Abstract
Objective
Cardiovascular (CV) disease is leading cause of mortality in rheumatoid arthritis (RA). Dysfunction of the vascular endothelium is a hallmark of most conditions that are associated with atherosclerosis and is therefore an early feature in atherogenesis. Biomarkers for rapid evolution of CV complications would be highly desirable for risk stratification. Finally, predictive biomarkers for cardiovascular risk would allow tailoring therapy to the individual. We assessed endothelial function and atherosclerosis utilizing carotid intima-media thickness (CIMT) in RA in context of clinical and laboratory markers in Indian RA population.
Methods
We performed a prospective study of 35 consecutive RA patients and 25 age- and sex matched healthy controls. Patients with traditional CV risk factors were excluded. Flow mediated dilatation (FMD) as measures of endothelial function and CIMT as measures of atherosclerosis were assessed. Disease-specific measures, inflammatory measures, serum cytokines, serum nitrite, lipids and endothelial progenitor cells (EPCs) were estimated.
Results
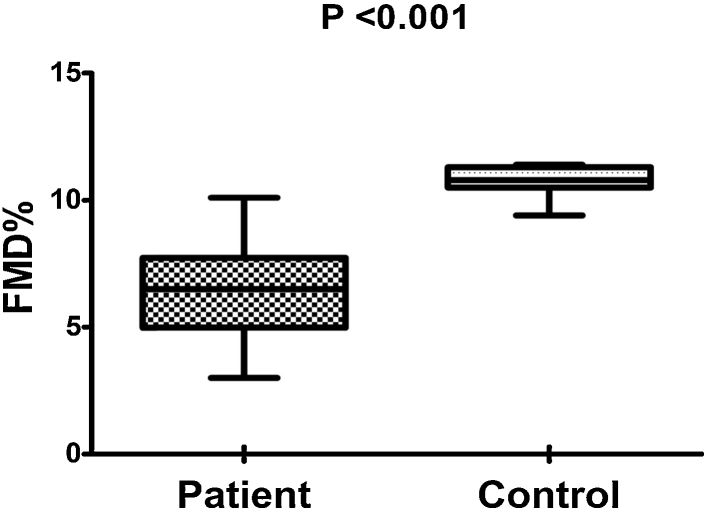
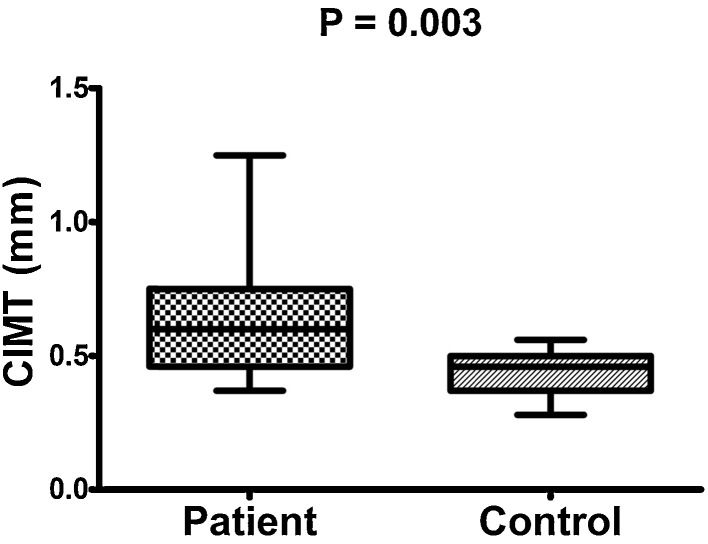
FMD was significantly lower in RA (6.53% ± 1.81%) compared to controls (10.77% ± 0.53%; p < 0.001). CIMT (mm) was significantly increased in RA (0.62 ± 0.17) vs. controls (0.043 ± 0.07; p = 0.003). In RA patients, FMD% inversely correlated with CIMT, CRP, DAS-28, TNF-α, serum nitrite and positively correlated with EPC. CIMT correlated with age, DAS-28, IL-6, HDL, LDL, and inversely correlated with EPC.
Conclusions
In the present study, FMD and CIMT were impaired in RA, indicating endothelial dysfunction and accelerated atherosclerosis respectively. CRP, TNF-α, serum nitrite, DAS-28 and depleted EPC population predicted endothelial dysfunction. Age, IL-6, HDL, LDL and depleted EPC population predicted accelerated atherosclerosis.
Keywords: Rheumatoid arthritis, Atherosclerosis, Carotid intima media thickness, Flow mediated dilatation, Inflammatory cytokines
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory, autoimmune disease that affects the synovial tissues, leading to cause pain and joint destruction. The prevalence rate of RA in Indian population is approximately 0.75% and it is quite similar to developed countries.1 These findings are keeping with fact that Indian population is genetically closer to the Caucasians than to other ethnic groups.1 Patients with RA have a higher risk of cardiovascular disease (CV); over the past decade it has become clear that CV disease is the major cause of death in RA.2 Increased cardiovascular risk in RA patients, as mortality is primarily due to CV events rather than disease per se.3 The increased cardiovascular mortality risk in patients with RA cannot be entirely explained by traditional CV risk factors, suggesting that the systemic inflammation that characterizes RA may cause endothelial dysfunction and accelerate atherosclerosis.2 The expression of pro-inflammatory cytokines and inflammatory mediators plays a crucial role in atherosclerotic process in rheumatic patients.4
Endothelial dysfunction is a barometer of CV health an important sign of early atherosclerosis and can be assessed non-invasively by flow mediated vasodilatation (FMD) of peripheral arteries.5 Increased carotid intima-media thickness (CIMT), determined by high-resolution B-mode ultrasound,6 is regarded as an early indicator of atherosclerosis and future cardiovascular disease.7 Both methods are closely related to endothelial damage and are early markers of atherosclerosis.8
There has been several studies has been shown altered endothelial function and increased CIMT from other countries in RA patients.9, 10, 11, 12, 13, 14, 15, 16 However, there are only a few studies assessing the endothelial function and CIMT in RA patients from India.17, 18, 19, 20, 21 The prevalence of coronary artery disease (CAD) in Indian population is nearly 10 times higher than that seen 40 years ago.22 In a recent study, the prevalence of CAD in migrant Indians in Canada was more than twice of that observed in the Caucasian population.23 It has been demonstrated that both traditional and nontraditional risk factors contribute to the pathogenesis of cardiovascular disease in RA patients.
Thus, we examined the endothelial function and carotid intima-media thickness in active RA patients from India who did not have traditional cardiovascular risk factors with well matched healthy individuals. We also measured disease activity, serum cytokines, endothelial progenitor cells and serum nitrite and studied their possible correlation with FMD and CIMT to evaluate the potential risk factors for endothelial dysfunction and atherosclerosis related to RA especially in the context of Indian population.
2. Materials and methods
2.1. Patients and control
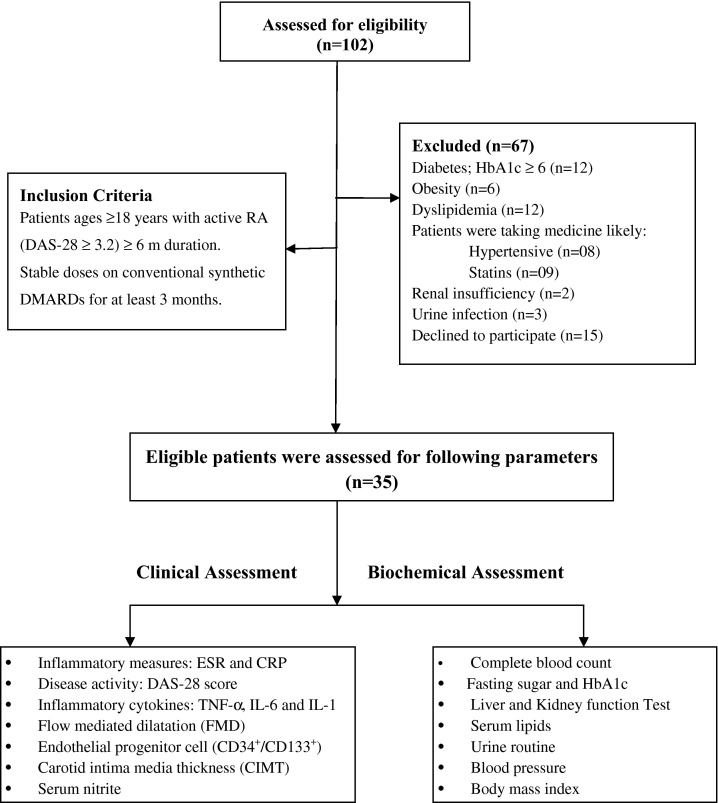
We performed a prospective study of 35 consecutive RA patients meeting 2010 Rheumatoid Arthritis Classification Criteria,24 compared with age and sex matched healthy controls (n = 25) from among the clinic staff and patient attendants were recruited. This study was carried out out-patient rheumatology clinic in Chandigarh, India during October 2012 to March 2014. Written informed consent was taken from all patients and the control group and the Institutional Clinical Ethics Committee (ICEC) approved the study protocol. In all of them, a detailed history was taken and a trough physical examination and following laboratory investigations were carried out: complete blood count, fasting sugar and HbA1c level, liver and kidney function test, serum lipids, urine analysis and electrocardiogram (Fig. 1). Patients and controls having known risk factors for atherosclerosis were excluded, including ages between >18 and 60 years, did not have any other autoimmune disease, diabetes mellitus as diagnosed according to World Health Organization criteria (WHO, 1985), dyslipidemia (total cholesterol 240 mg/dl, low-density lipoprotein cholesterol 160 mg/dl), obesity (body mass index ≥30) or hypothyroidism, past or present smokers, renal insufficiency, liver disease. Coronary artery disease, stroke or hypertension as defined by blood pressure >140/90 mm Hg or history of use of antihypertensive medication. Patients taking medication likely to affect endothelial function (anti-TNF-α inhibitors, beta blockers, angiotensin converting enzyme inhibitor, angiotensin receptor blocker, statins, aldosterone antagonist, peroxisome proliferator-activated receptor and steroids) were also excluded from the study. Detailed patients and healthy controls characteristics are depicted in Table 1. RA patients were on stable dose of combination of synthetic disease modifying anti-rheumatic drugs (DMARDs) for at least 3 months prior to study enrollment.
Fig. 1.
Recruitment flow diagram of rheumatoid arthritis patients.
Table 1.
Demographic, clinical and biochemical characteristics of the study subjects.
| Variable | Rheumatoid arthritis (n = 35) | Healthy subjects (n = 25) | p Value |
|---|---|---|---|
| Age (years) | 46.0 ± 9.1 | 42.8 ± 7.6 | 0.15 |
| Gender: M/F (n) | 12/23 | 9/16 | – |
| BMI (kg/m2) | 24.1 ± 3.9 | 23.2 ± 2.6 | 0.30 |
| Smoking (n) | 0 | 0 | – |
| Systolic BP (mm Hg) | 125.0 ± 20.9 | 118.8 ± 8.7 | 0.16 |
| Diastolic BP (mm Hg) | 79.3. ± 8.3 | 76.4 ± 8.4 | 0.27 |
| Disease duration (years) | 8.5 ± 5.0 | – | – |
| Fasting glucose (mg/dl) | 102.1 ± 8.4 | 96.3 ± 6.2 | 0.26 |
| HbA1c (%) | 5.44 ± 0.4 | 5.26 ± 0.7 | 0.23 |
| Serum creatinine (μmol/l) | 0.89 ± 0.8 | 0.82 ± 0.6 | 0.27 |
| Total cholesterol (mg/dl) | 166.6 ± 18.8 | 162.2 ± 15.7 | 0.42 |
| HDL cholesterol (mg/dl) | 42.4 ± 3.3 | 44.1 ± 3.8 | 0.09 |
| LDL cholesterol (mg/dl) | 95.9 ± 15.9 | 89.49 ± 11.86 | 0.26 |
| Triglycerides (mg/dl) | 121.5 ± 26.7 | 118.2 ± 20.3 | 0.45 |
| ESR (mm 1st h) | 36.64. ± 9.42 | 17.6 ± 4.64 | <0.001* |
| CRP (mg/dl) | 13.09 ± 8.26 | 3.98 ± 1.14 | <0.001* |
| DAS-28 | 4.10 ± 0.64 | – | – |
| FMD% | 6.53 ± 1.81 | 10.77 ± 0.53 | <0.001* |
| CIMT (mm) | 0.62 ± 0.017 | 0.43 ± 0.007 | 0.003* |
| EPC% | 0.021 ± 0.007 | 0.044 ± 0.01 | <0.001* |
| S. Nitrite | 5.76 ± 1.0 | 1.92 ± 0.64 | <0.01* |
| TNF-α (pg/ml) | 4.02 ± 0.89 | 3.13 ± 0.99 | <0.001* |
| IL-6 (pg/ml) | 12.40 ± 3.91 | 4.01 ± 0.83 | <0.001* |
| IL-1 (pg/ml) | 243.8 ± 59.5 | 88.72 ± 17.9 | <0.001* |
Values are mean ± SD.
p-value <0.05.
BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; DAS-28, disease activity score in 28 joints count; FMD, flow mediated dilation; CIMT, carotid intima media thickness; EPCs, Endothelial progenitor cells; TNF-α, tumor necrosis factor-a; IL-6, interleukin-6; IL-1, interleukin-1.
2.2. Assessment of endothelial function
Subjects were studied in the morning, after overnight fasting, in a quiet room with controlled temperature. With the subjects lying supine and their arms in a comfortable position, we assessed endothelial function by brachial artery flow-mediated dilatation (FMD) by using, AngioDefender (Everist Genomics, Ann Arbor, MI, United States). The AngioDefender device uses a novel, proprietary software algorithm to analyze pulse wave data collected before and after Brachial Artery (BA) occlusion by an upper arm sphygmomanometric cuff. At the end, of the testing procedure (∼15 min), the maximal relative post-occlusion change in the diameter of the BA relative to baseline is calculated and expressed as a percentage of flow mediated dilation (%FMD). AngioDefender test results are not dependent on user technique or operator proficiency.5 Mean of the two measures were considered for analysis.
2.3. Assessment of carotid intima-media thickness
All subjects were examined using a high-resolution Doppler ultrasound (HD 11 XE ultrasound machine, Philips Medical System) using a 13–5 MHz linear array transducer in the supine position. The CCA intima-media thickness (IMT) was defined as the average of the maximum IMT of the near and of the far wall measurements in the distal CCA (1 cm proximal to the carotid bulb). Intima media thickness was measured at three points on the far walls of both the left and the right common carotid arteries (CCA). The three locations were then averaged to produce the mean IMT for each side. All images of the carotid arteries were recorded on the hard disk of the ultrasound system for subsequent analysis and evaluated by a well experienced radiologist who was blinded to the clinical characteristics of the participants.6 The subjects had fasted overnight and they were studied in the morning between 9 and 11 AM.
2.4. Assessment of EPC population through flow cytometry analysis
After patients and control subjects fasted overnight, peripheral blood was taken at rest, in the morning, at forearm, together with routine analysis. EPCs were quantified by fluorescence-activated cell sorting (FACS) calibur flow cytometer (Canto II; BD Biosciences, San Jose, CA).
FACS analysis was performed with utilization of the following three markers:
-
1.
Fluorescein isothiocyanate (FITC) anti-CD45 (BD Sciences, San Jose, CA)
-
2.
Phycoerythrin (PE) anti-CD34 (BD Sciences, San Jose, CA)
-
3.
Allophycocyanin (APC) anti-CD133 (Miltenyi Biotec, Bergisch Gladbach, Germany)
Peripheral blood in EDTA (200 μL) was labeled with a panel of above mentioned antibodies and incubated for 1 h at room temperature. After conjugation, red blood cells were lysed with ammonium chloride for 15 min at room temperature. Thereafter, cells were washed and resuspended in 500 μl phosphate buffered saline (PBS; Seromed). Appropriate sequential analysis was used to enumerate total EPCs and to exclude debris. Putative EPCs were defined as positive for anti-CD34 and anti-CD133. At least 250,000–500,000 cells per sample were acquired. Data were analyzed with Cell Quest software (Becton Dickinson). Results are expressed as % cells gated.25
2.5. Serum nitrite estimation
Nitric oxide was directly determined as nitrite by a spectrophotometric method,26 using serum samples collected after overnight fasting. Gaseous NO free radical is rapidly metabolized to nitrate or nitrite, hence measured nitrite concentration is taken as an index of NO production. Nitrite is also not influenced by dietary variation.10 Samples for serum nitrite were estimated in duplicate. Mean of the two measured value was considered for analysis.
2.6. Clinical and biochemical assessment
Patients underwent clinical evaluation at time of recruitment. Disease activity was measured using the 28-joint disease activity score (DAS28). Biochemical assessment including a complete blood count, liver function tests, renal function test, vitamin B12, thyroid stimulating hormone, blood sugar, HbA1c and ‘serum lipids were determined using standard commercial kits and urine analysis to detect proteinuria, hematuria and cellular casts. Inflammatory markers i.e. Erythrocyte sedimentation rate (ESR) was measured using Westergren method and C-reactive protein (CRP) level using standard commercial kits. Inflammatory cytokines; tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1 were also assessed in all subjects by enzyme-linked immunosorbent assays (Hiss Diagnostics GmbH, Freiburg, Switzerland). All patients underwent clinical and biochemical assessment at the time of recruitment. Clinical, biochemical, FMD and CIMT assessments were carried out on the same day of recruitment after overnight fasting. Blood samples drawn for determination of serum cytokines and nitrite were stored at −80 °C before laboratory testing.
2.7. Statistical analysis
Continuous data are expressed as the mean ± standard deviation (SD). Patients and healthy control subjects were compared using unpaired Student's t-test for continuous variables and chi-squared test for categorical variables. Pearson correlation coefficients were calculated for RA patients to study the relationship between endothelial function, CIMT and clinical and biochemical disease variables. Two-sided p-values of less than 0.05 were considered statistically significant. The statistical analysis was carried out using Sigmastat 5.5 for Windows 7.
3. Results
The recorded baseline characteristics in the RA patients and healthy control individuals comprised traditional cardiovascular risk factors are summarized in Table 1. There were no significant differences in age, sex, and mean BMI values between RA patients and healthy controls. All study subjects were free from any traditional cardiovascular risk factors and cardiovascular disorders.
The clinical index of disease activity (DAS-28) was 4.10 ± 0.64 (Table 1). There were significant differences in the ESR and CRP levels between the RA patients and the controls (both p < 0.001). The mean serum level of proinflammatory cytokines i.e. TNF-α, IL-6 and IL-1 in RA patients was higher than that in healthy controls (All p < 0.001, Table 1). As regards the FMD (6.53 ± 1.81% vs. 10.77 ± 0.53%, Fig. 2), CIMT (0.62 ± 0.017 mm vs. 0.43 ± 0.007 mm, p = 0.003, Fig. 3) and EPC% (0.021 ± 0.007 vs. 0.044 ± 0.01, p < 0.001) revealed significantly abnormal values in RA patients compared with controls (Table 1).
Fig. 2.
Comparison between mean flow mediated dilatation (FMD) of both patients and controls.
Fig. 3.
Comparison between mean carotid intima-media thickness (CIMT) of both patients and controls.
Table 2 shows negative correlations between FMD% and CRP, DAS-28, TNF-α, serum nitrite, as well as CIMT. In contrast, significant positive correlations were found between CIMT and age, DAS-28, IL-6, HDL, as well as LDL. EPC level significantly correlated with FMD and CIMT. However, no significant correlation was demonstrated between FMD% and age, disease duration and BMI. Moreover, there was no significant correlation of CIMT with disease duration and BMI.
Table 2.
Correlations of clinical, laboratory and inflammatory biomarkers with FMD and CIMT in RA patients.
| FMD% |
CIMT |
|||||
|---|---|---|---|---|---|---|
| r value | p Value | 95% CI for mean | r value | p Value | 95% CI for mean | |
| Age | −0.295 | 0.09 | 0.57–0.04 | 0.564 | <0.001 | 0.27–0.75 |
| BMI | −0.07 | 0.660 | 0.40–0.26 | 0.152 | 0.39 | 0.46–0.19 |
| DD | −0.001 | 0.994 | 0.34–0.33 | 0.230 | 0.192 | 0.11–0.52 |
| ESR | −0.117 | 0.510 | 0.23–0.43 | 0.071 | 0.690 | 0.39–0.27 |
| CRP | −0.347 | 0.02 | 0.63–0.07 | 0.297 | 0.071 | 0.08–0.59 |
| DAS-28 | −0.424 | 0.01 | 0.66–0.10 | 0.383 | 0.02 | 0.05–0.63 |
| TNF-α | −0.489 | 0.003 | 0.70–0.17 | 0.314 | 0.07 | 0.02–0.58 |
| IL-6 | −0.267 | 0.127 | 0.55–0.07 | 0.344 | 0.04 | 0.007–0.61 |
| IL-1 | −0.156 | 0.377 | 0.46–0.19 | 0.186 | 0.291 | 0.16–0.01 |
| CIMT | −0.387 | 0.02 | 0.64–0.05 | – | – | – |
| EPC% | 0.523 | 0.001 | 0.22–0.73 | −0.352 | 0.04 | 0.61–0.01 |
| S. Nitrite | −0.342 | 0.04 | 0.60–0.03 | 0.122 | 0.491 | 0.22–0.44 |
| TC | −0.052 | 0.78 | 0.39–0.30 | 0.347 | 0.06 | 0.008–0.62 |
| HDL | −0.167 | 0.370 | 0.49–0.19 | 0.407 | 0.02 | 0.06–0.66 |
| LDL | −0.051 | 0.787 | 0.39–0.30 | 0.430 | 0.01 | 0.08–0.68 |
| TG | −0.107 | 0.566 | 0.25–0.44 | 0.272 | 0.13 | 0.09–0.57 |
Bold values are statistically significant, p < 0.05. Values were calculated using Pearson's correlation coefficient. BMI, body mass index; DD, disease duration; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; DAS-28, disease activity score in 28 joints count; TNF-α, tumor necrosis factor-a; IL-6, interleukin-6; IL-1, interleukin-1; CIMT, carotid intima media thickness; EPCs, endothelial progenitor cells; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides.
4. Discussion
Patients with RA are more prone to accelerated atherosclerosis and Asian Indians as an ethnic group are predisposed to a high risk of premature atherosclerosis.27 Increased prevalence of endothelial dysfunction and accelerated atherosclerosis has been reported in Indian RA patients.17, 18, 20, 21 However, there is sparse data regarding the predictors of endothelial dysfunction and accelerated atherosclerosis among asymptomatic active RA patients in Indian population.19 To the best of our knowledge, this is the first study to investigate the potential biomarkers of endothelial dysfunction and accelerated atherosclerosis in active RA patients in Indian population. These results may help to explain the increased cardiovascular morbidity and mortality observed in RA patients and possibly also acts as potential therapeutic targets to prevent/treat CV risk in RA. Result of the present study demonstrate that active RA patients in the absence of traditional CV risk factors (hypertension, hypercholesterolemia, diabetes, obesity and smoking) having altered endothelial function and accelerated atherosclerosis exhibited by decreased FMD and increased CIMT compared to age and sex matched healthy controls. Increased level of CRP, TNF-α, serum nitrite, DAS-28 and depleted endothelial progenitor cells (EPCs) predicted endothelial dysfunction. Increased age, IL-6, LDL, decreased HDL level and depleted EPC population predicted accelerated atherosclerosis.
Results of our study implicate that FMD and CIMT were significantly higher in active RA patients in absence of CV risk factors than that in healthy controls. Our result was the same supporting the earlier evidence had shown increased FMD and CIMT.11, 12, 13, 14, 17, 18, 20, 21 Earlier evidence suggests that Caucasian individuals without diabetes, chronic kidney disease or cardiovascular events, who had long-standing RA exhibited of endothelial dysfunction.9 An another classical study by Gonzalez-Juanatey et al. has shown increased frequency of severe subclinical atherosclerotic findings in long-term actively treated RA patients without clinically evident atherosclerotic disease and absence of classic CV risk factors from northwest Spain.10 In addition, a meta-analysis of different studies confirmed that CIMT is increased in patients with RA.16 Impaired FMD and increased CIMT in DMARD naive early RA patients has also been shown in Indian population without clinically evident atherosclerotic disease.19 However, in our study we have recruited those patients who have established and active RA in the absence of CV risk factor and we also measured inflammatory cytokines, serum nitrite and EPC population.
Early endothelial dysfunction indicated by FMD may precede manifest atherosclerosis indicated by CIMT. Therefore, the more pronounced endothelial dysfunction may lead to more accelerated atherosclerosis.8 In conformity with this observation, FMD% significantly correlated with CIMT in our study when the. In this regard, in Spanish individuals with RA, CIMT was negatively associated with FMD when the time from disease diagnosis ranged from 7.5 to 19.7 years.15
On Pearson correlation analysis, CRP, DAS-28, TNF-α, serum nitrite and EPC population predicted endothelial dysfunction independent of traditional cardiovascular risk factors. In our study the level of CRP (13.09 mg/dl) is very high compared to the healthy controls (3.98 mg/dl) and it significantly correlated with FMD. It has been shown that high level of CRP in RA is an independent predictor for cardiovascular morbidity and mortality28 and it can also develop a secondary immune cell activation, which may result in atherogenesis.29
Earlier evidence suggests that endothelial dysfunction in RA patients might be explained by disease activity measured by DAS-28.11 Similarly, in our study we found significant association between disease activity and FMD suggesting that inflammation plays a crucial role in the pathogenesis of cardiovascular disease in patients with RA. TNF-α plays an important role in the initiation and progression of inflammation in RA as well as in the mechanisms implicated the accelerated atherosclerosis in these patients.30 The proinflammatory cytokine, TNF-α also degrades endothelial nitric oxide synthase (eNOS) mRNA and impaired NO bioavailability.31, 32 This impaired NO bioavailability is a primary manifestation of endothelial dysfunction, leading to impairment in EDV.33 Multiple studies have demonstrated that endothelial nitric oxide synthase (eNOS) protein expression is reduced via TNF-α induced inhibition of eNOS promoter activity and mRNA destabilization.32 A key role of TNF-α in atherosclerosis is supported by the observation that biologic DMARD, anti TNF-α agent improves endothelial dysfunction and improves atherosclerosis.34 A beneficial effect of combination of synthetic DMARDs (methotrexate (MTX), hydroxychloroquine, and sulfasalazine) on FMD and CIMT has been shown in RA patients.35 Our observations suggest that increased level of TNF-α and serum nitrite significantly linked with FMD implicates that increased TNF-α and nitrite level as a mediator of endothelial dysfunction in RA. We did not find any significant correlation with IL-6 and IL-1. It may be due to the small RA population. Dessein et al. reported that endothelial dysfunction in RA was significantly associated with IL-6 and suggested IL-6 known to be biomarker of endothelial dysfunction.13
In the current study we also measured EPCs and found depleted EPC population in RA patients. EPCs are bone marrow derived stem cells which have reparative potential in overcoming the endothelial damage and protect against atherosclerotic vascular disease.36 Earlier, these cells have been studied in RA and recently studied by us in ankylosing spondylitis (AS).25, 36 Herbrig et al. have shown a clear positive correlation between endothelial dysfunction and depletion of EPCs.36 The study by Yiu et al. demonstrated that RA patients with atherosclerosis have significantly lower level of EPCs than those without.37 The result of our study thus confirmed that EPCs population is closely associated with endothelial dysfunction and increased CIMT and contributes to atherosclerosis in RA patients.
CIMT was found to be positively correlated with age of the patient in the present study suggesting that age is one of the risk factor for carotid atherosclerotic disease. However, we did not find any correlation of CIMT with disease duration, ESR and CRP as it was available in only 35 patients: therefore it did not assume significance, possibly due to low number of patients. Several researchers provide evidence for greater CIMT with longer disease duration,2, 38 while others do not find such an association.17, 39 In a study by Giles et al., increasing severity of RA was associated with a high prevalence of increase of CIMT level.39 Similarly, in our study we found significant positive correlation between CIMT and increased DAS-28 Score. We also found a significant correlation between CIMT and increased level of LDL and decreased level of HDL suggesting their role in the pathogeneses of atherosclerosis.
The role of proinflammatory cytokines IL-6 in the pathogenesis of RA has been well demonstrated. In the current study we have found that IL-6 significantly correlated with CIMT level and this observation suggests the role of IL-6 in the progression of atherosclerosis in patients with RA. Nunez, et al. has shown increased level of IL-6 significantly associated with increased carotid IMT.40 Inhibition of IL-6 has been reported to suppress atherogenesis in experimental animal model.41 There are no human studies of the effects of IL-6 inhibition on atherosclerosis.
This study design had several strengths, notably analysis of well characterized population and measurement of many inflammatory biomarkers reported to be associated with endothelial dysfunction and increased CIMT. The study also has its limitations, including the cross-sectional design and the relatively low number of study subjects. Therefore, we cannot exclude the possibility that markers showing weaker, non significant associations with atherosclerosis, may also contribute to a relationship between inflammation and atherosclerosis.
5. Conclusions
Our study on Indian patients have shown decreased FMD and increased CIMT in well characterized RA population without traditional cardiovascular risk factors, indicating early endothelial dysfunction and accelerated atherosclerosis, respectively.
Our data suggest that early determination of FMD% and CIMT may be useful tools to assess cardiovascular risk in RA. CRP, TNF-α, serum nitrite, DAS-28 and depleted EPC population predicted endothelial dysfunction. Age, IL-6, HDL, LDL and depleted EPC population predicted accelerated atherosclerosis. Therapy that effectively suppresses inflammation and stimulation of EPCs may be beneficial to prevent and manage early endothelial dysfunction and accelerated atherosclerosis related to RA.
Funding
No funding sources.
Conflicts of interest
The authors have none to declare.
Acknowledgements
We are very grateful to University Grant Commission, New Delhi (Govt. of India) for providing the research fellowship [No. F.10-15/2007 (SA-I)].
References
- 1.Malaviya A.N., Kapoor S.K., Singh R.R., Kumar A., Pande I. Prevalence of rheumatoid arthritis in the adult Indian population. Rheumatol Int. 1993;13:131–134. doi: 10.1007/BF00301258. [DOI] [PubMed] [Google Scholar]
- 2.Hand R. Rheumatoid arthritis – at the cross roads of inflammation and atherosclerosis! J Assoc Physicians India. 2013;61:529. [PubMed] [Google Scholar]
- 3.Pal G.K. Association of cardiovascular risks with sympathovagal imbalance in rheumatoid arthritis. Indian J Med Res. 2012;134:547–548. [PMC free article] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Garg N., Krishan N., Syngle A. Rosuvastatin improves endothelial dysfunction in ankylosing spondylitis. Clin Rheumatol. 2015;34:1065–1071. doi: 10.1007/s10067-015-2912-3. [DOI] [PubMed] [Google Scholar]
- 6.Huang K., Zou C.C., Yang X.Z., Chen X.Q., Liang L. Carotid intima media thickness and serum endothelial marker levels in obese children with metabolic syndrome. Arch Pediatr Adolesc Med. 2010;164:846–851. doi: 10.1001/archpediatrics.2010.160. [DOI] [PubMed] [Google Scholar]
- 7.Bots M.L., Grobbee D.E. Intima media thickness as a surrogate marker for generalized atherosclerosis. Cardiovasc Drugs Ther. 2002;16:341–351. doi: 10.1023/a:1021738111273. [DOI] [PubMed] [Google Scholar]
- 8.Staub D., Meyerhans A., Bundi B., Schmid H.P., Frauchiger B. Prediction of cardiovascular morbidity and mortality: comparison of the internal carotid artery resistive index with the common carotid artery intima-media thickness. Stroke. 2006;37:800–805. doi: 10.1161/01.STR.0000202589.47401.c6. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Juanatey C., Testa A., Garcia-Castelo A. HLA-DRB1 status affects endothelial function in treated patients with rheumatoid arthritis. Am J Med. 2003;1(114):647–652. doi: 10.1016/s0002-9343(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Juanatey C., Llorca J., Testa A., Revuelta J., Garcia-Porrua C., Gonzalez-Gay M.A. Increased prevalence of severe subclinical atherosclerotic findings in long-term treated rheumatoid arthritis patients without clinically evident atherosclerotic disease. Medicine (Baltimore) 2003;82:407–413. doi: 10.1097/01.md.0000101572.76273.60. [DOI] [PubMed] [Google Scholar]
- 11.Vaudo G., Marchesi S., Gerli R. Endothelial dysfunction in young patients with rheumatoid arthritis and low disease activity. Ann Rheum Dis. 2004;63:31–35. doi: 10.1136/ard.2003.007740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerli R., Sherer Y., Vaudo G. Early atherosclerosis in rheumatoid arthritis: effects of smoking on thickness of the carotid artery intima media. Ann N Y Acad Sci. 2005;1051:281–290. doi: 10.1196/annals.1361.069. [DOI] [PubMed] [Google Scholar]
- 13.Dessein P.H., Joffe B., Singh S. Biomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritis. Arthrits Res Ther. 2005;7:R634–R643. doi: 10.1186/ar1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerekes G., Szekanecz Z., Der H. Endothelial dysfunction and atherosclerosis in rheumatoid arthritis: a multiparametric anal using imaging techniques and laboratory markers of inflammation and autoimmunity. J Rheumatol. 2008;35:398–406. [PubMed] [Google Scholar]
- 15.Gonzalez-Juanatey C., Llorca J., Gonzalez-Gay M.A. Correlation between endothelial function and carotid atherosclerosis in rheumatoid arthritis patients with long standing disease. Arthritis Res Ther. 2011;13:R101. doi: 10.1186/ar3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Sijl A.M., Peters M.J., Knol D.K. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum. 2011;40(5):389–397. doi: 10.1016/j.semarthrit.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Grover S., Sinha R.P., Singh U., Tewari S., Aggarwal A., Misra R. Subclinical atherosclerosis in rheumatoid arthritis in India. J Rheumatol. 2006;33:244–247. [PubMed] [Google Scholar]
- 18.Mahajan V., Handa R., Kumar U. Assessment of atherosclerosis by carotid intimomedial thickness in patients with rheumatoid arthritis. JAPI. 2008;56:587–590. [PubMed] [Google Scholar]
- 19.Adhikari M.C., Guin A., Chakarborty S., Ghosh A. Subclinical atherosclerosis and endothelial dysfunction in patients with early rheumatoid arthritis as evidenced by measurement of carotid intima-media thickness and flow-mediated vasodilatation: an observational study. Semin Arthritis Rheum. 2012;41:669–675. doi: 10.1016/j.semarthrit.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Deo S.S., Chogle A.R., Mistry K.J., Shetty R.R., Nadkar U.L. Increased prevalence of subclinical atherosclerosis in rheumatoid arthritis patients of Indian descent. Exp Clin Cardiol. 2012;17:20–25. [PMC free article] [PubMed] [Google Scholar]
- 21.Mohan A., Sada S., Kumar B.S. Subclinical atherosclerosis in patients with rheumatoid arthritis by utilizing carotid intimamedia thickness as a surrogate marker. Indian J Med Res. 2014;140:379–386. [PMC free article] [PubMed] [Google Scholar]
- 22.Mohan V., Deepa R. Risk factors for coronary artery disease in Indians. J Assoc Phys India. 2004;52:95–97. [PubMed] [Google Scholar]
- 23.Anand S.S., Yusuf S., Vuksan V. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE) Lancet. 2000;356:279–284. doi: 10.1016/s0140-6736(00)02502-2. [DOI] [PubMed] [Google Scholar]
- 24.Aletaha D., Neogi T., Silman A.J. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 25.Verma I., Syngle A., Krishan P. Endothelial progenitor cell biology in ankylosing spondylitis. Int J Rheum Dis. 2014;18:336–340. doi: 10.1111/1756-185X.12487. [DOI] [PubMed] [Google Scholar]
- 26.Sastry K.V., Moudgal R.P., Mohan J., Tyagi J.S., Rao G. Spectrophotometric determination of serum nitrite and nitrate by copper–cadmium alloy. Anal Biochem. 2002;306:79–82. doi: 10.1006/abio.2002.5676. [DOI] [PubMed] [Google Scholar]
- 27.Misra A., Khurana L. The metabolic syndrome in South. Asians: epidemiology, determinants, and prevention. Metab Syndr Relat Disord. 2009;7:497–514. doi: 10.1089/met.2009.0024. [DOI] [PubMed] [Google Scholar]
- 28.Goodson N.J., Symmons D.P., Scott D.G., Bunn D., Lunt M., Silman A.J. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293–2299. doi: 10.1002/art.21204. [DOI] [PubMed] [Google Scholar]
- 29.Keeling S.O., Landewe R., van der Heijde D. Testing of the preliminary OMERACT validation criteria for a biomarker to be regarded as reflecting structural damage endpoints in rheumatoid arthritis clinical trials: The example of C-reactive protein. J Rheumatol. 2007;34:623–633. [PubMed] [Google Scholar]
- 30.Bacon P.A., Raza K., Banks M.J., Townend J., Kitas G.D. The role of endothelial cell dysfunction in the cardiovascular mortality of RA. Int Rev Immunol. 2002;21:1–17. doi: 10.1080/08830180210413. [DOI] [PubMed] [Google Scholar]
- 31.Yoshizumi M., Perrella M.A., Burnett J.C., Lee M.E. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res. 1993;73:205–209. doi: 10.1161/01.res.73.1.205. [DOI] [PubMed] [Google Scholar]
- 32.Steyers C.M., Miller F.J. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15:11324–11349. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura M., Yoshida H., Arakawa N., Saitoh S., Satoh M., Hiramori K. Effects of tumor necrosis factor-alpha on basal and stimulated endothelium-dependent vasomotion in human resistance vessel. J Cardiovasc Pharmacol. 2000;36:487–492. doi: 10.1097/00005344-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Hurlimann D., Forster A., Noll G. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106:2184–2187. doi: 10.1161/01.cir.0000037521.71373.44. [DOI] [PubMed] [Google Scholar]
- 35.Kisiel B., Kruszewski R., Juszkiewicz A. Methotrexate, cyclosporine A, and biologics protect against atherosclerosis in rheumatoid arthritis. J Immunol Res. 2015 doi: 10.1155/2015/759610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herbrig K., Haensel S., Oelschlaegel U., Pistrosch F., Foerster S., Passauer J. Endothelial dysfunction in patients with rheumatoid arthritis is associated with a reduced number and impaired function of endothelial progenitor cells. Ann Rheum Dis. 2006;65:157–163. doi: 10.1136/ard.2005.035378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yiu K.H., Wang S., Mok M.Y. Role of circulating endothelial progenitor cells in patients with rheumatoid arthritis with coronary calcification. J Rheumatol. 2010;37:529–535. doi: 10.3899/jrheum.090782. [DOI] [PubMed] [Google Scholar]
- 38.Del Rincon I., Free man G.L., Haas R.W., O’Leary D.H., Escalante A. Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum. 2005;52:3413–3423. doi: 10.1002/art.21397. [DOI] [PubMed] [Google Scholar]
- 39.Giles J.T., Post W., Blumenthal R.S., Bathon J.M. Therapy insight: managing cardiovascular risk in patients with rheumatoid arthritis. Nat Clin Pract Rheumatol. 2006;2:320–329. doi: 10.1038/ncprheum0178. [DOI] [PubMed] [Google Scholar]
- 40.Rho Y.H., Chung C.P., Oeser A. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61:1580–1585. doi: 10.1002/art.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nunez A.L., Figuroa S.M., Munoz D.A. Correlation of carotid intima media thickness with interleukin-6, tumor necrosis factor-α and anti-cyclic citrullinated peptides in rheumatoid arthritis. Arthritis Rheum. 2013;65(suppl 10):410. [Google Scholar]