Abstract
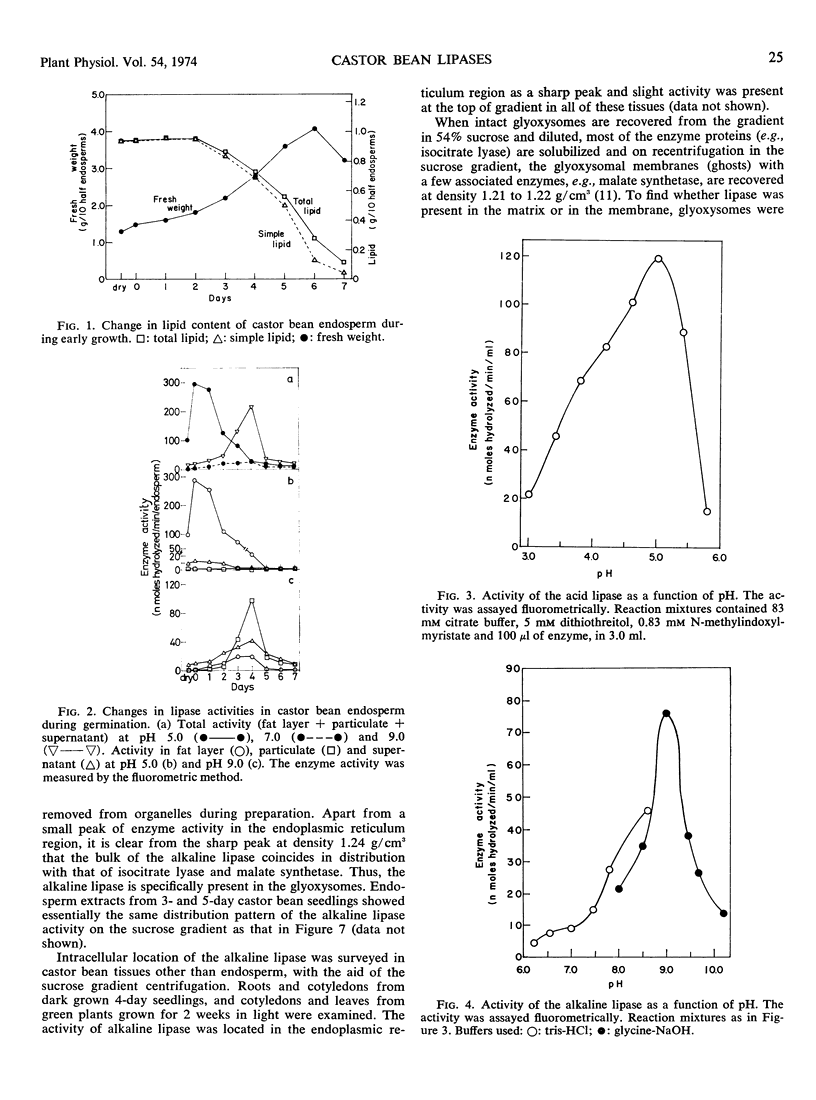
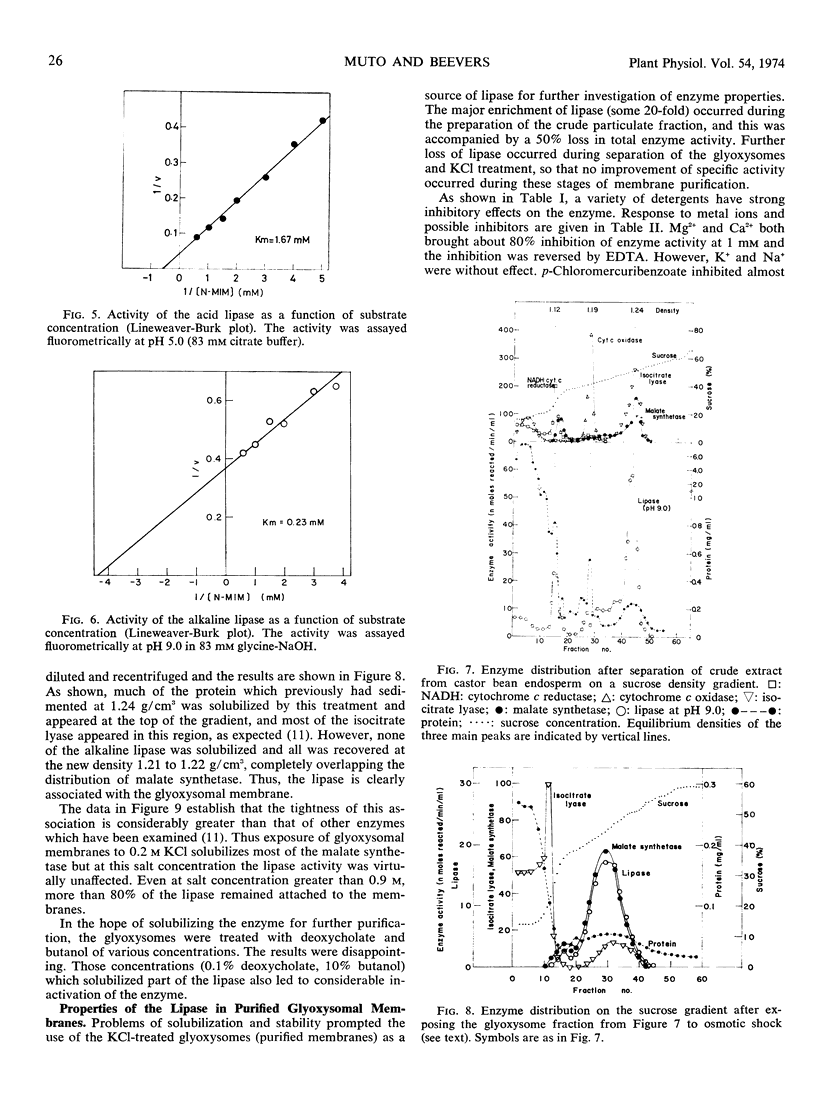
Two lipases were found in extracts from castor bean (Ricinus communis L.) endosperm. One, with optimal activity at pH 5.0 (acid lipase), was present in dry seeds and displayed high activity during the first 2 days of germination. The second, with an alkaline pH optimum (alkaline lipase), was particularly active during days 3 to 5. When total homogenates of endosperm were fractionated into fat layer, supernatant, and particulate fractions, the acid lipase was recovered in the fat layer, and the alkaline lipase was located primarily in the particulate fraction. Sucrose density gradient centrifugation showed that the alkaline lipase was located mainly in glyoxysomes, with some 30% of the activity in the endoplasmic reticulum. When glyoxysomes were broken by osmotic shock and exposed to KCl, which solubilizes most of the enzymes, the alkaline lipase remained particulate and was recovered with the glyoxysomal “ghosts” at equilibrium density 1.21 g/cm3 on the sucrose gradient. Association of the lipase with the gly-oxysomal membrane was supported by the responses to detergents and to butanol. The alkaline lipase hydrolyzed only monosubstituted glycerols. The roles of the two lipases in lipid utilization during germination of castor bean are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEEVERS H. Metabolic production of sucrose from fat. Nature. 1961 Jul 29;191:433–436. doi: 10.1038/191433a0. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Guilbault G. G., Hieserman J. Fluorometric substrate for sulfatase and lipase. Anal Chem. 1969 Dec;41(14):2006–2009. doi: 10.1021/ac50159a034. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Localization of enzymes within microbodies. J Cell Biol. 1973 Aug;58(2):379–389. doi: 10.1083/jcb.58.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton D., Stumpf P. K. Fat Metabolism in Higher Plants. XXXVII. Characterization of the beta-Oxidation Systems From Maturing and Germinating Castor Bean Seeds. Plant Physiol. 1969 Apr;44(4):508–516. doi: 10.1104/pp.44.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. The origin and turnover of organelle membranes in castor bean endosperm. Plant Physiol. 1973 Jan;51(1):61–65. doi: 10.1104/pp.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katocs A. S., Jr, Calvert D. N., Lech J. J. Characterization of two monoglyceride hydrolyzing enzymes in rat adipose tissue. Biochim Biophys Acta. 1971 Mar 10;227(3):608–617. doi: 10.1016/0005-2744(71)90011-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Beevers H. Intracellular distribution of enzymes of the cytidine diphosphate choline pathway in castor bean endosperm. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2429–2432. doi: 10.1073/pnas.69.9.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer H. H., Totten C. Studies on seeds: v. Microbodies, glyoxysomes, and ricinosomes of castor bean endosperm. Plant Physiol. 1970 Dec;46(6):794–799. doi: 10.1104/pp.46.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Borgström B. The acid lipase of castor beans. Positional specificity and reaction mechanism. Biochim Biophys Acta. 1971 Jan 13;227(1):106–115. doi: 10.1016/0005-2744(71)90172-0. [DOI] [PubMed] [Google Scholar]
- ORY R. L., ST ANGELO A. J., ALTSCHUL A. M. Castor bean lipase: action on its endogenous substrate. J Lipid Res. 1960 Apr;1:208–213. [PubMed] [Google Scholar]
- Ory R. L., Yatsu L. Y., Kircher H. W. Association of lipase activity with the spherosomes of Ricinus communis. Arch Biochem Biophys. 1968 Feb;123(2):255–264. doi: 10.1016/0003-9861(68)90132-x. [DOI] [PubMed] [Google Scholar]
- VAUGHAN M., BERGER J. E., STEINBERG D. HORMONE-SENSITIVE LIPASE AND MONOGLYCERIDE LIPASE ACTIVITIES IN ADIPOSE TISSUE. J Biol Chem. 1964 Feb;239:401–409. [PubMed] [Google Scholar]
- Vigil E. L. Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol. 1970 Sep;46(3):435–454. doi: 10.1083/jcb.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. P. Isolation and characterization of four lipolytic preparations from rat skeletal muscle. J Lipid Res. 1968 Mar;9(2):200–206. [PubMed] [Google Scholar]


