Abstract
Introduction
Crocus sativus L. (saffron) has many biological effects such as antioxidant property. The present study investigated the immunomodulatory effects of the aqueous saffron extract on streptozotocin (STZ)-induced diabetic rats.
Materials and methods
In this study, the rats were divided into the following groups of 9 animals each: control, untreated diabetic, three saffron extract-treated diabetic groups. Diabetes was induced by STZ in rats. Saffron was administered 3 days after STZ administration; these injections were continued to the end of the study (4 weeks). At the end of the 4-week period, blood was drawn for biochemical assays and the abdominal aorta was removed for detecting the inflammatory cytokines expression.
Results
We found that saffron decreased blood glucose, malondialdehyde, nitric oxide, total lipids, triglycerides, cholesterol levels significantly (p < 0.01) and increased glutathione level, catalase, and superoxide dismutase activities in the saffron-treated diabetic groups compared with the untreated groups, in a dose dependent manner (p < 0.05, p < 0.01, p < 0.001). On the other hand, saffron-treated diabetic rats inhibited the expression of inflammatory cytokines in the abdominal aorta versus the untreated diabetic rats.
Conclusion
Our results validate the use of saffron as a treatment against diabetes mellitus and its vascular complications.
Keywords: Diabetes mellitus, Saffron, TNF-α, IL-6, Lipid profile, Oxidant, Free radical, Antioxidant, Aorta
1. Introduction
Diabetes mellitus (DM) is a chronic disease that occurs when the pancreases do not produce enough insulin or alternatively, when the body cannot effectively use the insulin it produces, and its incidence is considered to be high (4–5%) all over the world.1 DM is the principal factor responsible for renal failure, blindness and non-traumatic amputations; the connection between diabetes with poor metabolic control and the high prevalence of mortality due to coronary heart disease, retinopathy, nephropathies and neuropathies has been well established.2, 3, 4 Furthermore, diabetes mellitus in turn leads to hyperlipidemia to cardiovascular morbidity and mortality. Nevertheless, control of plasma glucose and lipid concentrations inhibit micro-vascular complications.5 Hyperglycemia, the hallmark of diabetes, is believed to generate reactive oxygen species (ROS) that eventually lead to oxidative stress and microvascular complications in several organs. Vascular complications are the major cause of morbidity and mortality in patients with DM, which include macrovascular and microvascular disorders.6 Macrovascular injury includes coronary artery disease, atherosclerosis, and peripheral vascular disease.2 The cardinal pathological mechanism of macrovascular disease is the process of atherosclerosis, resulting in narrowed arteries throughout the body.7 Accumulating evidence suggests that inflammation plays a central role in the pathophysiology of diabetes-accelerated atherosclerosis, starting from initiation through progression and, ultimately, the thrombotic complications of atherosclerosis, and thus can be used to predict future cardiovascular risk by monitoring inflammatory biomarkers and to serve as a therapeutic target for atherosclerotic diseases.8 Supra physiological levels of glucose are notorious to provoke these changes in the vascular causing diabetic vasculopathy.9 Besides, growing evidence suggest that elevation in circulating lipids may also contribute to vascular disease progression.10 There is overwhelming evidence that inflammatory injury play a crucial role in the development of accelerated atherosclerosis and endothelial dysfunction in DM.11 Activation of proinflammation genes thought to induce the transcription of a large range of genes implicated in vascular inflammation, including cytokines [e.g. tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1beta (IL-1β)], adhesion molecules [e.g. inter cellular adhesion molecule-1 (ICAM-1)], chemokines, and other growth factors,12 which suggest that some of these genes are a key regulator in the initiation and development of vascular inflammation. Emerging evidence now indicates that DM is an inflammatory disease and that certain inflammatory markers seem to play major roles in the pathophysiology of diabetic vascular complications.13 The mechanisms responsible are suggested to be hyperglycemia-induced ROS generation of AGEs, activation of proinflammatory cascades and stimulation of glomerular mesangial cells to produce extracellular matrix proteins.14
Among the cytokines, TNF-α can promote local ROS generation resulting in alterations in glomerular permeability leading to albuminuria.15 There is enough evidence that serum and urinary TNF-α levels are significantly upregulated in various complication of DM including diabetes nephropathy (DN).16 More recent studies in diabetic patients demonstrate significant association between DN and IL-6, another proinflammatory cytokine. IL-6 mediates glomerular basement membrane thickening, a crucial lesion of DN, and is a strong predictor of progression of diabetes complications.17, 18, 19
Moreover, implication of oxidative stress in the pathogenesis of diabetes is suggested, not only by oxygen free radical generation, but also due to nonenzymatic protein glycosylation, auto-oxidation of glucose, impaired glutathione metabolism, alteration in antioxidant enzymes and lipid peroxides formation.20 Cellular defense against free radical injury is provided by enzymatic (catalase (CAT), superoxide dismutases (SOD), and glutathione peroxidase) and nonenzymatic (gluthatione (GSH), α-tocopherol, vitamin C, and urate) free radical scavenging systems, present in the cell.21 Although, there is treatment available for diabetes, the drugs used are associated with undesirable side effects and high cost for patients, which in recent years have led to intense research in alternative therapies such as medicinal plants that provide an effective, reliable and cheap treatment option. Recent overwhelming attention to plant products and alternative medicine has encouraged plant chemists, pharmacologists, biochemists, and molecular biologists to combine their efforts in a search for natural agents that can limit free radical mediated injuries during and following diabetes mellitus, for better therapeutic management of diabetes.
Crocus sativus L. (saffron) is a perennial stem less herb which belongs to the Iridaceae family. It is widely cultivated in Iran and other countries.22 In traditional medicine, as well as in modern pharmacology, saffron has been used in the treatment of numerous diseases. It was reported that saffron and its constituents have antitumor,23 anti-inflammatory, antinociceptive,24 antioxidant,25 antidepressant,26 hypolipidemic,27 and could improve memory as well learning abilities in rats.28, 29 Evidence showed that saffron and its constituents reduced lipid peroxidation in various tissues25, 30, 31 following oxidative damages in rats. Furthermore radical scavenging effect of saffron extract and its bioactive constituents, safranal and crocin have been shown previously using DPPH (1,1-diphenyl-2-picryl-hydrazyl) radical scavenging test.32
Thus, the present study was designed to evaluate the effects of saffron on the plasma levels of glucose, lipids, oxidant antioxidant balance and also, we shall investigate the changes of proinflammatory genes (TNF-α and IL-6) expression in the abdominal aorta to verify the protective effects of saffron on vascular complication of DM in rats.
2. Materials and methods
2.1. Chemicals
All purified enzymes, coenzymes, substrates, standards, buffers, kits and other chemicals were purchased from Sigma Chemicals Corporation, MO, USA.
2.2. Animals
Adult male Wistar rats (weight 250–300 g) were provided by the university experimental animal care center. Rats were kept in their own cages at constant room temperature (21 ± 2 °C) under a normal 12-h light/dark cycle with free access to food and water. These conditions were maintained constant throughout the experiments. The animals were housed according to regulations for the Welfare of experimented animals. The study was conducted in Mashhad Medical University Experimental Animal Research Laboratory. Protocols were approved by the Ethical Committee (The Ethical Research Committee of Mashhad University of Medical Sciences).
2.3. Plant
The saffron used in this study was collected from a private garden and identified by botanists in the herbarium of Ferdowsi University of Mashhad (specimen number 293-0303-1).
2.4. Preparation of aqueous saffron extract
Aqueous extract of saffron was prepared by maceration method. Briefly, 8 g of stigma powder was macerated in 300 ml distilled water for 72 h with continuous shaking in the refrigerator. Supernatant was separated by centrifuging and transferred to a freeze drier (FD-5003-BT, DENA, Iran). After 24 h, lyophilized powder of extract was available. The aqueous extract was dissolved in saline (0.9% NaCl). Saline (0.9% NaCl) was used as negative control. The extract yield was 54% (w/w).
2.5. Streptozotocin-induced diabetic rats
On the first day of the study, the diabetic groups were given streptozotocin (STZ) (dissolved in freshly prepared 0.01 M citrate buffer, pH 4.5) in a single intraperitoneal (i.p.) injection at a dose of 60 mg/kg for induction of diabetes. Blood was extracted from the tail vein for measuring the fasting glucose concentration 72 h after streptozotocin injection. Rats with blood glucose levels higher than 250 mg/dl were accepted as being diabetic.
2.6. Experimental design
45 male Wistar albino rats were randomly allotted to five experimental groups (n = 9 per group) as follows: group 1, control (C); group 2, diabetic (D); group 3, diabetic and saffron extract-treated (10 mg/kg/day) (D + Saf1); group 4, diabetic and saffron extract-treated (20 mg/kg/day) (D + Saf2); group 5, diabetic and saffron extract-treated (40 mg/kg/day) (D + Saf3). In the control groups (C), physiological saline (i.p.) was injected as vehicle. Saffron extract (i.p. injection) was administered to the treatment groups from 3 days after STZ administration; these injections were continued to the end of the study (for 4 weeks). Blood glucose level and body weights were recorded at weekly intervals. At the end of the 4-week period, animals were killed by Pentobarbital overdose (150 mg/kg, i.p.), and blood was subsequently collected from the retro orbital sinus and the abdominal aorta was removed, cleaned, dried and homogenized in 0.1 M Tris–HCl buffer, pH 7.4. Blood, plasma, and aorta homogenate were used for the following investigations. Blood and sera were separated by centrifugation at 3000 rpm for 10 min for glucose, lipid profile, malondialdehyde (MDA), GSH, CAT, SOD and nitric oxide (NO) assays.
2.7. Measurement of blood glucose
Glucose concentrations were measured with the Ames One Touch glucometer (One-Touch Basic; Lifescan, Johnson and Johnson, New Brunswick, NJ) in rat tail vein blood. Blood glucose was estimated using the diagnostic kits (Pars Azmoon kit, IRI) on an automatic analyzer (Abbott, model Alcyon 300, USA).
2.8. Measurement of serum lipid profile
The concentrations of glucose, total lipids, triglycerides, total cholesterol, low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol in serum were estimated by using diagnostic kits (Pars Azmoon kit, IRI) on an automatic analyzer (Abbott, model Alcyon 300, USA).
2.9. Measurement of serum reduced glutathione (GSH)
GSH was determined by the method of Ellman.33 To the homogenate was added 10% of trichloracetic acid (TCA) and centrifuged. 1.0 ml of supernatant was treated with 0.5 ml of Ellman's reagent (19.8 mg of 5,5′-dithiobisnitro benzoic acid (DTNB) in 100 ml of 0.1% sodium nitrate) and 3.0 ml of phosphate buffer (0.2 M, pH 8.0). The absorbance was read at 412 nm.33
2.10. Measurement of serum thiobarbituric acid reactive substance (TBARS)
The formation of lipid peroxides was measured in the serum. The formation of MDA, an end product of fatty acid peroxidation was measured spectrophotometrically at 532 nm by using a thiobarbituric acid reactive substance (TBARS), essentially by the method of Genet et al.34 Results are expressed as nmole of MDA formed/mg protein.34
2.11. Measurement of serum superoxide dismutase (SOD) activity
The activity of SOD was determined by a method using inhibition of pyrogallol autoxidation at pH 8.35 The specific activity of SOD is expressed as units per mg protein per minute.
2.12. Measurement of serum catalase (CAT) activity
Catalase activity was assayed by H2O2 consumption, following Aebi's36 method and modified by Pieper et al.37 Briefly, ethanol was added (1:100, v/v) to the supernatants and incubated for 30 min in an ice bath. 1% Triton X-100 (1:10, v/v) (Sigma Chemicals Corporation, MO, USA) was then added to the homogenates. This solution was placed in an ice bath for an additional 15 min. 500 μl of this solution was placed into a glass cuvette and 250 μl of 30 mM H2O2 (Sigma Chemicals Corporation, MO, USA) in phosphate buffer (50 mM, pH 7.0) was then added to start the reaction. After 15 s the absorbance at 240 nm was read every 15 s for 45 s. Catalase activity was expressed as mmol H2O2/min/mg protein. An enzyme unit was defined as the amount of enzyme that catalyzes the release of one μmol of H2O2 per min. Specific activity was calculated in terms of units per mg of protein. The assay was performed at 25 °C.36, 37
2.13. Measurement of serum nitric oxide (NO)
NO levels are determined spectrophotometrically by measuring the accumulation of its stable degradation products, nitrite and nitrate. The serum nitrite level was determined by the Griess reagent according to Hortelano et al.38 The Griess reagent, a mixture (1:1) of 1% sulfanilamide in 5% phosphoric acid and 0.1% 1-naphthyl ethylenediamine gives a red violent diazo color in the presence of nitrite. The color intensity was measured at 540 nm. Results were expressed as mol/l using a NaNO2 calibration graph.38
2.14. Measurement of serum protein content
Protein content was determined by the method of Lowry et al. (1951), using bovine serum albumin (BSA) as a standard.39
2.15. RNA extraction and RT-PCR analysis
Expression of proinflammatory genes (TNF-α and IL-6) were examined using reverse transcriptase-PCR (RT-PCR). Samples of 0.5–1 cm obtained from the abdominal aorta of rats of the experimental groups were deposited in RNAlater™ solution (Sigma Chemicals Corporation, MO, USA). Then it was stored at −70 °C for further processing. The samples were processed by the TRizol LS Reagent™ (Sigma Chemicals Corporation, MO, USA) technique. The assay was performed according to the protocol provided by the manufacturer. Briefly, 250 μl of TRIzol LS Reagent was added for each 50 mg of tissue and they were homogenized. RNA was extracted from each homogenate by adding 50 μl chloroform per 250 μl of the TRIzol LS Reagent used. Shake tubes vigorously by vortexing (15 s) and incubate them at room temperature for 2 min. Centrifuge the samples at 13,200 × g for 10 min at 4 °C. Transfer the aqueous phase to a clean tube. Precipitate the RNA from the aqueous phase by adding100 μl of isopropyl alcohol per 250 μl of the TRIzol LS Reagent used for the initial homogenization followed by vigorous vortexing (15 s) and incubate at room temperature for 10 min. Centrifuge at 13,200 × g for 10 min at 4 °C. Remove the supernatant. Wash the RNA pellet by adding100 μl of 75% ethanol per 250 μl of the TRIzol LS Reagent used for the initial homogenization. Mix the samples by vortexing (15 s) followed incubation at room temperature (2 min). Then samples were centrifugated at 90 × g for 10 min at 4 °C. Decant the supernant, remove it as much as possible without disturbing the pellet. Dry the RNA pellet at room temperature. Finally, resolubilize the pelletin50 μl of H2O with diethyl pyrocabonate (DEPC). The expected values of the A260/280 ratio of the total RNA isolated were obtained by spectrometer. RNA extraction was evaluated by 1% agarose gel electrophoresis. Bands (RNA18s and 28 s) of approximately 2 and 5 kilobase (kb) length were visualized under light UV (transilluminator BioRads).
2.16. Statistical analysis
All experiments were carried out at least in duplicate. Each group consisted of eight rats. One way analysis of variance (ANOVA) was performed and Tukey post hoc test was used for multiple comparisons. Statistical analyses were performed using the InStat 3.0 program. The results are expressed as mean ± SEM. The results originated from analysis of serum and tissues. Linear correlation tests were also performed. Differences of p < 0.05 were considered significant.
3. Results
3.1. Body weight
During the 4-week (experimental period), there was a weight loss in untreated diabetic rats compared with normal healthy rats (control) (p < 0.001) (Table 1). However, at the end of the experimental treatment period there was an elevation in body weight of saffron extract (20 and 40 mg/kg)-treated diabetic rats compared to untreated diabetic rats (p < 0.05 and p < 0.01, respectively), but the elevated body weight in the saffron extract (10 and 20 mg/kg)-treated diabetic groups was significantly lower than the control group (p < 0.05 and p < 0.01, respectively) (Table 1). At the highest saffron extract dose (40 mg/kg) there were no significant differences in body weight compared to control rats after the 4-week experimental period (Table 1).
Table 1.
Body weight in control (C), untreated diabetic rats (D), saffron extract (10 mg/kg/day)-treated diabetic (D + Saf1), saffron extract (20 mg/kg/day)-treated diabetic (D + Saf2) and saffron extract (40 mg/kg/day)-treated diabetic (D + Saf3) rats during 4 weeks of study.
| Days | 0 | 7 | 14 | 21 | 28 |
|---|---|---|---|---|---|
| C | 200 ± 17 | 242 ± 13 | 267 ± 19 | 275 ± 17 | 291 ± 21 |
| D | 211 ± 10 | 201 ± 19* | 195 ± 14* | 187 ± 11** | 178 ± 12*** |
| D + Saf1 | 218 ± 12 | 210 ± 8* | 223 ± 9* | 230 ± 11* | 233 ± 17** |
| D + Saf2 | 215 ± 7 | 218 ± 11 | 222 ± 13 | 229 ± 6*+ | 237 ± 10*+ |
| D + Saf3 | 219 ± 10 | 225 ± 11 | 242 ± 12+ | 255 ± 18++ | 263 ± 15++ |
Each measurement has been done at least in triplicate and the values are the mean ± SEM for nine rats in each group.
Significantly different from normal rats (*p < 0.05, **p < 0.01, ***p < 0.001).
Significantly different from STZ-treated rats (+p < 0.05, ++p < 0.01).
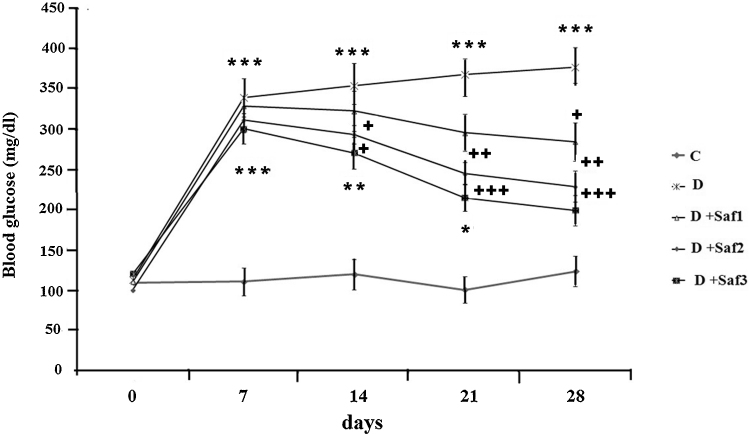
STZ-diabetic rats exhibited significant (p < 0.001) hyperglycemia compared to the control rats (Fig. 1). After 4 weeks the saffron extract dose dependently decreased blood glucose levels in the diabetic rats compared to the untreated diabetic rats (Fig. 1). Saffron extract (10 mg/kg/day) significantly decreased glucose in STZ diabetic rats only at the 4th week of the study (p < 0.05), while at 20 mg/kg/day saffron extract reduced blood glucose significantly at the 2nd, 3rd and 4th week from induction of diabetes compared with untreated diabetic rats (p < 0.05 and p < 0. 01, respectively). At the highest dose of saffron extract (40 mg/kg/day) blood glucose of diabetic rats was significantly reduced beginning from the 2nd week of treatment (p < 0.05, p < 0.001) (Fig. 1).
Fig. 1.
Effect of saffron on blood glucose level (mg/dl). Control (C), untreated diabetic rats (D), saffron extract (10 mg/kg/day)-treated diabetic (D + Sf1), saffron (20 mg/kg/day)-treated diabetic (D + Sf2) and saffron (40 mg/kg/day)-treated diabetic (D + S3) rats during 4 weeks of study (n = 9, for each group). Values are the mean ± SEM. Statistical significance for the difference between the data of the control group vs other groups: *p < 0.05; **p < 0.01, ***p < 0.001. Statistical significance for the difference between the data of untreated diabetic group vs treated groups: +p < 0.05; ++p < 0.01, +++p < 0.001.
3.2. Modulation of lipid profile by saffron extract
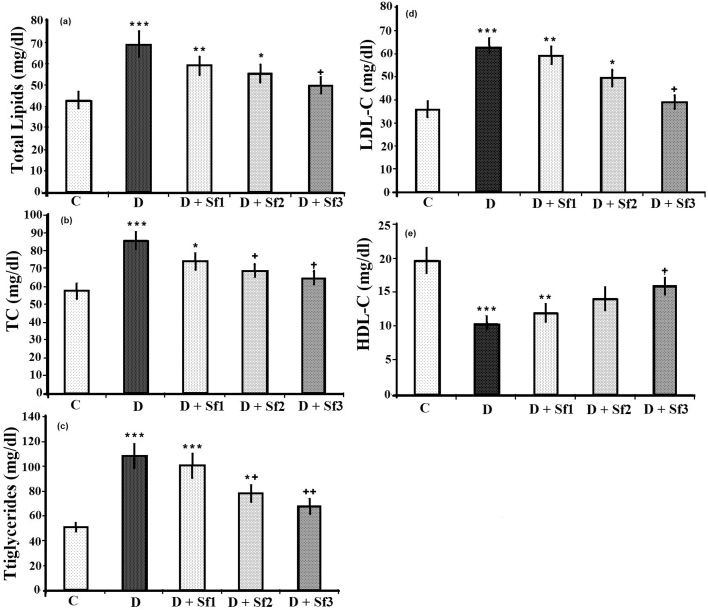
STZ-injected rats showed significant increases in the serum levels of total lipids, triglycerides, total cholesterol and LDL-cholesterol (LDL-C), and significantly decreased serum HDL-cholesterol (HDL-C) level compared to the control group (p < 0.001) (Fig. 2). Saffron extract dose-dependently reduced the serum levels of total lipids, triglycerides, total cholesterol and LDL-C, and increased serum HDL-C level during the experimental period (p < 0.05). At the highest saffron extract dose (40 mg/kg/day) there was no significant difference in total lipid, cholesterol, LDL-C and HDL-C levels between the STZ-treated rats and the control rats (Fig. 2).
Fig. 2.
Effect of saffron extract on plasma lipid profiles (mg/dl). (a) total lipid, (b) total cholesterol (TC), (c) triglycerides, (d) LDL-C, (e) HDL-C, in control (C), untreated diabetic rats (D), saffron (10 mg/kg/day)-treated diabetic (D + Sf1), saffron (20 mg/kg/day)-treated diabetic (D + Sf2) and saffron (40 mg/kg/day)-treated diabetic (D + Sf3) rats during 4 weeks of study (n = 9, for each group). Values are the mean ± SEM. Statistical significance for the difference between the data of the control group vs other groups: *p < 0.05; **p < 0.01, ***p < 0.001. Statistical significance for the difference between the data of untreated diabetes group vs treated groups: +p < 0.05; ++p < 0.01.
3.3. Modulation of MDA value by saffron extract
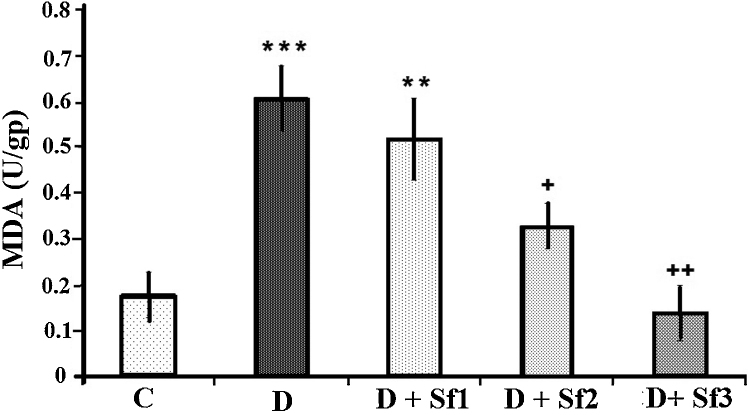
STZ injection produced significant changes in oxidative stress parameters in the serum of diabetic rats 4 weeks after diabetes induction, as shown by increased lipid peroxidation product (MDA) compared to control group (p < 0.001) (Fig. 3). Saffron extract (20 and 40 mg/kg/day) significantly decreased the serum MDA compared with the untreated diabetic groups (p < 0.05 and p < 0.01, respectively) (Fig. 3). In addition, the MDA levels in animals which were administrated with high saffron extract concentration (40 mg/kg/day) were significantly lower than the diabetic rats receiving low saffron extract concentration (10 mg/kg/day) (0.14 ± 0.04 vs 0.52 ± 0.09, p < 0.01) and MDA levels in diabetic rats treated with the medium concentration (20 mg/kg/day) were significantly lower than the low saffron extract treated diabetic rats (0.14 ± 0.04 vs 0.33 ± 0.05, p < 0.05). Furthermore, there was no significant difference between control rats and the high dose saffron extract treated diabetic rats (Fig. 3).
Fig. 3.
Effect of saffron extract on MDA. Serum MDA level (U/gp) in control (C), untreated diabetic rats (D), saffron (10 mg/kg/day)-treated diabetic (D + Sf1), saffron (20 mg/kg/day)-treated diabetic (D + Sf2) and saffron (40 mg/kg/day)-treated diabetic (D + Sf3) rats during 4 weeks of study (n = 9, for each group). Values are the mean ± SEM. Statistical significance for the difference between the data of the control group vs other groups: *p < 0.05; **p < 0.01, ***p < 0.001. Statistical significance for the difference between the data of untreated diabetes group vs treated groups: ++p < 0.01.
3.4. Effect of saffron extract on GSH, SOD, CAT
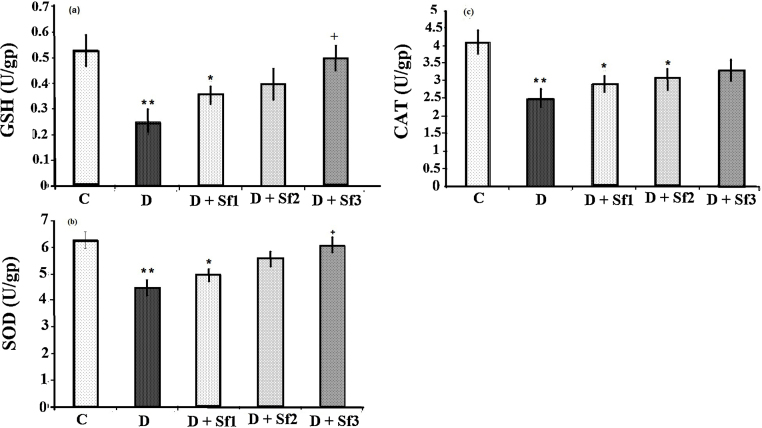
GSH, SOD and CAT activities were decreased in the STZ-diabetic group compared with the control group (p < 0.01) (Fig. 4). Saffron extract (10, 20 and 40 mg/kg/day) treated diabetic rats had significantly increased serum GSH and SOD compared with the untreated diabetic rats (p < 0.05). In addition, there was not a significant difference in CAT activity between diabetic rats treated with high saffron extract concentration (40 mg/kg/day) and the control group. The GSH, SOD and CAT activities in saffron extract (10 and 20 mg/kg/day)-treated diabetic rats were slightly higher than the untreated diabetic group, but they were not statistically significant (Fig. 4). The effects on GSH, SOD and CAT were dose dependent, the activities of serum GSH and SOD in animals administrated with the high saffron extract dose (40 mg/kg) being significantly greater than that in the rats receiving low saffron extract concentration (10 mg/kg) (0.5 ± 0.26 vs 0.36 ± 0.03, p < 0.05) (6.1 ± 0.29 vs 4.97 ± 0.09, p < 0.05), respectively.
Fig. 4.
Effect of saffron extract on serum GSH (a), SOD (b) and CAT (c) (U/gp). Control (C), untreated diabetic rats (D), saffron (10 mg/kg/day)-treated diabetic (D + Sf1), saffron (20 mg/kg/day)-treated diabetic (D + Sf2) and saffron (40 mg/kg/day)-treated diabetic (D + S3) rats during 4 weeks of study (n = 9, for each group). Values are the mean ± SEM. Statistical significance for the difference between the data of control vs other groups: *p < 0.05, **p < 0.01. Statistical significance for the difference between the data of diabetes vs treated groups: +p < 0.05.
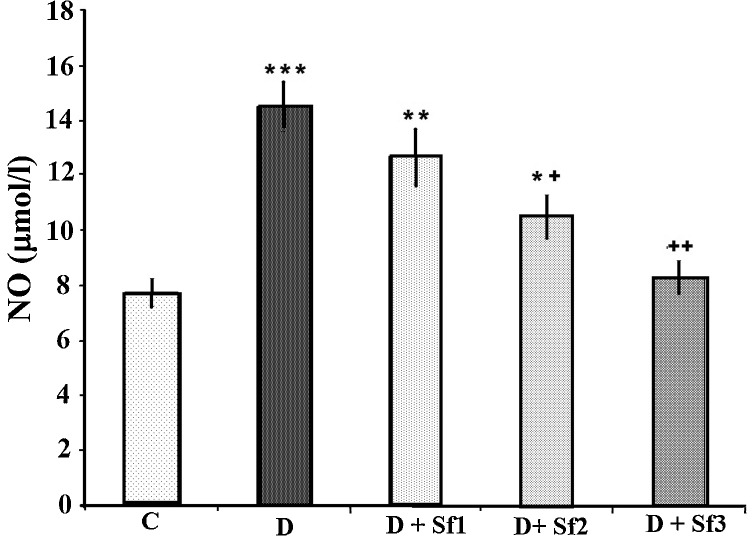
3.5. Modulation of NO value by saffron extract
STZ injection produced a significant increase of NO compared to the control group (p < 0.001) (Fig. 5). Saffron extract (20 and 40 mg/kg/day)-treated diabetic rats had significantly decreased serum NO compared with the untreated diabetic rats (p < 0.05, p < 0.01, respectively). The effects on NO were dose dependent, serum NO levels of animals having been administrated high saffron extract dose being significantly lower than those in the diabetic rats receiving the low saffron extract dose (8.3 ± 0.6 vs 12.7 ± 1.0, p < 0.05); and NO levels in diabetic rats treated with a medium concentration (20 mg/kg) were slightly lower than the low dose of saffron extract-treated diabetic rats (10.5 ± 0.8 vs 12.7 ± 1.0, p < 0.001).
Fig. 5.
Effect of saffron extract on serum NO (μM/l). Control (C), untreated diabetic (D), diabetic and (10 mg/kg/day) saffron (20 mg/kg/day)-treated diabetic (D + Sf1), saffron (20 mg/kg/day)-treated diabetic (D + Sf2) and saffron (40 mg/kg/day)-treated diabetic (D + Sf3) rats during 4 weeks of study (n = 9, for each group). Values are the mean ± SEM. Statistical significance for the difference between the data of control vs other groups: *p < 0.05; **p < 0.01, ***p < 0.001. Statistical significance for the difference between the data of untreated diabetes vs treated groups: +p < 0.05; ++p < 0.01.
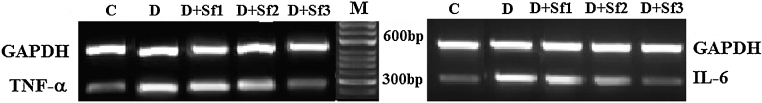
3.6. Inhibition of inflammatory cytokines expression by saffron extract
To further determine whether saffron extract regulates inflammatory cytokines in diabetic rats, we determined the expression of inflammatory cytokines from abdominal aorta (Fig. 6) in the absence or presence of the different concentrations of saffron extract and then we determined the expression levels of TNF-α and IL-6 by using the RT-PCR analysis. The control rats did not show expression of inflammatory cytokines due to the absence of this condition; by contrast the diabetic rats expressed the both inflammatory cytokines (TNF-α and IL-6). With respect to groups treated with saffron extracts, the low concentration of saffron extract (10 mg/kg/day) only slightly inhibited expression of the inflammatory cytokine sand, treatment of the diabetic rats with the highest concentration of saffron extract (40 mg/kg/day) significantly down-regulated the levels of the pro-inflammatory cytokine, so that, it was found be comparable with the control animal sand. As the results shown in Fig. 6, saffron treatment profoundly reduced TNF-α and IL-6 mRNA expression in the diabetic rats in a dose dependent manner. GAPDH was used as a loading control. All treatments expressed the constitutive GAPDH gen (Table 2).
Fig. 6.
Effect of saffron extract on proinflammatory genes (TNF-α and IL-6) in abdominal aorta. Control (C), untreated diabetic (D), diabetic and (10 mg/kg/day) saffron (20 mg/kg/day)-treated diabetic (D + Sf1), saffron (20 mg/kg/day)-treated diabetic (D + Sf2) and saffron (40 mg/kg/day)-treated diabetic (D + Sf3) rats during 4 weeks of study (n = 9, for each group).
Table 2.
Primers of the polymerase chain reaction (PCR) assay. IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
| Sense (5′–3′) | Anti-sense (3′–5′) | Size (bp) | |
|---|---|---|---|
| IL-6 | GGGACTGATGTTGTTGACAG | TGTTCTTCACAAACTCCAGG | 304 |
| TNF-α | CCCAGACCCTCACACTCAGAT | TTGTCCCTTGAAGAGAACCTG | 215 |
| GAPDH | ACCACCATGGAGAAGGCTGG | CTCAGTGTAGCCCAGGATGC | 528 |
4. Discussion
The incidence of DM is sharply increasing worldwide which represents a critical burden or patients and for the society as well due to micro- and macro-vascular complications. The risk factors for the development of vascular disease in subjects with DM are obesity, the presence of poor glycemic control, dyslipidemia, imbalance of oxidant/antioxidant, and inflammation. Our present data indicate that intraperitoneal injection of saffron extract significantly ameliorated the adverse metabolic effects in rats treated with STZ. Despite glucose-lowering effect of saffron extract was not observed for control rats (data was not shown), we found saffron extract mildly caused a decrease in fast blood glucose in DM rats, which was in agreement with several studies.40, 41 This change in blood glucose caused by saffron extract could contribute to the improvement in endothelial cell dysfunction, which is induced by hyperglycemia through multiple pathways including enhanced glycolysis, the buildup of glycolytic intermediates, and AGE-modification of proteins.42 In addition, we observed saffron extract attenuated lipid abnormalities including TC, TG, LDL-C in DM rats, that may due to increased plasma lipid uptake by the liver and adipose tissue or by decreased hepatic cholesterol genesis and fatty acid synthesis.41 Although, saffron extract treatment did not significantly influence body weight within groups of non diabetic (data was not shown) but, the saffron extract significantly enhanced body weight in diabetic rats at the end of experimental period. Therefore, the saffron extract injection after STZ treatment resulted in decrease in serum glucose level, and also improved lipid profile as well as body weight as compared with untreated diabetic rats. Furthermore, the saffron extract treatment of diabetic rats were observed to significantly recover the decreased levels of GSH, CAT and SOD, as well as the decline in the lipid profiles and the oxidative stress parameters MDA and NO compared with untreated diabetic group. These results are compatible with the findings reported by other investigators using saffron and its active constituent, crocin, which improved oxidative damage due to ischemia and injury in rats.24, 28, 29 The results in the current study are consistent with amelioration of oxidative stress in the STZ diabetic rats. The effects observed in lipid profile and hyperglycemia that induced by diabetes in rats in the present study are similar to the findings reported by other investigations using STZ to induce diabetes in rats, accompanied by an increase in the susceptibility to lipid peroxidation.43, 44
Oxidative stress plays an important role in the complications and the pathogenesis of diabetes.
Hyperglycemia results in overproduction of oxygen free radicals, which contributes to the complications of diabetes and its progression.43 Several studies have shown that STZ produces imbalance between plasma oxidant and antioxidant content, resulting in the development of DM and its complications.
In our experimental model of diabetes mellitus, it was seen that STZ administration led to a significant decrease in plasma GSH content and CAT and SOD activities, accompanied by a significant increase in MDA, NO and overall lipid profile, indicating an increased plasma oxidative stress which may also occur in the different tissues in STZ treated rats. Increasing evidence suggests that oxidative stress and changes in nitric oxide formation or action play major roles in the onset of diabetic complications such as atherosclerosis. The improvement of variable measurements in STZ-diabetic rats after saffron extract treatment might suggest a protective influence of saffron against STZ action that could be mediated through suppression of oxygen free radicals induced by STZ. A stimulating effect of the formation of GSH by saffron extract was observed in the present study. The GSH reacts with free radicals and is a crucial substrate for glutathione peroxidase and glutathione-S-transferase, which take part in the cellular defense mechanisms against intermediate oxygen products.45, 46 It may be relevant that the ratio of GSH/GSSG plays a important role in glucose homeostasis of diabetes because thiol groups are critical in intracellular and membrane redox state.45 Saffron extract induced an increase in plasma GSH content, which might enhance the GSH/GSSG ratio and decrease lipid peroxidation, hence aldehydic concentration, and therefore improve serum glucose regulation. Parallel to these events, plasma CAT and SOD activity were increased in saffron extract treated diabetic rats as compared with untreated diabetic rats. SOD is responsible for removal of superoxide radicals and catalase decomposes hydrogen peroxide to water and oxygen; thus, these enzymes may contribute to the modulation of redox state of plasma.48 This observation perfectly agrees with those of Kianbakht et al. (2011) who observed that saffron, crocin and safranal may effectively control glycemia in the alloxan induced diabetes rat model.47, 48 The results of the present study indicate that saffron extract is also effective to prevent hyperlipidemia due to diabetes. Saffron extract inhibits elevation of the serum lipid profile by controlling oxidative and nitrosative systems. Saffron has been reported to help lower cholesterol and keep cholesterol at healthy levels.49 In agreement with previous studies, one mechanism for the hypolipidemic effect of saffron extract has proposed inhibitory effects on the levels of MDA, oxygen free radical and activating superoxide dismutase.50 Reactive oxygen species are increased by hyperglycemia. Hyperglycemia, which occurs during diabetes (both type 1 and type 2) and, to a lesser extent, during insulin resistance, causes oxidative stress. Elevated glucose in diabetes may also react with lipids, resulting in the generation of ROS.51 Diabetes mellitus often includes lipid abnormalities such as elevated LDL-C and cholesterol, and such effects were shown in this in vivo study. These abnormalities may be further exacerbated by the increased oxidizing environment which enhances the formation of oxidized LDLs (oxLDLs), glycated LDL and oxysterols (formed from the oxidation of cholesterol). It has been suggested that these oxidized lipid products can bind to specific receptor proteins or activate inflammatory proteins which generate ROS.51
In our study, prooxidant- antioxidant balance was evaluated by measuring MDA and NO levels and enzymatic antioxidants in the diabetic rats. Increased MDA, NO and decreased SOD, GST and CAT activities indicated that the balance changed toward prooxidation in STZ-induced diabetic rats. Saffron extract treatment of diabetic rats, which restored SOD, GST and CAT activities, may be due to a decrease in free radical generation by saffron extract and increased antioxidant defenses (12). Saffron extract showed good antioxidant activity in vivo.52, 53 Saffron administration regulates the expression of antioxidant related genes, and consequently oxidant levels in diabetic animals. In biological systems saffron shows its antioxidant impact via stabilizing membranes,29 inhibiting ROS and reducing peroxidation of unsaturated membrane fatty acids.25 Saffron has been known to function as a radical scavenger inhibiting lipid peroxidation in vivo and in vitro. It also has been reported that saffron supplementation could decrease lipid peroxidation.32, 54 Saffron modulates antioxidant gene expression and also upregulates mitochondrial antioxidant genes, leading to a lower mitochondrial oxygen radical generation, which may be responsible at least in part for the improved hyperglycemia, hyperlipidemia and oxidative stress seen in the present study in STZ induced diabetic rats. Additionally, increased SOD, GST and CAT levels after saffron extract treatment may play an additional role in decreasing oxidative stress. The present study elucidated the antihyperglycemia, hypolipidemic and protective potential of saffron extract treatment on activities of antioxidant enzymes (SOD, GST, CAT), lipid peroxidation levels and serum NO content in the STZ diabetic rat model. In confirm with our current data, it has been shown that aqueous extract of saffron ameliorates isoproterenol-induced myocardial injury via strengthening antioxidant defense system.55
It is well known that obese, hyperlipidemia, and imbalance of oxidant/antioxidant exhibit a state of chronic low grade inflammation, and such activation of inflammatory pathways have also been proposed as a major driver of obese and insulin resistance.56, 57 The cytokines are involved in the chronic inflammatory processes of the vessels wall, promoting lipid accumulation with consequent development of vasculopathy. Our data suggested that saffron extract inhibited production of the inflammatory mediatorsIL-6 and TNF-α in DM rats. Our results and findings of other laboratories58 indicated that diabetes represented a multifactorial vasculopathy involving various aspects of inflammatory and metabolic syndrome based on a combination of hyperglycemia, hyperlipidemia, and obesity. This might help explain why DM showed enhanced atherosclerotic plaque burden with more distal plaques compared to DM.59 Thus, identifying that a novel compound like saffron extract not only inhibit vascular inflammation, but also attenuate the hyperglycemia and hyperlipidemia classically associated with atherosclerotic plaque formation holds great promise for the prevention of vascular complications in patients with DM.
Atherosclerosis, as well as vasculopathy in type2 diabetes, is a chronic inflammatory disease, and pathological and epidemiological evidence suggest that proinflammatory cytokines play a key role or chest rating the pathological processes underlying the development of the atherosclerotic plaque.60 TNF-α impairs vascular endothelial cells and affects endothelial permeability possibly through an activation of tyrosine kinases both in vitro and in vivo. TNF-α may stimulate endothelial cells to upregulate adhesion molecule such as ICAM-1 which coincide with the earliest phases of local endothelial activation and contribute to endothelial–monocyte interactions in early atherogenesis.61 Induction of CAM-1 by TNF-α enhances the adhesion of neutrophils to endothelial cells, which initiate and aggravate the cascade of other inflammatory mediators.62, 63 Therefore, we detected the expression levels of TNF-α and IL-6 in abdominal aorta among the experimental groups. Our data demonstrated that the increases in the expression levels of TNF-α and IL-6 in abdominal aorta of untreated diabetic rats, but saffron extract decreased dose dependently the expression of TNF-α and IL-6 in aorta of the treated diabetic rats. We speculated that the vascular protective effects of saffron might be correlated with diminution of inflammation response. Thus our findings would be of great significance, showing that saffron extract can abrogate the development of a vascular injury microenvironment in the vascular wall of DM rats. But the mechanisms by which saffron exert anti-inflammatory effects is not clear. However, it is logical to hypothesize that saffron appears to regulate high glucose-induced proinflammatory mediator production. This view is in line with other study showing that saffron blocked TNF-α and IL-6 in endothelial of abdominal aorta to inhibit upregulation of inflammatory mediators.64 Saffron and its main ingredient (safranal) are antioxidants; it seems that inhibitory effects of saffron are also mainly attributed to its antioxidant action. Finally, our studies showed saffron suppressed and further down-regulated the mRNA expression of TNF-α and Il-6 indicating that that saffron inhibited the activation of inflammatory signaling induced by high glucose that would mimic the effect of vasculopathy in diabetes.
Conflicts of interest
The authors have none to declare.
Acknowledgment
The authors would like to thank Research Affairs of Neyshabur University of Medical Sciences for financially supporting this work.
References
- 1.Tousoulis D., Kampoli A.M., Stefanadis C. Diabetes mellitus and vascular endothelial dysfunction: current perspectives. Curr Vasc Pharmacol. 2012;10:19–32. doi: 10.2174/157016112798829797. [DOI] [PubMed] [Google Scholar]
- 2.Calcutt N.A., Cooper M.E., Kern T.S., Schmidt A.M. Therapies for hyperglycemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov. 2009;8:417–429. doi: 10.1038/nrd2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunning T. Preiodontal disease, the overlooked the diabetes complication. Nephrol Nurs J. 2009;36(5):489–495. [PubMed] [Google Scholar]
- 4.Chaiyasut C., Kusirisin W., Lailerd N., Lerttrakarnnon P., Suttaji M., Srichairatanakool S. Effects of phenolic compounds of fermented Thai indigenous plants on oxidative stress in streptozotocin-induced diabetic rats. J Evid Based Complementary Altern Med. 2011;3:1–10. doi: 10.1155/2011/749307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownlee M. A radical explanation for glucose induced beta cell dysfunction. J Clin Invest. 2013;112:1788–1790. doi: 10.1172/JCI20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvin E., Coresh J., Golden S.H., Boland L.L., Brancati F.L.M.W. Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2005;28:1965–1973. doi: 10.2337/diacare.28.8.1965. [DOI] [PubMed] [Google Scholar]
- 7.Fowler M.J. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82. [Google Scholar]
- 8.Maiti R., Agrawal N.K. Atherosclerosis in diabetes mellitus: role of inflammation. Indian J Med Sci. 2007;61:292–306. [PubMed] [Google Scholar]
- 9.Hamed E.A., Zakary M.M., Abdelal R.M., Moneim E.A. Vasculopathy in type 2 diabetes mellitus: role of specific angiogenic modulators. J Physiol Biochem. 2011;67:339–349. doi: 10.1007/s13105-011-0080-8. [DOI] [PubMed] [Google Scholar]
- 10.Ravid M., Brosh D., Ravid-Safran D., Levy Z., Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med. 1998;158:998–1004. doi: 10.1001/archinte.158.9.998. [DOI] [PubMed] [Google Scholar]
- 11.Csiszar A., Pacher P., Kaley G., Ungvari Z. Role of oxidative and nitrosative stress, longevity genes and poly(ADP-ribose) polymerase in cardiovascular dysfunction associated with aging. Curr Vasc Pharmacol. 2005;3:285–291. doi: 10.2174/1570161054368616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funk S.D., Yurdagul A., Orr A.W. Hyperglycemia and endothelial dysfunction in atherosclerosis: lessons from type 1 diabetes. Int J Vasc Med. 2012;2012:569654. doi: 10.1155/2012/569654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Natarajan R., Nadler J.L. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 14.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz A., Bustos C., Alonso J. Involvement of tumor necrosis factor-alpha in the pathogenesis of experimental and human glomerulonephritis. Adv Nephrol Necker Hosp. 1995;24:53–77. [PubMed] [Google Scholar]
- 16.Navarro J.F., Mora C., Macia M., Garcia J. Inflammatory parameters are independently associated with urinary albumin excretion in type 2 diabetes mellitus. Am J Kidney Dis. 2003;42:53–61. doi: 10.1016/s0272-6386(03)00408-6. [DOI] [PubMed] [Google Scholar]
- 17.Nosadini R., Velussi M., Brocco E. Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes. 2000;49:476–484. doi: 10.2337/diabetes.49.3.476. [DOI] [PubMed] [Google Scholar]
- 18.Chang Y.C., Chuang L.M. The role of oxidative stress in the pathogenesis of type 2 diabetes: from molecular mechanism to clinical implications. Am J Transl Res. 2010;2:316–331. [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg I.J. Diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab. 2009;88:965–971. doi: 10.1210/jcem.86.3.7304. [DOI] [PubMed] [Google Scholar]
- 20.Strain J.J. Disturbances of micronutrient and antioxidant status in diabetes. Proc Nutr Soc. 1991;50:591–604. doi: 10.1079/pns19910073. [DOI] [PubMed] [Google Scholar]
- 21.Samarghandian S., Borji A., Tabasi S.H. Effects of Cichorium intybus Linn on blood glucose, lipid constituents and selected oxidative stress parameters in streptozotocin-induced diabetic rats. Cardiovasc Hematol Disord Drug Targets. 2013;13:231–236. doi: 10.2174/1871529x13666131129103139. [DOI] [PubMed] [Google Scholar]
- 22.Rios J.L., Recio M.C., Giner R.M., Manez S. An update review of saffron and its active constituents. Phytother Res. 1996;10:189–193. [Google Scholar]
- 23.Samarghandian S., Borji A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacogn Res. 2014;6:99–107. doi: 10.4103/0974-8490.128963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosseinzadeh H., Younesi H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farahmand S.K., Samini F., Samini M., Samarghandian S. Safranal ameliorates antioxidant enzymes and suppresses lipid peroxidation and nitric oxide formation in aged male rat liver. Biogerontology. 2013;14:63–71. doi: 10.1007/s10522-012-9409-0. [DOI] [PubMed] [Google Scholar]
- 26.Samarghandian S., Borji S., Farahmand S.K., Afshari R., Davoodi S. Crocus sativus L. (Saffron) stigma aqueous extract induces apoptosis in alveolar human lung cancer cells through caspase-dependent activation. Biomed Res Int. 2013;2013:417928. doi: 10.1155/2013/417928. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Sheng L., Qian Z., Zheng S., Xi L. Mechanism of hypolipidemic effect of crocin in rats: crocin inhibits pancreatic lipase. Eur J Pharmacol. 2006;543:116–122. doi: 10.1016/j.ejphar.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 28.Hosseinzadeh H., Ziaei T. Effects of Crocus sativus stigma extract and its constituents, crocin and safranal, on intact memory and scopolamine-induced learning deficits in rats performing the Morris water maze task. J Med Plants. 2006;5:40–50. [Google Scholar]
- 29.Hosseinzadeh H., Sadeghnia H.R., Ghaeni F.A., Motamed Shariaty V.S., Mohajeri S.A. Effects of saffron (Crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypo perfusion in rats. Phytother Res. 2012;26:381–386. doi: 10.1002/ptr.3566. [DOI] [PubMed] [Google Scholar]
- 30.Samarghandian S., Afshari R., Sadati A. Evaluation of lung and bronchoalveolar lavage fluid oxidative stress indices for assessing the preventing effects of safranal on respiratory distress in diabetic rats. Sci World J. 2014;2014:2–6. doi: 10.1155/2014/251378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samarghandian S., Afshari J.T., Davoodi S. Suppression of pulmonary tumor promotion and induction of apoptosis by Crocus sativus L. extraction. Appl Biochem Biotechnol. 2011;164:238–247. doi: 10.1007/s12010-010-9130-x. [DOI] [PubMed] [Google Scholar]
- 32.Assimopoulou A.N., Sinakos Z., Papageorgiou V.P. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;19:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 33.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 34.Genet S., Kale R.K., Baquer N.Z. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: effect of vanadate and fenugreek (Trigonellafoenum graecum) Mol Cell Biochem. 2002;236:7–12. doi: 10.1023/a:1016103131408. [DOI] [PubMed] [Google Scholar]
- 35.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1979;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 36.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 37.Pieper G.M., Jordan M., Dondlinger L.A., Adams M.B., Roza A.M. Peroxidative stress in diabetic blood vessels. Reversal by pancreatic islet transplantation. Diabetes. 1995;44:884–889. doi: 10.2337/diab.44.8.884. [DOI] [PubMed] [Google Scholar]
- 38.Hortelano S., Dewez B., Genaro A.M., Díaz-Guerra M.J., Bosca L. Nitric oxide is released in regenerating liver after partial hepatectomy. Hepatology. 1995;21:776–786. [PubMed] [Google Scholar]
- 39.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 40.Arrick D.M., Sun H., Patel K.P., Mayhan W.G. Chronic resveratrol treatment restores vascular responsiveness of cerebral arterioles in type1 diabetic rats. Am J Physiol Heart Circ Physiol. 2011;301:H696–H703. doi: 10.1152/ajpheart.00312.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su H.C., Hung L.M., Chen J.K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:E1339–E1346. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- 42.Funk S.D., Yurdagul J.R., Orr A.W. Hyperglycemia and endothelial dysfunction in atherosclerosis: lessons from type 1 diabetes. Int J Vasc Med. 2012;2012:569654. doi: 10.1155/2012/569654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Budin S., Othman F., Louis S.A., Bakar M., Das S., Mohamed J. The effects of palm oil to cotrienol rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics. 2009;64:235–244. doi: 10.1590/S1807-59322009000300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yazdanparast R., Ardestani A., Jamshidi S. Experimental diabetes treated with Achillea santolina: effect on pancreatic oxidative parameters. J Ethnopharmacol. 2007;112:13–18. doi: 10.1016/j.jep.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 45.Soto C.P., Perez B.L., Favari L.P., Reyes J.L. Prevention of alloxan-induced diabetes mellitus in the rat by silymarin. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119:125–129. doi: 10.1016/s0742-8413(97)00198-9. [DOI] [PubMed] [Google Scholar]
- 46.El-Missiry M.A. Enhanced testicular antioxidant system by ascorbic acid in alloxan diabetic rats. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;124:233–237. doi: 10.1016/s0742-8413(99)00070-5. [DOI] [PubMed] [Google Scholar]
- 47.Kianbakht S., Hajiaghaee R. Anti-hyperglycemic effects of saffron and its active constituents, crocin and safranal, in alloxan-induced diabetic rats. J Med Plants. 2011;10:39–43. [Google Scholar]
- 48.Rahbani M., Mohajeri D., Rezaie A., Doustar Y., Nazeri M. Attenuation of oxidative stress of hepatic tissue by ethanolic extract of saffron (dried stigmas of Crocus sativus L.) in streptozotocin (STZ)-induced diabetic rats. Afr J Pharm Pharmacol. 2011;5:2166–2173. [Google Scholar]
- 49.Arasteh A., Aliyev A., Khamnei S., Delazar A., Mesgari M., Mehmannavaz Y. Effects of hydromethanolic extract of saffron (Crocus sativus) on serum glucose, insulin and cholesterol levels in healthy male rats. J Med Plants Res. 2010;4:397–402. [Google Scholar]
- 50.Xiang M., Yang M., Zhou C., Liu J., Li W., Qian Z. Crocetin prevents AGEs-induced vascular endothelial cell apoptosis. Pharmacol Res. 2006;54:268–274. doi: 10.1016/j.phrs.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Johansen J.S., Harris A.K., Rychly D.J., Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bharti S., Golechh M., Kumari S., Siddiqui K.M., Arya D.S. Akt/GSK-3b/eNOS phosphorylation arbitrates safranal-induced myocardial protection against ischemia–reperfusion injury in rats. Eur Nutr. 2012;51:719–727. doi: 10.1007/s00394-011-0251-y. [DOI] [PubMed] [Google Scholar]
- 53.Halataei B.A., Khosravi M., Arbabian S. Saffron (Crocus sativus) aqueous extract and its constituent crocin reduces stress-induced anorexia in mice. Phytother Res. 2011;25:1833–1838. doi: 10.1002/ptr.3495. [DOI] [PubMed] [Google Scholar]
- 54.Papandreou M.A., Tsachaki M., Efthimiopoulos S., Cordopatis P., Lamari F.N., Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav Brain Res. 2011;219:197–220. doi: 10.1016/j.bbr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Sachdeva J., Tanwar V., Golechha M. Crocus sativus L. (saffron) attenuates isoproterenol-induced myocardial injury via preserving cardiac functions and strengthening antioxidant defense system. Exp Toxicol Pathol. 2012;64(6):557–564. doi: 10.1016/j.etp.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Farb M.G., Bigornia S., Mott M. Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. J Am Coll Cardiol. 2011;58:232–237. doi: 10.1016/j.jacc.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hancu N., Netea M.G., Baciu I. High glucose concentrations increase the tumor necrosis factor-alpha production capacity by human peripheral blood mononuclear cells. Rom J Physiol. 1998;35:325–330. [PubMed] [Google Scholar]
- 58.de Zeeuw D., Bakker S.J.L. Does the metabolic syndrome add to the diagnosis and treatment of cardiovascular disease? Nat Clin Pract Cardiovasc Med. 2008;5:S10–S14. doi: 10.1038/ncpcardio1271. [DOI] [PubMed] [Google Scholar]
- 59.Burke A.P., Kolodgie F.D., Zieske A. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 60.Csiszar A., Labinskyy N., Podlutsky A. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cybulsky M.I., Gimbrone M.A., Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 62.Clausell N., deLima V.C., Molossi S. Expression of tumour necrosis factor alpha and accumulation of fibronectin in coronary artery restenotic lesions retrieved by atherectomy. Br Heart J. 1995;73:534–539. doi: 10.1136/hrt.73.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashizume M., Mihara M. Atherogenic effects of TNF-alpha and IL-6 via upregulation of scavenger receptors. Cytokine. 2012;58:424–430. doi: 10.1016/j.cyto.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Csiszar A., Smith K., Labinskyy N., Orosz Z., Rivera A., Ungvari Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappa B inhibition. Am J Physiol Heart Circ Physiol. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]