in plasma DNA of pancreatic cancer patients: in relation to cigarette smoking. Pancreas. 2007;34:55–62. doi: 10.1097/01.mpa.0000246665.68869.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.He W.S., Bishop K.S. The potential use of cell-free-circulating-tumor DNA as a biomarker for prostate cancer. Expert Rev Mol Diagn. 2016;16:839–852. doi: 10.1080/14737159.2016.1197121. [DOI] [PubMed] [Google Scholar]
- 119.Cherepanova A.V., Tamkovich S.N., Bryzgunova O.E., Vlassov V.V., Laktionov P.P. Deoxyribonuclease activity and circulating DNA concentration in blood plasma of patients with prostate tumors. Ann NY Acad Sci. 2008;1137:218–221. doi: 10.1196/annals.1448.016. [DOI] [PubMed] [Google Scholar]
- 120.Delgado P.O., Alves B.C., Gehrke Fde S., Kuniyoshi R.K., Wroclavski M.L., Del Giglio A. Characterization of cell-free circulating DNA in plasma in patients with prostate cancer. Tumour Biol. 2013;34:983–986. doi: 10.1007/s13277-012-0634-6. [DOI] [PubMed] [Google Scholar]
- 121.Kinde I., Bettegowda C., Wang Y., Wu J., Agrawal N., Shih Ie M. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med. 2013;5:167ra4. doi: 10.1126/scitranslmed.3004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murtaza M., Dawson S.J., Tsui D.W., Gale D., Forshew T., Piskorz A.M. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108–112. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 123.Sikora K., Bedin C., Vicentini C., Malpeli G., D'Angelo E., Sperandio N. Evaluation of cell-free DNA as a biomarker for pancreatic malignancies. Int J Biol Markers. 2015;30:e136–e141. doi: 10.5301/jbm.5000088. [DOI] [PubMed] [Google Scholar]
- 124.Laird P.W. The power and the promise of DNA methylation markers. Nat Rev Cancer. 2003;3:253–266. doi: 10.1038/nrc1045. [DOI] [PubMed] [Google Scholar]
- 125.Feinberg A. DNA methylation in cancer: three decades of discovery. Genome Med. 2014;6:1–4. doi: 10.1186/gm553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jin H., Ma Y., Shen Q., Wang X. Circulating methylated DNA as biomarkers for cancer detection. methylation-from DNA, RNA and histones to diseases and treatment. Intech. 2013:137–152. [Google Scholar]
- 127.Miotto E., Sabbioni S., Veronese A., Calin G.A., Gullini S., Liboni A. Frequent aberrant methylation of the CDH4 gene promoter in human colorectal and gastric cancer. Cancer Res. 2004;64:8156–8159. doi: 10.1158/0008-5472.CAN-04-3000. [DOI] [PubMed] [Google Scholar]
- 128.Grady W.M., Rajput A., Lutterbaugh J.D., Markowitz S.D. Detection of aberrantly methylated hMLH1 promoter DNA in the serum of patients with microsatellite unstable colon cancer. Cancer Res. 2001;61:900–902. [PubMed] [Google Scholar]
- 129.Zou H.Z., Yu B.M., Wang Z.W., Sun J.Y., Cai H., Gao F. Detection of aberrant p16 methylation in the serum of colorectal cancer patients. Clin Cancer Res. 2002;8:188–191. [PubMed] [Google Scholar]
- 130.Lecomte T., Berger A., Zinzindohoue F., Micard S., Landi B., Blons H. Detection of free-circulating tumor-associated DNA in plasma of colorectal cancer patients and its association with prognosis. Int J Cancer. 2002;100:542–548. doi: 10.1002/ijc.10526. [DOI] [PubMed] [Google Scholar]
- 131.Ebert M.P., Model F., Mooney S., Hale K., Lograsso J., Tonnes-Priddy L. Aristaless-like homeobox-4 gene methylation is a potential marker for colorectal adenocarcinomas. Gastroenterology. 2006;131:1418–1430. doi: 10.1053/j.gastro.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 132.Lofton-Day C., Model F., Devos T., Tetzner R., Distler J., Schuster M. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin Chem. 2008;54:414–423. doi: 10.1373/clinchem.2007.095992. [DOI] [PubMed] [Google Scholar]
- 133.Warren J.D., Xiong W., Bunker A.M., Vaughn C.P., Furtado L.V., Roberts W.L. Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 2011;9:133. doi: 10.1186/1741-7015-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.De Vos T., Tetzner R., Model F., Weiss G., Schuster M., Distler J. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem. 2009;55:1337–1346. doi: 10.1373/clinchem.2008.115808. [DOI] [PubMed] [Google Scholar]
- 135.Silva J., Dominguez G., Gonzalez R., Garcia J., Corbacho C., Provencio M. Aberrant DNA methylation of the p16INK4a gene in plasma DNA of breast cancer patients. Br J Cancer. 1999;80:1262–1264. doi: 10.1038/sj.bjc.6690495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Esteller M., Sanchez-Cespedes M., Rosell R., Sidransky D., Baylin S.B., Herman J.G. Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients. Cancer Res. 1999;59:67–70. [PubMed] [Google Scholar]
- 137.El Messaoudi S., Rolet F., Mouliere F., Thierry A.R. Circulating cell free DNA: preanalytical considerations. Clin Chim Acta. 2013;424:222–230. doi: 10.1016/j.cca.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 138.Wang W.Y., Barratt B.J., Clayton D.G., Todd J.A. Genome-wide association studies: theoretical and practical concerns. Nat Rev Genet. 2005;6:109–118. doi: 10.1038/nrg1522. [DOI] [PubMed] [Google Scholar]
- 139.Xu J., Mo Z., Ye D., Wang M., Liu F., Jin G. Genome-wide association study in Chinese men identifies two new prostate cancer risk loci at 9q31.2 and 19q13.4. Nat Genet. 2012;44:1231–1235. doi: 10.1038/ng.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Forshew T., Murtaza M., Parkinson C., Gale D., Tsui D.W.Y., Kaper F. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012;4:136ra68. doi: 10.1126/scitranslmed.3003726. [DOI] [PubMed] [Google Scholar]
- 141.Ashida A., Sakaizawa K., Mikoshiba A., Uhara H., Okuyama R. Quantitative analysis of the BRAF mutation in circulating tumor-derived DNA in melanoma patients using competitive allele-specific TaqMan PCR. Int J Clin Oncol. 2016;21:981–988. doi: 10.1007/s10147-016-0976-y. [DOI] [PubMed] [Google Scholar]
- 142.Hindson C.M., Chevillet J.R., Briggs H.A., Gallichotte E.N., Ruf I.K., Hindson B.J. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods. 2013;10:1003–1005. doi: 10.1038/nmeth.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Newman A.M., Bratman S.V., To J., Wynne J.F., Eclov N.C., Modlin L.A. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bratman S.V., Newman A.M., Alizadeh A.A., Diehn M. Potential clinical utility of ultrasensitive circulating tumor DNA detection with CAPP-Seq. Expert Rev Mol Diagn. 2015;15:715–719. doi: 10.1586/14737159.2015.1019476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Newman A.M., Lovejoy A.F., Klass D.M., Kurtz D.M., Chabon J.J., Scherer F. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol. 2016;34:547–555. doi: 10.1038/nbt.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chan K.C., Jiang P., Zheng Y.W., Liao G.J., Sun H., Wong J. Cancer genome scanning in plasma: detection of tumor-associated copy number aberrations, single-nucleotide variants, and tumoral heterogeneity by massively parallel sequencing. Clin Chem. 2013;59:211–224. doi: 10.1373/clinchem.2012.196014. [DOI] [PubMed] [Google Scholar]
- 147.Vogelstein B., Kinzler K.W. Digital PCR. Proc Natl Acad Sci U S A. 1999;96:9236–9241. doi: 10.1073/pnas.96.16.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gray E.S., Rizos H., Reid A.L., Boyd S.C., Pereira M.R., Lo J. Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget. 2015;6:42008–42018. doi: 10.18632/oncotarget.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tsao S.C., Weiss J., Hudson C., Christophi C., Cebon J., Behren A. Monitoring response to therapy in melanoma by quantifying circulating tumour DNA with droplet digital PCR for BRAF and NRAS mutations. Sci Rep. 2015;5:11198. doi: 10.1038/srep11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang A., Zhang X., Zhou S.L., Cao Y., Huang X.W., Fan J. Detecting circulating tumor DNA in hepatocellular carcinoma patients using droplet digital PCR is feasible and reflects intratumoral heterogeneity. J Cancer. 2016;7:1907–1914. doi: 10.7150/jca.15823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Laurent-Puig P., Pekin D., Normand C., Kotsopoulos S.K., Nizard P., Perez-Toralla K. Clinical relevance of KRAS-mutated subclones detected with picodroplet digital PCR in advanced colorectal cancer treated with anti-EGFR therapy. Clin Cancer Res. 2015;21:1087–1097. doi: 10.1158/1078-0432.CCR-14-0983. [DOI] [PubMed] [Google Scholar]
- 152.Denis J.A., Patroni A., Guillerm E., Pépin D., Benali-Furet N., Wechsler J. Droplet digital PCR of circulating tumor cells from colorectal cancer patients can predict KRAS mutations before surgery. Mol Oncol. 2016;10:1221–1231. doi: 10.1016/j.molonc.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mishima C., Kagara N., Matsui S., Tanei T., Naoi Y., Shimoda M. Promoter methylation of TRIM9 as a marker for detection of circulating tumor DNA in breast cancer patients. Springerplus. 2015;4:635. doi: 10.1186/s40064-015-1423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Balgkouranidou I., Chimonidou M., Milaki G., Tsaroucha E., Kakolyris S., Georgoulias V. SOX17 promoter methylation in plasma circulating tumor DNA of patients with non-small cell lung cancer. Clin Chem Lab Med. 2016;54:1385–1393. doi: 10.1515/cclm-2015-0776. [DOI] [PubMed] [Google Scholar]
- 155.Wang J., Qin Y., Li B., Sun Z., Yang B. Detection of aberrant promoter methylation of GSTP1 in the tumor and serum of Chinese human primary hepatocellular carcinoma patients. Clin Biochem. 2006;39:344–348. doi: 10.1016/j.clinbiochem.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 156.Feehery GR, Pradhan S. Method for enriching methylated CpG sequences. US Patent Application 2013;US8367331.
- 157.Herman J.G., Graff J.R., Myöhänen S., Nelkin B.D., Baylin S.B. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Licchesi J.D., Herman J.G. Methylation-specific PCR. Methods Mol Biol. 2009;507:305–323. doi: 10.1007/978-1-59745-522-0_22. [DOI] [PubMed] [Google Scholar]
- 159.Kimler B., Fackler M.J., Metheny T., Phillips T., Sukumar S., Fabian C. Consistency of quantitative multiplexed – methylation specific PCR (QM-MSP) performed on breast epithelial cells acquired by random periareolar fine needle aspiration (RPFNA) of women at high risk for development of breast cancer. Cancer Res. 2007;67 [Google Scholar]
- 160.Bailey V.J., Zhang Y., Keeley B.P., Yin C., Pelosky K.L., Brock M. Single-tube analysis of DNA methylation with silica superparamagnetic beads. Clin Chem. 2010;56:1022–1025. doi: 10.1373/clinchem.2009.140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wong I.H., Lo Y.M., Zhang J., Liew C.T., Ng M.H., Wong N. Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients. Cancer Res. 1999;59:71–73. [PubMed] [Google Scholar]
- 162.Eads C.A., Danenberg K.D., Kawakami K., Saltz L.B., Blake C., Shibata D. MethyLight: a high-throughput assay to measure DNA methylation. Nucl Acids Res. 2000;28:E32. doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bailey V.J., Keeley B.P., Zhang Y., Ho Y.P., Easwaran H., Brock M.V. Enzymatic incorporation of multiple dyes for increased sensitivity in QD-FRET sensing for DNA methylation detection. ChemBioChem. 2010;11:71–74. doi: 10.1002/cbic.200900630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guzzetta A.A., Pisanic Ii T.R., Sharma P., Yi J.M., Stark A., Wang T.H. The promise of methylation on beads for cancer detection and treatment. Expert Rev Mol Diagn. 2014;14:845–852. doi: 10.1586/14737159.2014.943665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fackler M.J., Lopez Bujanda Z., Umbricht C., Teo W.W., Cho S., Zhang Z. Novel methylated biomarkers and a robust assay to detect circulating tumor DNA in metastatic breast cancer. Cancer Res. 2014;74:2160–2170. doi: 10.1158/0008-5472.CAN-13-3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li Y., Zhu J., Geng T., Ning L., Li Q., Ye M. The DNA methylome of human peripheral blood mononuclear cells. PLoS Biol. 2010;8:549–552. doi: 10.1371/journal.pbio.1000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Urich M.A., Nery J.R., Lister R., Schmitz R.J., Ecker J.R. MethylC-seq library preparation for base-resolution whole-genome bisulfite sequencing. Nat Protoc. 2015;10:475–483. doi: 10.1038/nprot.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Smith Z.D., Gu H.C., Bock C., Gnirke A., Meissner A. High-throughput bisulfite sequencing in mammalian genomes. Methods. 2009;48:226–232. doi: 10.1016/j.ymeth.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Serre D., Lee B.H., Ting A.H. MBD-isolated genome sequencing provides a high-throughput and comprehensive survey of DNA methylation in the human genome. Nucl Acids Res. 2010;38:391–399. doi: 10.1093/nar/gkp992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Clark C., Palta P., Joyce C.J., Scott C., Grundberg E., Deloukas P. A comparison of the whole genome approach of MeDIP-Seq to the targeted approach of the infinium humanmethylation450 BeadChip® for methylome profiling. PLoS One. 2012;7:e50233. doi: 10.1371/journal.pone.0050233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Taiwo O., Wilson G.A., Morris T., Seisenberger S., Reik W., Pearce D. Methylome analysis using MeDIP-seq with low DNA concentrations. Nat Protoc. 2012;7:617–636. doi: 10.1038/nprot.2012.012. [DOI] [PubMed] [Google Scholar]
- 172.Chan K.C., Jiang P., Chan C.W., Sun K., Wong J., Hui E.P. Noninvasive detection of cancer-associated genome-wide hypomethylation and copy number aberrations by plasma DNA bisulfite sequencing. Proc Natl Acad Sci U S A. 2013;110:18761–18768. doi: 10.1073/pnas.1313995110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wen L., Li J., Guo H., Liu X., Zheng S., Zhang D. Genome-scale detection of hypermethylated CpG islands in circulating cell-free DNA of hepatocellular carcinoma patients. Cell Res. 2015;25:1250–1264. doi: 10.1038/cr.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rand K., Qu W., Ho T., Clark S.J., Molloy P. Conversion-specific detection of DNA methylation using real-time polymerase chain reaction (ConLight-MSP) to avoid false positives. Methods. 2002;27:114–120. doi: 10.1016/s1046-2023(02)00062-2. [DOI] [PubMed] [Google Scholar]
- 175.Ilie M., Hofman V., Long E., Bordone O., Selva E., Washetine K. Current challenges for detection of circulating tumor cells and cell-free circulating nucleic acids, and their characterization in non-small cell lung carcinoma patients. What is the best blood substrate for personalized medicine? Ann Transl Med. 2014;2:107. doi: 10.3978/j.issn.2305-5839.2014.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gormally E., Caboux E., Vineis P., Hainaut P. Circulating free DNA in plasma or serum as biomarker of carcinogenesis: practical aspects and biological significance. Mutat Res. 2007;635:105–117. doi: 10.1016/j.mrrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 177.Schwarzenbach H., Muller V., Milde-Langosch K., Steinbach B., Pantel K. Evaluation of cell-free tumour DNA and RNA in patients with breast cancer and benign breast disease. Mol Biosyst. 2011;7:2848–2854. doi: 10.1039/c1mb05197k. [DOI] [PubMed] [Google Scholar]
- 178.Park J.L., Kim H.J., Choi B.Y., Lee H.C., Jang H.R., Song K.S. Quantitative analysis of cell-free DNA in the plasma of gastric cancer patients. Oncol Lett. 2012;3:921–926. doi: 10.3892/ol.2012.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Salvianti F., Pinzani P., Verderio P., Ciniselli C.M., Massi D., De Giorgi V. Multiparametric analysis of cell-free DNA in melanoma patients. PLoS One. 2012;7:e49843. doi: 10.1371/journal.pone.0049843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ha T.T., Huy N.T., Murao L.A., Lan N.T., Thuy T.T., Tuan H.M. Elevated levels of cell-free circulating DNA in patients with acute dengue virus infection. PLoS One. 2011;6:e25969. doi: 10.1371/journal.pone.0025969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Huang C.H., Tsai M.S., Hsu C.Y., Chen H.W., Wang T.D., Chang W.T. Circulating cell-free DNA levels correlate with postresuscitation survival rates in out-of-hospital cardiac arrest patients. Resuscitation. 2012;83:213–218. doi: 10.1016/j.resuscitation.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 182.Iriyama C., Tomita A., Hoshino H., Adachi-Shirahata M., Furukawa-Hibi Y., Yamada K. Using peripheral blood circulating DNAs to detect CpG global methylation status and genetic mutations in patients with myelodysplastic syndrome. Biochem Biophys Res Commun. 2012;419:662–669. doi: 10.1016/j.bbrc.2012.02.071. [DOI] [PubMed] [Google Scholar]
- 183.Kwee S., Song M.A., Cheng I., Loo L., Tiirikainen M. Measurement of circulating cell-free DNA in relation to 18F-fluorocholine PET/CT imaging in chemotherapy-treated advanced prostate cancer. Clin Transl Sci. 2012;5:65–70. doi: 10.1111/j.1752-8062.2011.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Klein C.A. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 185.Ma M., Zhu H., Zhang C., Sun X., Gao X., Chen G. “Liquid biopsy”-ctDNA detection with great potential and challenges. Ann Transl Med. 2015;3:235. doi: 10.3978/j.issn.2305-5839.2015.09.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pan H., Chen L., Dogra S., Teh A.L., Tan J.H., Lim Y.I. Measuring the methylome in clinical samples: improved processing of the infinium human methylation450 beadchip array. Epigenetics. 2012;7:1173–1187. doi: 10.4161/epi.22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mack S.C., Witt H., Piro R.M., Gu L., Zuyderduyn S., Stutz A.M. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506:445–450. doi: 10.1038/nature13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hudecova I. Digital PCR analysis of circulating nucleic acids. Clin Biochem. 2015;48:948–956. doi: 10.1016/j.clinbiochem.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 189.Jansen M., Martens J., Helmijr J., Beaufort C., Van M., Krol N. Cell-free DNA mutations as biomarkers in breast cancer patients receiving tamoxifen. Oncotarget. 2016;7:43412–43418. doi: 10.18632/oncotarget.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Souverijn J.H. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;371:187. doi: 10.1056/NEJMc1405215. [DOI] [PubMed] [Google Scholar]
- 191.Church T.R., Wandell M., Lofton-Day C., Mongin S.J., Burger M., Payne S.R. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63:317–325. doi: 10.1136/gutjnl-2012-304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ilse P., Biesterfeld S., Pomjanski N., Wrobel C., Schramm M. Analysis of SHOX2 methylation as an aid to cytology in lung cancer diagnosis. Cancer Genomics Proteomics. 2014;11:251–258. [PubMed] [Google Scholar]
- 193.Brown P. The Cobas(®) EGFR mutation test v2 assay. Future Oncol. 2016;12:451–452. doi: 10.2217/fon.15.311. [DOI] [PubMed] [Google Scholar]
- 194.Kopreski M., Benko F., Kwee C., Leitzel K., Eskander E., Lipton A. Detection of mutant K-ras DNA in plasma or serum of patients with colorectal cancer. Br J Cancer. 1997;76:1293. doi: 10.1038/bjc.1997.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yamada T., Nakamori S., Ohzato H., Oshima S., Aoki T., Higaki N. Detection of K-ras gene mutations in plasma DNA of patients with pancreatic adenocarcinoma: correlation with clinicopathological features. Clin Cancer Res. 1998;4:1527–1532. [PubMed] [Google Scholar]
- 196.Sanchez-Cespedes M., Monzo M., Rosell R., Pifarre A., Calvo R., Lopez-Cabrerizo M.P. Detection of chromosome 3p alterations in serum DNA of non-small-cell lung cancer patients. Ann Oncol. 1998;9:113–116. doi: 10.1023/a:1008230331221. [DOI] [PubMed] [Google Scholar]