Abstract
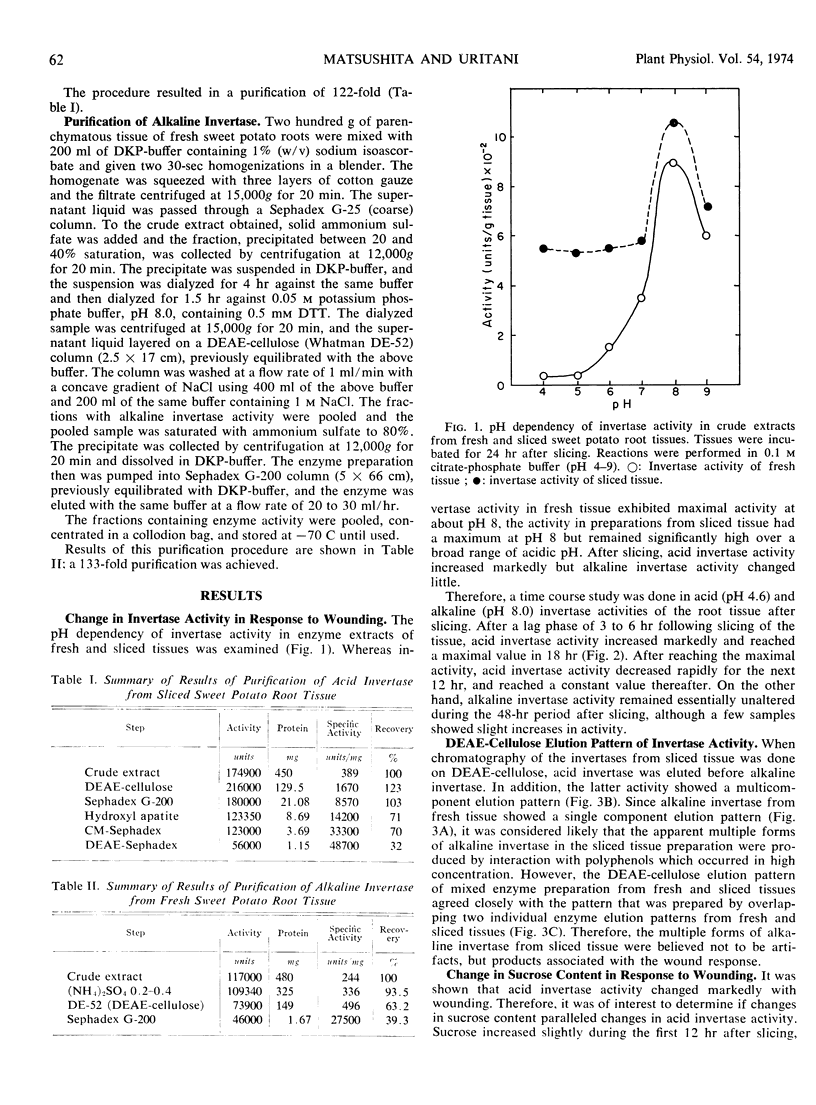
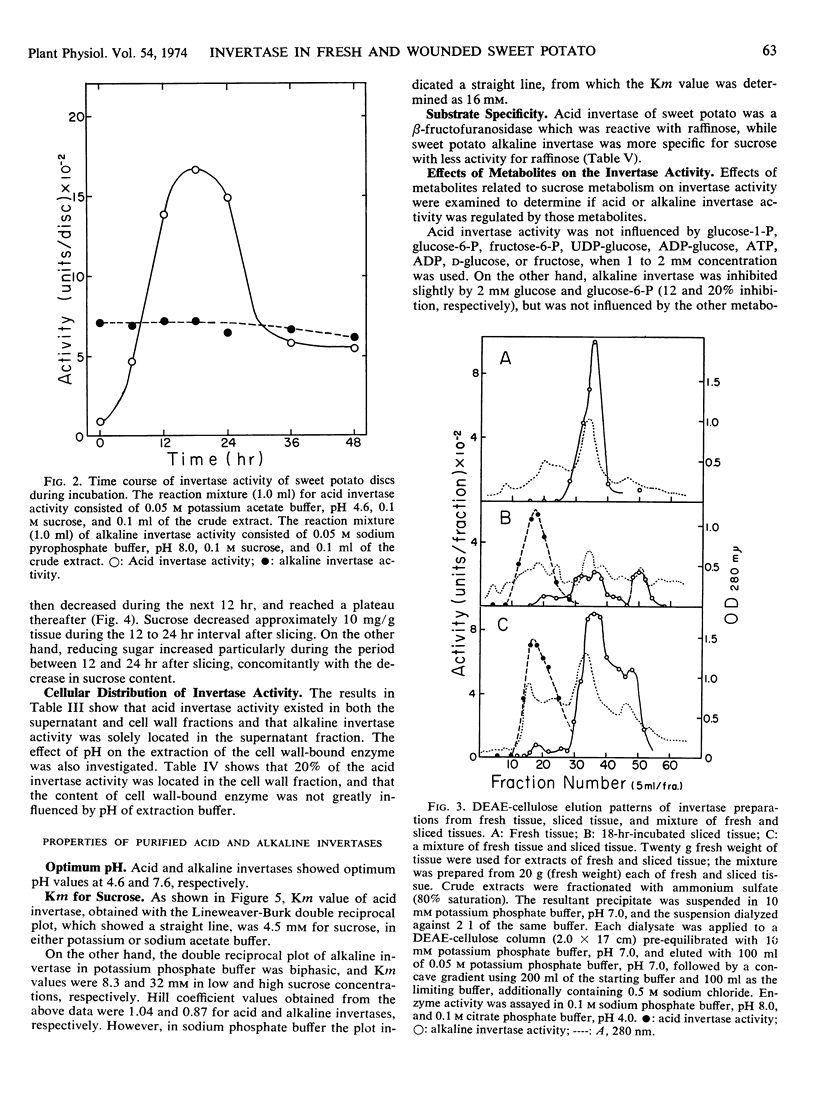
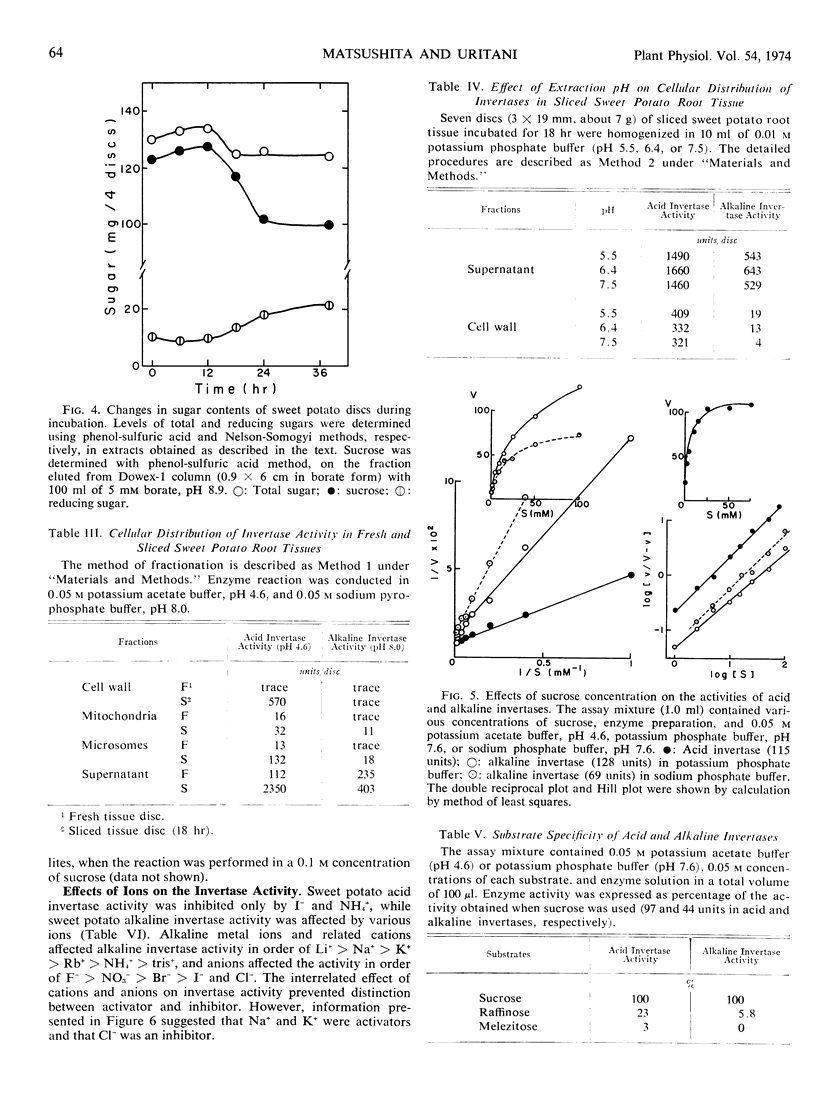
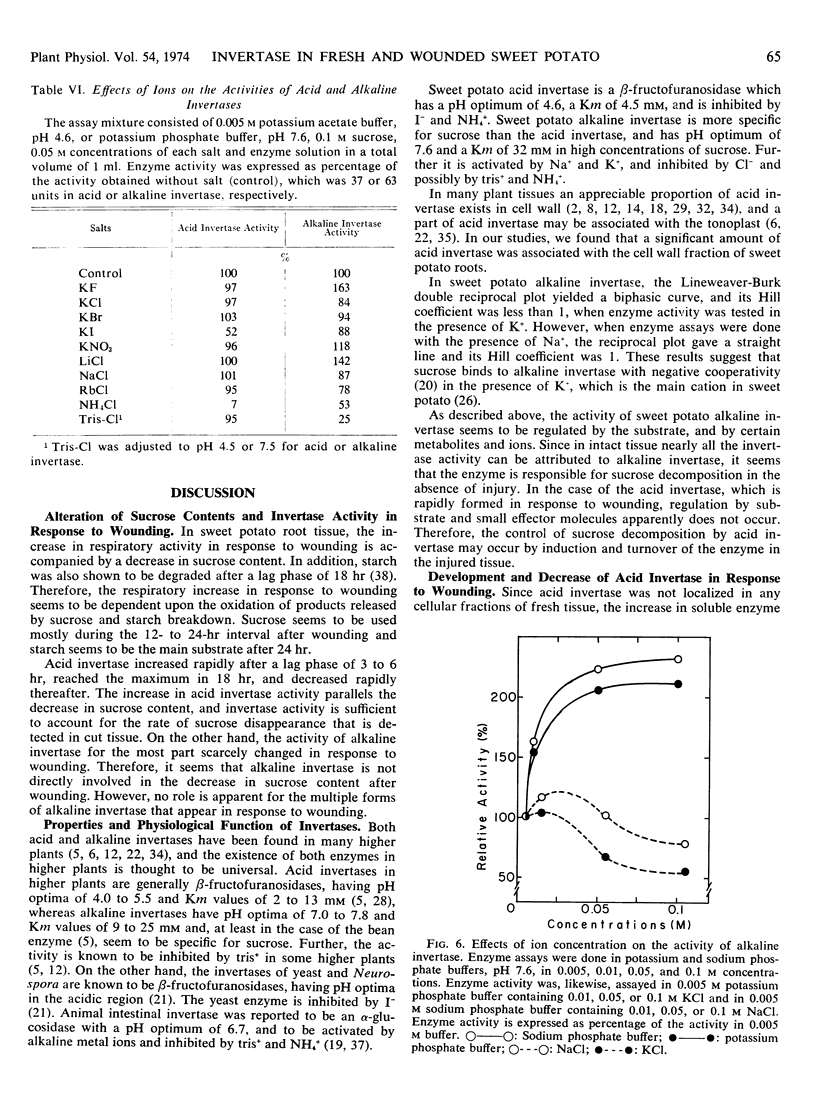
When root tissue of sweet potato (Ipomoea batatas Lam.) was sliced, acid invertase activity, initially absent in freshly sliced tissue, appeared after a 3- to 6-hour lag phase, rapidly reached a maximum in 18 hours, and thereafter decreased. The increase in invertase activity was accompanied by a decrease in sucrose content of the root tissue. Alkaline invertase activity was present in fresh root tissue, but changed little after wounding. Acid invertase in wounded tissue and alkaline invertase in fresh tissue were purified and their properties were investigated. The acid invertase was a ß-fructofuranosidase and was unaffected by substrate or by any of the cations and several metabolites. The alkaline invertase was more specific for sucrose, was inhibited by glucose and glucose 6-phosphate, and displayed non-Michaelis-Menten kinetics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akazawa T., Uritani I. Pattern of Carbohydrate Breakdown in Sweet Potato Roots Infected With Ceratocystis fimbriata. Plant Physiol. 1962 Sep;37(5):662–670. doi: 10.1104/pp.37.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W. N. Beta-fructofuranosidase from grape berries. II. Solubilization of a bound fraction. Biochim Biophys Acta. 1966 Oct 17;128(1):124–129. doi: 10.1016/0926-6593(66)90148-2. [DOI] [PubMed] [Google Scholar]
- Asahi T., Honda Y., Uritani I. Increase of Mitochondrial Fraction in Sweet Potato Root Tissue after Wounding or Infection with Ceratocystis fimbriata. Plant Physiol. 1966 Sep;41(7):1179–1184. doi: 10.1104/pp.41.7.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Greenshields R. N. The partial purification and some properties of two sucrases of Phaseolus vulgaris. Biochem J. 1964 Aug;92(2):357–364. doi: 10.1042/bj0920357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN J., HALL M. A. EFFECT OF GROWTH HORMONES ON THE DEVELOPMENT OF INVERTASE ASSOCIATED WITH CELL WALLS. Nature. 1964 Jan 18;201:296–297. doi: 10.1038/201296b0. [DOI] [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Sacher J. A., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. I. Studies on Enzymes of the Cycle. Plant Physiol. 1963 May;38(3):338–343. doi: 10.1104/pp.38.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo H., Yang S. F. Ethylene-enhanced Synthesis of Phenylalanine Ammonia-Lyase in Pea Seedlings. Plant Physiol. 1971 Jun;47(6):765–770. doi: 10.1104/pp.47.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes T. A., Nelson O. E. An invertase inactivator in maize endosperm and factors affecting inactivation. Plant Physiol. 1971 May;47(5):629–634. doi: 10.1104/pp.47.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes T. A., Nelson O. E. Invertase Activity in Normal and Mutant Maize Endosperms during Development. Plant Physiol. 1971 May;47(5):623–628. doi: 10.1104/pp.47.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolínská J., Semenza G. Studies on intestinal sucrase and on intestinal sugar transport. V. Isolation and properties of sucrase-isomaltase from rabbit small intestine. Biochim Biophys Acta. 1967 Sep 12;146(1):181–195. doi: 10.1016/0005-2744(67)90085-x. [DOI] [PubMed] [Google Scholar]
- Minamikawa T., Uritani I. Phenylalanine ammonia-lyase in sliced sweet potato roots. J Biochem. 1965 May;57(5):678–688. [PubMed] [Google Scholar]
- Murata T., Akazawa T. Enzymic mechanism of starch synthesis in sweet potato roots. I. Requirement of potassium lons for potassium ions for starch synthetase. Arch Biochem Biophys. 1968 Sep 10;126(3):873–879. doi: 10.1016/0003-9861(68)90481-5. [DOI] [PubMed] [Google Scholar]
- Palmer J. M. The influence of growth regulating substances on the development of enhanced metabolic rates in thin slices of beetroot storage tissue. Plant Physiol. 1966 Sep;41(7):1173–1178. doi: 10.1104/pp.41.7.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. Separation and properties of potato invertase and invertase inhibitor. Arch Biochem Biophys. 1966 Mar;113(3):667–674. doi: 10.1016/0003-9861(66)90246-3. [DOI] [PubMed] [Google Scholar]
- Sacher J. A., Hatch M. D., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. III. Physical & Metabolic Aspects of Cycle in Immature Storage Tissues. Plant Physiol. 1963 May;38(3):348–354. doi: 10.1104/pp.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon L. M., Uritani I., Imaseki H. De novo synthesis of peroxidase isozymes in sweet potato slices. Plant Physiol. 1971 Apr;47(4):493–498. doi: 10.1104/pp.47.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesue Y. Purification and properties of rabbit intestinal sucrase. J Biochem. 1969 Apr;65(4):545–552. doi: 10.1093/oxfordjournals.jbchem.a129048. [DOI] [PubMed] [Google Scholar]



