Abstract
T3, the active form of thyroid hormone, binds nuclear receptors that regulate the transcription of a large number of genes in many cell types. Unraveling the direct and indirect effect of this hormonal stimulation, and establishing links between these molecular events and the developmental and physiological functions of the hormone, is a major challenge. New mouse genetics tools, notably those based on Cre/loxP technology, are suitable to perform a multiscale analysis of T3 signaling and achieve this task.
Thyroid hormones (THs, including T3 and T4, the low activity precursor of T3) exert a pleiotropic influence on development and adult homeostasis in all vertebrates. In humans, early T3 deficiency, ie, congenital hypothyroidism, broadly alters development, the most visible consequences being mental retardation and skeletal growth defects. Adult hypothyroidism also has a detrimental influence on hepatic metabolism, heart rate, fertility, and water balance, among other physiological processes. T3 acts mainly by binding to nuclear receptors (thyroid hormone receptor [TR]α1, TRβ1, and TRβ2, collectively called TRs, respectively encoded by the THRA and THRB genes). TRs are ligand-dependent transcription factors present in all cell types, which directly regulate the transcription of a large number of genes (1). They do so by binding to DNA response elements that are mainly related to the DR4 consensus element (5′-AGGTCANNNNAGGTCA-3′), recognized by a TR/retinoid X receptor heterodimer. This response element is also recognized by other nuclear receptors (liver X receptor, farnesoid X receptor, etc), raising many possibilities, for cross talks (2). Unliganded DNA-bound TRs recruit transcription corepressors on the chromatin. Upon ligand binding, corepressors tend to be released and coactivators recruited (3). The balance between corepressors and coactivators association is thus defined by T3 concentration. Several alternative possibilities have been proposed to this canonical model (3–5). Among these, the so-called “nongenomic” pathways remain a controversial issue, recently discussed by others (6–8). There are, to our knowledge, no published evidence for TH response in mice knocked out for both THRA and THRB (9). Therefore the possibility remains that the nongenomic responses to TH are not independent of the canonical pathway, but reflect a rapid modulation of the canonical pathway by posttranslational modifications of TRs or its cofactors.
Establishing a link between the known physiological and developmental functions of T3 and gene regulations exerted by TRs is a major challenge. The gap between the findings in animal studies and observations in cultured cells or in vitro systems, needs to be filled. A recent effort to review and standardize the tools and approaches used in cellular and animal models, as well as recognize their limitations, is an important step (124). Another promising approach to address the gap is to perform a genome-wide analysis of gene expression after pharmacologic treatments in genetically manipulated animal models. Recent years witnessed a rapid accumulation of such transcriptome data and led to the identification of a large number of putative TR target genes in various cell types. However, up to now, this did not bring much clarification on T3 mode of action. We argue here that this difficulty is due to our limited understanding of the cellular response of many cell types to TH. Without this knowledge, it is difficult to distinguish between the primary transcriptional response of TR-expressing cells and the secondary consequences on the cellular environment. The main difficulty is not to identify more T3-regulated genes, but to unravel the direct and indirect influence exerted by TH in vivo on cell types that are in permanent interaction.
Direct and Indirect Effect of TH Treatments
TR target genes are defined as genes for which transcription initiation is modulated by the physical interaction of liganded or unliganded TRs with regulatory DNA sequences found in enhancers, promoters, and introns. However, most of the investigations aiming at recognizing TR target adopt an indirect approach, based on comparative RNA analysis. RNAs are extracted from tissues prepared from hypothyroid and euthyroid animals, or preferably hypothyroid animals in which TH levels are restored for a few hours. In such settings, the transactivation of TR target genes is accompanied by 3 types of indirect effects.
Systemic effect of TH
Among the TR target genes are genes encoding transcription factors and cofactors. For example Dbp, Klf9 (10), and Hairless (11) are well-characterized TR target genes in several cell types, and their products exert a regulation on gene expression rapidly after T3 stimulation. What we call secondary target genes are the genes that are quickly regulated in the T3 sensitive cells by these cascades of gene regulation events subsequent to T3 stimulation. One way to limit secondary response is to introduce cycloheximide, which inhibits mRNA translation. However, due to its toxicity, this treatment is only possible for a short time, and is used only for cultured cells. Based on the criterion of cycloheximide sensitivity, secondary response already represents 20% of the T3-induced changes in gene expression within 3 hours (12).
Local effects vs cell-autonomous effects
The direct and secondary responses to T3 described above are cell autonomous. However, some of the genes that are directly and indirectly regulated by T3 encode secreted proteins or influence their production: extracellular matrix components, growth factors, etc. T3 can thus modify the cellular microenvironment and have a local, but not cell-autonomous, influence. For example, in the granular cell progenitors of cerebellum, Ccdn2 encoding cyclinD2 is up-regulated by T3, but this regulation is relayed by neurotrophins. A likely candidate is Neutrophin 3 (13), which is encoded by Ntf3, a gene that is also unlikely to be itself a TR target gene (14). The relation between Ccdn2 up-regulation and T3 is thus very indirect and not fully understood.
Systemic effects correspond to the many circumstances in which T3 exerts a long-distance indirect influence, far from the T3-responsive cells. One obvious possibility, which will not be discussed in detail, comes from the fact that T3 exerts a global influence on energy metabolism in many tissues, including adipose tissue, liver, and muscle. The feedback regulation of the hypothalamus-pituitary-thyroid (HPT) axis also reflects the possibility for T3 to exert long-distance influence: T3 down-regulates TSH production by pituitary, and the change in TSH circulating level is sensed by thyrocytes in the thyroid gland. Although this is a matter of controversy, TSH might also act on other cell types expressing the TSH receptor, like in bone (15, 16). Prolonged disruption of the HPT axis induces several other changes in serum composition, altering, in particular, glycemia and the circulating level of cholesterol. Although this is not documented, the serum levels of other bioactive iodinated compounds might be also affected. These include thyronamines (17) and 3,5-diiodo-l-thyronine a compound the biosynthesis pathway of which is unknown and should not be confused with the inactive 3,3′-diiodo-l-thyronine, the product of T3 deiodination (18). T3 in the hypothalamus also regulates the activity of the autonomous nervous system, therefore contributing at the central level to setting the sympathetic tune of peripheral organs stimulation. Additional indirect effects would be worth considering. For example, cerebellum hypotrophy, delay of granule cell migration, increased cell death, and stunted Purkinje cell arborization are all neurodevelopmental defects associated with congenital hypothyroidism. Strikingly, these can also be induced by a hepatocyte-specific mutation of Pex5, disrupting peroxisomal fatty acid β-oxidation only in these cells (19). Similarly osteocalcin secretion by bone, which is T3 sensitive (20), influences fertility (21) and brain development (22). One may therefore suspect that some of the neurodevelopmental defects associated with congenital hypothyroidism result from initial defects taking place in liver, bone, or placenta (23–25).
Experimental Strategies to Recognize Direct and Indirect Influence of TH in Vivo
Chromatin occupancy by TR
The current trend is to define direct TR targets by addressing chromatin occupancy of regulatory sequences by immune-precipitation of cross-linked TRs containing complexes. Due to the difficulty of producing antibodies of sufficient quality, this has been done only occasionally on defined genomic regions (14, 26–29). Furthermore, the 2 first genome-wide studies, which both used tagged version of TRs, revealed that the situation will remain ambiguous in many cases, for 2 reasons. First, TRs can probably regulate transcription when bound at a very long distance from the transcription start site of a target gene. Second, in many cases, for unknown reasons, TR binding does not lead to the regulation of the closest neighboring genes (30, 31). The presence of a TR-binding site in the region surrounding the transcription start site is thus not sufficient to recognize TR target genes. This type of analysis also indicates that the vast majority of the consensus DNA sequences for TR binding present in the genome are not occupied, explaining why bioinformatics is, at least for the moment, unable to identify TR target genes (14). Future progress will rely on high-throughput mutagenesis assays, and complementary techniques based on deep sequencing such as GRO-Seq (Gene Run-On followed by deep DNA sequencing), which identifies mRNAs during the course of transcription (32).
Primary cell cultures
An ancient, but still valid, strategy to separate the cell-autonomous response to T3 from the other influences is to use primary cell cultures (although “cell-type autonomous response” would be a more accurate description because such analyses are not performed on single-cells). In such settings, cell-cell interactions are limited, and a single chosen cell type is usually favored by the culture conditions. This approach is limited, however, to few well-characterized cell types, and there is a general suspicion that cells will not behave in culture as they do in their natural environment. This is notably the case for neurons and hepatocytes, because their in vitro behavior is significantly modified by culture conditions (33). In this respect, the relevance of primary cell cultures is nevertheless less disputable than the one of immortalized cell lines. They can be considered as a useful complement to in vivo exploration and efficient tools for a first identification of putative TR target genes.
Changing TH levels and local distribution
Several tricks can be used to alter TH signaling in a restricted manner and thus help to distinguish between the direct and indirect effects of T3 in animals. One way is to introduce moderate alteration of TH signaling. In this case, compensatory mechanisms can restore normal level of TH signaling in some, but not all, tissues. Inducing a transient maternal hypothyroxynemia, ie, low T4 level with nearly normal level of T3, by a low-iodine diet can be regarded as a way to reduce TH signaling level in a limited number of fetal organs. Due to the variable ability of tissues to metabolize TH, this has a differential effect on tissue T3 content. When this status is maintained for few days only during fetal development, several neuronal migration processes are impaired in the cortex, but neuronal differentiation is only delayed (34–36). These subtle interventions of TH signaling demonstrated that T3 exerts several distinct influences on cortical migration and differentiation of cortical neurons. It is also conceivable to use synthetic TR ligands (37) to exert a limited perturbation of TH signaling in specific organs (38). Only a few of such ligands have been obtained. Due to their relative selectivity for TRβ1/2 and their preferential uptake by the liver, GC-1 (39) and KB2115 (40) can influence liver metabolism without altering heart rate. However, using these molecules to analyze the direct and indirect influence of TH signaling requires a precise assessment of possible side effects.
Local delivery of T3 can be performed either by direct injection or implantation of minipumps. With the help of stereotaxy, it is feasible to stimulate TH signaling in specific brain areas in rats, and also, to some extent, in mice. This key innovation has allowed the demonstration that, within the hypothalamus, T3 triggers 2 completely different responses: in the arcuate nucleus, T3 injection triggers a rapid activation of the mammalian target of rapamycin pathway, whereas in the ventromedial nucleus, it induces the slow onset of the AMP-activated protein kinase pathway. The effect of T3 in arcuate nucleus is related to food intake, whereas in the ventromedial nucleus it specifically regulates the sympathetic stimulation of brown adipose tissue and heat production (41, 42). In a third hypothalamic nucleus, the paraventricular nucleus, T3 activates the sympathetic stimulation of glucose production by the liver (43). A population of parvalbumin-expressing neurons that requires TRα1/T3 signaling for their differentiation has recently been identified in the anterior hypothalamus. Specific ablation of these cells results in hypertension and temperature-dependent tachycardia, indicating a role in the central autonomic control of blood pressure and heart rate (44). These experiments outline the paramount importance of T3 function in the hypothalamus and suggest that many peripheral functions of T3 are, at least in part, relayed by the hypothalamus. Somatic gene delivery in hypothalamus has been performed, using adenovirus (41) or naked DNA (45) to manipulate local T3 signaling by overexpressing or knocking down TRs and getting clues on the underlying gene regulations.
Introducing germline mutations in mice to alter TH transport and metabolism
Mouse genetics offers a number of possibilities to manipulate the local TH signaling. Many germline mutations have been produced in mice, altering the TH signaling level at various levels: TH production, transport, deiodination, and TR-mediated transcriptional response. Some of these germline mutations have a highly specific influence on TH signaling, which is limited compared with TH-deficient mice. First, mutations of genes encoding transporters have moderate consequences on mouse development and homeostasis. Oatp1c1 knockout reduces TH content only in brain and has limited developmental consequences (46). Mct8 knockout also limits the entry of TH in brain while increasing the circulating level in blood (47). Interestingly, only a small fraction of T3-responsive genes in mouse brain are sensitive to Mct8 knockout, an alternative pathway involving local deiodination of T4 compensating for the transporter defect (48). Surprisingly, serum and brain T4 content were found to be normalized in the Mct10/Mct8 double-knockout mice, whereas the hyperthyroid condition in liver, kidneys, and thyroid gland was aggravated (49). This suggests that in these organs, both transporters contribute to the TH efflux. However, like many other TH transporters, MCT10 is not TH specific.
One straightforward way to alter TH signaling in a limited and highly specific manner is to modify deiodinase expression levels. Type 1 deiodinase is present mainly in liver and has a dual effect on T3 level, converting T4 into T3, and then T3 into T2. Its elimination by gene knockout has no obvious effect on the circulating level of T3 (50). Type 2 deiodinase, encoded by Dio2, is found in several tissues and performs a local conversion of T4 into T3. Dio2 knockout has a moderate effect on development, visible in muscles (51) or inner ear (52) where local conversion of T4 is required to maintain normal concentration of T3. The impaired thermogenesis of these knockout mice correlates with their high susceptibility to obesity when placed on a high-fat diet (53). As expected, the lack of type 3 deiodinase, the enzyme that catabolizes both T4 and T3, results in neonatal thyrotoxicosis. This is followed later by an surprising central hypothyroidism that persists throughout life (54). However in these Dio3 knockout adult mice, T3 tends to accumulate over time in the anterior cortex and other specific brain areas (55). The same progressive accumulation of T3 takes place in the heart, resulting in restrictive cardiomyopathy (56).
Introducing germline mutations in mice to alter cellular response to T3
An alternative way to alter TH signaling is to mutate genes encoding the TRs. Whereas THRA expression is ubiquitous, THRB expression occurs at late developmental stages in a limited number of tissues. THRB knockout results in elevated TH levels, indicating a predominant function in feedback regulation of the HPT axis (57). Normalizing TH levels with drug treatment in these mice allows distinguishing between the consequences of impaired TRβ1/2-mediated response and those of increased T3 availability. The expression of the TRβ2 isotype is restricted to a few cell types in retina, hypothalamus, pituitary, and inner ear. The selective elimination of TRβ2 has thus less pleiotropic consequences and was used to prove the implication of this receptor in HPT axis regulation (58) and differentiation of retina cone photoreceptors (52). For reasons that have been discussed elsewhere (59), the first reported THRA knockout mutation was lethal (60). This is an exception, however, and, despite ubiquitous and early THRA expression, knockout normally leads to a milder phenotype than complete TH deficiency, which is lethal a few weeks after birth (61). The attenuated phenotype of THRA knockout compared with hypothyroidism received 2 explanations: first, the functions of THRA and THRB are sometimes redundant, as shown by combining both mutations (62, 63). Second, most manifestations of TH deficiency during development are consequences of the presence of unliganded TRα1 on target genes, which exerts a negative influence on gene expression. This repression of transcription is lost in knockouts. This is evidenced by the paradoxical improvement of phenotype provided by THRA knockout in TH-deficient mice (64, 65). Accordingly, unlike knockouts, the THRA “knock-in” germline mutations, which change TRα1 reading frame to turn the receptor into a constitutive repressor, can lead to a phenotype that is very similar to congenital hypothyroidism. Several of these point mutations have been produced, which either reduce the affinity for T3 or prevent coactivators recruitment, but preserve DNA binding (reviewed in Reference 66).
The residual sensitivity of one of these TRα1 mutant receptors (TR1αR384C) to T3 binding (67) has been cleverly used to differentiate between direct and indirect effect of T3. The analysis of this model provides a good illustration of the difficulties in interpretation inherent to this type of model. Adult behavior reveals signs of high anxiety in TR1αR384C/+ mice, notably demonstrated by the reduced exploration of open arms in an elevated plus maze (68). However, the mice have difficulties in balancing, due to altered cerebellum development, as shown by a rotarod test. Such locomotion troubles introduce a confounding factor on the elevated plus maze test. However, when treated with high doses of TH at early stages, proper cerebellum development and locomotion are restored. The T3-rescued TR1αR384C/+ mice still display altered behavior on the elevated plus maze, reinforcing the interpretation that impairing TH signaling in adults results in high anxiety. When born from THRB knockout mothers, TR1αR384C/+ mice receive high TH levels during fetal growth, and their development is also improved (67). Furthermore, the TR1αR384C/+ mice are hypermetabolic. This was originally attributed to a defect in sympathetic stimulation of the brown adipose tissue (69). More recent and convincing evidence suggests that the initial defect is permanent vasodilation (70), which favors caloric exchanges. When mice are raised below thermoneutrality, the hyperactivity of the brown adipose tissue, triggered by sympathetic stimulation, is thus a compensatory response required to counterbalance excessive heat dissipation. Deeper analysis of endothelial cells may thus be the way to pinpoint the origin of hypermetabolism.
The genes encoding TR coactivators and corepressors have also been knocked out. However, these cofactors usually interact with many other nuclear receptors and transcription factors. Few studies, with the exception discussed below (71), have tried to precisely isolate the influence of these knockouts on TH signaling. For example, knocking out the Ncoa1 gene, encoding the steroid receptor coactivator 1 histone-acetyl-transferase coactivator, alters the HPT axis. It does so by reducing the pituitary sensitivity to TH, leading to the proposal that TSH down-regulation requires the presence of this coactivator (72). Reciprocally, one would expect that eliminating nuclear receptor corepressor (NCoR) or silencing mediator of retinoid and thyroid hormone receptor (SMRT), the two main TR corepressors that possess the opposite histone deacetylase activity, would increase TH sensitivity of pituitary. This was actually observed for a mutation eliminating the domain of SMRT, which interacts with nuclear receptors. This mutation also entails a reduction of hypothyroidism-induced hypercholesterolemia (73). In the C57/Bl6 genetic background, this SMRT mutation provokes a respiratory distress syndrome that is often lethal after birth. Making the mice hypothyroid at late gestational stage favors survival. This observation led to the still isolated proposal that enhanced TH signaling compromises type I pneumocytes differentiation and lung maturation (74). The NcorΔID mutation has a more specific effect than a complete NcoR knockout, which leads to embryonic lethality. The NcorΔID mutation prevents the interaction with unliganded TRs but preserves ability of NCoR to form complexes with some other nuclear receptors. The mutation has very limited consequences. It mainly decreases serum TH levels while increasing tissue sensitivity to T3 (71). It also counteracts the negative effect of TR mutations (75, 76).
In summary, 20 years after the initial knockouts, many ambiguities remain regarding the primary defects induced by the mutations that alter the T3/TR signaling pathways, and phenotype analysis keeps bringing unexpected results.
Generation of somatic mutations by Cre/loxP recombination
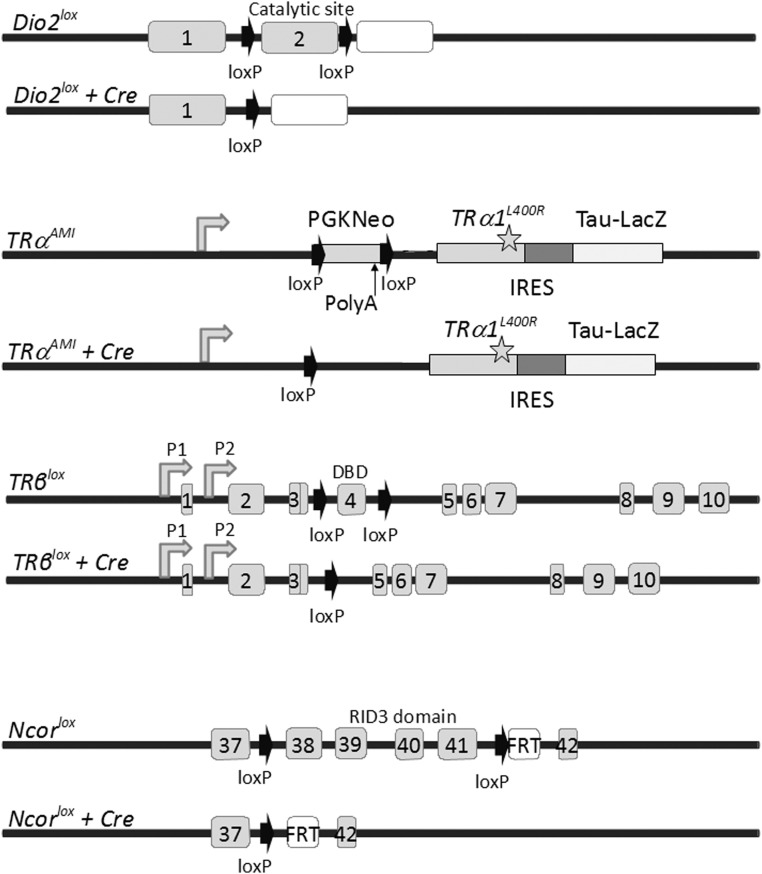
A major breakthrough in mouse genetics was the development of the Cre/loxP technology, which was initially promoted to study the in vivo function of other broadly expressed nuclear receptors (77, 78). This approach is safer than overexpression of mutant receptors from tissue-specific promoters (79) that could be suspected to create artifactual cross talks. A collective effort keeps producing a number of well-characterized transgenic mice expressing the Cre recombinase or its tamoxifen-inducible version Cre-ERT2 (80) with a well-characterized temporal and spatial pattern (81). This is a crucial technical point because the recombination pattern does not always reflect the previously reported expression pattern of the driver promoter. Combined with the so-called “floxed” alleles, in which loxP sequences are introduced in the targeted locus, these can provide a plethora of new models suitable to unravel direct and indirect T3 influences. Appropriate control mice are also necessary, because prolonged expression of Cre at high levels per se can have side effects (82). To date, only 4 floxed alleles have been produced for genes exerting a specific function in TH signaling: Dio2, THRA, THRB, Ncor1 (Figure 1). The TRα1L400R mutation is encoded by the TRαAMI allele. Its expression requires the Cre-mediated deletion of a stop cassette. The L400R amino acid substitution was chosen to maximize the dominant-negative effect based on structural studies (83). In vitro experiments suggest that TRα1L400R can exert dominant-negative activity toward intact TRα1 but also, to a lesser extent, toward TRβ1/2. However, systematic survey of cell types in which TRβ1/2 has a predominant function, as defined by knockout studies, suggests that in these cells TRβ1/2 function is not impaired. This includes the retina, in which opsin cone expression is not altered, and inner ear (84). In juvenile liver, in which TRβ1 is thought to be predominant, genes encoding phosphoenolpyruvate carboxykinase and pyruvate kinase are nevertheless down-regulated after ubiquitous TRα1L400R expression. However, these alterations are not cell-autonomous consequences of the mutation (85). After Cre -mediated deletion, the NcoR1lox allele produces the NCoRΔID mutant corepressors, which are unable to interact with TRs. As expected, liver-specific recombination limits the down-regulation exerted by TRs in case of TH deficiency (86) and protects against the deleterious effect of the TRα1PV and TRβ1PV mutations (75, 76). Floxed alleles have been produced for other genes involved in TH signaling, such as the steroid receptor coactivator 1 coactivator (87) but, to our knowledge, not yet used to specifically assess T3 response. In the following section, we review some recent contributions illustrating the potential of the Cre/LoxP approach to analyze the various levels of T3 action in vivo.
Figure 1.
Floxed alleles of genes with a specific function in TH signaling and their Cre-mediated recombination products. Cre recombination deletes the Dio2lox from the exon encoding the catalytic domain of type 2 deiodinase (90). Elimination of Dio2 prevents the local deiodination of T4 and produces a local T3 deficiency, in several tissues, including several brain areas, skeletal muscle, skin, pituitary, inner ear, and adipose tissues. The TRαAMI allele (84) carries a floxed cassette with polyadenylation signal, preventing expression of a downstream cDNA encoding the TRα1L400R dominant-negative mutant receptor receptor (mutation is indicated by the star). Cre-mediated recombination eliminates the cassette and triggers TRα1L400R expression in virtually any cell type because Thra expression is nearly ubiquitous. The IRES-TauLacZ cassette was introduced to monitor recombination by testing for β-galactosidase activity, which is, however, too low for easy detection. Therefore, assessment of recombination usually relies on the presence of a supplementary reporter transgene (122, 123). Because a strong phenotype is observed in heterozygous mice, new Cre/TRαAMI combinations can be generated within a single mouse generation. TRβlox (101) allows the elimination of exon 4 encoding the DNA-binding domain (DBD) shared by TRβ1 and TRβ2 and introduction of a frameshift for translational arrest. P1 and P2 are transcription promoters for TRβ1 and TRβ2 mRNA transcription. It can be used to eliminate TRβ1/2-mediated response in cells that express this receptor: hepatocytes, cardiomyocytes, pituitary thyrotropes, retina photoreceptors, etc. Cre recombination of the Ncor1lox allele (86) eliminates only exons encoding the RID3 domain of the corepressor that is required for TR interaction without changing the reading frame. Therefore, the NCoR1ΔID mutation preserves interactions with some other nuclear receptors and has limited developmental consequences. The mutation increases the sensitivity to T3 stimulation of the cells, such as hepatocytes, in which NcoR is a predominant corepressor. IRES, internal ribosome entry site; PGK, phosphoglycerate kinase; RID, receptor interaction domain.
Somatic Mutations Generated by Cre/loxP Recombination Highlight Mechanisms Underlying Development and Adult Maintenance by TH
HPT axis
For a long time, the molecular mechanisms underlying the negative regulation exerted by T3 on TSH production by pituitary thyrotroph cells has been a matter of controversy. It seems paradoxical that liganded TRs can exert a negative influence on the transcription of the 2 Cga and Tshb genes, which encode the 2 subunits of TSH. In vivo analysis is complicated by the fact that TRH (a hypothalamic peptide) production is also repressed by T3 and exerts an important control on TSH production by thyrotroph cells. In the absence of appropriate primary cell cultures, different cell lines have been used to study TSH regulation. The less questionable model is the TαT1.1 cell subclone of the TαT1 mouse thyrotroph cell line in which the physiological regulation of Tshb (but not of Cga) by T3 is maintained (88). In these cells, TRβ1/2 is bound to the Tshb proximal promoters. Although present in the cells, TRα1 can bind to the same DNA sequences only after TRβ1/2 depletion. This observation fits nicely with in vivo data, which indicate that THRA knockout has no influence on Tshb expression level, and TSH circulating level, unless THRB is knocked out (62, 63). As expected, T3 treatment is unable to suppress Tshb expression and TSH production in transgenic mice expressing a dominant-negative mutation of TRβ1, even when expression of this mutation is only in thyrotroph cells. In these mice, the excess in TSH production should result in increased activity of the thyroid gland. Surprisingly, however, the serum T4 level remains within normal range. This suggests that the TSH produced in excess is inactive and that TSH bioactivity is determined by a factor extrinsic to the pituitary, probably TRH. Whereas both TRH and TSH levels are increased in hypothyroid animals, only TSH production is increased here, and this increase alone seems insufficient to stimulate thyrocyte function (89). The Ncor1lox allele was used to generate mice with a mutation restricted to anterior pituitary. These mice have reduced Tshb expression. Therefore the down-regulation of Tshb is a cell-autonomous consequence of NcoR1 mutation (29). Although this would require direct demonstration by biochemical means, this observation strongly suggests that unliganded TRβ1/2 recruits the NcoR corepressor to transactivate Tshb.
Cre/loxP recombination was also used to specifically eliminate type 2 deiodinase from pituitary cells (90)(Table 1). In these mice, the serum levels of T4 and TSH are increased, but T3 level is not. This suggests that intrapituitary T4 deiodination is required for proper feedback regulation of TSH secretion. This also implies that thyrotroph cells mainly sense the circulating level of T4, rather than T3. Further analysis confirmed the reduced sensitivity of thyrotroph cells to T4 but intact TRH sensitivity. In hypothalamus, in which deiodination is preserved, the increase in T4 level results in the expected decrease in TRH gene expression. This seems again to decrease the TSH bioactivity, probably by impeding posttranslational maturation events, such as glycosylation (91). Finally, it was recently found that the specific elimination of THRB from thyrocytes was sufficient to alter the HPT axis. This somatic mutation decreases thyroid gland size and activity. Although the underlying mechanism is not understood, this indicates that a direct response of thyrocytes to T3 can participate in the HPT regulation, a possibility that has been previously overlooked (92). Surprisingly T3 level remains within normal range in the serum of these animals, whereas T4 level is increased. Due to intrapituitary T4 deiodination, the excess of T4 results in decreased TSH level that, in turn, alters thyrocytes activity. Therefore although the mutation is restricted to thyrocytes, some of the observed changes are not cell-autonomous consequences of the mutation but secondary to decreased stimulation by TSH. Accordingly, after TH treatment, TSH level is suppressed, in both mutant and control mice, and several differences in mRNA levels are erased. However, lower levels of Dio1 and Dio2 expression in thyrocytes persist after TH treatment. This suggests that impaired deiodination is a cell-autonomous consequence of THRB mutation in thyrocytes, and a possible primary cause of the HPT axis phenotype.
Table 1.
A Collection of Mouse Models With Somatic Mutations Altering TH Signaling
| Floxed Gene | Cre and Driving Promoter | Cell Types | Direct Effect | Reference |
|---|---|---|---|---|
| Dio2 | CGA-Cre | Pituitary cells | High TSH level | 90 |
| Dio2 | GFAP-Cre | Astrocytes | Normal TSH level. Increased fatty acid oxidation for energy expenditure. | 90 |
| Dio2 | Fabp4-Cre | Adipocytes | Increased carbohydrate oxidation for energy expenditure | 121 |
| Dio2 | MLC-Cre | Myocytes | No metabolic phenotype | 121 |
| NcoR | CGA-Cre | Pituitary cells | Low TSH level | 29 |
| Thra | CAG-Cre-ERT | All cells after tamoxifen treatment | Reduced postnatal growth. | 84 |
| Thra | Nestin-Cre | Whole brain | Lethality | 110 |
| Thra | Cnp-Cre | Oligodendrocytes and neurons | OPCs proliferation in adult brain | 115 |
| Thra | PDGFRα-Cre-ERT2 | Several cell types after tamoxifen treatment | Delayed myelination | 115 |
| Thra | Ptf1a-Cre | GABAergic neurons including Purkinje cells | Altered neuronal differentiation. | 110 |
| Thra | L7-Cre | Purkinje cells after P8 | Reduced synaptic density | 110 |
| Thra | Otx2-Cre-ERT2 | Granular cells | No effect | 110 |
| Thra | Glast-Cre-ERT2 | Astrocytes and Bergmann glia | Altered Bergmann glia | 110 |
| Thra | Thyr-Cre | Thyrocytes | Low T4 level | 92 |
| Thra | Amh-Cre | Sertoli cells | Testis development | 119 |
| Thra | Math1-Cre-ERT | Inner ear hair cells | Increased auditory response | 102 |
| Thrb | Prestin-Cre | Inner ear hair cells | Decreased auditory response | 101 |
| Thrb | Math1-Cre-ERT | Inner ear hair cells | Decreased auditory response | 102 |
| Thrb | MHCα-Cre | Cardiomyocytes | No effect | 120 |
GFAP, glial fibrillary acid protein; MHC, myosin heavy chain; PDGFR, platelet-derived growth factor receptor.
Hearing onset
Knocking out Dio2 in mice results in low local TH signaling and impairs auditory function. Histologic analysis reveals retarded differentiation of the cochlear inner sulcus and sensory epithelium and deformity of the tectorial membrane (93). The local deiodation of T4 is thus required soon after birth for proper inner ear maturation. Reciprocally increasing the local level of T3 by Dio3 knockout also compromises hearing onset, by inducing premature cochlear differentiation (94). A proper timing of T3 stimulation is thus required for cochlea postnatal maturation. Like human patient with 2 THRB-null alleles, THRB knockout mice are deaf (95). This phenotype correlates with retarded cochlear development. Therefore, the phenotype of THRB knockout mice does not result from increased level of circulating TH, but rather from the inability of some cochlear cells to respond to T3. As expected, combining THRB knockout with Dio3 knockout reverses the cochlear phenotype of Dio3 knockout mice from a premature state to a state of delayed differentiation (94). By contrast, the combination of THRA and Dio3 knockout fails to provide a similar improvement (96).
Because THRB knockout is sufficient to cause deafness, THRA auditory function has often been disregarded. However, both THRA and THRB are expressed in the ear, notably in the outer hair cells of the developing inner ear. THRA is specifically required for the proper expression of Kcnq4, encoding a potassium channel, in these cells (84, 97). If oligodendrocytes differentiation is mainly a TRα1 function (98), THRA mutations may also influence auditory function by delaying auditory nerve myelination (99). Following the general rule, according to which THRA knockout phenotype is attenuated compared with the one of knock-in mutations, the defects are much more pronounced in mice with the dominant-negative TRα1PV mutation. Although THRA-knockout mice have no hearing alteration, TRα1PV/+ mice are deaf. In the middle ear of adult TRα1PV/+ mice, persistence of mesenchyme is observed, and ossicles are enlarged (100). Therefore, deafness may reflect the general necessity of TRα1 function for proper bone ossification, not for neural cell differentiation.
The respective functions of THRA and THRB in hair cells were specifically investigated using Cre/loxP recombination. Prestin-Cre was used to produce a hair cell-specific deletion of THRB after postnatal day 11. The mutant mice display a delayed expression of the gene encoding large-conductance voltage and Ca2+-activated potassium channel (BK channel). However they have normal hearing, indicating that the origin of hearing loss in THRB-knockout mice manifests before postnatal day 11, or in cell types that are not hair cells (101). To address the possibility for an earlier intervention of THRB in hair cells, Math1-CreERT2 was used and tamoxifen treatment was administered at postnatal day 3. The mice with hair cell-specific ablation of TRβ1/2 from P3 also display normal hearing and delayed BK channel expression. However, they have slightly stronger outer hair-cell function and slightly reduced amplitudes of auditory brainstem responses. This is an additional indication that the function of THRB in hair cells is unrelated to the deafness observed in THRB knockout. Hair cell-specific TRα1L400R expression has very subtle consequences that point to an opposite function: the timing of BK channel expression is not modified, but outer hair-cell function is slightly reduced, and auditory brainstem response is slightly enhanced. Therefore, TRα1 and TRβ1/2 seem to play opposing roles in hair cells. However, although both receptors are expressed in hair cells and required for their timely differentiation, alteration of TH signaling in these cells is not sufficient to cause deafness (102). It seems rather that the key TRα1 function is in middle ear development, whereas abnormal structure of the tectorial membrane and disturbed mechanical performance seem to be the primary cause of the deafness observed in THRB knockout. These conclusions would require direct genetic demonstration and the selective introduction of THRA and THRB mutations in the corresponding cell types.
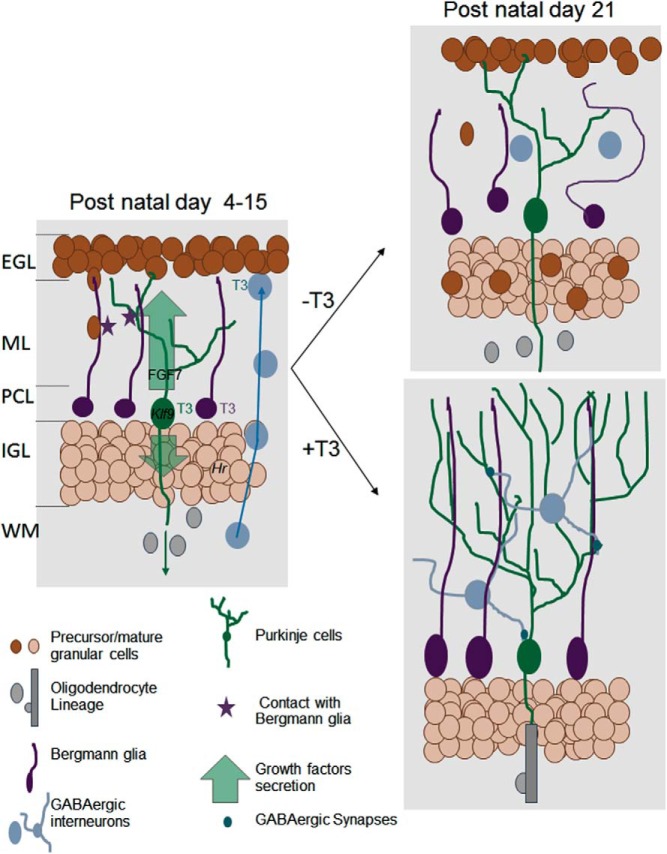
Cerebellum postnatal development (Figure 2)
Figure 2.
Direct and indirect influence of T3 on postnatal cerebellum development. The cerebellum cortex has a layered structured (EGL, external granular layer; ML, molecular layer; PCL, Purkinje cells layer; IGL, inner granular layer; WM, white matter). During early postnatal development (left), T3 cell-autonomous function is mainly to activate the differentiation of Purkinje cells (green), Bergmann glia (the cerebellum radial astrocytes, in purple), and probably also γaminobutyric acid (GABA)ergic interneurons (blue). The underlying genetic regulation is, for a large part, still unknown. Among few well-characterized TR target genes are Klf9, a transcription factor involved in Purkinje cells maturation, and Hr, a gene encoding a transcription corepressor in postmitotic granule cells the neurodevelopmental function of which is unclear. The T3-stimulated Purkinje cells secrete a number of neurotrophins and growth factors, including FGF7. These secreted factors stimulate myelin formation by oligodendrocytes in the WM and the cell-cycle exit of granule cell precursors in EGL. Direct contacts with Bergmann glia fibers guide granule cells inward migration. They also favor the establishment of synapses between Purkinje cells and GABAergic interneurons. Three weeks after birth (right, bottom), the terminal differentiation of all cell types and synaptogenesis are normally achieved. Purkinje cells axons are myelinated and EGL has disappeared. In absence of T3 stimulation (right, top), Purkinje cell arborization is reduced, Bergmann glia extension are disorganized, and cell bodies are not anchored in the PCL. Granule cell precursors migration is impaired. They accumulate in EGL and eventually die. Terminal differentiation of GABAergic interneurons is also impaired, and density of GABAergic synapses is low. In the WM, oligodendrocytes differentiation and myelin formation are delayed. FGF, fibroblast growth factor.
Rodent cerebellum development, which takes place mainly after birth, is highly sensitive to TH deficiency (103). THRA expression takes place in all cerebellar cell layers and cell types but is higher in postmitotic neurons (104). THRB is mainly, or only, expressed in the Purkinje cells layer (105). In TH-deficient animals, the dendritic arborization of Purkinje cells, a cerebellum-specific neuronal type, is reduced. The radial fibers of Bergmann glia, which serve as tracks for the inward migration of granule cell progenitors, are disorganized. The proliferation of granule cell progenitors is slow, and their inward migration is impaired. The terminal maturation of GABAergic interneurons is retarded (106). The TRβΔ337T mutation, which introduces a dominant-negative mutant TRβ in all THRB-expressing cells, alters the HPT axis regulation and reproduces part of this phenotype (107). Whereas some of the cytological alterations observed in TRβΔ337T/+ mice are likely to be cell-autonomous consequences of the inability of Purkinje cells to respond to T3, others may be due rather to the excess of T3, which overstimulates the remaining TRα1. This is the case for a transient foliation defect of the cerebellum. This feature was reported in hyperthyroid rats (108) and Dio3 knockout mice, in which it is reversed by knocking out THRA (96). It might reflect a premature cell-cycle exit of granule cell progenitors.
Early histologic examination of THRA knockout proved that absence of TRα1 is not sufficient to clearly alter neurodevelopment. This indicates that the cytological defects observed in TH-deficient animals mainly reflect the negative influence of unliganded TRα1 in cerebellar cells (64). In line with this hypothesis, the cerebellum phenotype of mice expressing TRα1L400R from the very beginning of development in all tissues is identical to the one of hypothyroid mice (109). Several Cre/TRαAMI combinations were used to distinguish between several simultaneous influences of the TRα1L400R mutation (110). In agreement with primary culture data (111), early postnatal differentiation of Purkinje cells was found to be directly controlled by the T3/TRα1 pathway. This would suggest that differentiation of Purkinje cells is a multistep process (112) and that differentiation arrest occurs at a later stage in mice carrying TRβΔ337T mutation (see above) (107). It is difficult, however, to rule out that the mutant receptors, TRα1L400R or TRβΔ337T, exert their dominant-negative activity on both TRα1 and TRβ1.
Interestingly, maturation of Purkinje cells is marked by the loss of axon regeneration capacity, which can be evidenced after in vitro axotomy. The loss of regenerative capacity is delayed by expressing TRα1L400R in Purkinje cells. This effect is mediated by a down-regulation of the Kfl9 gene transcription (113). The other cell type in which TRα1L400R expression has a clear cell-autonomous effect is Bergmann glia, in which radial organization is altered. By contrast, the defect of inward migration of granule cell progenitors appears to be a consequence secondary to the defects in Purkinje cells and Bergmann glia differentiation. Purkinje cells secrete a number of growth factors and neurotrophins that are required for the cell-cycle exit of granule cell progenitors that precedes their migration. Among these diffusible factors, Fgf7 is under T3 control. The alteration of Bergmann glia fibers directly impacts radial migration of granule cells. That the most visible histologic sign of TH deficiency in cerebellum, ie, the persistence of granule cell progenitors in the external granular layer, is not a direct consequence of deregulation of TR target genes is in agreement with the low expression of THRA is these cells (104). The case of oligodendrocyte precursor cells (OPCs) appears fairly complex. Classical primary culture experiments have demonstrated that the differentiation of these cells into mature oligodendrocytes, which ensure axon myelination, is strictly T3/TRα1 dependent (114). However, during cerebellum postnatal development, the delayed differentiation of OPCs in mutant mice is clearly not a cell-autonomous consequence of TRα1L400R expression, but rather an indirect consequence of altered secretion of neurotrophic and growth factors by neurons (115). This discrepancy can be explained, however, if one takes into account the likely existence of at least 2 distinct population of OPCs: one is active in newborn animals and ensures the initial myelination process, whereas the other is responsible for myelin renewal in adult brain (116). Primary cultures seem to correspond to adult OPC population. Only these later OPCs would directly depend on T3 for cell-cycle exit and differentiation. Accordingly, the cerebellar white matter of mice expressing TRα1L400R in OPCs only is progressively invaded by slow-cycling OPCs over time.
Future Directions
The above genetic exploration illustrates the importance of a multiscale analysis of specific aspects of mouse phenotypes before focusing on gene expression analysis. It also underscores the interest of the new generation of mouse models that provides the opportunity to fill the gap between gene expression studies and physiological/developmental studies. Several attempts have already been made to identify TR target genes from whole-cerebellum analyses that were met with limited success (14, 28, 117, 118). Retrospectively, this is not surprising because the cell-autonomous response to T3 is limited to a small fraction of the cell population and is different in different cell types. The auditory function studies were focused on hair cells, but mouse genetics indicates that future investigations should also include the cells surrounding the mechanosensory epithelium and the middle ear. In this review we considered only a few examples of T3/TR-dependent functions out of many for which investigations can benefit from this genetic approach. To be exhaustive, we should mention, however, testis development, in which the cell-autonomous influence of TRα1 on the postnatal differentiation of Sertoli cells was demonstrated (119); the heart in which a specific deletion of THRB in cardiac myocites has no visible consequence (120); and the energy expenditure, as the respective contribution of brain astrocytes, skeletal muscles, and adipose tissues was analyzed using the floxed Dio2 allele (121). Overall, a vision emerges in which T3 coordinates cells and tissue behaviors, not by acting independently on each cell type, but by controlling the differentiation or function of “master cell types,” which exert an important influence on their local or distant environment. This is unexpected considering the broad distribution of the TRs and their ligand. Once these cell master types are identified, genome-wide analyses of gene expression will certainly be more rewarding. In a more general manner, current progress in the genetic analysis of TH signaling in vivo provides an original experimental approach by which to understand the basic mechanisms of communication between neighboring cells and distant organs, which orchestrate development and play a major role in the maintenance of homeostasis.
Acknowledgments
We thank J. Burden for reading the manuscript.
This work was supported by Agence Nationale pour la Recherche (Thyrogenomics2 contract).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by Agence Nationale pour la Recherche (Thyrogenomics2 contract).
Footnotes
- HPT
- hypothalamus-pituitary-thyroid
- NCoR
- nuclear receptor corepressor
- OPC
- oligodendrocyte precursor cell
- SMRT
- silencing mediator of retinoid and thyroid hormone receptor
- TH
- thyroid hormone
- TR
- thyroid hormone receptor.
References
- 1. Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81(3):1097–1142. [DOI] [PubMed] [Google Scholar]
- 2. Mangelsdorf DJ, Thummel C, Beato M, et al. . The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perissi V, Rosenfeld MG. Controlling nuclear receptors: the circular logic of cofactor cycles. Nat Rev Mol Cell Biol. 2005;6(7):542–554. [DOI] [PubMed] [Google Scholar]
- 4. Lee S, Privalsky ML. Heterodimers of retinoic acid receptors and thyroid hormone receptors display unique combinatorial regulatory properties. Mol Endocrinol. 2005;19(4):863–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mengeling BJ, Lee S, Privalsky ML. Coactivator recruitment is enhanced by thyroid hormone receptor trimers. Mol Cell Endocrinol. 2008;280(1–2):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Storey NM, Gentile S, Ullah H, et al. . Rapid signaling at the plasma membrane by a nuclear receptor for thyroid hormone. Proc Natl Acad Sci USA. 2006;103(13):5197–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blanchet E, Bertrand C, Annicotte JS, et al. . Mitochondrial T3 receptor p43 regulates insulin secretion and glucose homeostasis. FASEB J. 2012;26(1):40–50. [DOI] [PubMed] [Google Scholar]
- 8. Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31(2):139–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Das B, Matsuda H, Fujimoto K, Sun G, Matsuura K, Shi YB. Molecular and genetic studies suggest that thyroid hormone receptor is both necessary and sufficient to mediate the developmental effects of thyroid hormone. Gen Comp Endocrinol. 2010;168(2):174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dugas JC, Ibrahim A, Barres BA. The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Mol Cell Neurosci. 2012;50(1):45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thompson CC. Thyroid hormone-responsive genes in developing cerebellum include a novel synaptotagmin and a hairless homolog. J Neurosci. 1996;16(24):7832–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin JZ, Sieglaff DH, Yuan C, et al. . Gene specific actions of thyroid hormone receptor subtypes. PLoS One. 2013;8(1):e52407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poguet AL, Legrand C, Feng X, et al. . Microarray analysis of knockout mice identifies cyclin D2 as a possible mediator for the action of thyroid hormone during the postnatal development of the cerebellum. Dev Biol. 2003;254(2):188–199. [DOI] [PubMed] [Google Scholar]
- 14. Quignodon L, Grijota-Martinez C, Compe E, et al. . A combined approach identifies a limited number of new thyroid hormone target genes in post-natal mouse cerebellum. J Mol Endocrinol. 2007;39(1):17–28. [DOI] [PubMed] [Google Scholar]
- 15. Abe E, Marians RC, Yu W, et al. . TSH is a negative regulator of skeletal remodeling. Cell. 2003;115(2):151–162. [DOI] [PubMed] [Google Scholar]
- 16. Bassett JH, O'Shea PJ, Sriskantharajah S, et al. . Thyroid hormone excess rather than thyrotropin deficiency induces osteoporosis in hyperthyroidism. Mol Endocrinol. 2007;21(5):1095–1107. [DOI] [PubMed] [Google Scholar]
- 17. Piehl S, Hoefig CS, Scanlan TS, Köhrle J. Thyronamines–past, present, and future. Endocr Rev. 2011;32(1):64–80. [DOI] [PubMed] [Google Scholar]
- 18. Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. Metabolic effects of thyroid hormone derivatives. Thyroid. 2008;18(2):239–253. [DOI] [PubMed] [Google Scholar]
- 19. Krysko O, Hulshagen L, Janssen A, et al. . Neocortical and cerebellar developmental abnormalities in conditions of selective elimination of peroxisomes from brain or from liver. J Neurosci Res. 2007;85(1):58–72. [DOI] [PubMed] [Google Scholar]
- 20. Freitas FR, Capelo LP, O'Shea PJ, et al. . The thyroid hormone receptor β-specific agonist GC-1 selectively affects the bone development of hypothyroid rats. J Bone Miner Res. 2005;20(2):294–304. [DOI] [PubMed] [Google Scholar]
- 21. Karsenty G, Oury F. Regulation of male fertility by the bone-derived hormone osteocalcin. Mol Cell Endocrinol. 2014;382(1):521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oury F, Khrimian L, Denny CA, et al. . Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155(1):228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan SY, Vasilopoulou E, Kilby MD. The role of the placenta in thyroid hormone delivery to the fetus. Nat Clin Pract Endocrinol Metab. 2009;5(1):45–54. [DOI] [PubMed] [Google Scholar]
- 24. Loubière LS, Vasilopoulou E, Bulmer JN, et al. . Expression of thyroid hormone transporters in the human placenta and changes associated with intrauterine growth restriction. Placenta. 2010;31(4):295–304. [DOI] [PubMed] [Google Scholar]
- 25. Vasilopoulou E, Loubiere LS, Heuer H, et al. . Monocarboxylate transporter 8 modulates the viability and invasive capacity of human placental cells and fetoplacental growth in mice. PLoS One. 2013;8(6):e65402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Compe E, Malerba M, Soler L, Marescaux J, Borrelli E, Egly JM. Neurological defects in trichothiodystrophy reveal a coactivator function of TFIIH. Nat Neurosci. 2007;10(11):1414–1422. [DOI] [PubMed] [Google Scholar]
- 27. Bilesimo P, Jolivet P, Alfama G, et al. . Specific histone lysine 4 methylation patterns define TR-binding capacity and differentiate direct T3 responses. Mol Endocrinol. 2011;25(2):225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dong H, Yauk CL, Rowan-Carroll A, et al. . Identification of thyroid hormone receptor binding sites and target genes using ChIP-on-chip in developing mouse cerebellum. PLoS ONE. 2009;4(2):e4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Costa-e-Sousa RH, Astapova I, Ye F, Wondisford FE, Hollenberg AN. The thyroid axis is regulated by NCoR1 via its actions in the pituitary. Endocrinology. 2012;153(10):5049–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chatonnet F, Guyot R, Benoît G, Flamant F. Genome-wide analysis of thyroid hormone receptors shared and specific functions in neural cells. Proc Natl Acad Sci USA. 2013;110(8):E766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramadoss P, Abraham BJ, Tsai L, et al. . Novel mechanism of positive versus negative regulation by thyroid hormone receptor β 1 (TRβ1) identified by genome-wide profiling of binding sites in mouse liver. J Biol Chem. 2014;289(3):1313–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hah N, Kraus WL. Hormone-regulated transcriptomes: Lessons learned from estrogen signaling pathways in breast cancer cells. Mol Cell Endocrinol. 2014;382(1):652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Menjo M, Yamaguchi S, Murata Y, et al. . Responsiveness to thyroid hormone is enhanced in rat hepatocytes cultured as spheroids compared with that in monolayers: altered responsiveness to thyroid hormone possibly involves complex formed on thyroid hormone response elements. Thyroid. 1999;9(9):959–967. [DOI] [PubMed] [Google Scholar]
- 34. Lavado-Autric R, Ausó E, Garcia-Velasco JV, et al. . Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest. 2003;111(7):1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ausó E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145(9):4037–4047. [DOI] [PubMed] [Google Scholar]
- 36. Cuevas E, Ausó E, Telefont M, Morreale de Escobar G, Sotelo C, Berbel P. Transient maternal hypothyroxinemia at onset of corticogenesis alters tangential migration of medial ganglionic eminence-derived neurons. Eur J Neurosci. 2005;22(3):541–551. [DOI] [PubMed] [Google Scholar]
- 37. Tancevski I, Rudling M, Eller P. Thyromimetics: a journey from bench to bed-side. Pharmacol Ther. 2011;131(1):33–39. [DOI] [PubMed] [Google Scholar]
- 38. Grijota-Martínez C, Samarut E, Scanlan TS, Morte B, Bernal J. In vivo activity of the thyroid hormone receptor β- and α-selective agonists GC-24 and CO23 on rat liver, heart, and brain. Endocrinology. 2011;152(3):1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiellini G, Apriletti JW, Yoshihara HA, Baxter JD, Ribeiro RC, Scanlan TS. A high-affinity subtype-selective agonist ligand for the thyroid hormone receptor. Chem Biol. 1998;5(6):299–306. [DOI] [PubMed] [Google Scholar]
- 40. Ladenson PW, Kristensen JD, Ridgway EC, et al. . Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med. 2010;362(10):906–916. [DOI] [PubMed] [Google Scholar]
- 41. López M, Varela L, Vázquez MJ, et al. . Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16(9):1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Varela L, Martínez-Sánchez N, Gallego R, et al. . Hypothalamic mTOR pathway mediates thyroid hormone-induced hyperphagia in hyperthyroidism. J Pathol. 2012;227(2):209–222. [DOI] [PubMed] [Google Scholar]
- 43. Klieverik LP, Janssen SF, van Riel A, et al. . Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc Natl Acad Sci USA. 2009;106(14):5966–5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mittag J, Lyons DJ, Sällström J, et al. . Thyroid hormone is required for hypothalamic neurons regulating cardiovascular functions. J Clin Invest. 2013;123(1):509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Decherf S, Seugnet I, Kouidhi S, Lopez-Juarez A, Clerget-Froidevaux MS, Demeneix BA. Thyroid hormone exerts negative feedback on hypothalamic type 4 melanocortin receptor expression. Proc Natl Acad Sci USA. 2010;107(9):4471–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mayerl S, Visser TJ, Darras VM, Horn S, Heuer H. Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology. 2012;153(3):1528–1537. [DOI] [PubMed] [Google Scholar]
- 47. Heuer H, Visser TJ. The pathophysiological consequences of thyroid hormone transporter deficiencies: Insights from mouse models. Biochim Biophys Acta. 2013;1830(7):3974–3978. [DOI] [PubMed] [Google Scholar]
- 48. Morte B, Ceballos A, Diez D, et al. . Thyroid hormone-regulated mouse cerebral cortex genes are differentially dependent on the source of the hormone: a study in monocarboxylate transporter-8- and deiodinase-2-deficient mice. Endocrinology. 2010;151(5):2381–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Muller J, Mayerl S, Visser TJ, et al. . Tissue-specific alterations in thyroid hormone homeostasis in combined Mct10 and Mct8 deficiency. Endocrinology. 2013;155(1):315–325. [DOI] [PubMed] [Google Scholar]
- 50. Schneider MJ, Fiering SN, Thai B, et al. . Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147(1):580–589. [DOI] [PubMed] [Google Scholar]
- 51. Marsili A, Tang D, Harney JW, et al. . Type II iodothyronine deiodinase provides intracellular 3,5,3′-triiodothyronine to normal and regenerating mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2011;301(5):E818–E824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ng L, Hurley JB, Dierks B, et al. . A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27(1):94–98. [DOI] [PubMed] [Google Scholar]
- 53. Castillo M, Hall JA, Correa-Medina M, et al. . Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes. 2011;60(4):1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116(2):476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hernandez A, Quignodon L, Martinez ME, Flamant F, St Germain DL. Type 3 deiodinase deficiency causes spatial and temporal alterations in brain T3 signaling that are dissociated from serum thyroid hormone levels. Endocrinology. 2010;151(11):5550–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ueta CB, Oskouei BN, Olivares EL, et al. . Absence of myocardial thyroid hormone inactivating deiodinase results in restrictive cardiomyopathy in mice. Mol Endocrinol. 2012;26(5):809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Forrest D, Hanebuth E, Smeyne RJ, et al. . Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor β: evidence for tissue-specific modulation of receptor function. EMBO J. 1996;15(12):3006–3015. [PMC free article] [PubMed] [Google Scholar]
- 58. Abel ED, Ahima RS, Boers ME, Elmquist JK, Wondisford FE. Critical role for thyroid hormone receptor β2 in the regulation of paraventricular thyrotropin-releasing hormone neurons. J Clin Invest. 2001;107(8):1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Flamant F, Samarut J. Thyroid hormone receptors: lessons from knockout and knock-in mutant mice. Trends Endocrinol Metab. 2003;14(2):85–90. [DOI] [PubMed] [Google Scholar]
- 60. Fraichard A, Chassande O, Plateroti M, et al. . The T3R α gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. EMBO J. 1997;16(14):4412–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet. 1998;19(1):87–90. [DOI] [PubMed] [Google Scholar]
- 62. Gauthier K, Plateroti M, Harvey CB, et al. . Genetic analysis reveals different functions for the products of the thyroid hormone receptor α locus. Mol Cell Biol. 2001;21(14):4748–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Göthe S, Wang Z, Ng L, et al. . Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev. 1999;13(10):1329–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Morte B, Manzano J, Scanlan T, Vennström B, Bernal J. Deletion of the thyroid hormone receptor α 1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc Natl Acad Sci USA. 2002;99(6):3985–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Flamant F, Poguet AL, Plateroti M, et al. . Congenital hypothyroid Pax8(−/−) mutant mice can be rescued by inactivating the TRα gene. Mol Endocrinol. 2002;16(1):24–32. [DOI] [PubMed] [Google Scholar]
- 66. Flamant F, Gauthier K. Thyroid hormone receptors: the challenge of elucidating isotype-specific functions and cell-specific response. Biochim Biophys Acta. 2013;1830(7):3900–3907. [DOI] [PubMed] [Google Scholar]
- 67. Tinnikov A, Nordström K, Thorén P, et al. . Retardation of post-natal development caused by a negatively acting thyroid hormone receptor α1. EMBO J. 2002;21(19):5079–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Venero C, Guadaño-Ferraz A, Herrero AI, et al. . Anxiety, memory impairment, and locomotor dysfunction caused by a mutant thyroid hormone receptor α1 can be ameliorated by T3 treatment. Genes Dev. 2005;19(18):2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sjögren M, Alkemade A, Mittag J, et al. . Hypermetabolism in mice caused by the central action of an unliganded thyroid hormone receptor α1. EMBO J. 2007;26(21):4535–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Warner A, Rahman A, Solsjö P, et al. . Inappropriate heat dissipation ignites brown fat thermogenesis in mice with a mutant thyroid hormone receptor α1. Proc Natl Acad Sci USA. 2013;110(40):16241–16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Astapova I, Vella KR, Ramadoss P, et al. . The nuclear receptor corepressor (NCoR) controls thyroid hormone sensitivity and the set point of the hypothalamic-pituitary-thyroid axis. Mol Endocrinol. 2011;25(2):212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Weiss RE, Xu J, Ning G, Pohlenz J, O'Malley BW, Refetoff S. Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone. EMBO J. 1999;18(7):1900–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nofsinger RR, Li P, Hong SH, et al. . SMRT repression of nuclear receptors controls the adipogenic set point and metabolic homeostasis. Proc Natl Acad Sci USA. 2008;105(50):20021–20026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pei L, Leblanc M, Barish G, et al. . Thyroid hormone receptor repression is linked to type I pneumocyte-associated respiratory distress syndrome. Nat Med. 2011;17(11):1466–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fozzatti L, Kim DW, Park JW, Willingham MC, Hollenberg AN, Cheng SY. Nuclear receptor corepressor (NCOR1) regulates in vivo actions of a mutated thyroid hormone receptor α. Proc Natl Acad Sci USA. 2013;110(19):7850–7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fozzatti L, Lu C, Kim DW, et al. . Resistance to thyroid hormone is modulated in vivo by the nuclear receptor corepressor (NCOR1). Proc Natl Acad Sci USA. 2011;108(42):17462–17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Laryea G, Schütz G, Muglia LJ. Disrupting hypothalamic glucocorticoid receptors causes HPA axis hyperactivity and excess adiposity. Mol Endocrinol. 2013;27(10):1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7:e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schreiber AM, Mukhi S, Brown DD. Cell-cell interactions during remodeling of the intestine at metamorphosis in Xenopus laevis. Dev Biol. 2009;331(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24(1):71–80. [DOI] [PubMed] [Google Scholar]
- 81. Murray SA. Mouse resources for craniofacial research. Genesis. 2011;49(4):190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Karaca M, Maechler P. Development of mice with brain-specific deletion of floxed Glud1 (glutamate dehydrogenase 1) using Cre recombinase driven by the nestin promoter. Neurochem Res. 2014;39(3):456–459. [DOI] [PubMed] [Google Scholar]
- 83. Darimont BD, Wagner RL, Apriletti JW, Stallcup MR, Kushner PJ, Baxter JD, Fletterick RJ, Yamamoto KR. Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev. 1998;12(21):3343–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Quignodon L, Vincent S, Winter H, Samarut J, Flamant F. A point mutation in the activation function 2 domain of thyroid hormone receptor α1 expressed after CRE-mediated recombination partially recapitulates hypothyroidism. Mol Endocrinol. 2007;21(10):2350–2360. [DOI] [PubMed] [Google Scholar]
- 85. Vujovic M, Nordström K, Gauthier K, et al. . Interference of a mutant thyroid hormone receptor α1 with hepatic glucose metabolism. Endocrinology. 2009;150(6):2940–2947. [DOI] [PubMed] [Google Scholar]
- 86. Astapova I, Lee LJ, Morales C, Tauber S, Bilban M, Hollenberg AN. The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proc Natl Acad Sci USA 2008;105(49):19544–19549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yamada T, Kawano H, Sekine K, et al. . SRC-1 is necessary for skeletal responses to sex hormones in both males and females. J Bone Miner Res. 2004;19(9):1452–1461. [DOI] [PubMed] [Google Scholar]
- 88. Chiamolera MI, Sidhaye AR, Matsumoto S, et al. . Fundamentally distinct roles of thyroid hormone receptor isoforms in a thyrotroph cell line Are due to differential DNA binding. Mol Endocrinol. 2012;26(6):926–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Abel ED, Kaulbach HC, Campos-Barros A, et al. . Novel insight from transgenic mice into thyroid hormone resistance and the regulation of thyrotropin. J Clin Invest. 1999;103(2):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fonseca TL, Correa-Medina M, Campos MP, et al. . Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J Clin Invest. 2013;123(4):1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schaaf L, Theodoropoulou M, Gregori A, et al. . Thyrotropin-releasing hormone time-dependently influences thyrotropin microheterogeneity–an in vivo study in euthyroidism. J Endocrinol. 2000;166(1):137–143. [DOI] [PubMed] [Google Scholar]
- 92. Selmi-Ruby S, Bouazza L, Obregon MJ, et al. . The targeted inactivation of TRβ gene in thyroid follicular cells suggests a new mechanism of regulation of thyroid hormone production. Endocrinology. 2014;155(2):635–646. [DOI] [PubMed] [Google Scholar]
- 93. Ng L, Goodyear RJ, Woods CA, et al. . Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci U S A. 2004;101(10):3474–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ng L, Hernandez A, He W, et al. . A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology. 2009;150(4):1952–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Forrest D, Erway LC, Ng L, Altschuler R, Curran T. Thyroid hormone receptor β is essential for development of auditory function. Nat Genet. 1996;13(3):354–357. [DOI] [PubMed] [Google Scholar]
- 96. Peeters RP, Hernandez A, Ng L, et al. . Cerebellar abnormalities in mice lacking type 3 deiodinase and partial reversal of phenotype by deletion of thyroid hormone receptor α1. Endocrinology. 2013;154(1):550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Winter H, Braig C, Zimmermann U, et al. . Thyroid hormone receptors TRα1 and TRβ differentially regulate gene expression of Kcnq4 and prestin during final differentiation of outer hair cells. J Cell Sci. 2006;119(Pt 14):2975–2984. [DOI] [PubMed] [Google Scholar]
- 98. Billon N, Jolicoeur C, Tokumoto Y, Vennström B, Raff M. Normal timing of oligodendrocyte development depends on thyroid hormone receptor α 1 (TRα1). EMBO J. 2002;21(23):6452–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Knipper M, Bandtlow C, Gestwa L, et al. . Thyroid hormone affects Schwann cell and oligodendrocyte gene expression at the glial transition zone of the VIIIth nerve prior to cochlea function. Development. 1998;125(18):3709–3718. [DOI] [PubMed] [Google Scholar]
- 100. Cordas EA, Ng L, Hernandez A, Kaneshige M, Cheng SY, Forrest D. Thyroid hormone receptors control developmental maturation of the middle ear and the size of the ossicular bones. Endocrinology. 2012;153(3):1548–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Winter H, Rüttiger L, Muller M, et al. . Deafness in TRβ mutants is caused by malformation of the tectorial membrane. J Neurosci. 2009;29(8):2581–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dettling J, Franz C, Zimmermann U, et al. . Autonomous functions of murine thyroid hormone receptor TRα and TRβ in cochlear hair cells. Mol Cell Endocrinol. 2014;382(1):26–37. [DOI] [PubMed] [Google Scholar]
- 103. Anderson GW. Thyroid hormone and cerebellar development. Cerebellum. 2008;7(1):60–74. [DOI] [PubMed] [Google Scholar]
- 104. Wallis K, Dudazy S, van Hogerlinden M, Nordström K, Mittag J, Vennström B. The thyroid hormone receptor α1 protein is expressed in embryonic postmitotic neurons and persists in most adult neurons. Mol Endocrinol. 2010;24(10):1904–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bradley DJ, Young WS 3rd, Weinberger C. Differential expression of α and β thyroid hormone receptor genes in rat brain and pituitary. Proc Natl Acad Sci U S A. 1989;86(18):7250–7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Manzano J, Bernal J, Morte B. Influence of thyroid hormones on maturation of rat cerebellar astrocytes. Int J Dev Neurosci. 2007;25(3):171–179. [DOI] [PubMed] [Google Scholar]
- 107. Portella AC, Carvalho F, Faustino L, Wondisford FE, Ortiga-Carvalho TM, Gomes FC. Thyroid hormone receptor β mutation causes severe impairment of cerebellar development. Mol Cell Neurosci. 2010;44(1):68–77. [DOI] [PubMed] [Google Scholar]
- 108. Lauder JM, Altman J, Krebs H. Some mechanisms of cerebellar foliation: effects of early hypo- and hyperthyroidism. Brain Res. 1974;76(1):33–40. [DOI] [PubMed] [Google Scholar]
- 109. Fauquier T, Romero E, Picou F, et al. . Severe impairment of cerebellum development in mice expressing a dominant-negative mutation inactivating thyroid hormone receptor α1 isoform. Dev Biol. 2011;356(2):350–358. [DOI] [PubMed] [Google Scholar]
- 110. Fauquier T, Chatonnet F, Picou F, et al. . Purkinje cells and Bergmann glia are primary targets of the TRα1 thyroid hormone receptor during mouse cerebellum postnatal development. Development. 2014;141(1):166–175. [DOI] [PubMed] [Google Scholar]
- 111. Heuer H, Mason CA. Thyroid hormone induces cerebellar Purkinje cell dendritic development via the thyroid hormone receptor α1. J Neurosci. 2003;23(33):10604–10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Boukhtouche F, Brugg B, Wehrle R, et al. . Induction of early Purkinje cell dendritic differentiation by thyroid hormone requires RORα. Neural Dev. 2010;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Avci HX, Lebrun C, Wehrlé R, et al. . Thyroid hormone triggers the developmental loss of axonal regenerative capacity via thyroid hormone receptor α1 and krüppel-like factor 9 in Purkinje cells. Proc Natl Acad Sci USA. 2012;109(35):14206–14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Raff M. Intracellular developmental timers. Cold Spring Harb Symp Quant Biol. 2007;72:431–435. [DOI] [PubMed] [Google Scholar]
- 115. Picou F, Fauquier T, Chatonnet F, Flamant F. A bimodal influence of thyroid hormone on cerebellum oligodendrocyte differentiation. Mol Endocrinol. 2012;26(4):608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bouslama-Oueghlani L, Wehrlé R, Sotelo C, Dusart I. Heterogeneity of NG2-expressing cells in the newborn mouse cerebellum. Dev Biol. 2005;285(2):409–421. [DOI] [PubMed] [Google Scholar]
- 117. Takahashi M, Negishi T, Tashiro T. Identification of genes mediating thyroid hormone action in the developing mouse cerebellum. J Neurochem. 2008;104(3):640–652. [DOI] [PubMed] [Google Scholar]
- 118. Chatonnet F, Guyot R, Picou F, Bondesson M, Flamant F. Genome-wide search reveals the existence of a limited number of thyroid hormone receptor α target genes in cerebellar neurons. PLoS One. 2012;7(5):e30703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fumel B, Guerquin MJ, Livera G, et al. . Thyroid hormone limits postnatal Sertoli cell proliferation in vivo by activation of its α1 isoform receptor (TRα1) present in these cells and by regulation of Cdk4/JunD/c-myc mRNA levels in mice. Biol Reprod. 2012;87(1):16, 1–9. [DOI] [PubMed] [Google Scholar]
- 120. Makino A, Suarez J, Wang H, Belke DD, Scott BT, Dillmann WH. Thyroid hormone receptor-β is associated with coronary angiogenesis during pathological cardiac hypertrophy. Endocrinology. 2009;150(4):2008–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Fonseca TL, Werneck-De-Castro JP, Castillo M, et al. . Tissue-specific inactivation of type II deiodinase reveals multi-level control of fatty acid oxidation by thyroid hormone in the mouse [published online January 31, 2014]. Diabetes. doi:10.2337/db13-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21(1):70–71. [DOI] [PubMed] [Google Scholar]
- 123. Srinivas S, Watanabe T, Lin CS, et al. . Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bianco AC, Anderson G, Forrest D. American thyroid association guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid. 2014;24:88–168. [DOI] [PMC free article] [PubMed] [Google Scholar]