Abstract
Background:
Patients with type 2 diabetes (T2DM) have an increased prevalence of dyslipidemia, which contributes to their high risk of cardiovascular diseases (CVDs). This study is an attempt to determine the correlation between hemoglobin A1c (HbA1c) and serum lipid profile and to evaluate the importance of HbA1c as an indicator of dyslipidemia in Afghani patients with T2DM.
Methods:
A total of 401 Afghani patients with T2DM (men, 175; women, 226; mean age, 51.29 years) were included in this study. The whole blood and sera were analyzed for fasting blood sugar (FBS), HbA1c, total cholesterol (TC), triglycerides (TGs), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C). Dyslipidemia was defined according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines. Diabetes was defined as per American Diabetes Association criteria. The correlation of FBS, HbA1c with lipid ratios and individual lipid indexes were analyzed. The statistical analysis was done by SPSS statistical package version 16.0.
Results:
The mean age ± standard deviation of male and female patients were 51.71 ± 11.70 and 50.97 ± 10.23 years respectively. There was a significant positive correlation between HbA1c, TC, TG, LDL-C and LDL-C/HDL-C ratio. The correlation between HbA1c and HDL-C was negative and was statistically nonsignificant. Furthermore, HbA1c was found to be a predictor of hypercholesterolemia, LDL-C and TG via a linear regression analysis. Patients with HbA1c value greater than 7.0% had significantly higher value of cholesterol, LDL-C, and LDL-C/HDL-C ratio compared with patients with an HbA1c value up to 7.0%.
Conclusions:
Apart from a reliable glycemic index, HbA1c can also be used as a predictor of dyslipidemia and thus early diagnosis of dyslipidemia can be used as a preventive measure for the development of CVD in patients with T2DM.
Keywords: Afghanistan, cardiovascular diseases, diabetes mellitus, dyslipidemia, HbA1c, serum lipids
Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder that occurs as a result of a complex interaction of genetic, environmental factors, and lifestyle choices. Morbidity and mortality due to DM are increasing because of changes in behavior such as an unhealthy diet, physical inactivity, being overweight, obesity and tobacco use.1,2
T2DM is a rapidly growing public health problem worldwide, with a significant impact on health, quality of life and the healthcare system of the countries. According to current statistics from the International Diabetes Federation (IDF), 415 million adults have DM worldwide; this figure is expected to reach the 642 million mark by 2040, which means every 11th individual will be diagnosed as having T2DM.3 The proportion of people with T2DM is increasing in most countries.3 Nearly 80% of people with the disease live in low- and middle-income countries.4
Pakistan and Afghanistan have been included in the Middle East and North African region by the IDF; this region is approximately 9.7% of the total regional population, which means around 37 million people in the region are living with T2DM.5
Due to the political instability in Afghanistan, very limited data are available regarding the prevalence of DM in the country, its complications and mortality rates. The prevalence of DM in the adult population after the world population adjustment has been estimated to be 8.6% and 9.9% in 2010 and 2030 respectively.1,6
Annually, 5% of all deaths globally are related to DM and its complications.7 DM was responsible for 342,000 deaths in 2015 in this part of the world.5 Over half (51.3%) of all deaths from DM in the region occurred in people under 60 years of age. These early deaths may be the result of a combination of factors: the rapidly changing environments and lifestyles in the region, late diagnoses, and healthcare systems that are not equipped to provide optimal management of the increasing numbers of people with DM.8
DM is a metabolic disorder that has a long-term impact on different body systems, contributing to the huge burden of morbidity associated with it. Cardiovascular disease (CVD) is one group among the spectrum of diseases; altered lipid metabolism in DM contributes to atherosclerosis.9 This greatly increases the risk of CVD compared with people without DM. The prevalence of CVD is higher in people with T2DM and is also the top killer in this population.4
DM is an independent risk factor for CVD and it potentiates the effects of other common risk factors such as smoking, hypertension and dyslipidemia.10
HbA1c predicts the risk of developing diabetic complications in patients with DM.7 Apart from classical risk factors like dyslipidemia, elevated HbA1c is an independent risk factor for CVD. It is estimated that there is an 18% increased risk of CVD for each 1% rise in absolute HbA1c levels in the diabetic population. This positive correlation between HbA1c and CVD has been demonstrated in nondiabetic cases, even within the normal range of HbA1c.7,11
The aim of this study was to assess the relationship between glycemic control (HbA1c) and serum lipid profile as well as to evaluate the importance of HbA1c as an indicator of dyslipidemia in patients with T2DM in an Afghani population.
Material and methods
Study design and location
A cross-sectional study was conducted from April 2015 to August 2015. Afghani patients with T2DM visiting an outpatient department (diabetic clinic) in the Northwest General Hospital & Research Center, Hayatabad Peshawar, Khyber Pakhtunkhwa, Pakistan were included in the study. This is a 220-bed tertiary care hospital, a Venture of Alliance Healthcare (PVT) Ltd, located in the historic city of Peshawar. Its catchment area is all over the province of Khyber Pakhtunkhwa and nearby border areas of Afghanistan. The study protocol was approved by the ethics committee of the Hospital. Informed written consent was obtained from all the patients after explaining the study objectives.
Patients and methods
Venous blood samples were collected from a total of 401 Afghani patients with T2DM, of which there were 175 men and 226 women. The serum was used for analyzing fasting blood sugar (FBS), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TGs), and low-density lipoprotein cholesterol (LDL-C) using an auto analyzer (Roche/Hitachi 912/Modular Analyzers: CAN 435, Germany). HbA1c was estimated using an ARCHITECT c4000 analyzer (Abbott Diagnostics, USA).
For the serum lipid reference level, the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guideline was referred. According to these guidelines, hypercholesterolemia is defined as TC greater than 200 mg/dl, high LDL-C when the value is over 100 mg/dl, hypertriglyceridemia when TG is greater than 150 mg/dl and low HDL-C as a value less than 40 mg/dl. Dyslipidemia was defined as the presence of one or more abnormal serum lipid concentrations.12 DM was defined as per American Diabetes Association criteria.13
All patients were categorized into two groups according to their HbA1c levels. The data were evaluated via the SPSS statistical package version 16.0. Pearson’s correlation test was performed to examine various correlations. Independent sample Student’s t test (two tailed) was used to compare means of different parameters. All p values less than 0.05 were considered statistically significant. The value of HbA1c was given as a percentage of the total hemoglobin and values of all other parameters were given in mg/dl. All values are expressed as mean ± standard deviation (SD) or standard error of the mean.
Results
A total of 401 Afghani patients with T2DM (men, 175; Women; 226; mean age, 51.29 years) were included in this study. The mean age ± SD of male and female patients were 51.71 ± 11.70 and 50.97 ± 10.23 years respectively. The mean ± SD of FBS, HbA1c, TC, TG, HDL-C and LDL-C were 230.54 ± 100.58, 9.84 ± 2.47, 206 ± 53.51, 256 ± 143.2, 40.85 ± 13.62, and 118.81 ± 51.78 respectively.
Hypercholesterolemia was found in 50.1% of patients; similarly hypertriglyceridemia was found in 74.8% of patients. Abnormal LDL-C levels were found in 62.8% of patients and HDL-C was less than 40 mg/dl in 51.9% of the study sample. Of the 401 patients, 48 (11.97%) had only one abnormal lipid profile parameter, 44 (10.97%) had two abnormal lipid parameters, and 208 (51.87%) had more than two abnormal lipid profile parameters. According to the NCEP ATP III guideline, 118 of 226 women (52.21%) and 83 of 175 men (47.42 %) were found to have dyslipidemia.
The mean value of FBS, HbA1c, TC, HDL-C and LDL-C were slightly higher in female compared with male patients, but the differences were not statistically significant. Although the mean levels of TC and the TC/HDL-C ratio were slightly lower in women than in men, these differences were statistically nonsignificant, as shown in Table 1.
Table 1.
Serum lipid profile parameter results for male and female patients with T2DM.
| Parameters | Male patients (n = 175) |
Female patients (n = 226) |
p value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | ||
| Age (years) | 51.71 | 11.70 | 22–92 | 50.97 | 10.23 | 17–81 | 0.49 |
| FBS (mg/dl) | 219.64 | 96.52 | 95–580 | 239.15 | 103.18 | 95–500 | 0.07 |
| HbA1c (%) | 9.79 | 2.66 | 5.50–16.80 | 9.94 | 2.36 | 5.50–16.50 | 0.53 |
| Cholesterol (mg/dl) | 204.08 | 54.46 | 88–365 | 208.04 | 52.96 | 96–321 | 0.46 |
| TG (mg/dl) | 266.03 | 156.72 | 51–1046 | 249.53 | 131.79 | 45–779 | 0.25 |
| HDL-C (mg/dl) | 39.68 | 14.25 | 13–98 | 41.75 | 13.08 | 18–98 | 0.13 |
| LDL-C (mg/dl) | 115.10 | 55.35 | 20–295 | 121.67 | 48.77 | 23–256 | 0.20 |
| TC/HDL-C | 5.64 | 2.21 | 1.70–15.38 | 5.40 | 2.03 | 1.59–15.45 | 0.27 |
FBS, fasting blood sugar; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol SD, standard deviation; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglyceride.
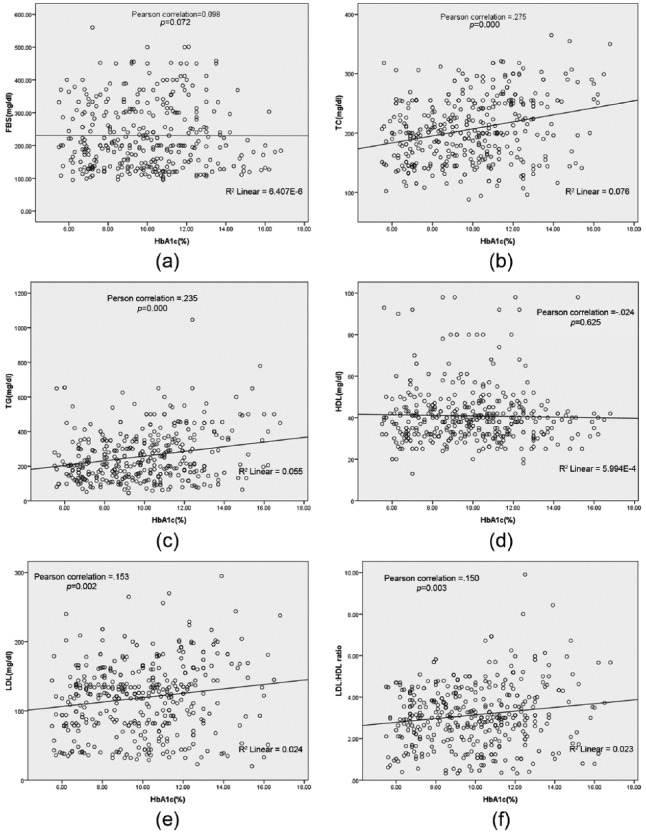
The age and FBS of patients did not show any significant correlation with HbA1c (r = 0.063, p = 0.206; r = 0.098, p = 0.072 respectively). There was a significant positive correlation between HbA1c and TC (r = 0.257, p = 0.000). HbA1c also demonstrated a significant correlation with TG (r = 0.235, p = 0.000). The correlation between HbA1c and HDL-C was negative and was found to be statistically nonsignificant (r = −0.024, p = 0.625). Furthermore, it was found that HbA1c was positively and significantly related to LDL-C (r = 0.153, p = 0.002) and LDL-C/HDL-C ratio (r = 0.150, p = 0.003). HbA1c was also found to be a predictor of hypercholesterolemia (p = 0.000, R2 = 0.078), LDL-C (p = 0.002, R2 = 0.002), and TG (p = 0.000, R2 = 0.055) validated by a linear regression analysis as shown in Figure 1. However, HDL-C (p = 0.625, R2 = 0.001) and FBS (p = 0.963, R2 = 0.000) did not show any significant association with HbA1c in the regression analysis.
Figure 1.
Correlations between hemoglobin A1c, FBS and lipid profile panel. FBS, fasting blood sugar; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglyceride.
Patients were divided into two groups as per their glycemic index (HbA1c); the first group consisted of patients with HbA1c values up to 7.0% and the second group consisted of patients with HbA1c values greater than 7.0%. Patients with HbA1c values greater than 7.0% had significantly higher values of cholesterol (p = 0.004), LDL-C (p = 0.002), LDL-C/HDL-C ratio (p = 0.024), FBS (p = 0.64), TG (p = 0.097), HDL-C (p = 0.334), and risk ratio (p = 0.58), compared with the first group, as shown in Table 2.
Table 2.
Biochemical parameters categorized by patient’s hemoglobin A1c.
| Parameters | HbA1c ⩽ 7.0(n = 66) |
HbA1c > 7.0 (n = 335) |
p value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | SEM | Mean | Range | SD | SEM | ||
| Age (years) | 49.39 | 13.18 | 17–81 | 1.62 | 51.67 | 19–92 | 10.36 | 0.57 | 0.12 |
| FBS (mg/dl) | 224.4 | 88.77 | 95–400 | 12.31 | 231.35 | 95–560 | 102.62 | 6.05 | 0.64 |
| Cholesterol (mg/dl) | 189.03 | 46.12 | 102–318 | 5.68 | 209.72 | 88–365 | 54.36 | 2.97 | 0.004* |
| TG (mg/dl) | 229.97 | 144.04 | 69–654 | 17.73 | 262.00 | 45–1046 | 142.72 | 7.79 | 0.097 |
| HDL-C (mg/dl) | 39.36 | 15.25 | 13–93 | 1.85 | 41.14 | 18–98 | 13.34 | 0.73 | 0.334 |
| LDL-C (mg/dl) | 101.08 | 47.19 | 35–240 | 5.81 | 122.30 | 20–295 | 51.99 | 2.84 | 0.002* |
| TC/HDL- C (risk ratio) | 5.37 | 2.32 | 1.59–15.38 | 0.29 | 5.53 | 1.65–15.45 | 2.07 | 0.11 | 0.58 |
| LDL-C/HDL-C | 2.76 | 1.23 | 0.39–4.70 | 0.15 | 3.21 | 0.33–9.90 | 1.53 | 0.08 | 0.024* |
Statistically significant.
FBS, fasting blood sugar; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol SD, standard deviation; SEM, standard error of the mean; TC, total cholesterol; TG, triglyceride.
Discussion
The association of DM and CVD is well known and this relationship has been much discussed over the past few decades.14 Both lipid profile and DM have been shown to be important predictors of metabolic disturbances, including dyslipidemia, hypertension, CVD, and hyperinsulinemia.15
People with T2DM have a higher cardiovascular morbidity and mortality, and are disproportionately affected by CVD compared with subjects without DM. Early detection and treatment of dyslipidemia associated with DM may be one step in reducing the CVD risk.16
In our study, the correlation of HbA1c and lipid profile in T2DM was evaluated. Interestingly, 52.21% of women and 47.42% of men were found to have dyslipidemia according to the NCEP ATP III guideline. These findings are consistent with some previous studies.7,12,13,17 Hyperlipidemia in women may be attributed to the effects of sex hormones on body fat distribution, which leads to differences in changed lipoproteins.18
We observed that the correlation between HbA1c and HDL-C was negative, however there was a positive, significant correlation between HbA1c and TC, LDL-C, and TGs. Various studies have described this before.7,19–21
A rather interesting finding was that HbA1c was found to be a predictor of hypercholesterolemia by linear regression analysis.
In one Pakistani study by Firdous and colleagues, 38% of people with DM were found to have high TG levels. In our study, women with DM were found to have slightly higher LDL levels compared with men, which is consistent with previous studies.7,12,22
It is suggested that insulin resistance has a central role in the development of diabetic dyslipidemia. One of the causes is increased free fatty acid release from insulin-resistant fat cells.23 If the glycogen stores are adequate, these free fatty acids promote TG production which further stimulates Apolipoprotein (Apo-B) and very low density lipoprotein.24
According to the Diabetes Complications and Control Trial, HbA1c is the gold standard of glycemic control.25 Various studies have shown an HbA1c of up to 7.0% may reduce the risk of cardiovascular complications.7,11
In our study, we divided patients into two groups as per the HbA1c values. Those with HbA1c greater than 7.0% exhibited a significant increase in TC, LDL-C, TG, and LDL-C/HDL-C ratio compared with those with HbA1c up to 7.0%. Improving glycemic control may substantially reduce the risk of cardiovascular events. It has been projected that a decrease in the HbA1c value by 0.2% could lower the mortality by 10%.26
The results of our study suggest the importance of glycemic control in managing dyslipidemia and further reducing the risk for CVD in patients with T2DM, as shown by the significant association of HbA1c with various lipid parameters.
Data from the Afghani population are lacking due to political unrest in the form of war and a paucity of structured health care. At the Northwest General Hospital & Research Center, Peshawar we treat some of the patients from across the border. We hope that by doing this study the world will get some insight into the diabetic status of the Afghani population.
Conclusion
In our study we have seen a significant correlation between HbA1c and various circulating lipid parameters. We noted a significant difference in lipid parameters in two groups (⩽7.0% and >7.0%) of HBA1c. This may indicate that HbA1c can be used as a potential biomarker for predicting dyslipidemia in patients with T2DM in addition to glycemic control. Hence, early diagnosis can be accomplished through relatively inexpensive blood testing and may be utilized for screening high-risk patients with DM for timely intervention with lipid-lowering drugs.
Acknowledgments
We are thankful to Northwest General Hospital & Research Centre, Peshawar, Pakistan for providing ethical approval of data collection and publishing this article. We would also like to thank Waheed Iqbal and Muhammad Nasir from the Department of Pharmacy Services for data entry and cleaning.
Footnotes
Authors Contribution: Substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work: Iftikhar Ali, Arshad Hussain, Muhammad Ijaz; drafting the work and revising it critically for important intellectual content: Arshad Hussain, Iftikhar Ali, Muhammad Ijaz, Afaq Rahim; final approval of the version to be published: Arshad Hussain, Iftikhar Ali, Afaq Rahim. This article has not been presented or published in any conference/journal
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Arshad Hussain, Department of Medicine & Allied, Northwest General Hospital & Research Center, Peshawar 25100, Khyber Pakhtunkhwa, Pakistan.
Iftikhar Ali, Department of Pharmacy, University of Swabi, 23430, Khyber Pakhtunkhwa, Pakistan Department of Pharmacy Services, Northwest General Hospital & Research Center, Peshawar 25100, Khyber Pakhtunkhwa, Pakistan.
Muhammad Ijaz, Department of Medicine & Allied, Northwest General Hospital & Research Center, Peshawar 25100, Khyber Pakhtunkhwa, Pakistan.
Afaq Rahim, Department of Medicine & Allied, Northwest General Hospital & Research Center, Peshawar 25100, Khyber Pakhtunkhwa, Pakistan.
References
- 1. Saeed KMI, Asghar RJ, Sahak MN, et al. Prevalence and risk factors associated with diabetes mellitus among Kabul citizens—Afghanistan, 2012. Int J Diabetes Dev Ctries 2015; 35: 297–303. [Google Scholar]
- 2. WHO. Diabetes: fact sheet, http://www.who.int/mediacentre/factsheets/fs312/en/ (2009, 20 September 2016).
- 3. International Diabetes Federation. IDF Diabetes Atlas. 7th ed., http://www.diabetesatlas.org/ (2016, 14 September 2016).
- 4. Tabish SA. Is diabetes becoming the biggest epidemic of the twenty-first century? Int J Health Sci 2007; 1: V–VIII. [PMC free article] [PubMed] [Google Scholar]
- 5. International Diabetes Federation. Middle East and North Africa at a glance. IDF Diabetes Atlas. 6th ed., http://www.idf.org/sites/default/files/attachments/MENA%20factsheet.pdf (2014, accessed 25 August 2016).
- 6. Saeed KMI. Prevalence and predictors of diabetes mellitus in Jalalabad City, Afghanistan-2013. Iran J Diabetes Obes 2014; 1: 1–8. [Google Scholar]
- 7. VinodMahato R, Gyawali P, Raut PP, et al. Association between glycaemic control and serum lipid profile in type 2 diabetic patients: glycated haemoglobin as a dual biomarker. Biomed Res 2011; 22: 375–380. [Google Scholar]
- 8. International Diabetes Federation. Middle East and North Africa. IDF Diabetes Atlas. 7th ed., http://www.worlddiabetesfoundation.org/sites/default/files/Middle-east.pdf (2015, 25 August 2016).
- 9. Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, et al. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes 2014; 5: 444–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zahidullah M, Aasim M, Khan I, et al. Evaluation of patients with coronary artery disease for major modifiable risk factors for ischemic heart disease. J Ayub Med Coll Abbottabad 2012; 24: 102–105. [PubMed] [Google Scholar]
- 11. Syed I, Khan WA. Glycated haemoglobin – a marker and predictor of cardiovascular disease. J Pak Med Assoc 2011; 61: 690–695. [PubMed] [Google Scholar]
- 12. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 13. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33(Suppl. 1): S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dixit AK, Dey R, Suresh A, et al. The prevalence of dyslipidemia in patients with diabetes mellitus of Ayurveda Hospital. J Diabetes Metab Disord 2014; 13: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldberg IJ. Diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab 2001; 86: 965–971. [DOI] [PubMed] [Google Scholar]
- 16. Khaw K, Wareham N, Bingham S, et al. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141: 413–420. [DOI] [PubMed] [Google Scholar]
- 17. Wexler DJ, Grant RW, Meigs JB, et al. Sex disparities in treatment of cardiac risk factors in patients with type 2 diabetes. Diabetes Care 2005; 28: 514–520. [DOI] [PubMed] [Google Scholar]
- 18. Sibley SD, Thomas W, de Boer I, et al. Gender and elevated albumin excretion in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort: role of central obesity. Am J Kidney Dis 2006; 47: 223–232. [DOI] [PubMed] [Google Scholar]
- 19. Andersen G, Christiansen J, Mortensen H, et al. Plasma lipid and lipoprotein in type 1 diabetic children and adolescent in relation to metabolic regulation, obesity and genetic hyperlipoprotenimia. Acta Paediatr Scand 1983; 72: 361–365. [DOI] [PubMed] [Google Scholar]
- 20. Erciyas F, Taneli F, Arslan B, et al. Glycemic control, oxidative stress, and lipid profile in children with type 1 diabetes mellitus. Arch Med Res 2004; 35: 134–140. [DOI] [PubMed] [Google Scholar]
- 21. Ohta T, Nishiyama S, Nakamura T, et al. Predominance of large low density lipoprotein particles and lower fractional esterification rate of cholesterol in high density lipoprotein in children with insulin-dependent diabetes mellitus. Eur J Pediatr 1998; 157: 276–281. [DOI] [PubMed] [Google Scholar]
- 22. Firdous S, Khan M. Comparison of patterns of lipid profile in type-2 diabetics and non-diabetics. Ann King Edward Med Coll 2007; 13: 84–87. [Google Scholar]
- 23. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest 2000; 106: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis 2015; 14: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch Public Health 2015; 73: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kishore P, Kim SH, Crandall JP. Glycemic control and cardiovascular disease: what’s a doctor to do? Curr Diab Rep 2012; 12: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]