Abstract
According to the recent definition proposed by the Consensus conference on Acute Dialysis Quality Initiative Group, the term cardio-renal syndrome (CRS) has been used to define different clinical conditions in which heart and kidney dysfunction overlap.
Type 1 CRS (acute cardio- renal syndrome) is characterized by acute worsening of cardiac function leading to AKI (5, 6) in the setting of active cardiac disease such as ADHF, while type – 2 CRS occurs in a setting of chronic heart disease.
Type 3 CRS is closely link to acute kidney injury (AKI), while type 4 represent cardiovascular involvement in chronic kidney disese (CKD) patients.
Type 5 CRS represent cardiac and renal involvement in several diseases such as sepsis, hepato – renal syndrome and immune – mediated diseases.
Keywords: Cardiorenal syndrome, Heart failure, Acute kidney injury, Chronic kidney disease, Sepsis
1. Background
According to the recent definition proposed by the Consensus conference on Acute Dialysis Quality Initiative Group,1 the term cardio-renal syndrome (CRS) has been used to define different clinical conditions in which heart and kidney dysfunction overlap. RS complexity needs to be explained starting by its pathogenesis and this is the aim of the following chapter.
The classification of CRS proposed in the Consensus Conference by the Acute Dialysis Quality Group essentially divides CRS in two main groups, cardio-renal and reno-cardiac CRS, on the basis of the primum movens of disease (cardiac or renal).Both cardio-renal and reno- cardiac CRS are then divided into acute and chronic, according to disease’s acuity of onset. Type 5 of CRS integrates simultaneous cardio- renal involvement induced by systemic disease (Table 1). In the following chapters it will be pointed up all novel approaches on CRS pathophysiological pathways mainly focused on immunologic and biometabolic new findings.
Table 1.
Classification of cardio-renal syndrome.
| Type | Denomination | Description | Example |
|---|---|---|---|
| 1 | Acute cardiorenal | Heart failure leading to AKI | Acute coronary syndrome leading to acute heart and kidney failure |
| 2 | Chronic cardiorenal | Chronic heart failure leading to CKD | Chronic heart failure |
| 3 | Acute nephrocardiac | AKD leading to acute heart failure | AKI related uremic |
| 4 | Chronic nephrocardiac | CKD leading to heart failure | Left ventricular hypertrophy and diastolic heart failure due to CKD |
| 5 | Secondary | Systemic disease leading to heart and kidney failure | Sepsis, vasculitis, diabetes mellitus, amyloidosis |
2. Type 1 cardio renal syndrome
Type 1 CRS occurs in about 25% of patients hospitalized for acute decompensated heart failure (ADHF).2, 3 Among these patients, underlying chronic kidney disease (CKD) is quite common and contributes to acute kidney injury (AKI) in 60% of all cases studied. AKI is an independent mortality risk factor in acute decompensated heart failure patients, including those with acute myocardial infarction (AMI) and/or reduced left ventricular ejection fraction.4
2.1. Pathophysiology
Type 1 CRS (acute cardio- renal syndrome) is characterized by acute worsening of cardiac function leading to AKI5, 6 in the setting of active cardiac disease such as ADHF. Preliminary observations highlight the importance of timing in the development of AKI and its early diagnosis (Fig. 1).
Fig. 1.
Timing of acute kidney injury in the setting of acute decompensated heart failure.
Hemodynamic mechanisms play a major role in type 1 CRS in presence of ADHF leading to decreased renal arterial flow and a consequent fall in glomerular filtration rate(GFR). Once hemodynamics have been restored, renal and cardiac parameters come back to normal.7 Different hemodynamic profiles have been proposed8: in “cold” pattern patients, reduction in effective circulation fluid volume (ECFV) represents the main hemodynamic change, while there is a marked increase in central venous pressure (CVP) in “wet” pattern patients.
“Cold” patients also present with decrease in renal blood flow related to the renin angiotensin-aldosterone system (RAAS) and systemic nervous system activation causing afferent vasoconstriction, decreased renal blood flow and decreased effective glomerular perfusion pressure. Patients who present with a “wet” hemodynamic profile display increased pulmonary and/or systemic congestion. In these patients, high CVP directly affects renal vein and kidney perfusion pressure9, 10; CVP increase also results in increased interstitial pressure with tubular’ collapse and progressive decline in GFR.11
“Warm and wet” patients represent the most frequent profile in acute and chronic advanced heart failure.12, 13 Mechanisms of increased CVP are quite similar to “cold” profile patients, but renal perfusion pressure is less affected because of higher arterial blood pressures.9
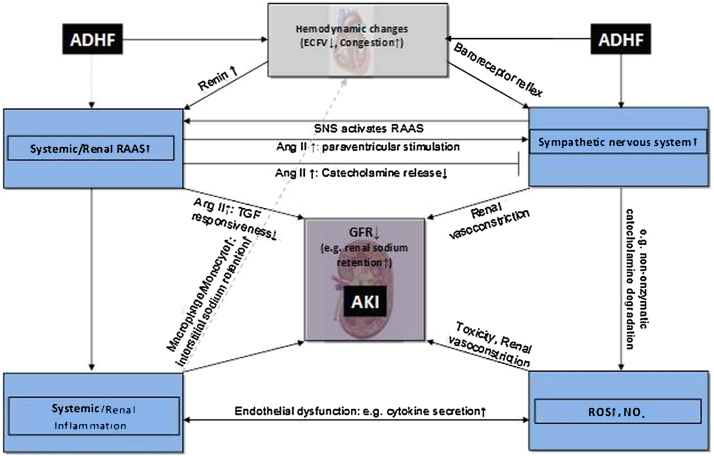
Non-hemodynamic mechanisms were also proposed as involved in type 1 CRS including sympathetic nervous system (SNS) and RAAS activation, chronic inflammation and imbalance in the proportion of reactive oxygen species (ROS)/nitric oxide (NO) production (Fig. 2). Patients with ADHF show more frequently defective regulation of monocyte apoptosis and activation of inflammatory pathways compared with healthy subjects.14, 15
Fig. 2.
Non-hemodynamic network of pathophysiological interactions in CRS type 1. Note the emerging potential role of macrophages/monocytes as mediator of sodium and fluid retention. Reproduced with permission from ADQI.
Several pathophysiological processes contribute to perpetuating AKI, including endothelial and epithelial cell death, and a primary role for apoptotic mechanisms due to renal ischemia, toxic injury, radiation and ureteral obstruction have been suggested in experimental models.16
Renal tubular epithelium is particularly vulnerable to ischemic injury resulting in cell death by apoptosis and necrosis with consequent loss of r epithelial cell structure and function.17 Renal tubular cells represent major site of cell damage during AKI with strong association between intra-renal inflammatory activity and renal cell apoptosis.18
Sera from type 1 CRS patients’ show high levels of proinflammatory cytokines and proapoptotic factors19 There are two main intracellular pathways for apoptosis (intrinsic and extrinsic), characterized by activation of different activator caspases19 and linked by caspase-3.19 Cleavage of caspase-3 and its activation causes DNA fragmentation, demolition of cellular cytoskeletal and nuclear proteins and consequent formation of apoptotic bodies.19, 20 Fragmentation of renal tubular cells genomic DNA represent a biochemical hallmark of apoptosis, an irreversible process leading to cell’s death.20 The final pathway of apoptotic process is characterized by phagocytosis of apoptotic bodies.20
Oxidative stress is a hallmark of type 1 CRS, as evidenced by a significant increase in circulating reactive oxygen species (ROS) and reactive nitrogen species (RNS), coupled with increased expression of interleukin-6 (IL-6). Increased levels of NADPH oxidase and myeloperoxydase (MPO), with upregulation of proinflammatory mediators via powerful oxidants such as peroxynitrite have also been recently demonstrated.21
MPO acts as primary enzyme in ROS generation by promoting hydrogen peroxide (H2O2) conversion into nitrogen dioxide (NO2) and other species involved in oxidative damage of several critical compounds (lipids, lipoproteins) implicated in the pathogenesis of atherosclerosis, cancer, diabetic vasculopathy and CKD.21, 22 Gut under-perfusion and endotoxin release in patients with ADHF have also been proposed as pathophysiologic mechanisms accelerating progression of HF and CRS.23
3. Type 2 cardio-renal syndrome
Type 2 Cardiorenal syndrome is characterized by chronic abnormalities in cardiac function leading to kidney injury or dysfunction. Chronic heart and kidney disease often coexist but large cohort studies assess the onset of one disease (e.g. chronic heart failure [HF]) subsequently describing the prevalence of the other (chronic kidney disease [CKD]).24, 25 CKD has been observed in 45-63% of CHF patients24, 25, 26 but it’s unclear how to classify these patients often including those ones shifting from a clinical condition of Type 1 CRS. to recognize these patients from Type 4 CRS (chronic reno-cardiac syndrome).27
3.1. Pathophysiology
Intrinsic to its definition, type-2 CRS is characterized by CKD onset in HF patients, but two fundamental features are proposed: CHF and CKD are to be simultaneously present and CHF causally underlies CKD occurrence or progression.28 Examples of type-2 CRS can be provided by “cyanotic nephropathy” occurring in patients with congenital heart disease, when heart disease clearly precedes kidney involvement or acute coronary syndrome leading to left ventricular dysfunction and onset/progression of co-existing CKD. Neuro-hormonal activation, renal hypoperfusion and venous congestion, inflammation, atherosclerosis and oxidative stress represent most important pathophysiological mechanisms of type 2 CRS. These mechanisms are operative in recurrent episodes of acute heart and/or kidney decompensation, which are associated with HF and CKD progression.29
In experimental studies, a reduction in glomerular plasma flow together with elevated intra-glomerular filtration pressure(efferent arteriolar constriction) is observed; if these changes persist (up to six months in experimental models) podocytes injury, focal and segmental glomerulosclerosis can occur, often related to local renal increase in sympathetic nervous system and RAAS activation.30
Kidneys of HF patients seems to release large amounts of circulating renin with consequent abnormal angiotensin II production, resulting in efferent arteriolar constriction and increase in oncotic pressure of peritubular capillaries.31 High venous pressure is described as an key factor in worsening GFR in HF patients, especially in those with preserved ejection fraction. Patients with decompoensated heart failure and venous congestion often have t with significant RAAS activation without decreased circulating volume as stimulus.32 Persistent RAAS and SNS activation could contribute to CKD progression in type 2 cardio renal syndrome.
Angiotensin II production and aldosterone release lead to distal nephron augmented sodium reabsorption and subsequent systemic pressure and volume overload. Increased aldosterone levels can also contribute to glomerular fibrosis due to up-regulation of transforming growth factor-β (TGF-β) and increased secretion of fibronectin.33, 34
Persistent inflammation triggered by ongoing cardiac decompensation is also responsible for CKD progression in ADHF.35
4. Type-3 cardiorenal syndrome
Type 3 cardio renal syndrome, also defined as acute reno-cardiac syndrome, occurs when acute kidney injury (AKI) contributes and/or precipitates development of acute cardiac injury. AKI may directly or indirectly produce an acute cardiac event; triggered by the inflammatory surge, oxidative stress and secretion of neurohormones following AKI.36, 37 Other triggers for cardiac injury and dysfunction include AKI related volume overload, metabolic acidosis and electrolytes disorders such as hyperkalemia and hypocalcemia. Acute, left ventricular dysfunction and accelerated fibrosis have been also described in patients with AKI.38 Lastly, AKI can affect cardiac function contributing to alterations in drug pharmacokinetics and dynamics (such as excretion of digoxin).
4.1. Pathophysiology
4.1.1. Direct AKI effects on heart function
Pathophysiological interactions between kidney and heart in AKI have been referred to “cardio-renal connectors”,39 l which include immune modulation (pro- and anti-inflammatory cytokines and chemokines release) and sympathetic nervous systems and RAAS hyperactivity, and activation of the coagulation cascade.
Circulating levels of tumor necrosis factor-alpha (TNFα), interleukin-1 (IL-1) and interleukin-6 (IL-6) seems to increase immediately after renal experimental ischemia and, together with other cytokines as well as and interferon- alfa (IFN-α), have direct cardio-depressant effects, such as reduction in left ventricular ejection fraction and elevation of left ventricular end diastolic and systolic volumes and areas.40, 41 Cytokines release can affect myocardial cells directly on their contractility or by close interactions with extracellular matrix leading to negative inotropic effects Cellular mechanisms involve secondary mediators such as sphingolipids, arachidonic acid and intracellular Ca2+ alterations.42
In animal models, infusion of TNF-α results in decrease of left ventricular diastolic pressure with secondary coronary vasoconstriction. Infusions cause time-dependent dysfunction (regional contractility alterations) of left ventricle and its dilation lasting up to 10 days.42 Several diastolic abnormalities are also observed, including slow relaxation of left ventricle and raised left atrium filling pressure to indicate an increase in left ventricle diastolic stiffness.43 In presence of renal ischemia, rat hearts show increased expression of adhesion molecules such as ICAM-1 together with myocardial apoptosis (this is not true in case of bilateral nephrectomy) to prove that systemic inflammation, and not AKI, plays an immediate role in myocardial damage and dysfunction.44 In animal experiments it has been shown that left ventricular dilation, increased left ventricular end diastolic and end systolic diameters, increased relaxation time and decreased fractional shortening can occur 48 h after renal injury.45
Hyperactivity of the SNS with abnormal secretion of norepinephrine impairs myocardial activity in several ways: direct norepinephrine effect, impairment in Ca2+ metabolism, increase in myocardial oxygen demand with potential evolution to myocardial ischemia, myocardial cells β1-adrenergic mediated apoptosis, stimulation of α1 receptors and, finally, activation of RAAS. Abnormal and uncontrolled RAAS activation leads to angiotensin II release with consequent systemic vasoconstriction and elevation of vascular resistance; in addition, angiotensin II itself directly promotes cellular hypertrophy and apoptosis.46 Increased RAAS activity could be accountable for diminished coronary response to adenosine, bradykinin and l-arginine.47 Other animal models exemplify how the inflammatory cascade of AKI can contribute to altered permeability of lung vessels, with resultant interstitial edema and micro-hemorrhage mediated by inflammatory mediators and altered expression of epithelial sodium channel and aquaporin-5.48
Myocardial cells apoptosis and neutrophil activation greatly contribute to the pathophysiologic pathways of cardiac injury following AKI, leading to lethal major cardiac events as can be seen in rat transgenic models.49 Cardiac myocyte apoptosis and neutrophil infiltration represent two of the most important contributors to the pathophysiology of myocardial infarction during AKI.50 The cardio-renal link between AKI and cardiac fibrosis is shown with the upregulation of beta-galactoside-binding lectin galectin-3, mRNA expression renal ischemia.It is also implicated in the development of myocardial fibrosis and heart failure in AKI, and its inhibition can delay progression of myocardial fibrosis.51
4.1.2. Indirect effects of AKI on heart function
As renal function declines, it can result in significant pathphysiological derangement, leading to cardiac injury. Oliguria can lead to sodium and water retention with consequent fluid overload and development of volume overload, hypertension, pulmonary edema and myocardial injury. Electrolytes imbalances (primarily hyperkalemia), can contribute to raised risk of fatal arrhythmias and sudden death. Acidemia also can worsen pulmonary vasoconstriction, increased right ventricular after load and contribute to a negative cardiac inotropic effect. Finally uremia itself can directly affect myocardial cells contractility through myocardial depressant factors and promoting pericardial effusions and pericarditis.52, 53
In response to systemic and renal hemodynamic changements, baroceptor and intrarenal chemoceptors lead to SNS and RAAS activation as described previously SNS activation directly affects intrarenal hemodynamics and stimulates renin incretion, and also causes cardiomyocyte apoptosis. Neuropeptide Y, a vascular growth factor accountable for neointimal formation and following vasoconstriction is also stimulated by RAAS activation.54, 55
4.1.3. Electrophysiological effects
Classical ECG changes in hyperkalemic patients are represented by tenting of T wave due to rapid and consistent elevation in extracellular potassium levels, leading to increased activity of potassium channel (and inactivation of sodium channel) with faster repolarization and predisposition to arrhythmias.56 Hyperkalemia reduces resting membrane potentials (both atrial and ventricular) and induces ST-T segment abnormalities (i.e. elevations in V1 and V2) simulating an ischemic pattern. In some patients, hyperkalemia can simulate a Brugada-like pattern, characterized by right bundle branch block and persistent ST-T segment elevation.56
5. Type-4 cardio renal syndrome
Type-4 CRS, also defined as chronic reno-cardiac disease, is characterized by cardiovascular involvement in patients affected by chronic kidney disease at any stage according to National Kidney Foundation (NKF) classification.It’s well established that renal dysfunction is an independent risk factor for cardiovascular disease with higher mortality risk for myocardial infection and sudden death in CKD.57
5.1. Pathophysiology
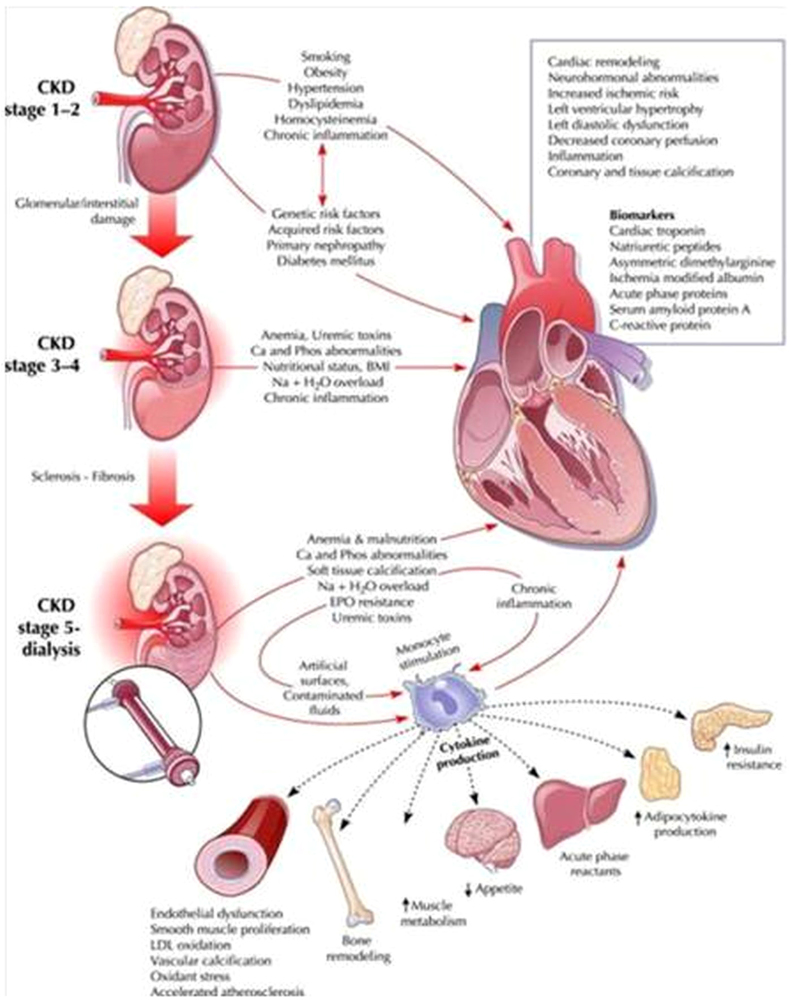
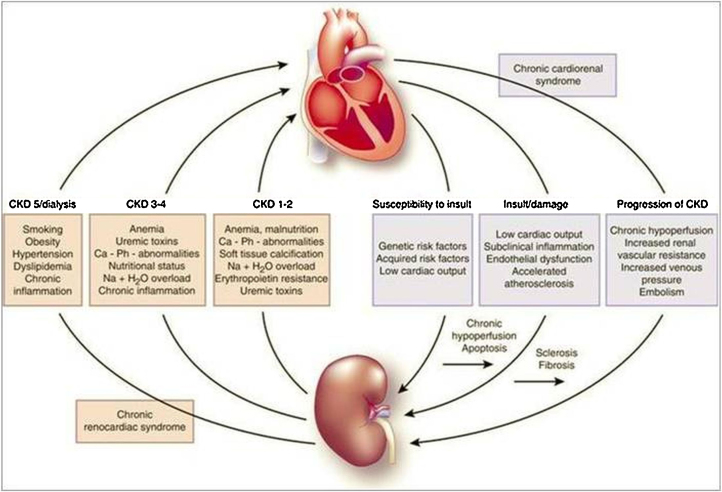
Fig. 3, Fig. 4 show close interactions between chronic kidney disease (CKD) and cardiovascular involvement. Chronic kidney disease independently accelerates ischemic heart disease and contributes to pressure and volume overload, leading to left ventricular hypertrophy.58
Fig. 3.
Pathophysiological pathways of type-4 cardiorenal syndrome. It has been highlighted the role of uremia in developing minor and major cardiovascular complications referring to main CKD-related cardiovascular risk factors such as secondary hyperparathyroidism, anemia, accelerated atherosclerosis and chronic inflammation.
Fig. 4.
Clinical correlation between kidney and heart disease. This is a summary of close relationship between renal failure main features and equivalent heart involvement with particular focus on uremia effects on systolic and diastolic left ventricular function.
Left ventricular hypertrophy (LVH) highly prevalent in patients starting hemodialysis. Pressure overload leading to LVH results from hypertension and calcific valvular disease as early as CKD-2, but is particularly prevalent in hemodialysis and pre-dialysis patients.59, 60 Hyperphosphatemia and secondary hyperparathyroidism can produce ossification of cardiac vessels and valves because of “osteoblastic” transformation of vascular smooth muscle cells.61 Congestive heart failure is exacerbated by volume overload central to CKD with underlying anemia of chronic disease and the presence of hemodialysis arteriovenous fistulae being common contributing factors.62, 63
Chronic inflammation, insulin resistance, hyperhomocysteinemia and malnutrition-inflammation associated dyslipidemiacan also contribute to accelerated cardiovascular disease in CKD. as GFR declines, gradual accumulation of a spectrum of toxins (β2 microglobulin, guanidines, phenols, indoles, aliphatic amines, furans, polyols, nucleosides, leptin, serum amyloid A protein, asymmetric dimethyl arginine, parathyroid hormone and erythropoiesis inhibitors) can occur,64, 65, 66 which contribute to the inflammatory milieu of progressive CKD. B-type natriuretic peptide (BNP) and related N-terminal pro BNP (NT-proBNP) are both elevated in CKD patients compared to age and sex matched cohorts wirth preserved renal function, reflecting myocardial cells injury due to hypertension, volume overload, LVH, cardiac remodelling and fibrosis.67, 68
5.1.1. Congestive heart failure and left ventricular hypertropphy
Echocardiographic abnormalities (impairment of ejection fraction, increased end systolic and end disatolic left ventricular diameter and volume) are frequently reported since early stages of CKD to ESKD. Incident dialysis patients show higher rates of systolic dysfunction (15%), LVH (74%) and left ventricular dilation (36%).69, 70 Pathophysiological mechanisms proposed includes pressure and volume overload in parallel with progressive GFR decline. Pressure overload is also execerbated by co-existing hypertension, valvular heart disease (accelerated by secondary hyperparathyroidism) and impaired vascular compliance. Consequent increase in cardiac workload leads to compensatory hypertrophy and excessive myocardial cells stress relative to increased oxygen demand resulting in myocyte fibrosis and death, cardiac chamber dilation and systolic dysfunction.70
Fibroblast growth factor-23 (FGF-23), member of fibroblast growth factor family (implicated in regulation, growth and differentiation of cardiac myocytes) has paracrine functions in kidneys because of its phosphaturic properties blocking vitamin D3 synthesis.71 During CKD progression, accumulation of phosphate leads to increase in FGF-23 secretion that promotes LVH and cardiac remodeling. Echocardiographic assays demonstrated a 5% LVMI (lef ventricular mass imdex) rise for every log increase in plasma FGF-23 levels.72
5.1.2. Cardiac arrhythmias and sudden cardiac death
CKD patients, especially those on hemodialysis, are more prone to develop arrhythmias, especially atrial fibrillation and ventricular tachyarrhythmias. Significant shifts of electrolytes and blood pressures/volumes levels are common in intra and inter-dialytic periods leading to myocardial cells mechanical (regional wall motion abnormalities) and arrythmogenic potential.73 Almost half of cardiovascular deaths in end-stage kidney disease population are related to cardiac arrhythmia or sudden death.73 Increased risk for sudden death seems to be particularly related to longer dialytic intervals in subjects undergoing thrice weekly hemodialysis treatment,because of extreme shifts of electrolytes and fluids.93 In the non dialysis CKD population, a 1.11 hazard ratio for sudden cardiac death exists for every 10 ml/min/1.73 m2 fall in GFR.74
Atrial fibrillation is a common arrhythmia in the CKD/ESKD population. In the Chronic Renal Insufficiency Cohort (CRIC) study, an 18% prevalence of atrial fibrillation was found.75 The incidence of atrial fibrillation (ECG-detected) correlates with the degree of CKD with a 4–5% prevalence in stage 4–5 CKD patients. After multivariate analysis, the odds ratios for ECG- defined atrial fibrillation were 2.20 in CKD stage 1–2 patients, 1.51 in CKD 3, and 2.86 in CKD 4–5 patients respectively, compared to control subjects with normal renal function,75 The burden of atrial fibrillation is complicated by the increased hemorrhagic risk in this population from anticoagulation.75
5.1.3. Coronary atherosclerotic heart disease
CKD patients present higher prevalence of coronary artery disease at angiographic evaluation with multivessel disease and ECG evidence of previous ischemia.76
Conchol et al. assessed CAD prevalence in early stages of CKD with coronary catheterization procedures in 261 patients with GFR between 30 and 90 ml/min More than half the patients with GFR <90 mls/min/1.73 m2 had a 70% stenosis in at least one coronary artery, and more than 84% patients with GFR < 30 ml/min/1.73 m2 showed significant CAD mainly involving the left coronary arterial territory.77
5.1.4. Uremia and cardiac fibrosis
End-stage CKD patients develop cardiac fibrosis similar to hypertensive and chronic ischemic heart disease patients in which endocardial and epicardial fibrosis predominate.78 Uremic toxins such as indoxyl sulfate and p-cresol can contribute to cardiac fibrosis in CKD patients. Indoxyl sulfate concentrations are 300 fold higher than control population and it directly contributes to cardiac fibrosis by synthesis of TGF-β, tissue inhibitor of metalloproteinase-1 (TIMP-1) and alpha-1 collagen.79, 80
Recent evidence shows up-regulation of galectin-3, a member of the β-galactoside-binding lectin family synthesized by macrophages, which interacts with extracellular matrix protein like laminin, synexin and integrins. Galectin-3 can bind to cardiac fibroblasts increasing collagen production in the myocardium. Lok et al. enrolled 232 stage 3–4 CKD patients and demonstrated that galectin-3 levels were independent predictors of cardiovascular mortality.80
6. Type-5 cardio renal syndrome
Type-5 CRS is a recently defined clinical syndrome and complete epidemiological data on this entity are still incomplete. Type-5 CRS occurs when cardiac and renal injury occur simultaneously. encompassing many clinical syndromes such as sepsis, and drug toxicity where heart and kidney are involved secondary to a common underlying pathological trigger.1
6.1. Pathophysiology
The pathophysiology of CRS-5 depends on the underlying disease. Acute CRS-5 results from systemic processes e.g. sepsis, infections, drugs, toxins and connective tissue disorders such as lupus, Wegener’s granulomatosis, and sarcoidosis. The temporal course of the development of CRS 5 is variable. For example, in sepsis induced acute CRS-5, there is a fulminant disease process with an acute impact on both the kidney and the heart, with obvious clinical manifestations. On the other hand in cirrhosis, CRS-5 has a more insidious onset and the kidney and cardiac dysfunction may develop slowly, until a crucial point is reached and full decompensation occurs.
Acute CRS-5 develops into four following steps and it can be hyper-acute (0–72 h after diagnosis), acute (3–7 days), sub-acute (7–30 days) and chronic (over 30 days) (Table 2).
Table 2.
Temporal considerations in pathophysiology of CRS-5.
| Attribute | CRS5 Acute (Sepsis) (Fig. 1) | CRS5 Chronic (Cirrhosis) (Fig. 2) |
|---|---|---|
| Time for organ dysfunction | Short: hours to days | Long: weeks to months |
| Underlying organ function | May be superimposed on underlying cardiac and kidney disease | Heart and kidney have adaptive responses that fail over time |
| Sequence of organ involvement | Generally simultaneous or in close proximity to each other | One organ precedes the other e.g. cardiac dysfunction precedes renal in cirrhosis |
| Underlying disease | Systemic event contributes to CRS5 | Precipitating events can transition to an acute deterioration in CRS5 e.g. GI bleed can precipitate hepatorenal syndrome |
| Pathophysiology | Direct effects on organs | Failure of adaptive responses over time |
| Mechanisms | Determined by underlying disease | Determined by adaptive changes |
| Reversibility | Possible with control of sepsis and organ support | Limited unless there is replacement of diseased organ e.g. liver transplant |
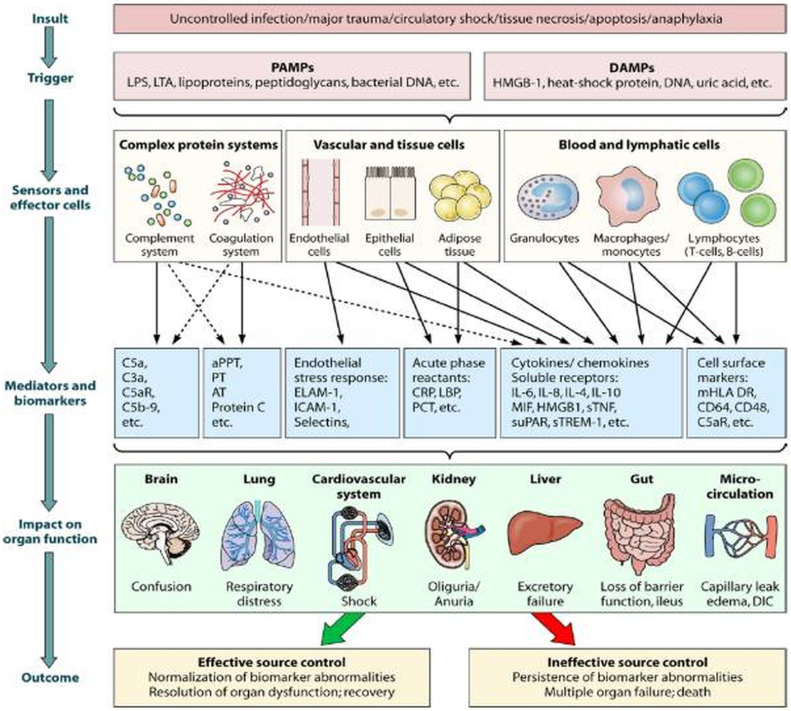
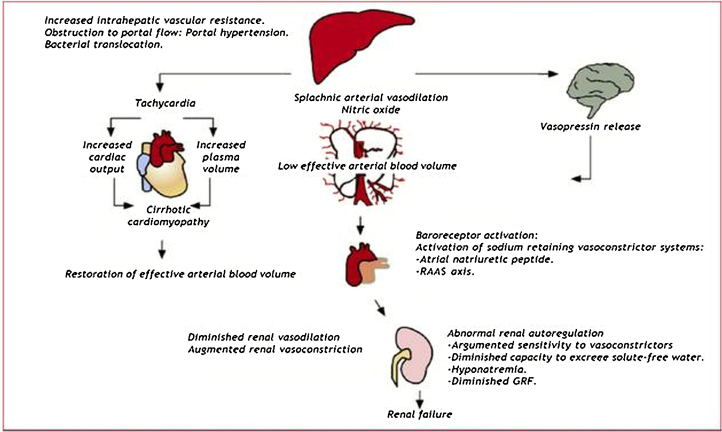
Chronic CRS-5 (i.e. CRS in cirrhotic patients) presents time sequence quite variable because in most cases of CRS-5 there is an underlying condition and related precipitating event leading to attention. For instance, cirrhotic patients are subject to infections and an acute CRS-5 can overlap a chronic process. Krag et al. described the cardiorenal link in advance cirrhotic patients underlying how these patients, together with splancnic vasodilation and activation of vasoconstrictive systems, present an impaired contractile responsiveness to stress and altered diastolic relaxation. Altered diastolic function could be accountable as a major determinant of kidney function and survival in cirrhotic patients.81 The mechanisms invoked in acute and chronic forms of CRS-5 are described in Fig. 5, Fig. 6.
Fig. 5.
Pathophysiology of sepsis induced organ dysfunction. It has been focused attention on immunologic pathways leading to toxic damage on target organs since complement and coagulation cascade activation and endothelial and epitelial damage.
Fig. 6.
Pathophysiology of cirrhosis induced CRS-5.
Pathophysiological changes in sepsis related CRS 5 depend on systemic effects of the sepsis itself, and also, from direct cross-talk between the damaged heart and kidney.In early stages of sepsis microcirculation is often initially involved des pite normal systemic hemodynamics82, 83 and strongly correlates with morbidity and mortality rates.
Sepsis associated cardiomyopathy represents one of main predictors of mortality in septic patients.84Both the left and right ventricle can be injured with dilation and decreased ejection fraction, often unresponsive to fluid and catecholamine therapy.85 Septic cardiomyopathy, when severe, can mimic cardiogenic shock but it is usually reversible.86 Myocardial blood flow and oxygen consumption do not seem involved in pathophysiology of septic cardiomyopathy.87 Pro-inflammatory mediators and complement factors have been proposed as crucial actors in the development of cardiac involvement during sepsis.88, 89
In sepsis associated AKI, there are clear changes in intra-parenchymal blood flow independent of systemic hemodynamic changes linked to the septic process.90, 91 Recent experimental data have compared two different sepsis models in pigs in which, irrespective of systemic hemodynamics, only pigs developing septic AKI demonstrated increased renal vascular resistance and early rises in pro-inflammatory cytokines (IL-6) and oxidative stress markers.91
Sepsis, is able to affect the autonomic nervous system (ANS), RAAS and hypothalamus-pituitary gland-adrenal gland axis (HPA) independently which can impact, in several and distinctive steps, cardiac and/or renal function. Severity of ANS dysfunction correlates with morbidity and mortality92, 93; autonomic dysfunction can be assessed by observing decreased heart rate variability (HRV), often associated with release of inflammatory biomarkers such as IL-6, IL.10 and C- reactive protein (CRP).94 It’s clear that during combined heart and kidney dysfunction, as in sepsis, several cellular and molecular changes occur in both tissues.Activation and induction of cytokines (TNF-α and IL-6) and leukocytes (macrophages, neutrophils and lymphocytes) is well documented both in heart and kidney during.95, 96 Myocardial contractility is significantly affected and muscle protein expression (actin and myosin) is abnormal in sepsis as well as membrane associated proteins, as dystrophin, normally regulating cell shape, mechanical strength and myocardial cells contractility.Mean amount of dystrophin and other similar glycoproteins are reduced in septic myocardium.97 Sepsis induces tubular damage in kidneys affected by increased secretion of lipopolysaccharide that alters HCO3 transport leading to abnormalities in urine acidification.98 Lipopolysaccharide also modifies megalin, a glomerular protein involved in increasing albuminuria, and consequently intrarenal inflammation.99
7. Therapeutic implications
According to pathophysiological pathways cardiac dysfunction may occur in any stage of AKI and CKD. European Society of Cardiology (ESC) and American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) guidelines for ADHF100, 101 have to be followed. Intravascular and extravascular volume control should be reached with diuretics and extracorporeal therapies. Prevention of left ventricular volume overload is critical to maintain adequate cardiac output and systemic perfusion.
Diuretics, especially loop diuretics, are the gold standard in ADHF and type 1-3 CRS therapy since they provide to reduce fluid overload and improve symptoms (beneficial effects on patients’ dispnea and edema); on the other side, inappropriate diuretic therapy can worse kidney’s injury during AKI.
Therefore, diuretic therapy has been associated with increased risk of death in AKI patients showing no benefits in kidneys’ function recovery.102 Despite of these clinical evidence, diuretics remains first choice tharapy in ADHF patients. Continuous infusion of furosemide has been recommended for improved efficacy as far as combination therapy thiazides diuretics. Once pharmacological treatment fails in AKI patients and oligo-anuric renal failure is established, renal replacement therapy has to been started and it represent a cornerstone in the management of severe kidney injury although several aspects of RRT remain still controversial. The timing of RRT initiation is strongly dependent by clear impairment of renal function with electrolytes and acid-base imbalancement, hpercreatininemia and severe fluid overload not responsive to pharmacological treatment.103
Concerning type-4 CRS, RENAAL study was one of the corner stone in this field of application. RENAAL investigators aimed to evaluate renoprotective effects of losartan in over 1500 type 2 diabetic patients with renal involvement without evidence of heart failure at baseline.104 Quite similar to RENAAL study, the Irbesartan Diabetic Nephropathy Trial (IDNT) study was designed to evaluate renoprotective effects of irbesartan versus amlodipine or placebo in over 1700 patients.105 Results showed how irbesartan group had lower incidence of heart failure compared to amlodipine and placebo group.105 The use of beta-blockers together with ACE inhibitors or Angiotensin II receptors blockers (ARBs) is associated with better cardiovascular and renal outcomes in elderly patients, also those with advanced CKD.106 In the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE), reduction in first heart failure episode was reported in cinacalcet group.107 Di Lullo et al. found that, treating pre-dialysis patients with sevelamer chloridrate (1600 mg/day), a calcium-free phasphate binder, both reduction on cardiac valve calcifications and delay in kidney function decline occured.108 Dyslipidemia represent another fundamental target to achieve in managing cardiovascular complications in CKD patients; SHARP trial actually represents largest trial on statin employment in CKD patients showing a significant benefit of the combination simvastatin/ezetimibe on major atherosclerotic events although all-cause mortality was unaffected.109
Finally in type-5 CRS maintaining hemodynamic stability and guarantee tissue perfusion are key points to prevent type-5 CRS in hyperacute phase of sepsis together with fluid control and correct antibiotic treatment. Fluid therapy must be carefully managed to avoid fluid overload and other iatrogenic complications.110
Since inflammation and immune disorders play an important role in the pathogenesis of sepsis, removal of cytokines and immunomodulation can be obtained with high permeability membranes.111 To manage heart complications, approach with fluid therapy together with vasopressors, vasodilators and inotropes is required for maintaining filling pressures; vasopressors should be carefully administered because of depressive effects on cardiac output. More recently levosimendan has to be proven to provide benefits in decompensated heart failure to increase ejection fraction and diuresis; levosimendan efficacy is still to be proven in prevention of type-5 CRS.110 Renal support include removal of any nephrotoxic drug and media, maintenance of adeguate perfusion pressure and, if indicated, early intervention with dialysis therapy.110
8. Summary
The pathophysiology and clinical impact of the various subtypes of cardiorenal syndrome exemplify the intricate cross talk between the heart and the kidney. Given the huge morbidity and mortality of the dual burden of these organ system afflictions, early recognition of the clinical phenotype of cardiorenal syndrome and interventions to slow down end organ damage are crucial in positively influencing the burden of this pathological symbiosis.
Authors declaration
All the authors declare that manuscript has never been submitted and it will never be to any other scientific journal.
Authors disclosure
All the authors have not anything to disclose.
Conflict of interest
All the authors have no conflict of interest to decline.
References
- 1.Ronco C. The cardiorenal syndrome: basis and common ground for a multidisciplinary patient-oriented therapy. Cardiorenal Med. 2011;1:3–4. doi: 10.1159/000323352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bargshaw S.M., Cruz D.M., Aspromonte N. Epidemiology of cardio –renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25:1406–1416. doi: 10.1093/ndt/gfq066. [DOI] [PubMed] [Google Scholar]
- 3.Damman K., Navis G., Voors A.A. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 4.McCullough P.A. Cardiorenal syndromes: pathophysiology to prevention. Int J Nephrol. 2010;2010:762590. doi: 10.4061/2011/762590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronco C. Cardiorenal syndromes: definition and classification. Contrib Nephrol. 2010;164:33–38. doi: 10.1159/000313718. [DOI] [PubMed] [Google Scholar]
- 6.Eren Z., Ozveren O., Buvukoner E., Kaspar E., Degertekin M., Kantarci G. A single-centre study of acute cardiorenal syndrome: incidence, risk factors and consequences. Cardiorenal Med. 2012;2:168–176. doi: 10.1159/000337714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanada S., Takewa Y., Mizuno T., Tsukiyan T., Taenaka Y., Tatsumi E. Effect of the technique for assisting renal blood circulation on ischemic kidney in acute cardiorenal syndrome. J Artif Organs. 2012;15:140–145. doi: 10.1007/s10047-011-0613-5. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson L.W., Perloff J.K. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884–888. [PubMed] [Google Scholar]
- 9.Mullens W., Abrahams Z., Francis G.S. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uthoff H., Breidthardt T., Klima T. Central venous pressure and impaired renal function in patients with acute heart failure. Eur J Heart Fail. 2011;13:432–439. doi: 10.1093/eurjhf/hfq195. [DOI] [PubMed] [Google Scholar]
- 11.Braam B., Cupples W.A., Joles J.A., Gaillard C. Systemic arterial and venous determinants of renal hemodynamics in congestive heart failure. Heart Fail Rev. 2012;17:161–175. doi: 10.1007/s10741-011-9246-2. [DOI] [PubMed] [Google Scholar]
- 12.De Silva R., Loh H., Rigby A.S. Epidemiology, associated factors, and prognostic outcomes of renal artery stenosis in chronic heart failure assessed by magnetic resonance angiography. Am J Cardiol. 2007;100:273–279. doi: 10.1016/j.amjcard.2007.02.098. [DOI] [PubMed] [Google Scholar]
- 13.Nohria A., Tsang S.W., Fang J.C. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41:1797–1804. doi: 10.1016/s0735-1097(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 14.Machnik A., Neuhofer W., Jantsch J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 15.Virzì G.M., Torregrossa R., Cruz D.N. Cardiorenal syndrome type 1 may Be immunologically mediated: a pilot evaluation of monocyte apoptosis. Cardiorenal Med. 2012;2:33–42. doi: 10.1159/000335499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havasi A., Borkan S.C. Apoptosis and acute kidney injury. Kidney Int. 2011;80:29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonventre J.V. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14(Suppl. 1):S55–61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 18.Akcay A., Nguyen Q., Edelstein C.L. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pastori S., Virzì G.M., Brocca A. Cardiorenal syndrome type-1: activation of dual apoptotic pathways. Cardiorenal Med. 2015;5:306–315. doi: 10.1159/000438831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virzì G.M., Clementi A., DeCal M. Oxydative stress: dual pathway induction in cardiorenal syndrome type 1 pathogenesis. Oxid Med Cell Longevity. 2015;2015 doi: 10.1155/2015/391790. [Article ID 391790] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama Y., Lindholm B., Stenvinkel P. Inflammation and oxidative stress in ESRD-the role of myeloperoxydase. J Nephrol. 2004;17(Suppl. (8)):S72–76. [PubMed] [Google Scholar]
- 23.Kraut E.J., Chen S., Hubbard N.E., Erickson K.I., Wisner D.H. Tumor necrosis factor depresses myocardial contractility in endotoxemic swine. J Trauma. 1999;46:900–906. doi: 10.1097/00005373-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Heywood J.T., Fonarow G.C., Costanzo M.R., Mathur V.S., Wigneswaran J.R., Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13(6):422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Hebert K., Dias A., Delgado M.C. Epidemiology and survival of the five stages of chronic kidney disease in a systolic heart failure population. Eur J Heart Fail. 2010;12(8):861–865. doi: 10.1093/eurjhf/hfq077. [DOI] [PubMed] [Google Scholar]
- 26.Cruz D.N., Bagshaw S.M. Heart-kidney interaction: epidemiology of cardiorenal syndromes. Int J Nephrol. 2010;2011:351291. doi: 10.4061/2011/351291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagshaw S.M., Cruz D.N., Aspromonte N. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25(5):1406–1416. doi: 10.1093/ndt/gfq066. [DOI] [PubMed] [Google Scholar]
- 28.Cruz D.N., Schmidt-Ott K.M., Vescovo G. Pathophysiology of cardiorenal syndrome type 2 in stable chronic heart failure: workgroup statements from the eleventh consensus conference of the acute dialysis quality initiative (ADQI) Contrib Nephrol. 2013;182:117–136. doi: 10.1159/000349968. [DOI] [PubMed] [Google Scholar]
- 29.Setoguchi S., Stevenson L.W., Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–266. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 30.Bongartz L.G., Cramer M.J., Doevendans P.A., Joles J.A., Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005;26(1):11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 31.Merrill A.J., Morrison J.L., Branno E.S. Concentration of renin in renal venous blood in patients with chronic heart failure. Am J Med. 1946;1(5):468. doi: 10.1016/0002-9343(46)90067-8. [DOI] [PubMed] [Google Scholar]
- 32.Kishimoto T., Maekawa M., Abe Y., Yamamoto K. Intrarenal distribution of blood flow and renin release during renal venous pressure elevation. Kidney Int. 1973;4(4):259–266. doi: 10.1038/ki.1973.112. [DOI] [PubMed] [Google Scholar]
- 33.Remuzzi G., Cattaneo D., Perico N. The aggravating mechanisms of aldosterone on kidney fibrosis. J Am Soc Nephrol. 2008;19(8):1459–1462. doi: 10.1681/ASN.2007101079. [DOI] [PubMed] [Google Scholar]
- 34.Onozato ML, Tojo A, Kobayashi N, Goto A, Matsuoka H, Fujita T. Dual blockade of aldosterone and angiotensin II additively suppresses TGF-beta and NADPH. [DOI] [PubMed]
- 35.Colombo P.C., Ganda A., Lin J. Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev. 2012;17(2):177–190. doi: 10.1007/s10741-011-9261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yap S.C., Lee H.T. Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology. 2012;116(May (5)):1139–1148. doi: 10.1097/ALN.0b013e31824f951b. [DOI] [PubMed] [Google Scholar]
- 37.Prabhu S.D. Cytokine-induced modulation of cardiac function. Circ Res. 2004;95(December (12)):1140–1153. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 38.Kingma J.G., Jr., Vincent C., Rouleau J.R., Kingma I. Influence of acute renal failure on coronary vasoregulation in dogs. J Am Soc Nephrol. 2006;17(May (5)):1316–1324. doi: 10.1681/ASN.2005101084. [DOI] [PubMed] [Google Scholar]
- 39.Bongartz L.G., Cramer M.J., Doevendans P.A., Joles J.A., Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005;26(January (1)):11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 40.Ma X.L., Lefer D.J., Lefer A.M., Rothlein R. Coronary endothelial and cardiac protective effects of a monoclonal antibody to intercellular adhesion molecule-1 in myocardial ischemia and reperfusion. Circulation. 1992;86(September (3)):937–946. doi: 10.1161/01.cir.86.3.937. [DOI] [PubMed] [Google Scholar]
- 41.Blake P., Hasegawa Y., Khosla M.C., Fouad-Tarazi F., Sakura N., Paganini E.P. Isolation of myocardial depressant factor(s) from the ultrafiltrate of heart failure patients with acute renal failure. ASAIO J. 1996;42(September–October (5)):M911–915. doi: 10.1097/00002480-199609000-00127. [DOI] [PubMed] [Google Scholar]
- 42.Prabhu S.D. Cytokine-induced modulation of cardiac function. Circ Res. 2004;95(December (12)):1140–1153. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 43.Edmunds N.J., Lal H., Woodward B. Effects of tumour necrosis factor-alpha on left ventricular function in the rat isolated perfused heart: possible mechanisms for a decline in cardiac function. Br J Pharmacol. 1999;126(January (1)):189–196. doi: 10.1038/sj.bjp.0702294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rauchhaus M., Doehner W., Francis D.P. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102(December (25)):3060–3067. doi: 10.1161/01.cir.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 45.Chuasuwan A., Kellum J.A. Cardio-renal syndrome type 3: epidemiology, pathophysiology, and treatment. Semin Nephrol. 2012;32(January (1)):31–39. doi: 10.1016/j.semnephrol.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Kajstura J., Cigola E., Malhotra A. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol. 1997;29(March (3)):859–870. doi: 10.1006/jmcc.1996.0333. [DOI] [PubMed] [Google Scholar]
- 47.Kingma J.G., Jr., Vincent C., Rouleau J.R., Kingma I. Influence of acute renal failure on coronary vasoregulation in dogs. J Am Soc Nephrol. 2006;17(May (5)):1316–1324. doi: 10.1681/ASN.2005101084. [DOI] [PubMed] [Google Scholar]
- 48.Nath K.A., Grande J.P., Croatt A.J. Transgenic sickle mice are markedly sensitive to renal ischemia-reperfusion injury. Am J Pathol. 2005;166(April (4)):963–972. doi: 10.1016/S0002-9440(10)62318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly K.J. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14(June (6)):1549–1558. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 50.Bryant D., Becker L., Richardson J. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–1381. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y.H., D'Ambrosio M., Liao T.D. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circul Physiol. 2009;296(February (2)):H404–H412. doi: 10.1152/ajpheart.00747.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Deyn P.P., vanholder R., D’Hooge R. Nitric oxide in uremia: effects of several potentially toxic guanidino compounds. Kidney Int. 2003;(May (84)):S25–S28. doi: 10.1046/j.1523-1755.63.s84.9.x. [DOI] [PubMed] [Google Scholar]
- 53.Scheuer J., Stezoski W. The effects of uremic compounds on cardiac function and metabolism. J Mol Cell Cardiol. 1973;5:287–300. doi: 10.1016/0022-2828(73)90068-0. [DOI] [PubMed] [Google Scholar]
- 54.Jackson G., Gibbs C.R., Davies M.K., Lip G.Y. ABC of heart failure. Pathophysiol Br Med J. 2000;320:167–170. doi: 10.1136/bmj.320.7228.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li L., Lee E.W., Ji H., Zukowska Z. Neuropeptide Y-induced acceleration of postangioplasty occlusion of rat carotid artery. Arterioscler Thromb Vasc Biol. 2003;23:1204–1210. doi: 10.1161/01.ATV.0000071349.30914.25. [DOI] [PubMed] [Google Scholar]
- 56.Karnik J.A., Young B.S., Lew N.L. Cardiac arrest and sudden cardiac death in dialysis units. Kidney Int. 2001;60:350–357. doi: 10.1046/j.1523-1755.2001.00806.x. [DOI] [PubMed] [Google Scholar]
- 57.Redóna J., Cea-Calvob L., Lozanoc J.V. Kidney function and cardiovascular disease in the hypertensive population: the ERIC-HTA study. J Hypertens. 2006;24:663–669. doi: 10.1097/01.hjh.0000217848.10831.5f. [DOI] [PubMed] [Google Scholar]
- 58.Levin A., Singer J., Thompson C.R., Ross H., Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis. 1996;27(3):347–354. doi: 10.1016/s0272-6386(96)90357-1. [DOI] [PubMed] [Google Scholar]
- 59.Lezaic V., Tirmenstajn-Jankovic B., Bukvic D. Efficacy of hyperphosphatemia control in the progression of chronic renal failure and the prevalence of cardiovascular calcification. Clin Nephrol. 2009;71(1):21–29. doi: 10.5414/cnp71021. [DOI] [PubMed] [Google Scholar]
- 60.Di Lullo L., Floccari F., Santoboni A. Progression of cardiac valve calcification and decline of renal function in CKD patients. J Nephrol. 2013;26(July (4)):739–744. doi: 10.5301/jn.5000290. [DOI] [PubMed] [Google Scholar]
- 61.Olgaard K., Lewin E., Silver J. Calcimimetics, vitamin D and ADVANCE in the management of CKD-MBD. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfq862. [DOI] [PubMed] [Google Scholar]
- 62.MacRae J.M., Pandeya S., Humen D.P., Krivitski N., Lindsay R.M. Arteriovenous fistula-associated high-output cardiac failure: a review of mechanisms. Am J Kidney Dis. 2004;43(5):e17–e22. doi: 10.1053/j.ajkd.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 63.Di Lullo L., Floccari F., Polito P. Right ventricular diastolic function in dialysis patients could be affected by vascular access. Nephron Clin Pract. 2011;118:c258–c262. doi: 10.1159/000321867. [DOI] [PubMed] [Google Scholar]
- 64.Fort J. Chronic renal failure: a cardiovascular risk factor. Kidney Int. 2005;68(Suppl. 99):S25–S29. doi: 10.1111/j.1523-1755.2005.09906.x. [DOI] [PubMed] [Google Scholar]
- 65.Schiffrin E.L., Lipman M.L., Mann J.F. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116(1):85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 66.Bologa R.M., Levine D.M., Parker T.S. Interleukin-6 predicts hypoalbuminemia hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis. 1998;32(1):107–114. doi: 10.1053/ajkd.1998.v32.pm9669431. [DOI] [PubMed] [Google Scholar]
- 67.Maisel A.S., Katz N., Hillege H.L. Biomarkers in kidney and heart disease. Nephrol Dial Transplant. 2011;26(1):62–74. doi: 10.1093/ndt/gfq647. [DOI] [PubMed] [Google Scholar]
- 68.Foley R.N., Parfrey P.S., Sarnak M.J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(Suppl. 3 (5)):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 69.Harnett J.D., Foley R.N., Kent G.M. Congestive heart failure in dialysis patients: prevalence incidence, prognosis and risk factors. Kidney Int. 1995;47(3):884–890. doi: 10.1038/ki.1995.132. [DOI] [PubMed] [Google Scholar]
- 70.Foley R.N., Parfrey P.S., Harnett J.D. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. 1995;5(12):2024–2031. doi: 10.1681/ASN.V5122024. [DOI] [PubMed] [Google Scholar]
- 71.Jovanovich A., Ix J.H., Gottdiener J. Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis. 2013 Nov;231(1):114–119. doi: 10.1016/j.atherosclerosis.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shamseddin M.K., Parfrey P.S. Medscape: sudden cardiac death in chronic kidney disease: epidemiology and prevention. Nat Rev Nephrol. 2011;7(March (3)):145–154. doi: 10.1038/nrneph.2010.191. [DOI] [PubMed] [Google Scholar]
- 73.Chan C.T., Levin N.W., Chertow G.M. Determinants of cardiac autonomic dysfunction in ESRD. Clin J Am Soc Nephrol. 2010;5(10):1821–1827. doi: 10.2215/CJN.03080410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winkelmayer W.C., Patrick A.R., Liu J. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol. 2011;22(2):349–357. doi: 10.1681/ASN.2010050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boerrigter G., Costello-Boerrigter L.C., Abraham W.T. Cardiac resynchronization therapy improves renal function in human heart failure with reduced glomerular filtration rate. J Card Fail. 2008;14(7):539–546. doi: 10.1016/j.cardfail.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cai Q., Mukku V.K., Ahmad M. Coronary artery disease in patients with chronic kidney disease: a clinical update. Curr Cardiol Rev. 2013;9(November (4)):331–339. doi: 10.2174/1573403X10666140214122234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chonchol M., Whittle J., Desbien A., Orner M.B., Petersen L.A., Kressin N.R. Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol. 2008;28(2):354–360. doi: 10.1159/000111829. [DOI] [PubMed] [Google Scholar]
- 78.Kajstura J., Cigola E., Malhotra A. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol. 1997;29(March (3)):859–870. doi: 10.1006/jmcc.1996.0333. [DOI] [PubMed] [Google Scholar]
- 79.Kingma J.G., Jr., Vincent C., Rouleau J.R., Kingma I. Influence of acute renal failure on coronary vasoregulation in dogs. J Am Soc Nephrol. 2006;17(May (5)):1316–1324. doi: 10.1681/ASN.2005101084. [DOI] [PubMed] [Google Scholar]
- 80.Lok D.J., Lok S.I., Bruggink-André de la Porte P.W. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin Res Cardiol. 2013;102(February (2)):103–110. doi: 10.1007/s00392-012-0500-y. [DOI] [PubMed] [Google Scholar]
- 81.Krag A., Bendtsen F., Burroughs A.K., Møller S. The cardiorenal link in advanced cirrhosis. Med Hypotheses. 2012;79(July (1)):53–55. doi: 10.1016/j.mehy.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 82.Lundy D.J., Trzeciak S. Microcirculatory dysfunction in sepsis. Crit Care Clin. 2009;25(4):721–731. doi: 10.1016/j.ccc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Trzeciak S. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007;49(1):88–98. doi: 10.1016/j.annemergmed.2006.08.021. [98 e1–2] [DOI] [PubMed] [Google Scholar]
- 84.Jardin F. Sepsis-related cardiogenic shock. Crit Care Med. 1990;18(10):1055–1060. doi: 10.1097/00003246-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 85.Lambermont B. Effects of endotoxic shock on right ventricular systolic function and mechanical efficiency. Cardiovasc Res. 2003;59(2):412–418. doi: 10.1016/s0008-6363(03)00368-7. [DOI] [PubMed] [Google Scholar]
- 86.Parker M.M. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100(4):483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 87.Dhainaut J.F. Coronary hemodynamics and myocardial metabolism of lactate: free fatty acids, glucose, and ketones in patients with septic shock. Circulation. 1987;75(3):533–541. doi: 10.1161/01.cir.75.3.533. [DOI] [PubMed] [Google Scholar]
- 88.Kumar A. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183(3):949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Torre-Amione G. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27(5):1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 90.Benes J. Searching for mechanisms that matter in early septic acute kidney injury: an experimental study. Crit Care. 2011;15(5):R256. doi: 10.1186/cc10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bougle A., Duranteau J. Pathophysiology of sepsis-induced acute kidney injury: the role of global renal blood flow and renal vascular resistance. Contrib Nephrol. 2011;174:89–97. doi: 10.1159/000329243. [DOI] [PubMed] [Google Scholar]
- 92.Schmidt H. Autonomic dysfunction predicts both 1- and 2-month mortality in middle-aged patients with multiple organ dysfunction syndrome. Crit Care Med. 2008;36(3):967–970. doi: 10.1097/CCM.0B013E3181653263. [DOI] [PubMed] [Google Scholar]
- 93.Tateishi Y. Depressed heart rate variability is associated with high IL-6 blood level and decline in the blood pressure in septic patients. Shock. 2007;28(5):549–553. doi: 10.1097/shk.0b013e3180638d1. [DOI] [PubMed] [Google Scholar]
- 94.Mortensen E.M. Impact of previous statin and angiotensin II receptor blocker use on mortality in patients hospitalized with sepsis. Pharmacotherapy. 2007;27(12):1619–1626. doi: 10.1592/phco.27.12.1619. [DOI] [PubMed] [Google Scholar]
- 95.Chopra M. Modulation of myocardial mitochondrial mechanisms during severe polymicrobial sepsis in the rat. PLoS One. 2011;6(6):e21285. doi: 10.1371/journal.pone.0021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu M. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009;76(3):277–285. doi: 10.1038/ki.2009.157. [DOI] [PubMed] [Google Scholar]
- 97.Celes M.R., Prado C.M., Rossi M.A. Sepsis: going to the heart of the matter. Pathobiology. 2013;80(2):70–86. doi: 10.1159/000341640. [DOI] [PubMed] [Google Scholar]
- 98.Good D.W., George T., Watts B.A., 3rd Toll-like receptor 2 mediates inhibition of HCO(3)(−) absorption by bacterial lipoprotein in medullary thick ascending limb. Am J Physiol Renal Physiol. 2010;299(3):F536–44. doi: 10.1152/ajprenal.00108.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schreiber A. Acute endotoxemia in mice induces downregulation of megalin and cubilin in the kidney. Kidney Int. 2012;82(1):53–59. doi: 10.1038/ki.2012.62. [DOI] [PubMed] [Google Scholar]
- 100.Jessup M., Abraham W.T., Casey D.E. ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 101.Gheorghiade M., Zannad F., Sopko G. Failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 102.Mehta R.L., Pascual M.T., Soroko S.H. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288:2547–2553. doi: 10.1001/jama.288.20.2547. [DOI] [PubMed] [Google Scholar]
- 103.Bellomo R., Kellum J.A., Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 104.Brenner B.M., Cooper M.E., de Zeeuw D. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 105.Lewis E.J., Hunsicker L.G., Clarke W.R. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 106.Cice G., Ferrara L., D'Andrea A. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41(9):1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 107.EVOLVE Trial Investigators, Chertow G.M., Block G.A. Parfrey PS Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 108.Di Lullo L., Floccari F., Santoboni A. Progression of cardiac valve calcification and decline of renal function in CKD patients. J Nephrol. 2013;26(July (4)):739–744. doi: 10.5301/jn.5000290. [12] [DOI] [PubMed] [Google Scholar]
- 109.Sharp Collaborative Group Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J. 2010;160(November (5)):785–794. doi: 10.1016/j.ahj.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 110.Ronco C., Di Lullo L. Cardiorenal syndrome. Heart Fail Clin. 2014;10(April (2)):251–280. doi: 10.1016/j.hfc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 111.Nakamura M. Treatment of severe sepsis and septic shock by CHDF using a PMMA membrane hemofilter as a cytokine modulator. Contrib Nephrol. 2010;166:73–82. doi: 10.1159/000314855. [DOI] [PubMed] [Google Scholar]