Abstract
The concept of scleral stiffening therapies has emerged as a novel theoretical approach for treating the ocular disorders glaucoma and myopia. Deformation of specific regions of the posterior eye is innately involved in the pathophysiology of these diseases, and thus targeted scleral stiffening could resist these changes and slow or prevent progression of these diseases. Here, we present the first systematic screen and direct comparison of the stiffening effect of small molecule collagen cross-linking agents in the posterior globe, namely using glyceraldehyde, genipin and methylglyoxal (also called pyruvaldehyde). To establish a dose–response relationship, we used inflation testing to simulate the effects of increasing intraocular pressure in freshly harvested rat eyes stiffened with multiple concentrations of each agent. We used digital image correlation to compute the mechanical strain in the tissue as a metric of stiffness, using a novel treatment paradigm for screening relative stiffening by incubating half of each eye in cross-linker and using the opposite half as an internal control. We identified the doses necessary to increase stiffness by approximately 100%, namely 30 mM for glyceraldehyde, 1 mM for genipin and 7 mM for methylglyoxal, and we also identified the range of stiffening it was possible to achieve with such agents. Such findings will inform development of in vivo studies of scleral stiffening to treat glaucoma and myopia.
Keywords: biomechanics, rat, sclera, collagen, cross-linking, digital image correlation
1. Introduction
Vision loss has been ranked in patient surveys as the worst possible type of health outcome, equivalent to a diagnosis of cancer, HIV/AIDS and losing a limb [1]. It is therefore unfortunate that there is no known cure for glaucoma, the second leading cause of blindness [2], or myopia, the most common vision disorder [3] with incidence rates approaching 90% in some countries [4]. Although both diseases can be treated, these treatments are not successful in all patients and are not a true cure. In glaucoma, for example, 25–45% of patients continue to lose vision even with treatment [5–7]. At present, all therapies for glaucoma are based upon the notion of reducing intraocular pressure (IOP); when these approaches fail, there is no alternative treatment paradigm. Thus, there is significant clinical need for novel treatments for vision loss from glaucoma and myopia.
Some evidence suggests that stiffening the sclera may be a beneficial treatment for these diseases (reviewed extensively in [8]). In glaucoma, the elastic modulus of the peripapillary sclera (the region immediately surrounding the optic nerve) has been shown in computer models and physical tests to strongly influence deformation of the lamina cribrosa, the region where axonal damage first starts [8–13]. In myopia, the stiffness of the sclera may play a role as well, although conflicting data exist, warranting further study [14,15]. Finally, corneal stiffening is currently used as a clinical treatment for keratoconus [16], suggesting that the eye can tolerate local modulation of the stiffness of its collagenous tissues.
Pursuant from this evidence, in vivo testing of scleral stiffening therapies for disorders of the posterior eye is indicated. This requires dose–response relationships for suitable agents to be well understood. Collagen cross-linking agents have been reported in the orthopaedic and ophthalmic literature to modulate stiffness, and, based upon this evidence, three agents have emerged with potential for posterior eye scleral stiffening: glyceraldehyde [17–24], genipin [25–32] and methylglyoxal [20,31,33] (also called pyruvaldehyde). Glutaraldehyde is known to increase scleral stiffness [12,19] but is toxic in vivo [34], and riboflavin, used in the treatment of keratoconus, requires ultraviolet light to induce cross-linking [16], which adds complications for posterior eye delivery in a clinical setting.
Although these agents have been identified and studied in an ocular context, no studies to date have directly compared the dose–stiffening relationship of all these agents for sclera. A few studies have examined multiple agents [20,31] or more than two concentrations of a single agent [25,29,33] side by side, but the paucity of agents interrogated with identical testing methodologies limits the ability to widely compare the dose–stiffening relationship of scleral collagen cross-linking agents. Given the prevalence and acceptance of rodent models in pre-clinical studies of treatments for vision disorders, there is also significant need for a well-characterized dose of scleral stiffening agents to be used in animal trials. Here, we hypothesize that incubation in collagen cross-linking agents will locally reduce the strain in the sclera resulting from elevated IOP in a dose-dependent manner. In this study, our specific objective is to determine the dose–response of each agent's effect on scleral stiffness with a goal of approximately doubling scleral stiffness (roughly the magnitude observed in prior trials [19]) for future use in vivo.
2. Material and methods
2.1. Animals
Eyes were freshly harvested from a total of 67 euthanized male, retired breeder (approx. 9–12 months old) Brown Norway rats (Charles River Laboratories, Inc., Wilmington, MA) that were otherwise experimentally naive. Female retired breeders were not used in this initial study, as oestrogen is known to modulate collagen density and turnover with mechanical consequences [35], and female rats that have had numerous litters (such as retired breeders) may have atypical oestrogen levels. Further work will consider animals of both genders.
Based upon the results of preliminary studies performed during methods development, we used an a priori power analysis to estimate that we needed three rats/concentration/agent (nested 2-factor ANOVA (agent and concentration); α = 0.05; ratio of treatment effect to error effect size = 1.2; 95% power). To be conservative, we harvested five eyes per group and used all that were not excluded due to methodological problems (e.g. puncture while cleaning or air bubble when inflating) except for two groups where we harvested eight eyes (62.5 mM and 125 mM glyceraldehyde).
2.2. Tissue preparation
2.2.1. Stiffening agents
Stiffening agents and administered concentrations were chosen based on published studies [17–33]. We used three agents: genipin (078-03021; Wako Pure Chemical Industries Ltd, Richmond, VA), glyceraldehyde (G5001-5G; Sigma-Aldrich Corp., St. Louis, MO) and methylglyoxal (W296902-100G; Sigma-Aldrich Corp., St. Louis, MO). Several concentrations (table 1) of each agent were used to establish a dose–response curve of concentration and relative stiffness. All dilutions were made in phosphate-buffered saline (PBS) except for glyceraldehyde, which was made at stock concentration (500 mM) in deionized water to obtain an osmolality similar to extracellular fluid, then diluted further with PBS.
Table 1.
Concentrations of all stiffening agents tested.
| stiffening agent | concentration (mM) | concentration (% w/v in PBS) |
|---|---|---|
| genipin | 0.25, 0.50, 1.0, 7.5, 15, 30 | 0.06, 0.11, 0.23, 1.7, 3.4, 6.8 |
| glyceraldehyde | 10.0, 30.0, 62.5, 125 | 0.90, 2.7, 5.7, 11 |
| methylglyoxal | 3.5, 7.0, 14 | 0.25, 0.50, 1.0 |
2.2.2. Partial incubation technique
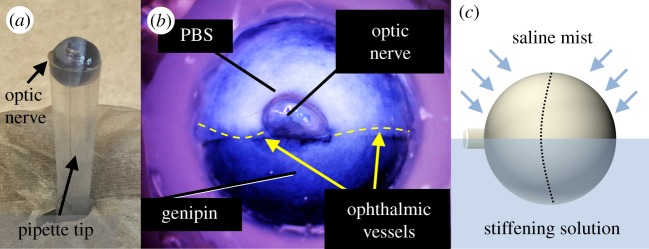
Intact eyes were incubated in stiffening agents overnight, such that half the sclera was immersed in the stiffening solution (treated) and the other half (control) was moistened by PBS. Freshly harvested rat eyes were cleaned under a dissecting microscope by carefully removing excess fat, connective tissue and musculature from the posterior sclera. A 3 ml polypropylene transfer pipette (225; Samco Thermo Scientific, Waltham, MA) was then trimmed to yield a cone approximately the diameter of the eye (approx. 6.5 mm). The eye was then gently placed into the cut pipette with the anterior–posterior axis (identified by the position of the optic nerve) parallel to the cut (figure 1a) with the ophthalmic blood vessels aligned with the cut and serving as natural landmarks to aid in identifying the scleral region exposed to stiffening agent. Two small (approx. 1 mm diameter) droplets of glue (Loctite Super Glue Ultragel Control; Henkel Corporation, Westlake, OH) were applied to the cornea with a toothpick, attaching the cornea to the pipette, and a third droplet was carefully applied to the face of the distal optic nerve so that no glue touched the sclera. Drops of PBS were applied to keep the eye moist during handling. Stiffening agent (table 1) was injected slowly into the pipette tip with a hypodermic needle until all air was evacuated.
Figure 1.
Eyes were partially immersed in cross-linking agents, exposing approximately half the eye to a stiffening agent overnight by mounting it in a trimmed pipette tip (a). Genipin, which is also used as a blue dye, provides a visual indicator of its location (b). This is closely localized to the treated region and demonstrates little evidence of wicking. Regions appearing blueish near the top of panel (b) are actually thin regions of translucent sclera where choroid is visible, not regions exposed to genipin. Eyes were then incubated overnight while misting the tissue-draped control half with PBS to keep it moist (c). Dashed line indicates the limbus.
Once the pipette was filled with the agent, a small rectangle (4 × 8 mm) of paraffin film was tightly wrapped around the opening at the bottom of the pipette to prevent any stiffening agent from leaking out. A Kimwipe was cut into a 5 × 5 cm cross shape, wetted with PBS, draped over the top of the eye and then wetted with PBS to maintain moisture in the region not immersed in stiffening solution. The entire assembly (pipette, eye and Kimwipe) was then placed into a PBS-filled 1.5 ml microtube with the dangling strips of the Kimwipe allowing PBS to wick up to keep the control portion of the eye moist. To further maintain physiological conditions overnight, the microtube was placed in a floating rack in a 37°C water bath (Precision Shallow Chamber Water Bath 280; Thermo Scientific, Waltham, MA) and misted from above (Monsoon RS400; Exo Terra, Mansfield, MA) every 3 min with PBS (figure 1c). Eyes were carefully removed from the tube the next day (approx. 16 h incubation time) and mounted for inflation testing.
2.3. Inflation testing
Stiffening agents were evaluated by comparing mechanical strain measurements (stiffened versus control regions) during whole globe inflation tests. We modulated the IOP of each eye while submerged in a PBS bath at physiological temperature. Calibrated stereo cameras (including compensation for refraction through PBS) imaged a speckle pattern on the surface of the eye throughout the inflation test, and three-dimensional digital image correlation (DIC) was used to quantify surface strain (Q-400 DIC; Dantec Dynamics, Holtsville, NY).
2.3.1. Testing chamber construction
The eye was submerged in a temperature-controlled, PBS-filled plastic chamber (Kritter Keeper; Lee's Aquarium & Pets, San Marcos, CA) during experimentation. To model physiological conditions ex vivo, the temperature of the PBS in the chamber was maintained at 37°C ± 2°C throughout the experiment by pumping saline through a thermoelectric heater assembly (LA-045-24-02-00-00; Laird Technologies, London, UK; temperature controller TC-XX-PR-59; measured by thermistor TC-NTC-1 immersed next to the eye) using a peristaltic pump (BT300 L; Golander LLC, Duluth, GA; pump head DT15-44; tubing no. 25 (ID 4.8 mm, OD 8 mm)) at 60 ml min−1. This low flow rate was selected so as not to produce any turbulence and resultant optical distortion in the PBS around the eye.
To avoid evaporation of PBS during experimentation, a 1/8″ thick borosilicate glass sheet was placed over the chamber and warmed to 70°C to prevent condensation (3682K25; McMaster Carr, Douglasville, GA; PID controller 36815K71). The mounted eye was then illuminated from above with dual gooseneck lighting (Mi-LED-US-DG; Dolan-Jenner Industries, Boxborough, MA).
An adjustable-height pressure reservoir [36] was connected to the base of the chamber through silicone tubing connected to a bulkhead fitting. This presented a female luer connection on the inside surface of the chamber where we could attach mounted eyes and modulate their IOP using hydrostatic pressure.
2.3.2. Mounting procedure
Prior to experimentation, custom-made mounting blocks (figure 2) were manufactured from acrylic sheets (8560K369; McMaster Carr, Douglasville, GA). A 1/4″ diameter ball end mill created a hemispherical cradle for rat eyes, and a thin channel was drilled through the block with a 1/16″ drill bit. This hole was widened opposite the indentation for the eye using a 3/16″ drill bit that could accept a luer fitting adaptor.
Figure 2.
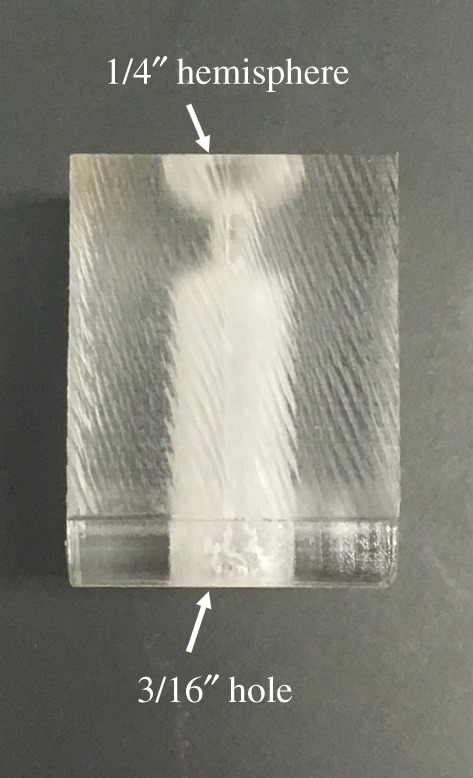
Side view of the acrylic mounting block. Eyes are placed in the hemisphere at the top, and a threaded luer fitting mates with the hole in the bottom.
Following overnight (16 h) incubation, the orientation of the eye relative to the solution was recorded. The cornea was blotted dry with a Kimwipe, and a small, continuous bead of gel superglue was applied along the inner rim of the mounting block hemisphere. The eye was then placed onto the hemisphere, cornea-side down, with the optic nerve centred upwards and excess glue was scraped away. The mounting block was marked with a waterproof marker to record the region of the eye that was incubated in stiffening solution.
In order for DIC to evaluate displacements, a speckle pattern must be applied to the tissue. For this study, the speckle pattern was applied to the posterior sclera with graphite powder (no. 970 PG; General Pencil Company, Inc., Redwood City, CA). Graphite was poured onto a fine mesh sieve (tensile bolting cloth no. 60; Amazon), and an airbrush was used to blow the powder through the sieve onto the external surface of the eye and allowed to dry briefly. This method was repeated until the graphite powder formed a speckle pattern that did not detach from the surface of the eye when submerged in PBS. Eyes were immersed in ice-cold PBS until testing began.
2.3.3. Experimental procedure
Prior to testing each day, the PBS chamber was filled and heated to temperature, and the intrinsic stereo-calibration parameters of the cameras were determined using a standardized chessboard calibration target. To inflate the eye, the cornea was punctured by inserting a 1 mm biopsy punch through the 3/16″ hole in the mounting block and twisting gently until slight collapse of the eye was observed. Care was taken to ensure that the eye did not detach from the mounting block, nor that the biopsy punch deeply penetrated the eye. A threaded male luer fitting (EW-45505-84; Cole-Parmer, Vernon Hills, IL) was then glued into the 3/16″ hole.
The pressure reservoir was set to the height corresponding to the baseline IOP of 3 mmHg (approximately the minimum necessary to prevent the eye globe from buckling under its own weight). PBS was injected through polyethylene tubing into the lumen of the mounting block to purge all air bubbles. The eye was then submerged in the PBS chamber 25 mm below the surface, imparting an external pressure of approximately 2 mmHg to the eye, and attached to the luer fitting at the base of the chamber connected to the pressure reservoir. Extrinsic camera calibration parameters were then determined after the eye was mounted to account for refraction through the borosilicate glass sheet and PBS [37].
Effective IOP was calculated by subtracting the external pressure on the eyes (2 mmHg from the tissue bath) from the internal hydrostatic pressure from the reservoir. Images were captured every 30 s at an exposure time of 20 ms for 30 min (see DIC system characterization results) at each of three pressures: 3 (low/hypotensive IOP), 13 (normal/normotensive IOP) and 28 mmHg (high/hypertensive IOP). The pressure reservoir was raised after each set of 60 images to the next height via a stepper motor at a speed of 5 mm s−1. Eyes were not preconditioned prior to inflation testing.
2.3.4. Strain calculation
Dantec's Istra 4D software (v. 4.4.1) was used to compute displacement and the resulting principal strains from the image dataset using DIC. Correlation settings were: 99-pixel facets, 45-pixel grid spacing, maximum permissible start point accuracy 0.2 pixels, residuum of 30 grey values and three-dimensional residuum of 1.1 pixels. All strain calculations were performed from smoothed displacement data using a two-dimensional bicubic spline function to the dataset. The grid reduction factor (minimizes the difference between the data point and the spline function) was set to 3 for displacement and 2 for contours, and the smoothness factor (straightens the filtered data) was set to 0 for both items.
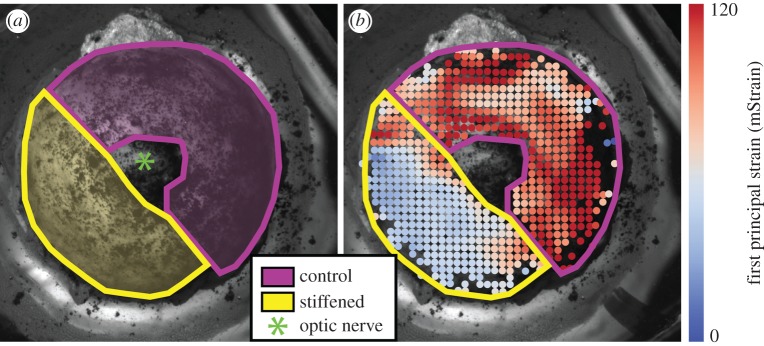
Strain was computed relative to the reference state (3 mmHg after 30 min). Exported strain data for each image were then segmented (figure 3) in custom Matlab software (R2016a; MathWorks, Natick, MA) by manually tracing the experimental and control regions of the posterior sclera (excluding the optic nerve) based upon the markings made on the mounting block prior to testing. Relative stiffness as a per cent change between Eexp (elastic modulus in the experimental region) and Econ (elastic modulus in the control region; see appendix A for derivation) was defined as
| 2.1 |
where ɛcon represents strain in the control region, and ɛexp represents strain in the stiffened region. The calculation was performed following outlier removal, as described in the Data analysis section.
Figure 3.
DIC was used to spatially resolve the surface strains in individual eyes. (a) The speckle pattern on the posterior sclera is overlaid with manually traced masks (made prior to calculating strain) denoting the locations treated with cross-linking agent or PBS as a control, taking care not to include the optic nerve. (b) We have overlaid these same masks on the computed surface strains at an inflation pressure of 13 mmHg (normotensive). Regions of comparatively low and high strain match closely with the treatment and control zones.
2.3.5. Data analysis
DIC data are noisy, particularly when dealing with small strains, as tiny errors in displacements become amplified in strain computations. Although smoothing displacements helps minimize this type of error, we required outlier detection to remove spurious data points. Having verified that the data were normally distributed within both the experimental and control regions of each eye at each time point (Anderson–Darling normality test, p > 0.05), the median absolute deviation (MAD) was calculated according to 1.4826 times the median of the absolute values of the difference between each data point and the median [38]. Any values that were more than two MADs away from the median were considered to be outliers and removed from the dataset.
We then computed the mean and standard deviation of the first principal Lagrange strain, as this metric is sensitive to deformation in the direction of local stretching, for a given control or experimental region at each time point. The primary deformation mode of a spherical eye is expected to be a hoop deformation, which would result in in-plane extension of the sclera; thus, the principal Lagrange strains should capture this effect. Following outlier removal, we used a weighted linear fit of this strain metric (weighted by 1/σ2) using Matlab's lmfit function using strains from the final 10 min at normotensive and hypertensive IOPs each. If the slope of this fit was above 0.5 millistrain (mStrain) per minute, we assumed the eye was creeping and had not reached its steady state, and thus the eye was discarded from further analysis (2 of 73 total inflation tests were excluded under this criterion). We then recorded the intercept of fits that were not excluded as well as the 95% confidence interval (CI) of the intercept of this fit as an indication of the uncertainty of the test.
Finally, we used equation (2.1) to compute the relative stiffness at normotensive and hypertensive IOPs for both the control and experimental halves of the eye. Using a nested 2-factor ANOVA (relative stiffening as a function of pressure nested within concentration; R v. 3.3.1), we compared the relative stiffness of each ocular region as a function of treatment and inflation pressure.
3. Results
3.1. Digital image correlation system characterization
We characterized two aspects of our inflation testing system. First, to estimate the baseline correlation noise of the system, we speckled a glass sphere of approximately the same radius as a rat eye (3.25 mm radius; 8996K25; McMaster-Carr), immersed it in our PBS bath and imaged it for 8 h. Noise was <2 mStrain, indicating this level as the minimum resolvable strain magnitude.
To study the viscoelastic relaxation of pressurized rat eyes, we also imaged an untreated pair of rat eyes at pressure levels corresponding to baseline/hypotensive, normotensive and hypertensive IOPs (3, 13 and 28 mmHg) for 2 h per pressure level. We fitted a standard Kelvin–Voigt model of viscoelastic relaxation
| 3.2 |
to this strain ɛ as a function of time t in Matlab with fitting constants A, C and τ, and we found that the time constant τ was approximately 1 min. Out of abundance of caution, specifically to avoid confounding our stiffness findings with biomechanical creep of the scleral shell, we thus maintained our treated eyes for 30 min at these same three pressure levels and only analysed data from the final 10 min of each pressure step.
3.2. Partial immersion of eyes in collagen cross-linking agents
Eyes were partially immersed in various stiffening agents overnight such that approximately half the eye was exposed to the collagen cross-linking agent and the other half to PBS as a control. Genipin, which is also used as a blue dye, acted as a visual reporter of its presence, confirming that the agent stayed constrained to the incubation region and did not diffuse or wick into the control region (figure 1b). We also visually confirmed that agents did not adversely affect the structure of the eye. In preliminary experiments (not shown), we incubated eyes overnight in 500 mM glyceraldehyde, as has been done previously [19–21]. However, the eyes were visibly dehydrated the following day. We calculated that the osmolarity of 500 mM glyceraldehyde is approximately 800 mOsm, whereas the osmolarity of aqueous humour and PBS is about 300 mOsm [39]. Thus, we diluted the glyceraldehyde and only used lower concentrations in these experiments.
3.3. Average strain magnitudes
In almost all eyes, the mean first and second principal strains (representing stretch in the direction of greatest local deformation and the stretch orthogonal to this direction, both tangent to the surface of the eye) in the control half of the eye were of the order of 40–150 mStrain at 13 and 28 mmHg, respectively, relative to the reference configuration at 3 mmHg. These strain values are well above the noise floor of our system. In the stiffened half of the eye, strains were lower, generally 10–50 mStrain, again above the noise floor. Strains stabilized within minutes of a change in pressure in all but two cases, and the difference in strain between baseline/hypotensive and normotensive pressures was always considerably larger than between the normotensive and hypertensive pressures (figure 4). Second principal strains in the posterior sclera were approximately half the magnitude of first principal strains, consistent with current understanding that there is a direction of preferential collagen fibre alignment but that the posterior sclera is quasi-transversely isotropic tangent to the scleral surface [40]. The distribution of strains within each region at any given time point followed a normal distribution (Anderson–Darling test; p > 0.05).
Figure 4.
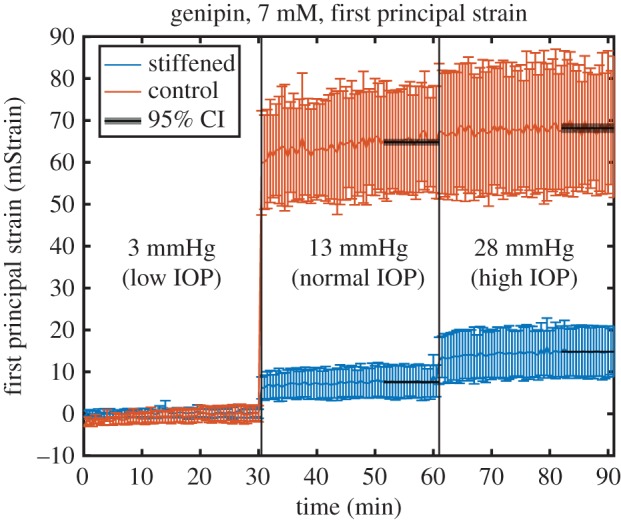
Representative plot of average first principal strain as a function of time from a single eye during our inflation experiment. Eyes were maintained for 30 min at each of three pressures representing different ranges of IOP. Strains were considerably higher in the control region of the eye than in the treated region, indicating that the treated region is stiffer. Black overlays represent the 95% CI about the mean during the final 10 min of each pressure step, when the eye reached steady state. Error bars: standard deviation over the interrogated region. Raw data to generate these figures are included in the electronic supplementary material.
3.4. Relative stiffening
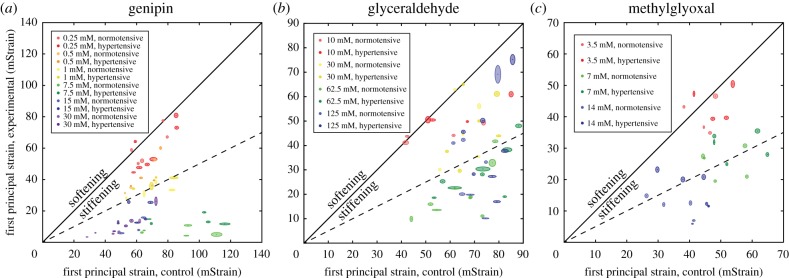
We observed a significant (p = 1.03 × 10−9) stiffening effect (relative stiffening as a function of pressure nested within concentration; table 2) pooled over all agents. All three agents demonstrated a dose-dependent stiffening effect where increasing the concentration of the solution increased the relative stiffness of the treated region. However, at very high concentrations (for genipin, above 7.5 mM; for glyceraldehyde, above 62.5 mM), increasing concentration did not increase stiffness. For genipin (figure 5a), we observed stiffness increases between 14.7% and 1320%. For glyceraldehyde (figure 5b), stiffness increased between 21.8% and 273%, and for methylglyoxal (figure 5c) stiffness increased between 11.9% and 310% at the concentrations included in these studies. In order to achieve a target increase in stiffness of approximately 100% [13], the appropriate dose for a rat eye overnight is therefore approximately 1 mM for genipin, 30 mM for glyceraldehyde and 7 mM for methylglyoxal.
Table 2.
Relative stiffening expressed as a percentage. Values are mean ± s.d.
| agent | concentration (mM) | 13 mmHg | 28 mmHg | number of eyes (n) |
|---|---|---|---|---|
| genipin | 0.25 | 15 ± 14 | 19 ± 17 | 6 |
| 0.50 | 64 ± 23 | 54 ± 23 | 4 | |
| 1.0 | 108 ± 28 | 86 ± 16 | 4 | |
| 7.5 | 1321 ± 703 | 577 ± 220 | 5 | |
| 15 | 503 ± 252 | 253 ± 130 | 4 | |
| 30 | 576 ± 164 | 348 ± 137 | 5 | |
| glyceraldehyde | 10.0 | 22 ± 26 | 20 ± 20 | 4 |
| 30.0 | 73 ± 64 | 55 ± 40 | 6 | |
| 62.5 | 273 ± 143 | 165 ± 75 | 8 | |
| 125 | 192 ± 214 | 131 ± 120 | 7 | |
| methylglyoxal | 3.5 | 12 ± 20 | 11 ± 19 | 4 |
| 7.0 | 108 ± 52 | 81 ± 38 | 5 | |
| 14 | 310 ± 222 | 160 ± 119 | 5 |
Figure 5.
Average first principal strains for control (horizontal axis) and stiffened (vertical axis) regions of eyes treated with (a) genipin, (b) glyceraldehyde or (c) methylglyoxal. Each dot represents the mean steady-state strain for one eye, and the surrounding oval represents the 95% CI of that point from linear fitting. Points falling below the unity line (black line) indicate that the treated eye has been stiffened relative to the control, and vice versa. The dotted line represents 100% stiffening. Eyes in red lie very close to the unity line, suggesting that this low dose has a minimal stiffening effect. Higher concentrations lie further from the unity line until reaching a maximum effective dose around 7 mM for genipin, 62.5 mM for glyceraldehyde and 14 mM for methylglyoxal. Higher concentrations do not further stiffen the experimental half of the eye but do reduce strain in the control portion of the eye, possibly as a result of diffusion into the internal tissues of the eye and cross-linking them.
4. Discussion
This study offers the first quantification of the efficacy of scleral stiffening agents in the rat eye, a common and important animal model of experimental glaucoma. It also offers the first demonstration of the efficacy of genipin and methylglyoxal in the rodent eye, an important milestone for use in mice, whose eyes have similar collagen composition to rats and are widely used in glaucoma and myopia research. We found that each agent is capable of stiffening the sclera by several hundred per cent but that there exists an upper bound to this stiffening effect. This quantification sets a range on the magnitude one might be able to achieve using collagen cross-linking approaches to scleral stiffening. Researchers investigating the physiological consequences of scleral stiffening using these agents should not expect to increase stiffness by more than several hundred per cent.
While we cannot determine the mechanism causing genipin to achieve its highest stiffness around 7 mM and glyceraldehyde around 30 mM from the data at hand, we hypothesize that the collagen cross-linking sites have become fully saturated at these higher concentrations. Thus, the presence of additional cross-linker may have no further effect. Although we did observe a drop in relative stiffness at the highest concentrations of genipin and glyceraldehyde in this study, this resulted from a decrease in strain in the control portion of the eye without a change in the treated portion. Thus, this phenomenon should not be interpreted as a drop in efficacy at the highest concentrations. Instead, it is likely that the agents at these very high concentrations diffused through the eye into the internal tissues or even into the control portions, potentially cross-linking them and reducing strain, thereby decreasing the relative stiffness of the eye.
Our novel approach to treating approximately half an eye with stiffening agent overnight while using the other half as a control provides a powerful tool for studying the efficacy of small molecule collagen cross-linking agents. Although these agents may diffuse outside the desired region of the eye at very high concentrations, we do not believe this is a problem at the more moderate concentrations examined in this study. Genipin yields a visible blue dye at sites where it is present (figure 1), and this colour change has previously been shown to correlate with scleral stiffness [28], suggesting its relevance as a visual reporter of cross-linking. Glyceraldehyde and methylglyoxal have approximately half the molecular weight of genipin, and thus they may diffuse slightly faster but are not expected to enter the control half of the eye considerably. Strain maps for these eyes similar to figure 3 show a relatively sharp line of demarcation between the two halves. To avoid confounding our analysis with any diffusion effects, however minor, we also avoided including regions closest to the line of demarcation when computing average strain. As shown in figure 3, the perimeters of the regions of interest do not overlap perfectly where they come closest to intersecting (there are no points included that are underneath the visible perimeters) in order to exclude strain measurements in the transition zone. Additionally, it is important to remember that the relatively higher strains in the stiffened region close to its boundary with the control region result from cross-linker not fully diffusing into this region, so we err on the side of under-diffusion, not cross-linker bleed-over. We also used outlier removal to eliminate any data points that deviated considerably from the median, such as those resulting from edge effects, and, by computing the mean relative stiffness from several hundred data points per region after outlier removal, the effect of any small bleed-over should be small.
Our method of using half of each eye as experimental and control groups to compute relative stiffening is especially powerful when we consider the inter-eye variability in strains in naive regions of eyes (and confirmed in fully untreated eyes, data not shown). Even in two eyes from a single rat, strains in PBS-treated regions can vary by a factor of approximately 3 (see dispersion of data points along the x-axis in figure 5). Thus, by using the two halves of each eye as an internal comparison, we can minimize the effects of inter-eye variability. While it is certainly true that strains are somewhat heterogeneous even within regions of a single eye (as in figure 3) our technique for computing relative strain allows us to only introduce intra-eye variability without adding the effect of inter-eye variability to each relative strain calculation.
An additional benefit of using half the eye as an internal control and making a relative comparison is that the need for preconditioning is greatly reduced. Prior work such as that of Wong et al. [31] used up to 10 cycles of preconditioning before the eye converged to a stable relationship between inflation and strain. With our testing methodology, we are comparing the relative stiffness of two halves of a single eye such that preconditioning effects, or lack thereof, should be approximately uniform between the two halves. Thus, the strain magnitudes quantified in this study may not exactly equal ocular strains in the rat eye at various magnitudes of IOP, but the relative stiffening effect should still be relevant to future in vivo studies in the rat.
This study focused exclusively on eyes ex vivo, although we took care to freshly harvest eyes and maintain them at physiological temperatures during testing. We treated eyes overnight in order to simulate the stiffening effect that might result if such agents were delivered to the posterior eye within Tenon's capsule. However, because this is a relatively un-explored frontier of ophthalmology, it is unclear what the body's clearance of such agents would be in vivo. Recently, Kimball et al. [19] investigated the efficacy of glutaraldehyde scleral stiffening in vivo in a mouse model of glaucoma and found that its use was detrimental to visual function. In an attempt to recreate the conditions of their study, we first attempted to study eyes incubated in 500 mM glyceraldehyde, identical to the Kimball et al. paper. However, eyes became significantly dehydrated and collapsed with this treatment, presumably from a significant osmolarity mismatch. Such an effect would clearly be problematic in vivo and could explain the negative findings of the Kimball study, but active transport of fluids in a living mouse also might be able to compensate for any osmolar mismatch. Further investigation is certainly warranted.
Eyes were freshly harvested from rats daily and randomized to a treatment agent and concentration. However, in an effort to ensure that our stiffening solutions were freshly mixed from a stock solution, all eyes studied in a single day (usually two or three pairs) were incubated in a single agent, although often at different concentrations of that agent. Thus, one limitation of the present work is that some treatments were from both eyes of a single rat. Although Brown Norway rats are an inbred strain and thus should have low genetic variability, as previously mentioned, in initial testing prior to this study using naive eyes (not shown), variability in average strain between two eyes from a single rat were high enough that, for this study, we assumed that each eye was an independent sample regardless of which rat it came from.
Although the eyes in this study were all studied within 24 h of harvest, another unknown factor for scleral stiffening therapies is the temporal efficacy of such agents. Previously, Wollensak & Iomdina [22] showed that glyceraldehyde increases scleral stiffness for at least eight months in rabbits, a promising finding. However, further work is necessary to characterize the temporal profile of the stiffening agents in this study, both in terms of how long the eye must be incubated in order to derive a stiffening effect as well as in terms of how long the eye maintains its increased stiffness before collagen turnover and remodelling negates the effects of treatment.
Eyes were incubated in agents for approximately 16 h each, with an unavoidable variability of several hours as a result of practical aspects of the eye mounting procedure, tissue cleaning, etc. Per Fick's law, the rate of diffusion into the tissue should drop as the concentration of cross-linker equalizes between the solution and the tissue. Additionally, we can approximately estimate an upper bound on the effects of different incubation times using the fact that the diffusion distance is proportional to the square root of elapsed time, so a deviation of 2 h less than our approximated 16 h would lead to a variation in the extent of cross-linking of roughly 8–9% 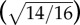 , considerably smaller than the stiffening effects of 100% or more observed in this study. Further study is certainly warranted to better characterize the dynamics of cross-linking treatments to ocular tissues, but, in this study, we attempted to characterize the role of collagen cross-linking at various starting concentrations with the understood limitation that some modest variability in stiffening may result from variations in tissue preparation.
, considerably smaller than the stiffening effects of 100% or more observed in this study. Further study is certainly warranted to better characterize the dynamics of cross-linking treatments to ocular tissues, but, in this study, we attempted to characterize the role of collagen cross-linking at various starting concentrations with the understood limitation that some modest variability in stiffening may result from variations in tissue preparation.
Having characterized these three collagen cross-linking agents ex vivo, our next step will be to deliver them to rats in vivo to answer the questions raised by this and other studies. Future work will need to characterize how well-tolerated these agents are by delicate neural tissues of the retina and optic nerve head, as well as by the scleral fibroblasts providing collagen turnover in the eye. If the stiffening agents have any sort of toxic effect to these components of the eye, it may be necessary to take care to use highly targeted delivery of such agents using novel drug delivery techniques. Such approaches might involve delivering agents with an activatable reservoir of cross-linker or by flushing away agents from undesired locations, but such techniques will need to be evaluated after determining whether scleral stiffening offers any benefit for glaucoma or myopia as well as which agents are the safest for in vivo use. It will also be important to quantify whether the same magnitude of relative stiffening for the concentrations of the agents measured here exists in vivo and how long the stiffening is maintained. Most importantly, future studies should build upon this foundation in order to evaluate the efficacy of various scleral stiffening approaches for ocular diseases such as glaucoma and myopia in order to improve our clinical ability to preserve vision.
5. Conclusion
Here, we have reported the first direct comparison of the dose–response relationship of three stiffening agents in sclera. All three collagen cross-linking agents examined in this study, genipin, glyceraldehyde and methylglyoxal, exhibited dose-dependent stiffening behaviour, with maximum relative stiffening of several hundred per cent at higher concentrations. Thus, all three agents can be titred to achieve a desired magnitude of stiffening. Future studies will examine the efficacy of these agents in vivo to ensure the stiffening effect is maintained in longitudinal studies and, more importantly, to assess whether scleral stiffening agents protect against vision loss in diseases like glaucoma and myopia.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Dr Jonathan Suever (Geisinger Health Systems) for providing Matlab segmentation code.
Appendix A. Relative stiffness in terms of strain derivation
Relative stiffness is defined as the stiffness of the experimental material relative to the control:
| A 1 |
where Eexp is the experimental effective modulus, and Econ is the control effective modulus.
By modelling the eye as a thin-walled pressure vessel and assuming a constant radius and thickness throughout the eye, we can compute the applied stress at each pressure step,
| A 2 |
where σ is the hoop stress in a sphere, P is the internal pressure, R is the radius and t is the thickness of the sphere (see the electronic supplementary material, figure).
Since the internal pressure applied is the same for the entire eye, the stresses in the experimental and control portions of the eye are the same,
| A 3 |
where σcon and σexp are the hoop stresses in the control and experimental portions of the eye, respectively.
We approximate the tissue behaviour as incrementally linear elastic within this loading regime to write
| A 4 |
where ɛcon and ɛexp are the control and experimental first principal strains, respectively.
Using equation (A 4), we can write
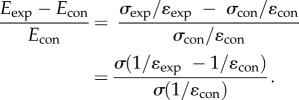 |
A 5 |
Simplifying by multiplying by ɛexp ɛcon, we obtain the relative stiffness equation in terms of strains at a given pressure step,
| A 6 |
Ethics
All procedures were approved by the Institutional Animal Care and Use Committee at the Georgia Institute of Technology, and all experiments were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
I.C.C., B.G.H., A.T.R. and S.A.S. collected data, performed data analysis, drafted the manuscript and designed the study. J.M.S. performed data analysis, drafted the manuscript and performed statistical analysis. C.R.E. designed the study, coordinated the study and drafted the manuscript. All authors gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
The authors gratefully acknowledge research support from the Department of Veterans Affairs (Career Development Award IK1 RX001791 to I.C.C.), the National Institutes of Health (R01 EY025286 to C.R.E.) and the Georgia Research Alliance (C.R.E.).
References
- 1.Scott AW, Bressler NM, Ffolkes S, Wittenborn JS, Jorkasky J. 2016. Public attitudes about eye and vision health. JAMA Ophthalmol. 134, 1111–1118. ( 10.1001/jamaophthalmol.2016.2627) [DOI] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. 2014. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121, 2081–2090. ( 10.1016/j.ophtha.2014.05.013) [DOI] [PubMed] [Google Scholar]
- 3.Foster PJ, Jiang Y. 2014. Epidemiology of myopia. Eye (Lond.) 28, 202–208. ( 10.1038/eye.2013.280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan IG, Ohno-Matsui K, Saw SM. 2012. Myopia. Lancet 379, 1739–1748. ( 10.1016/S0140-6736(12)60272-4) [DOI] [PubMed] [Google Scholar]
- 5.Anderson DR, Drance SM, Schulzer M, Collaborative Normal-Tension Glaucoma Study Group. 2001. Natural history of normal-tension glaucoma. Ophthalmology 108, 247–253. ( 10.1016/S0161-6420(00)00518-2) [DOI] [PubMed] [Google Scholar]
- 6.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial Group. 2002. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 120, 1268–1279. ( 10.1001/archopht.120.10.1268) [DOI] [PubMed] [Google Scholar]
- 7.Noecker RJ. 2006. The management of glaucoma and intraocular hypertension: current approaches and recent advances. Ther. Clin. Risk Manag. 2, 193–206. ( 10.2147/tcrm.2006.2.2.193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell IC, Coudrillier B, Ethier CR. 2014. Biomechanics of the posterior eye: a critical role in health and disease. J. Biomech. Eng. 136, 021005 ( 10.1115/1.4026286) [DOI] [PubMed] [Google Scholar]
- 9.Sigal IA, Flanagan JG, Ethier CR. 2005. Factors influencing optic nerve head biomechanics. Invest. Ophthalmol. Vis. Sci. 46, 4189–4199. ( 10.1167/iovs.05-0541) [DOI] [PubMed] [Google Scholar]
- 10.Anderson DR, Hendrickson A. 1974. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest. Ophthalmol. 13, 771–783. [PubMed] [Google Scholar]
- 11.Quigley H, Anderson DR. 1976. The dynamics and location of axonal transport blockade by acute intraocular pressure elevation in primate optic nerve. Invest. Ophthalmol. 15, 606–616. [PubMed] [Google Scholar]
- 12.Coudrillier B, et al. 2016. Effects of peripapillary scleral stiffening on the deformation of the lamina cribrosa. Invest. Ophthalmol. Vis. Sci. 57, 2666–2677. ( 10.1167/iovs.15-18193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coudrillier B, et al. 2016. Phase-contrast micro-computed tomography measurements of the intraocular pressure-induced deformation of the porcine lamina cribrosa. IEEE Trans. Med. Imaging 35, 988–999. ( 10.1109/TMI.2015.2504440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grytz R, Siegwart JT Jr. 2015. Changing material properties of the tree shrew sclera during minus lens compensation and recovery. Invest. Ophthalmol. Vis. Sci. 56, 2065–2078. ( 10.1167/iovs.14-15352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips JR, McBrien NA. 2004. Pressure-induced changes in axial eye length of chick and tree shrew: significance of myofibroblasts in the sclera. Invest. Ophthalmol. Vis. Sci. 45, 758–763. ( 10.1167/iovs.03-0732) [DOI] [PubMed] [Google Scholar]
- 16.Goldich Y, Marcovich AL, Barkana Y, Mandel Y, Hirsh A, Morad Y, Avni I, Zadok D. 2012. Clinical and corneal biomechanical changes after collagen cross-linking with riboflavin and UV irradiation in patients with progressive keratoconus: results after 2 years of follow-up. Cornea 31, 609–614. ( 10.1097/ICO.0b013e318226bf4a) [DOI] [PubMed] [Google Scholar]
- 17.Danilov NA, Ignatieva NY, Iomdina EN, Semenova SA, Rudenskaya GN, Grokhovskaya TE, Lunin VV. 2008. Stabilization of scleral collagen by glycerol aldehyde cross-linking. Biochim. Biophys. Acta 1780, 764–772. ( 10.1016/j.bbagen.2008.01.014) [DOI] [PubMed] [Google Scholar]
- 18.Hwang YJ, Granelli J, Tirumalasetty M, Lyubovitsky J. 2013. Microscopic imaging of glyceraldehyde-induced tissue glycation with intrinsic second harmonic generation and two-photon fluorescence contrasts. In Imaging, manipulation, and analysis of biomolecules, cells, and tissues (eds Farkas DL, Nicolau DV, Leif RC), pp. 858725–858727. San Francisco, CA: Society of Photo-Optical Instrumentation Engineers (SPIE). [Google Scholar]
- 19.Kimball EC, Nguyen C, Steinhart MR, Nguyen TD, Pease ME, Oglesby EN, Oveson BC, Quigley HA. 2014. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Exp. Eye Res. 128, 129–140. ( 10.1016/j.exer.2014.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spoerl E, Boehm AG, Pillunat LE. 2005. The influence of various substances on the biomechanical behavior of lamina cribrosa and peripapillary sclera. Invest. Ophthalmol. Vis. Sci. 46, 1286–1290. ( 10.1167/iovs.04-0978) [DOI] [PubMed] [Google Scholar]
- 21.Wollensak G, Iomdina E. 2008. Crosslinking of scleral collagen in the rabbit using glyceraldehyde. J. Cataract Refract. Surg. 34, 651–656. ( 10.1016/j.jcrs.2007.12.030) [DOI] [PubMed] [Google Scholar]
- 22.Wollensak G, Iomdina E. 2008. Long-term biomechanical properties after collagen crosslinking of sclera using glyceraldehyde. Acta Ophthalmol. 86, 887–893. ( 10.1111/j.1755-3768.2007.01156.x) [DOI] [PubMed] [Google Scholar]
- 23.Wollensak G, Spoerl E. 2004. Collagen crosslinking of human and porcine sclera. J. Cataract Refract. Surg. 30, 689–695. ( 10.1016/j.jcrs.2003.11.032) [DOI] [PubMed] [Google Scholar]
- 24.Mattson MS, Huynh J, Wiseman M, Coassin M, Kornfield JA, Schwartz DM. 2010. An in vitro intact globe expansion method for evaluation of cross-linking treatments. Invest. Ophthalmol. Vis. Sci. 51, 3120–3128. ( 10.1167/iovs.09-4001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avila MY, Navia JL. 2010. Effect of genipin collagen crosslinking on porcine corneas. J. Cataract Refract. Surg. 36, 659–664. ( 10.1016/j.jcrs.2009.11.003) [DOI] [PubMed] [Google Scholar]
- 26.Hrabchak C, Rouleau J, Moss I, Woodhouse K, Akens M, Bellingham C, Keeley F, Dennis M, Yee A. 2010. Assessment of biocompatibility and initial evaluation of genipin cross-linked elastin-like polypeptides in the treatment of an osteochondral knee defect in rabbits. Acta Biomater. 6, 2108–2115. ( 10.1016/j.actbio.2009.12.034) [DOI] [PubMed] [Google Scholar]
- 27.Hwang YJ, Larsen J, Krasieva TB, Lyubovitsky JG. 2011. Effect of genipin crosslinking on the optical spectral properties and structures of collagen hydrogels. ACS Appl. Mater. Interfaces 3, 2579–2584. ( 10.1021/am200416h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu TX, Luo X, Gu YW, Yang B, Wang Z. 2014. Correlation of discoloration and biomechanical properties in porcine sclera induced by genipin. Int. J. Ophthalmol. 7, 621–625. ( 10.3980/j.issn.2222-3959.2014.04.06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu TX, Wang Z. 2013. Collagen crosslinking of porcine sclera using genipin. Acta Ophthalmol. 91, e253–e257. ( 10.1111/aos.12172) [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Corpuz CC. 2015. Effects of scleral cross-linking using genipin on the process of form-deprivation myopia in the guinea pig: a randomized controlled experimental study. BMC Ophthalmol. 15, 89 ( 10.1186/s12886-015-0086-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong FF, Lari DR, Schultz DS, Stewart JM. 2012. Whole globe inflation testing of exogenously crosslinked sclera using genipin and methylglyoxal. Exp. Eye Res. 103, 17–21. ( 10.1016/j.exer.2012.06.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu B, Chow MJ, Zhang Y. 2011. Experimental and modeling study of collagen scaffolds with the effects of crosslinking and fiber alignment. Int. J. Biomater. 2011, 172389 ( 10.1155/2011/172389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart JM, Schultz DS, Lee OT, Trinidad ML. 2009. Exogenous collagen cross-linking reduces scleral permeability: modeling the effects of age-related cross-link accumulation. Invest. Ophthalmol. Vis. Sci. 50, 352–357. ( 10.1167/iovs.08-2300) [DOI] [PubMed] [Google Scholar]
- 34.Ballantyne B, Myers RC. 2001. The acute toxicity and primary irritancy of glutaraldehyde solutions. Vet. Hum. Toxicol. 43, 193–202. [PubMed] [Google Scholar]
- 35.Wei X, Cai SP, Zhang X, Li X, Chen X, Liu X. 2012. Is low dose of estrogen beneficial for prevention of glaucoma? Med. Hypotheses 79, 377–380. ( 10.1016/j.mehy.2012.05.041) [DOI] [PubMed] [Google Scholar]
- 36.Sherwood JM, Reina-Torres E, Bertrand JA, Rowe B, Overby DR. 2016. Measurement of outflow facility using iPerfusion. PLoS ONE 11, e0150694 ( 10.1371/journal.pone.0150694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunz C, Singh H. 2008. Hemispherical refraction and camera calibration in underwater vision. In OCEANS 2008, Quebec City, Canada, 15–18 September 2008, pp. 1–7. New York, NY: IEEE. [Google Scholar]
- 38.Leys C, Ley C, Klein O, Bernard P, Licata L. 2013. Detecting outliers: do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 49, 764–766. ( 10.1016/j.jesp.2013.03.013) [DOI] [Google Scholar]
- 39.Agarwal S, Agarwal A, Apple DJ. 2002. Textbook of ophthalmology. New Delhi, India: Jaypee Brothers. [Google Scholar]
- 40.Baumann B, Rauscher S, Glosmann M, Gotzinger E, Pircher M, Fialova S, Groger M, Hitzenberger CK. 2014. Peripapillary rat sclera investigated in vivo with polarization-sensitive optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 55, 7686–7696. ( 10.1167/iovs.14-15037) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.