Abstract
Purpose
To evaluate the efficacy of intravitreal aflibercept therapy in treatment-naïve Japanese patients with polypoidal choroidal vasculopathy (PCV) using Early Treatment Diabetic Retinopathy Study (ETDRS) letter scores.
Subjects and methods
This study was a prospective, nonrandomized, interventional exploratory clinical trial performed in an institutional setting. Patients with PCV were treated with intravitreal aflibercept 2 mg/0.05 mL every 2 months after 3 initial monthly doses, for 1 year. Visual acuity test using the ETDRS chart and indocyanine green angiography was performed at baseline and at 6 and 12 months after initiating the treatment, in addition to routine examinations performed at each visit. The main outcome measure was the proportion of patients who achieved <15 ETDRS letter score loss.
Results
Twenty-two patients were enrolled in this study. Nineteen (86%) patients were eligible for analysis. All the patients maintained their visual acuity (<15 ETDRS letter score loss) at 12 months. The ETDRS letter scores were 64.1 at baseline and 69.8 at 12 months (P<0.039). The polyps regressed completely in 14 (74%) patients at 12 months. Cataract progressed in 1 eye, but this progression was considered to be a senile change.
Conclusions
Japanese patients with treatment-naïve PCV, who were treated with intravitreal aflibercept every 2 months after 3 initial monthly doses, exhibited a significant increase in ETDRS letter scores and a high rate of polyp resolution at 12 months.
Keywords: aflibercept, polypoidal choroidal vasculopathy, Japanese, early treatment diabetic retinopathy study chart, polyp resolution
Introduction
Polypoidal choroidal vasculopathy (PCV) is a distinct clinical entity of age-related macular degeneration (AMD) characterized by a branching vascular network and polypoid structures derived from the choroid.1,2 The incidence of PCV in the Japanese population appears to be remarkably high.3 The principal therapies are laser photocoagulation, photodynamic therapy (PDT), anti-vascular endothelial growth factor (VEGF) agents, and combinations of these treatments. Efficacies of each monotherapy and combination therapy of PDT with anti-VEGF agents have been reported; however, the optimal treatment for PCV is still controversial. Although the LAPTOP trial4 showed that ranibizumab was superior to PDT for improving visual acuity, PDT was superior to bevacizumab or ranibizumab in terms of the polyp regression rate.5–7
Aflibercept (Eylea; Bayer HealthCare Pharmaceuticals, Berlin, Germany), one of the anti-VEGF agents, is a soluble decoy fusion protein that binds to members of the VEGF family.8,9 The VIEW 1 and 2 studies demonstrated that intravitreal aflibercept dosed every 2 months after 3 initial monthly doses had an efficacy and safety outcome similar to that of monthly ranibizumab.10 However, without analyzing the efficacy according to each AMD subtype, we cannot obtain any information about the visual outcomes of patients with PCV in the VIEW 1 and 2 studies. Recently, other studies have revealed that intra vitreal aflibercept dosed as needed11–13 or every 2 months11,14–16 after 3 initial monthly doses was effective for treatment-naïve patients with PCV.
Our major concern was whether aflibercept is as effective for PCV as it is for AMD. As the VIEW 1 and 2 studies was a randomized controlled trial (RCT) that provided strong clinical evidence, the results are worthy of being referenced. However, we cannot simply compare the visual outcome in the VIEW 1 and 2 studies with those of the other studies mentioned above because an Early Treatment Diabetic Retinopathy Study (ETDRS) chart was used in the VIEW 1 and 2 studies, while the logarithm of the minimum angle of resolution (logMAR) unit was used in the other studies.11–18 The concept of the ETDRS letter score was introduced by Gregori et al.19 Although the values are exchangeable between the ETDRS letter score and the logMAR unit, the former is more favorable than the latter not only in terms of statistical manipulation, but also in terms of the intuitive interpretation of visual acuity. Naturally, the ETDRS letter score has been applied to numerous ophthalmological RCTs in addition to the VIEW 1 and 2 studies. Therefore, use of the ETDRS chart is preferable in studies that aim to discuss visual outcomes using such RCTs for comparison.
We previously reported the logMAR visual acuity of the 16 patients with PCV who received intravitreal aflibercept for 6 months.11 In the present study, we investigated ETDRS letter scores for a larger number of the treatment-naïve Japanese patients with PCV who were treated with intravitreal aflibercept every 2 months after 3 initial monthly doses, for 12 months. We also investigated the regression rate of polyps as a secondary outcome measure.
Subjects and methods
This was a prospective, nonrandomized, interventional exploratory clinical trial performed in an institutional setting (UMIN 10461). The study protocol was conducted according to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Yokohama City University Medical Center. All the participants provided written informed consent. Patients were recruited between April 2013 and June 2014. Patients were followed up for 12 months. The inclusion criteria were, 1) age ≥50 years with active subfoveal lesions; juxtafoveal lesions with leakage affecting the fovea were also allowed; 2) the presence of polypoidal lesions identified using indocyanine green angiography (ICGA: SPECTRALIS Product Family Version 5.3; Heidelberg Engineering Inc., Dossenheim, Germany); 3) the presence of dye leakage observed using fluorescein angiography (FA: SPECTRALIS Product Family Version 5.3); 4) the presence of subretinal and/or intraretinal exudative changes observed using optical coherence tomography (OCT: Cirrus HD-OCT model 5000; Carl Zeiss Meditec, Dublin, USA); and 5) best-corrected decimal visual acuity between 0.7 and 0.1 (20/29–20/200 Snellen equivalent). Patients with any prior treatment for PCV in the study eye were excluded. The eligibility of the patients was determined at the registration center.
Patients received intravitreal aflibercept (2 mg/0.05 mL) 4 times every 2 months after 3 initial monthly doses (2q8). Patients were examined before initiating the treatment, at the time of each visit on which they received an injection, and 1 month after the seventh injection. Routine ocular examinations, that is, decimal visual acuity test, tonometry, slit-lamp and fundoscopic examination, and OCT, were performed at each visit. A visual acuity test using the ETDRS chart, FA, and ICGA was performed at baseline, and at 6 and 12 months after the first treatment. Polyp resolution was judged based on the ICGA findings. Adverse events were recorded at each visit. The primary endpoint analysis was the proportion of patients losing <15 ETDRS letters in a per-protocol set (PPS). The secondary endpoint analyses were as follows: changes in the ETDRS letter scores, complete regression rate of polypoidal lesions confirmed by ICGA, changes in central retinal thickness (CRT), and the proportion of patients maintaining exudative changes, that is, intraretinal and/or subretinal fluid observed using OCT. The full analysis set (FAS) included all patients who received at least 1 injection and had a baseline and at least 1 post-baseline BCVA assessment. PPS included all patients in the FAS who, 1) had not missed 2 consecutive injections before the administration of the seventh injection (per patient), and 2) did not have major protocol violations. The last-observation-carried-forward (LOCF) approach was used to impute missing values.
IBM SPSS Statistics 22.0 (International Business Machines Corp, Armonk, NY, USA) was used for the statistical analysis. The 90% confidence interval (CI) was calculated for the proportion of patients who lost <15 ETDRS letters. McNemar’s chi-square test was used to evaluate the regression rate of the polypoidal lesions and the proportion of patients with exudation. A paired t-test was used to evaluate the changes in visual acuity and the CRT. A value of P<0.05 was considered statistically significant.
Results
Patient demographics and baseline characteristics
Twenty-two patients (22 eyes) were enrolled in this study. Three patients (13.6%) missed 2 consecutive injections and were withdrawn from the study. One patient in the PPS (19 patients) missed 1 injection of aflibercept, so the LOCF method was used. The patient demographics and baseline characteristics are shown in Table 1.
Table 1.
Patient demographics and baseline characteristics
| Sex, n (%) | |
| Male | 15 (68.2) |
| Female | 7 (31.8) |
| Age, years | |
| Mean (SD) | 62.5 (8.8) |
| Range | 52–87 |
| ≥75, n (%) | 10 (45.5) |
| <75, n (%) | 12 (54.5) |
| Systemic diseases, n (%) | |
| DM | 3 (13.6) |
| HTN | 7 (31.8) |
| HL | 3 (13.6) |
| Laterality, n (%) | |
| R | 11 (50.0) |
| L | 11 (50.0) |
| BCVA, ETDRS letters | |
| Mean (SD) | 64.7 (14.8) |
| Range | 42–86 |
| CRT, μm | |
| Mean (SD) | 360 (85) |
| Range | 218–528 |
Abbreviations: BCVA, best-corrected visual acuity; CRT, central retinal thickness; DM, diabetes mellitus; ETDRS, Early Treatment Diabetic Retinopathy Study; HL, hyperlipidemia; HTN, hypertension; L, left eye; R, right eye; SD, standard deviation.
Visual outcome
All the cases in the PPS lost <15 ETDRS letters at the 12-month examination (90% CI 0.854–1.000) (Figure 1; P<0.046, binomial one-sided test). The ETDRS letter scores were 64.1, 69.7, and 69.8 letters at baseline and at 6 and 12 months after the first injection, respectively (Figure 2). The visual acuity had improved significantly at 12 months (P<0.039, paired t-test).
Figure 1.
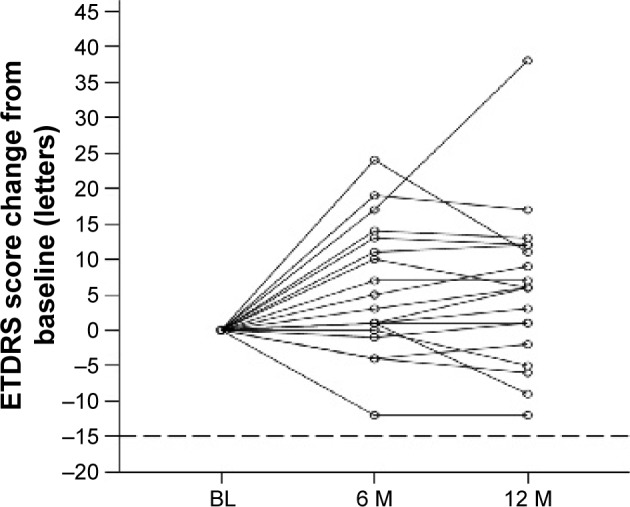
Changes in ETDRS letter scores from baseline.
Note: All the cases in the per-protocol set had lost <15 ETDRS letters at the 12-month examination (90% confidence interval, 0.854–1.000) (P<0.046, binomial one-sided test).
Abbreviations: BL, baseline; M, month(s); ETDRS, Early Treatment Diabetic Retinopathy Study.
Figure 2.
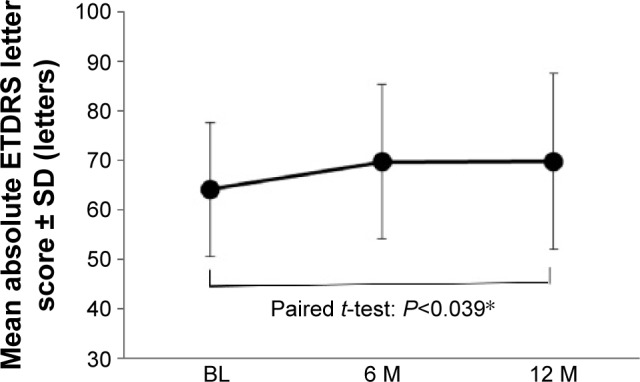
Mean absolute ETDRS letter score.
Note: The ETDRS letter scores were 64.1 and 69.8 letters at baseline and at 12 months, respectively (*P<0.039, paired t-test).
Abbreviations: BL, baseline; M, month(s); ETDRS, Early Treatment Diabetic Retinopathy Study; SD, standard deviation.
Polyp regression
Polyps were angiographically confirmed in 7 (37%) and 5 (26%) patients at 6 and 12 months, respectively. The proportion of the patients with polyps had significantly decreased at 6 and 12 months (Bonferroni-corrected P=0.0030 and 0.0010, respectively, McNemar’s chi-square test). Branching network vessels (BNV) were confirmed in all patients except 1 at baseline. The BNV persisted in all the patients who initially had BNV at 6 and 12 months.
OCT findings
CRTs (mean ± SD; µm) were 362±89, 260±84, 232±89, 231±76, and 226±70 at baseline and 1, 2, 6, and 12 months, respectively (Figure 3). The CRT was significantly reduced at 6 and 12 months (Bonferroni-corrected P<0.0001 and <0.0001, respectively; paired t-test). Thirteen (68%), 5 (26%), 5 (26%), and 2 (11%) patients showed exudative changes at 1, 2, 6, and 12 months, respectively. The proportion of patients with exudation was significantly reduced at 6 and 12 months (Bonferroni-corrected P=0.0010 and 0.0002, respectively; McNemar’s chi-square test).
Figure 3.
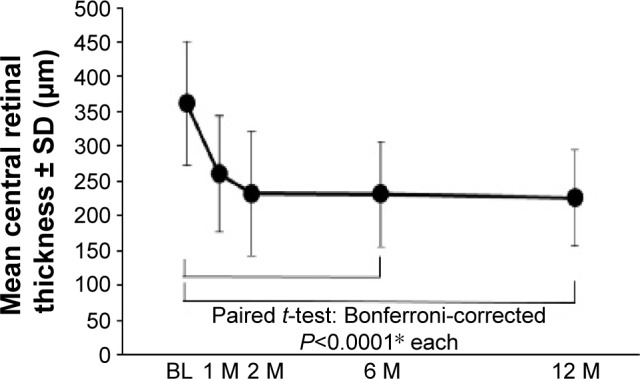
Mean central retinal thickness.
Note: The mean central retinal thickness decreased significantly at 6 and 12 months (*Bonferroni-corrected P<0.0001 and <0.0001, respectively; paired t-test).
Abbreviations: BL, baseline; M, month(s); SD, standard deviation.
Adverse events
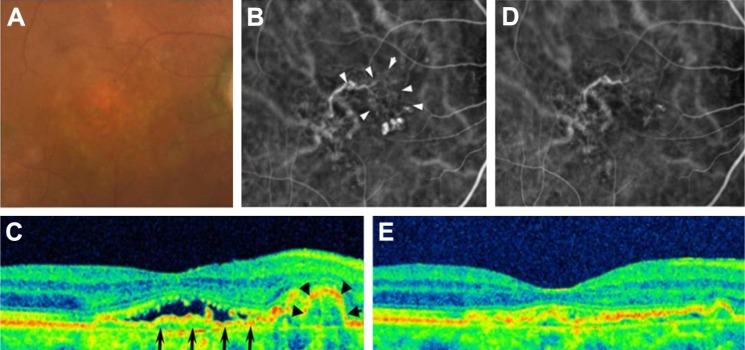
Cataract had progressed in 1 study eye at the 10-month visit. The ETDRS letter score of this patient had decreased by 12 letters at 12 months, although fundoscopic and OCT findings revealed no exudation. Severe adverse events, such as endophthalmitis or systemic arterial thromboembolitic events, did not occur in any of the patients during the study period. A representative case is shown in Figure 4.
Figure 4.
A representative case.
Notes: An 87-year-old man presented with reduced visual acuity in his right eye. His ETDRS letter score was 80 letters. The findings at presentation are shown in A, B, and C (A, fundus photograph; B, ICGA; and C, OCT). A mild color change in the macula is visible (A). Three polyps adjacent to the network vessel (arrowheads) are visible (B). A sharp RPE elevation (arrowheads) adjacent to a mild RPE elevation (arrows) with subretinal fluid is visible (C). The findings at 12 months of aflibercept therapy are shown in D and E (D, ICGA and E, OCT). All the polyps had disappeared, although the network vessels remained (D). A sharp RPE elevation and subretinal fluid resolved, though a mild irregularity of the RPE remained (E). The ETDRS letter score was 75 letters.
Abbreviations: ETDRS, Early Treatment Diabetic Retinopathy Study; ICGA, indocyanine green angiography; OCT, optical coherence tomography; RPE, retinal pigment epithelium.
Discussion
As our major concern was whether aflibercept was as effective for PCV as it is for AMD, we set the proportion of patients whose vision was maintained as the primary endpoint analysis, in accordance with VIEW 1 and 2 studies. In the present study, the proportion of patients with PCV whose vision was maintained at 12 months was 100%. This value tended to be greater in the present study than in the 2q8 group in VIEW 1 and 2 studies (which was ~95%). On the other hand, the mean ETDRS letter score change tended to be smaller in the present study (+5.7 letters in the present study vs +8.4 letters in the VIEW 1 and 2 2q8 arm). Several factors that influenced visual outcome during anti-VEGF therapy, such as age, baseline visual acuity, and choroidal neovascularization size, were reported. In general, patients presenting with good visual acuity at baseline were more likely to have good visual acuity after treatment, but the visual gain afforded by treatment was impacted by a ceiling effect.20 Considering that the baseline visual acuity was better than that in the VIEW 1 and 2 studies (55.7/51.6 letters in VIEW 1 and 2 2q8 arm vs 64.1 in the present study), our 2 results concerning visual acuity were compatible with the previously reported findings. If we limited the analysis to the 14 patients whose baseline ETDRS letter scores were between 73 and 25 (the same as the inclusion criteria for the VIEW 1 and 2 studies), the mean ETDRS letter score change at 12 months was +8.2, which was almost identical to that observed in the VIEW 1 and 2 2q8 arm. Taking the difference in the baseline visual acuity into consideration, the visual outcome in PCV patients who underwent 2q8 aflibercept therapy seemed comparable to that in AMD patients who underwent the same protocol.
On the other hand, the proportion of dry retina at 12 months tended to be greater in the present study than that in the VIEW 1/2 study (89% in the present study vs 63/72% in the VIEW 1 and 2 2q8 arm). For aflibercept therapy using the 2q8 regimen, the morphological improvement may be greater for patients with PCV than for those with AMD, though the reason for the difference is uncertain. Whether anti-VEGF therapy is equally effective for AMD and PCV remains controversial. Matsumiya et al21 reported that typical AMD patients showed greater vision improvement than PCV patients after 1 year of ranibizumab therapy. On the contrary, Oishi et al16 reported that visual acuity was better in PCV patients than in typical AMD patients after 12 months of aflibercept therapy. Yamamoto et al15 reported that an analysis of PCV patients with the same visual acuity inclusion criteria as those used in the VIEW 1 and 2 studies, and who were treated using the 2q8 aflibercept regimen for 1 year, exhibited a mean visual acuity change of +14.8 ETDRS letter score equivalent, which was greater than that observed in the VIEW 1 and 2 studies. More investigations are needed to determine whether the AMD subtype is associated with the efficacy of aflibercept therapy.
The complete polyp regression rate after 1 year of treatment was 74% in the present study. This rate was 55%–69% in other reports,15,16 in which PCV patients underwent treatment using the 2q8 aflibercept regimen. Baseline visual acuity in these reports was equivalent to 70–75 ETDRS letter scores. The rate of complete polyp regression tended to be greater and the baseline visual acuity tended to be lower in the present study than in these previous studies. As the study design (prospective vs retrospective) and the method used to assess visual acuity (ETDRS letter score vs logMAR unit) differed between our study and others, whether a lower baseline visual acuity is associated with a greater polyp regression rate remains uncertain. Further investigations are needed to clarify which factors affect the polyp regression rate. At present, the polyp regression rate after 1 year of aflibercept therapy seems to be superior to that after ranibizumab therapy (21%–29%)5,13 and comparable to that after PDT monotherapy (71%)5 and PDT combined with anti-VEGF agents (55%–85%).22,23 As PDT sometimes induces severe adverse events such as massive subretinal hemorrhage,24 aflibercept therapy seems to be preferable for the treatment of PCV patients.
One eye showed the progression of cataract and a loss of visual acuity. A slit-lamp examination revealed no mechanical injury of the crystalline lens except for the progression of nuclear sclerosis. We speculated that the crystalline lens of the patient incidentally developed a relatively rapid senile change during aflibercept therapy. In addition to the patient with cataract progression, 4 patients exhibited visual loss of 2–9 letters. Two out of the 4 patients exhibited no polyp on ICGA and no exudative changes on FA and OCT, 1 patient exhibited dye leakage on FA with polyp, and other 1 patient exhibited dye leakage on FA without polyp appearance.
As the recruitment period in this study partly coincided with that in our previous study,11 10 patients met inclusion criteria of both studies and were studied in both studies. In evaluating these 10 patients during the 6-month study period, we used the same images of FA, OCT, and ICGA in the 2 studies, although we evaluated these images separately in each study. We recognized that such a procedure was unusual. However, we chose to apply it to recruit as large number of patients as possible. We assume that the procedure did not affect the results of the present study. Protocol of the present study was separate from our previous study, that is, recruitment period, inclusion criterion of baseline visual acuity, method of visual acuity test, follow-up period, and statistical method were different. We assume that the present study was an independent clinical trial.
Results at 6 months in the present study were compared with results in our previous study. Visual acuity gain was 0.1 logMAR unit (5.0 ETDRS letter score equivalent) and 5.6 ETDRS letter score in our previous and present study, respectively. The rate of polyp regression was 75% and 63% in our previous and present study, respectively. Visual acuity gain and the rate of polyp regression were comparable in the 2 studies.
The present study had an important limitation. As the patients were recruited at a single institute, the sample size was relatively small. A study using the same protocol but with a large number of patients is needed to elucidate the efficacy of aflibercept for the treatment of PCV.
In conclusion, the administration of intravitreal aflibercept every 2 months after 3 initial monthly doses in treatment-naïve patients with PCV improved their visual acuity. A comparison of visual acuity between the present study and the VIEW 1 and 2 2q8 arm revealed that the efficacy of aflibercept for the treatment of PCV was comparable to that for the treatment of AMD. Using the ETDRS letter score enabled us to compare the visual outcomes of our study and the VIEW 1 and 2 2q8 arm directly.
Acknowledgments
This study was supported by funding from Bayer Yakuhin, Osaka, Japan.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV) Retina. 1990;10(1):1–8. [PubMed] [Google Scholar]
- 2.Yannuzzi LA, Ciardella A, Spaide RF, Rabb M, Freund KB, Orlock DA. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 1997;115(4):478–485. doi: 10.1001/archopht.1997.01100150480005. [DOI] [PubMed] [Google Scholar]
- 3.Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007;144(1):15–22. doi: 10.1016/j.ajo.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 4.Oishi A, Miyamoto N, Mandai M, et al. LAPTOP study: a 24-month trial of verteporfin versus ranibizumab for polypoidal choroidal vasculopathy. Ophthalmology. 2014;121(5):1151–1152. doi: 10.1016/j.ophtha.2013.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32(8):1453–1464. doi: 10.1097/IAE.0b013e31824f91e8. [DOI] [PubMed] [Google Scholar]
- 6.Lai TY, Lee GK, Luk FO, Lam DS. Intravitreal ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy. Retina. 2011;31(8):1581–1588. doi: 10.1097/IAE.0b013e31820d3f3f. [DOI] [PubMed] [Google Scholar]
- 7.Lai TY, Chan WM, Liu DT, Luk FO, Lam DS. Intravitreal bevacizumab (Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2008;92(5):661–666. doi: 10.1136/bjo.2007.135103. [DOI] [PubMed] [Google Scholar]
- 8.Economides AN, Carpenter LR, Rudge JS, et al. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat Med. 2003;9(1):47–52. doi: 10.1038/nm811. [DOI] [PubMed] [Google Scholar]
- 9.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heier JS, Brown DM, Chong V, et al. for VIEW 1 and VIEW 2 Study Groups Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, Yamane S, Taoka R, Arakawa A, Kadonosono K. Aflibercept for polypoidal choroidal vasculopathy: as needed versus fixed interval dosing. Retina. 2015;36(8):1527–1534. doi: 10.1097/IAE.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 12.Hara C, Sawa M, Sayanagi K, Nishida K. One-year results of intravitreal aflibercept for polypoidal choroidal vasuculopathy. Retina. 2016;36(1):37–45. doi: 10.1097/IAE.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 13.Cho HJ, Kim KM, Kim HS, et al. Intravitreal aflibercept and ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2016;165:1–6. doi: 10.1016/j.ajo.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Hosokawa M, Shiraga F, Yamashita A, et al. Six-month results of intravitreal aflibercept injections for patients with polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015;99(8):1087–1091. doi: 10.1136/bjophthalmol-2014-305275. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto A, Okada AA, Kano M, et al. One-year results of intravitreal aflibercept for polypoidal choroidal vasculopathy. Ophthalmology. 2015;122(9):1866–1872. doi: 10.1016/j.ophtha.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Oishi A, Tsujikawa A, Yamashiro K, et al. One-year result of aflibercept treatment on age-related macular degeneration and predictive factors for visual outcome. Am J Ophthalmol. 2015;159(5):853–860.e1. doi: 10.1016/j.ajo.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Koizumi H, Kano M, Yamamoto A, et al. Aflibercept therapy for polypoidal choroidal vasculopathy: short-term results of a multicentre study. Br J Ophthalmol. 2015;99(9):1284–1288. doi: 10.1136/bjophthalmol-2014-306432. [DOI] [PubMed] [Google Scholar]
- 18.Ijiri S, Sugiyama K. Short-term efficacy of intravitreal aflibercept for patients with treatment-naive polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253(3):351–357. doi: 10.1007/s00417-014-2707-2. [DOI] [PubMed] [Google Scholar]
- 19.Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing Snellen visual acuity measurements. Retina. 2010;30(7):1046–1050. doi: 10.1097/IAE.0b013e3181d87e04. [DOI] [PubMed] [Google Scholar]
- 20.Finger RP, Wickremasinghe SS, Baird PN, Guymer RH. Predictors of anti-VEGF treatment response in neovascular age-related macular degeneration. Surv Ophthalmol. 2014;59(1):1–18. doi: 10.1016/j.survophthal.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Matsumiya W, Honda S, Kusuhara S, Tsukahara Y, Negi A. Effectiveness of intravitreal ranibizumab in exudative age-related macular degeneration (AMD): comparison between typical neovascular AMD and polypoidal choroidal vasculopathy over a 1 year follow-up. BMC Ophthalmol. 2013;13:10. doi: 10.1186/1471-2415-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang HM, Koh HJ, Lee CS, Lee SC. Combined photodynamic therapy with intravitreal bevacizumab injections for polypoidal choroidal vasculopathy: long-term visual outcome. Am J Ophthalmol. 2014;157(3):598–606.el. doi: 10.1016/j.ajo.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Gomi F, Oshima Y, Mori R, et al. Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy. Retina. 2015;35(8):1569–1576. doi: 10.1097/IAE.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 24.Hirami Y, Tsujikawa A, Otani A, et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2007;27(3):335–341. doi: 10.1097/01.iae.0000233647.78726.46. [DOI] [PubMed] [Google Scholar]