Abstract
The coracoacromial ligament (CAL) was first described as a pain generator by Dr Charles Neer in the early 1970s. Since that time, considerable controversy regarding CAL management during acromioplasty has persisted. This review aims to better understand the role of the CAL in shoulder physiology and pathology. Sixty-six articles from 1958 to 2016 were identified using an electronic search of PubMed, Cochrane Library, AccessMedicine, and MD Consult for case series as well as cohort and prospective studies. The authors used “coracoacromial ligament” and “coracoacromial veil” as medical subject headings (MeSH). In addition, reference lists from all identified articles were reviewed for studies that the search terms may have omitted. The CAL plays an important role in shoulder biomechanics, joint stability, and proprioception. Morphological variance of the CAL is evident throughout the literature. Age-dependent changes due to chronic stress and cellular degradation cause thickening and stiffening of the CAL that may contribute to a spectrum of shoulder pathology from capsular tightness to rotator cuff tear arthropathy and impingement syndrome. The CAL is an integral component of the coracoacromial arch. CAL release during acromioplasty remains controversial. Future clinical outcomes research should endeavor to advance the understanding of the CAL to refine clinical and intraoperative decision making regarding its management.
Keywords: shoulder, coracoacromial ligament, rotator cuff arthropathy, impingement syndrome
The coracoacromial ligament (CAL) connects the acromion and coracoid process of the scapula, forming an osseoligamentous static restraint to superior humeral head displacement. Over the past 2 decades, biomechanical and clinical studies have furthered our understanding of its morphology and function, altering previous conclusions.§ The CAL has long been implicated as a pain generator in impingement syndrome as first described by Neer.44 Considerable controversy has surrounded the question of what to do with the CAL during acromioplasty due to increased risk of anterior and superior glenohumeral translation after release.∥ We aim to provide a comprehensive review of the anatomy and function of the CAL to better understand its role in the pathophysiology and treatment of impingement syndrome and rotator cuff arthropathy.
Methods
Three authors (A.R., G.G., and J.C.) independently searched the Medline, Cochrane Library, AccessMedicine, and MD Consult databases for case series, cohort studies, and prospective studies. “Coracoacromial ligament” and “coracoacromial veil” were used as medical subject headings (MeSH). In addition, reference lists from all identified articles were reviewed for studies that the search terms may have omitted. Ninety-four articles from 1958 to 2016 were identified and reviewed for possible inclusion. Inclusion criteria were studies with levels 1 through 5 evidence according to the American Academy of Orthopaedic Surgeons Evidence-Based Practice committee and publication in a peer-reviewed journal in the English language. Twenty-eight articles failed to meet our inclusion criteria and were excluded, leaving 66 articles for this analysis. There were 30 cadaveric studies, 25 clinical research articles, and 11 review articles included in this study.
Gross Anatomy
The CAL extends inferomedially from the inferior anterolateral surface of the acromion to the lateral border of the coracoid process. Together with the inferior aspect of the acromion and the coracoid process of the scapula, the CAL forms the coracoacromial arch that acts to limit superior displacement of the humeral head from the glenoid (Figure 1). A ligamentous connection between the CAL and the rotator interval capsule has been coined the “coracoacromial veil” and is thought to prevent inferior migration of the glenohumeral joint.39 The CAL is bordered superiorly by the clavicle and deltoid as well as inferiorly by the subacromial bursa and supraspinatus tendon. Superiorly, the CAL is continuous with the fascia of the deltoid at its insertion along the acromion.12,15,27 Near the coracoid, the CAL usually bifurcates into anterolateral band (ALB) and posteromedial band (PMB), which are often separated by a thin membrane.12,15,23,39,47,62 Fealy et al15 described the ALB as being thicker at the acromion than at the coracoid, with the PMB varying inversely. At its lateral edge, the CAL blends with fibers of the conjoined tendon formed by the constituent portions of the short head of the biceps and coracobrachialis.15,27 Medially, it is continuous with clavipectoral fascia.
Figure 1.
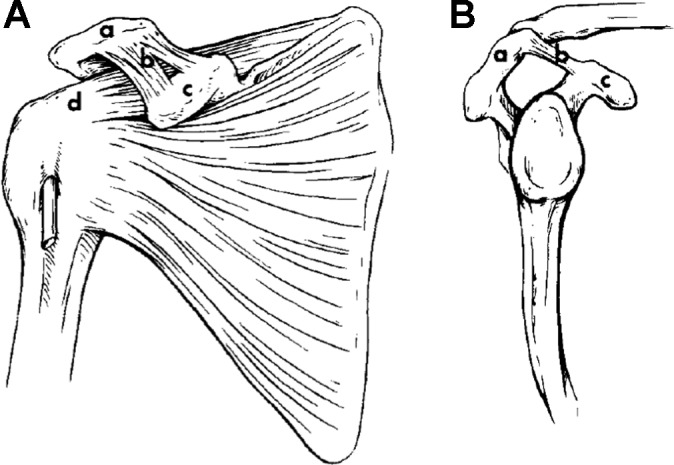
Anatomic schematic. (A) Anterior view of the coracoacromial arch: acromion (a), coracoacromial ligament (b), coracoid process (c), and supraspinatus insertion (d). (B) Lateral view of the coracoacromial arch: acromion (a), coracoacromial ligament (b), and coracoid process (c). (Reprinted with permission from Soslowsky et al.53)
Significant anatomic variation has been described. From birth the CAL can be undivided, bipartite, or multiple-banded.34 Further delineation reveals as many as 5 morphological subtypes, including broad-banded, quadrangular, Y-shaped, V-shaped, and multiple-banded.32,34 Broad-banded ligaments have a single ligament with roughly equal widths of insertion at the acromion and coracoid. Quadrangular ligaments are also single-banded, but the width of insertion at the coracoid is approximately 1.5 times greater than at the acromion.34 Y-shaped ligaments are confluent at the acromion but bifurcate along their length to form discrete insertions at the coracoid. Ligaments that have identifiably discrete insertions at both the acromion and coracoid are V-shaped. A last subtype includes all morphological variations in which more than 2 distinct bands exist, usually being confluent at the acromion and multipartite at the coracoid (Figure 2). Prevalence estimates vary by study but bipartite subtypes (Y- and V-shaped) are generally identified as being the most common, at 42% to 75% of all shoulders. The prevalence of undivided subtypes (broad and quadrangular) is estimated between 26% and 58%, while multiple-banded types make up a small minority (1%-15%). The relative prevalence of subtypes did not vary according to sex, age, or the presence of rotator cuff pathology.32,34,47 In a study of neonatal cadavers, all morphological types were present and equally prevalent. Morphological subtypes are identical bilaterally in as many as 45% to 64% of shoulders.
Figure 2.
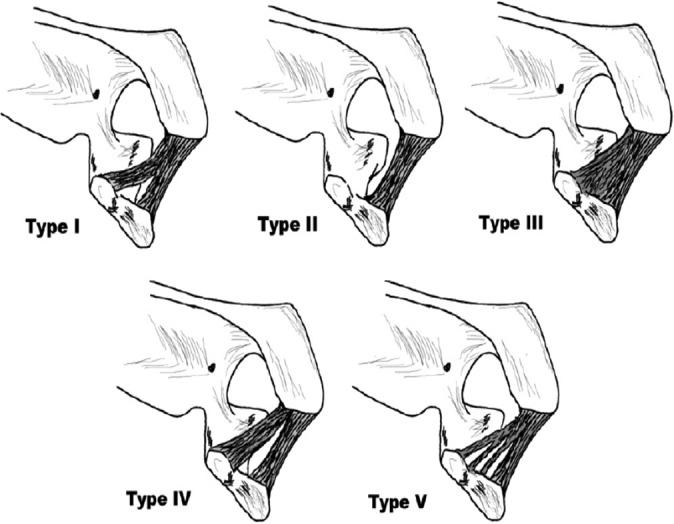
Schematic of coracoacromial ligament subtypes, including type I, Y-shaped; type II, broad-banded; type III, quadrangular; type IV, V-shaped; type V, multibanded. (Reprinted with permission from Kesmezacar et al.32)
While variations exist, the CAL is more often confluent at the acromion, with separation occurring more commonly at the coracoid. When bipartite or multiple-banded, the lateral band of the CAL is generally larger.32,34,53 Many authors agree that since significant variation is present at or around birth, final morphology may be due to developmental factors rather than degenerative changes.23,32,34,53
Histology and Microstructure
Like all other ligaments, the CAL is a soft collagenous tissue made up of fascicles containing elementary fibrils, reticular fibers, glycoproteins, and intervening fibroblasts. In particular, types I, III, and VI collagens along with proteoglycans, including chondroitin IV sulfate, keratan sulfate, dermatan sulfate, versican, tenascin, and cartilage oligomeric matrix protein are present throughout the CAL.40 Parallel collagen fibers are oriented along the ligament’s longitudinal axis, usually within wavy (>2 inflection points per 100 μm) bundles.33 Perhaps uniquely, the full length of the CAL is highly fibrocartilagenous, with areas of both uncalcified and calcified fibrocartilage. While often cited as a sign of degenerative change, the presence of fibrocartilage in both young specimens and those devoid of shoulder pathology implies that it is an integral component of the “normal” CAL. Thought to resist shear and compression forces, the fibrocartilage is concentrated most heavily at the entheses and is made up of type II collagen, link protein, and aggrecan, the latter of which is typical of articular cartilage.40 Along its posteroinferior surface at the acromial enthesis is a synovial layer coating the ligament’s fibrous core.46,51 In bipartite and multipartite ligaments, intervening ligamentous tissue thins progressively, resulting in formation of distinct bands of collagenous tissue.23 Concurrent along the midsubstance, there is reduced vascularization, reduced cell number, and some loss of collagen fiber orientation (Figure 3).
Figure 3.
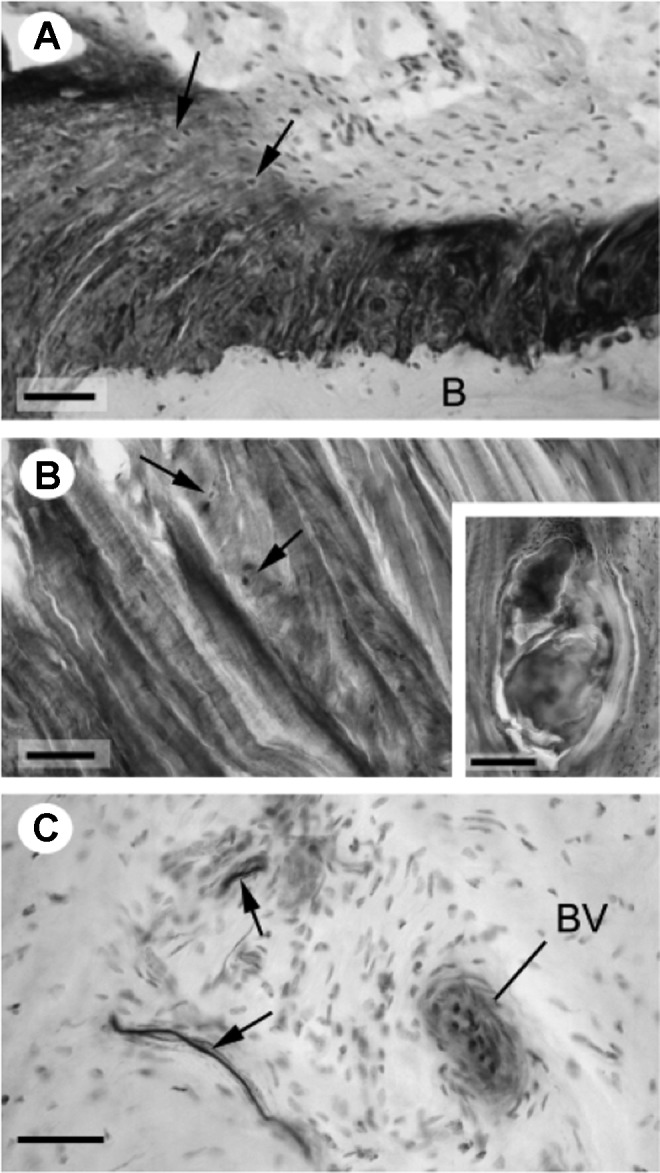
Histopathology. (A) Demonstrates fibrocartilage (arrows) at acromial enthesis. (B) Demonstrates fibrocartilage (arrows) at the mid-substance of the coracoacromial ligament. (C) Demonstrates the neurovascular bundle (BV) and nerve fibers (arrows) within the ligament. B, bony acromion (Reprinted with permission from Milz et al.38)
Investigation into alterations of “normal” CAL histology has revealed a number of consistent changes associated with age and shoulder pathology. Milz et al38 found that the distribution of fibrocartilage varied with age, as older (mean age, 74.7 years) specimens had increased concentrations of type II collagen and aggrecan along the CAL midsubstance when compared with younger (mean age, 24.2 years) specimens. Degenerative changes in fibrocartilage, including extracellular matrix degeneration, calcification, and ossification, were also associated with increasing age.40,46,51 Thus, increased exposure to compressive forces with age or repetitive activities may lead to metaplastic changes, calcium deposition, and increased fibrocartilage density along the midsubstance of the ligament, potentially contributing to shoulder pathology (Figure 4). In addition to changes in fibrocartilage composition and distribution, there are also characteristic changes in collagen fiber orientation and structure. CAL collagen fibers in young specimens are wavy (>2 inflection points per micrometer), whereas fibers in the elderly are straight (<2 inflection points per micrometer).57 Straightening results in increased elastic modulus as determined by scanning acoustic microscopy, translating into a stiffer ligament, which is associated with an increased frequency of rotator cuff tears.57 In specimens taken from patients with chronic impingement syndrome, there was fragmentation of collagen fibers resulting in fascicle size irregularity, fatty infiltration, and deposition of amorphous debris.51
Figure 4.
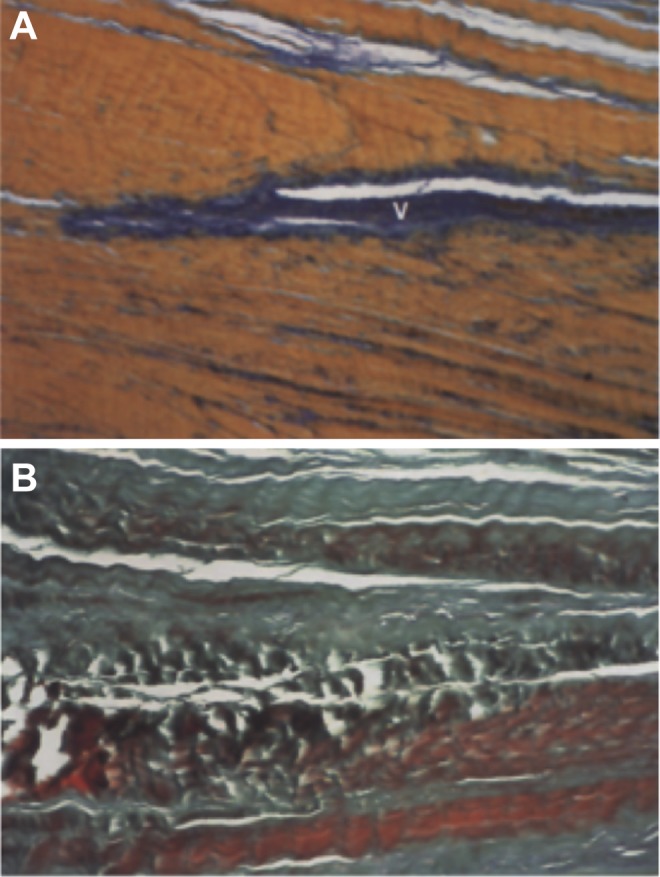
Comparison of histologic appearance with normal and degenerative coracoacromial ligament. (A) Normal appearance with parallel collagen bundles and elastic fibers. (B) Degenerative changes with loss of fiber orientation, decreased cell number, and overall disarray. V, blood vessel. (Reprinted with permission from Panni et al.46)
The blood supply originates from branches of the thoracoacromial artery entering the CAL primarily at the acromial and coracoid entheses. Microvessels course through superficial adipose tissue and within intervening areas of loose connective tissue separating adjacent collagen fibers. Adipose tissue is present in 2 forms, both intervening between adjacent fiber fascicles and lining the outermost layer of the ligament where it articulates with the subacromial bursa.40 Perhaps contributing to mechanosensory pathways, the adipose tissue is richly innervated with both free and encapsulated receptor nerve endings.9,36,40 The innervation of the CAL arises from branches of the suprascapular nerve that penetrate the ligament at both entheses. Nerve endings are most heavily concentrated in the acromial end of the CAL followed by the coracoid enthesis and midsubstance, respectively.36 The ligamentous substance of the CAL is heavily innervated with Pacinian corpuscles, Ruffini receptors, Golgi-tendon organ-like receptors, and free nerve endings.9,36 Additionally, Morisawa41 described the presence of “nontypical nerve endings,” including morphologically distinct Pacinian and Ruffini-like receptors and unclassifiable lamellated nerve endings (Figure 5). Both biomechanical and electrical stimulation studies have demonstrated the role of these nerve endings in dynamic stability of the shoulder.9,17 In particular, nonfocal electrical stimulation of the CAL resulted in inhibition of somatic control of ipsilateral shoulder muscles, likely via afferent inhibition of α-motor neurons.9
Figure 5.
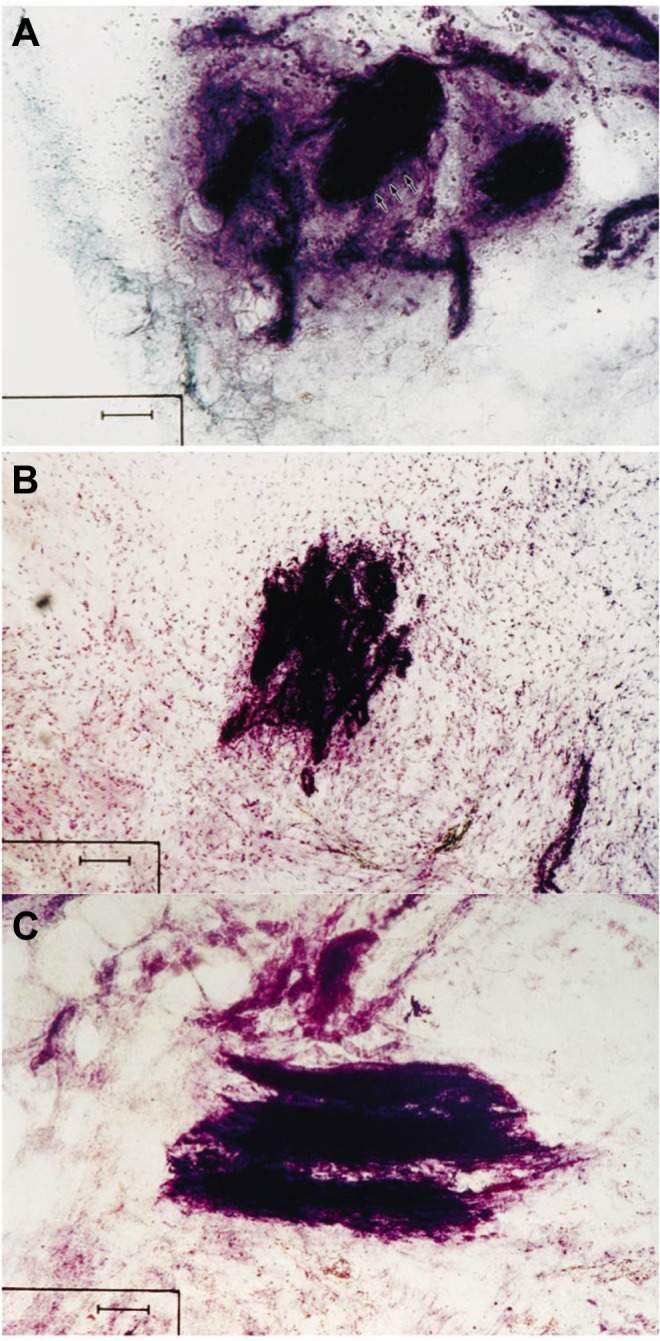
Histological images taken from coracoacromial ligament specimens. (A) Pacinian corpuscle. (B) Ruffini receptor has a dendritic structure. (C) Unclassifiable nerve ending. Scale bar represents 50 μm. (Reprinted with permission from Morisawa.41)
Function
The glenohumeral joint is uniquely dependent on muscles, tendons, and ligaments to maintain the humeral head in a functional position. The CAL is thought to play an important role in shoulder stability via both static restraint and dynamic interactions with other shoulder capsular elements including ligaments, muscles, and osseous structures. Simply by its position anterosuperior to the glenohumeral joint, it passively restricts upward displacement of the humeral head.4,41,47 The CAL also acts to transmit loads across the scapula. Serving as a tension band, forces exerted on the coracoid process by the coracobrachialis, pectoralis minor, and biceps (short head) muscles are transmitted to the acromion.18,55 Likewise, the acromial distortion due to forces exerted by the deltoid and trapezius muscles is limited by the action of the CAL.48 While of uncertain clinical significance, the CAL appears to serve as a dynamic brace within the shoulder girdle.
Given the high density of mechanoreceptors within the CAL, it seems reasonable to assume that it serves as a sensory organ, providing afferent static and dynamic proprioceptive signals. Pacinian corpuscles measure acceleration of the shoulder during movement, while Ruffini and Golgi tendon-organ receptors likely provide information about static position and angle of joint rotation.36 Proprioceptive information is then presumably integrated with other mechanosensory feeds to form feedback loops that affect shoulder muscle tone.9,36 Indeed, direct electrical stimulation of the CAL has been shown to inhibit voluntary control of shoulder muscles.9 Dynamic muscular control is essential to maintaining joint stability and preventing functional subacromial impingement, especially due to the relatively weak tensile strength of the CAL and other capsular ligaments that are often subjected to enormous directional forces.16,35,47,59
Imaging
As with many other structures of the glenohumeral joint, magnetic resonance imaging (MRI) is the modality of choice for visualizing the CAL.7 While the CAL can be visualized in the sagittal, coronal, or axial planes, Totterman et al56 reported that the CAL is best viewed in an oblique sagittal plane. The oblique sagittal view with proton-density and T2-weighted images is particularly useful for detecting thickening and hypertrophy of the CAL.30 When visualized with MRI, the fibrocartilagenous insertion of the CAL onto the inferior anterolateral surface of the acromion appears as a low signal density indenting on the overlying cuff. While this may be mistaken as a bone spur or enthesophyte, it is in fact a normal finding (Figure 6).30,50,56
Figure 6.
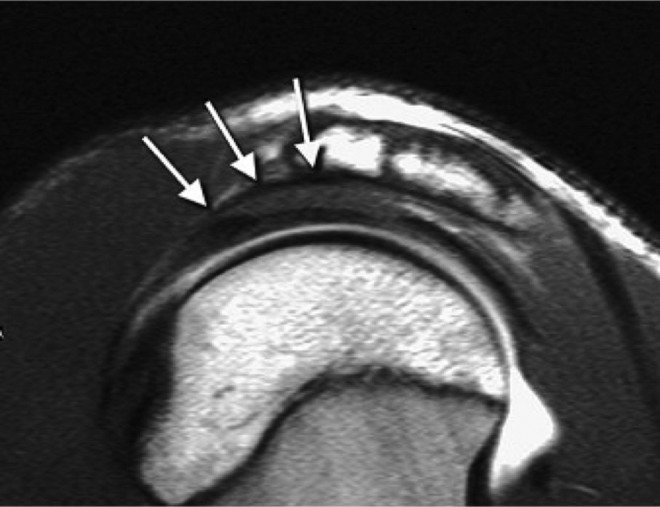
Parasagittal magnetic resonance image of the coracoacromial ligament attachment demonstrates the normal coracoacromial ligament, which may mimic an enthesophyte (white arrows). (Reprinted with permission from Rudez et al.50)
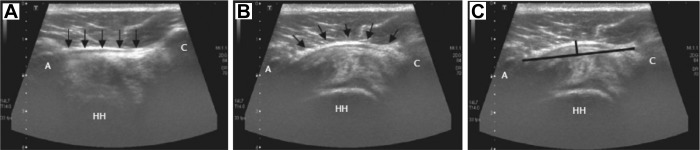
Other imaging modalities have been described for visualizing the CAL, including the use of conventional radiographs. It has been shown that conventional radiographs of the shoulder including a standard anterior-posterior view and lateral scapular view can detect enthesophyte formation within the CAL. The presence of enthesophytes within the CAL may be indicative of rotator cuff pathology.49 Wang et al59 detailed the use of high-resolution ultrasound for the visualization of the CAL, using an anterior-superior approach to accurately measure both its length and thickness. Ultrasound provides the distinct advantage of allowing dynamic visualization of the CAL during various provocative shoulder examination techniques. It has been hypothesized that bulging and elasticity of the CAL can be measured during impingement testing and used as a diagnostic tool for subacromial impingement syndrome (Figure 7).59,64
Figure 7.
Ultrasound measurement of displacement of coracoacromial ligament (CAL) during throwing motion. (A) CAL (indicated by arrows) at rest, (B) CAL bulging during shoulder abduction and internal rotation, (C) horizontal line connecting insertion points of CAL and vertical line measuring displacement of CAL. A, acromion; C, coracoid; HH, humeral head. (Reprinted with permission from Wu et al.63)
Pathologic Conditions
The CAL has long been implicated as contributing to the pathophysiology of pain associated with painful movement of the rotator cuff against the coracoacromial arch, or classic external impingement syndrome as first described by Neer.44 Contact between the coracoacromial arch and rotator cuff has been shown to be a physiologic phenomenon.6,59,63,65,66 However, many researchers have postulated that recurrent contact between the rotator cuff and CAL may lead to degenerative changes in both.11 By comparing shoulders with rotator cuff tears with age-matched controls, Kijima et al33 found an association between rotator cuff tears and straight collagen patterns, which have an increased elastic modulus (stiffness), within the substance of the CAL. This led them to theorize that increased stiffness of the CAL may raise contact pressures between the CAL and rotator cuff, resulting in pathologic degeneration.8 Dietrich et al10 recently compared CAL morphology in patients aged 20 to 60 years with and without shoulder pain due to subacromial impingement. Ultrasound evaluation showed that an anteriorly convex shape of the superficial contour of the CAL was more frequently seen in symptomatic patients.10
Posterior-inferior capsular tightness frequently seen in overhand-throwing athletes is a common finding in patients with external impingement syndrome.21,42,61 Several studies have examined the mechanical effects of posterior-inferior capsular tightness and have shown that the humeral head migrates anteriorly when the shoulder is put into flexion, abduction, and internal rotation.21 There is also a component of superior migration that occurs in flexion.21,42 As a restraint to superior translation of the humeral head, the CAL and coracoacromial arch experience significantly increased contact pressures in such circumstances.43 The clinical significance of anterior-superior humeral migration and resultant increased contact pressure on the CAL is not well understood. While repetitive use may lead to stiffness and eventual pathological changes within the ligament, contact force in itself does not appear to be pathologic.43,66 Some have proposed that posterior capsular tightness is one mechanism that may lead to impingement syndrome and rotator cuff pathology.26,43
Fiber disorganization within the CAL due to pathologic degeneration is also associated with enthesophyte formation.31 Spurs have been shown to form within the substance of the CAL, particularly at its acromial insertion (Figure 8).8,15,45–47,53,65 For those shoulders in which intraligament enthesophytes and/or rotator cuff pathologies were present, degenerative changes and differences in ligament geometry were usually localized to the lateral band only when multipartite.15,53 Of note, the preferential localization of changes in band architecture associated with shoulder pathology is not easy to determine. In addition to potential roles in degenerative rotator cuff pathology, CAL enthesopathy, independent of acromial angle, has been associated with impingement syndrome.37,38,40,47,65 A recent retrospective review evaluated the grade of CAL degeneration in patients with subacromial impingement.29 In this level 4 study, grades 1 and 2 CAL degeneration were strongly correlated with impingement syndrome, and partial CAL release was advocated by the authors particularly in the setting of bursal-sided partial rotator cuff tears.29 Yet others disagree with the notion that the degeneration and spurs contribute to impingement syndrome directly because they are usually contained within the substance of the CAL and do not impinge on adjacent structures.19,45
Figure 8.
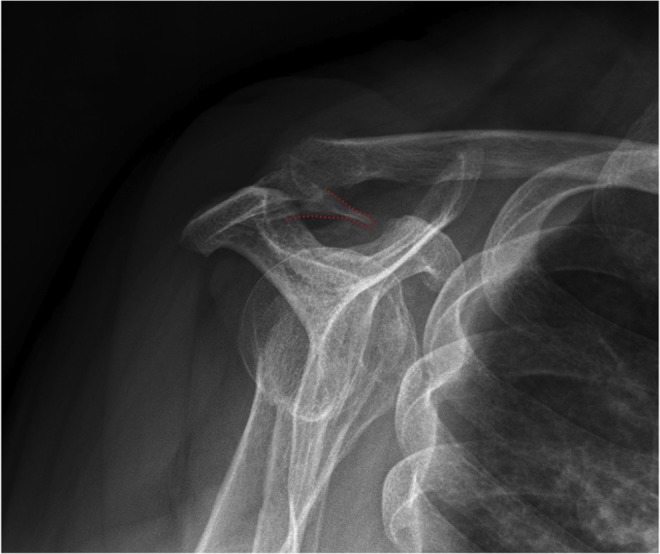
Plain radiography showing enthesophyte (dotted red outline) within coracoacromial ligament at acromial insertion. (Image courtesy of Matt Skalski, DC, DACBR, Radiopaedia.org [https://radiopaedia.org/cases/coracoacromial-ligament-ossification].)
As outlined above, the CAL and the coracoacromial arch serve as a static restraint to superior humeral head translation. Biomechanical and clinical studies have demonstrated increased anterior and superior glenohumeral translation after CAL release.¶ Budoff et al5 recently highlighted that CAL excision and acromioplasty increase rotator cuff force by 25% to 30% in order to maintain normal glenohumeral biomechanics. Additionally, several studies have also shown a position-dependent (0°-45° of shoulder abduction) increase in inferior translation after CAL release thought to be secondary to weakening of the coracohumeral ligament, although this has been subsequently refuted.35,41,60 Considerable controversy has surrounded what to do with the CAL during acromioplasty. Classically, the CAL has been released from its acromial insertion.1,3,22,44,47 Some authors have advocated preservation when possible and repair when indicated.2,14,24,27,58 Others, however, feel that damage to the CAL during acromioplasty, particularly the inferior acromial fibers, is inevitable.2,27 Although uncommon, one approach is to reconstruct the CAL through suture fixation of the detached portion of the ligament to the portion of the CAL remaining intact.2 Others have shown that direct release of the CAL from the anterior acromion precludes anatomic repositioning and have advocated subperiosteal elevation of the CAL, which has been shown to facilitate anatomic repair.52 A recent level 4 study evaluated patient-reported outcomes for impingement syndrome after arthroscopic subacromial decompression and CAL release without rotator cuff repair.28 Partial resection of the CAL was performed on all patients, and mean relative Constant scores at 20-year follow-up were 100.9%.28 Partial resection of the CAL has been reported elsewhere without any apparent postoperative complications related to its release.13,29 Interestingly, the CAL has been shown to reform spontaneously with the same orientation, gross appearance, and viscoelastic properties within a few years of release.20
In conclusion, the CAL is an integral component of the coracoacromial arch. Review of the literature demonstrates that the CAL plays an important role in shoulder biomechanics, joint stability, and proprioception. The CAL acts as a static restraint to superior humeral head migration and load distributor across the shoulder girdle. Additionally, it has a role in mechanosensory feedback loops that assist in dynamically stabilizing the shoulder through its full arc of motion. Age-dependent changes due to chronic stress and cellular degradation cause thickening and stiffening of the CAL that may contribute to a spectrum of shoulder pathology from capsular tightness to rotator cuff tear arthropathy and impingement syndrome. CAL release during acromioplasty remains controversial; however, partial release has been recently advocated in the literature with good long-term outcomes. Advances in our understanding of the CAL through clinical outcomes research will further contribute to clinical and intraoperative decision making in the future.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
References
- 1. Altchek DW, Warren RF, Wickiewicz TL, Skyhar MJ, Ortiz G, Schwartz E. Arthroscopic acromioplasty. Technique and results. J Bone Joint Surg Am. 1990;72:1198–1207. [PubMed] [Google Scholar]
- 2. Arrigoni P, Randelli P, Filiputti M, Cabitza P, Vaienti L. The CARE technique: arthroscopic CoracoAcromial ligament RE-attachment. Musculoskelet Surg. 2010;94(suppl 1): S65–S69. [DOI] [PubMed] [Google Scholar]
- 3. Bigliani LU, D’Alessandro DF, Duralde XA, McIlveen SJ. Anterior acromioplasty for subacromial impingement in patients younger than 40 years of age. Clin Orthop Relat Res. 1989;246:111–116. [PubMed] [Google Scholar]
- 4. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;330:13–30. [PubMed] [Google Scholar]
- 5. Budoff J, Lin C, Hong C, Chiang F, Su W. The effect of coracoacromial ligament excision and acromioplasty on the amount of rotator cuff force production necessary to restore intact glenohumeral biomechanics. J Shoulder Elbow Surg. 2016;25:967–972. [DOI] [PubMed] [Google Scholar]
- 6. Burns WC, 2nd, Whipple TL. Anatomic relationships in the shoulder impingement syndrome. Clin Orthop Relat Res. 1993;294:96–102. [PubMed] [Google Scholar]
- 7. Chaipat L, Palmer WE. Shoulder magnetic resonance imaging. Clin Sports Med. 2006;25:371–386. [DOI] [PubMed] [Google Scholar]
- 8. Chambler AF, Pitsillides AA, Emery RJ. Acromial spur formation in patients with rotator cuff tears. J Shoulder Elbow Surg. 2003;12:314–321. [DOI] [PubMed] [Google Scholar]
- 9. Diederichsen LP, Norregaard J, Krogsgaard M, Fischer-Rasmussen T, Dyhre-Poulsen P. Reflexes in the shoulder muscles elicited from the human coracoacromial ligament. J Orthop Res. 2004;22:976–983. [DOI] [PubMed] [Google Scholar]
- 10. Dietrich T, Jonczy M, Buck F, Sutter R, Puskas G, Pfirrmann W. Ultrasound of the coracoacromial ligament in asymptomatic volunteers and patients with shoulder impingement. Acta Radiol. 2016;57:971–977. [DOI] [PubMed] [Google Scholar]
- 11. Ecklund KJ, Lee TQ, Tibone J, Gupta R. Rotator cuff tear arthropathy. J Am Acad Orthop Surg. 2007;15:340–349. [DOI] [PubMed] [Google Scholar]
- 12. Edelson JG, Luchs J. Aspects of coracoacromial ligament anatomy of interest to the arthroscopic surgeon. Arthroscopy. 1995;11:715–719. [DOI] [PubMed] [Google Scholar]
- 13. Eid A, Dwyer A, Chambler A. Mid-term results of arthroscopic subacromial decompression in patients with or without partial thickness rotator cuff tears. Int J Shoulder Surg. 2012;6:86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fagelman M, Sartori M, Freedman, Patwardhan AG, Carandang G, Marra G. Biomechanics of coracoacromial arch modification. J Shoulder Elbow Surg. 2007;16:101–106. [DOI] [PubMed] [Google Scholar]
- 15. Fealy S, April EW, Khazzam M, Armengol-Barallat J, Bigliani LU. The coracoacromial ligament: morphology and study of acromial enthesopathy. J Shoulder Elbow Surg. 2005;14:542–548. [DOI] [PubMed] [Google Scholar]
- 16. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23:233–239. [DOI] [PubMed] [Google Scholar]
- 17. Fremerey R, Bastian L, Siebert WE. The coracoacromial ligament: anatomical and biomechanical properties with respect to age and rotator cuff disease. Knee Surg Sports Traumatol Arthrosc. 2000;8:309–313. [DOI] [PubMed] [Google Scholar]
- 18. Gallino M, Battiston B, Annaratone G, Terragnoli F. Coracoacromial ligament: a comparative arthroscopic and anatomic study. Arthroscopy. 1995;11:564–567. [DOI] [PubMed] [Google Scholar]
- 19. Gohlke F, Barthel T, Gandorfer A. The influence of variations of the coracoacromial arch on the development of rotator cuff tears. Arch Orthop Trauma Surg. 1993;113:28–32. [DOI] [PubMed] [Google Scholar]
- 20. Hansen U, Levy O, Even T, Copeland S. Mechanical properties of regenerated coracoacromial ligament after subacromial decompression. J Shoulder Elbow Surg. 2004;13:51–56. [DOI] [PubMed] [Google Scholar]
- 21. Harryman DT, 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA., 3rd Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72:1334–1343. [PubMed] [Google Scholar]
- 22. Hawkins RJ, Plancher KD, Saddemi SR, Brezenoff LS, Moor JT. Arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2001;10:225–230. [DOI] [PubMed] [Google Scholar]
- 23. Hockman DE, Lucas GL, Roth CA. Role of the coracoacromial ligament as restraint after shoulder hemiarthroplasty. Clin Orthop Relat Res. 2004;419:80–82. [DOI] [PubMed] [Google Scholar]
- 24. Holt EM, Allibone RO. Anatomic variants of the coracoacromial ligament. J Shoulder Elbow Surg. 1995;4:370–375. [DOI] [PubMed] [Google Scholar]
- 25. Hu X, Huang F, Zhong G, Cen S, Xiang Z, Li J. Biomechanical study on proximally based conjoined tendon transfer for coracoacromial ligament reconstruction as anterosuperior restraint of shoulder. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:1469–1473. [PubMed] [Google Scholar]
- 26. Huffman GR, Tibone JE, McGarry MH, Phipps BM, Lee YS, Lee TQ. Path of glenohumeral articulation throughout the rotational range of motion in a thrower’s shoulder model. Am J Sports Med. 2006;34:1662–1669. [DOI] [PubMed] [Google Scholar]
- 27. Hunt JL, Moore RJ, Krishnan J. The fate of the coracoacromial ligament in arthroscopic acromioplasty: an anatomical study. J Shoulder Elbow Surg. 2000;9:491–494. [DOI] [PubMed] [Google Scholar]
- 28. Jaegar M, Berndt T, Ruhmann O, Lerch S. Patients with impingement syndrome with and without rotator cuff tears do well 20 years after arthroscopic subacromial decompression. Arthroscopy. 2016;32:409–415. [DOI] [PubMed] [Google Scholar]
- 29. Kanatli U, Ayanoglu T, Aktas E, Ataoglu M, Ozer M, Cetinkaya M. Grade of coracoacromial ligament degeneration as a predictive factor for impingement syndrome and type of partial rotator cuff tear. J Shoulder Elbow Surg. 2016;25:1824–1828. [DOI] [PubMed] [Google Scholar]
- 30. Kaplan PA, Bryans KC, Davick JP, Otte M, Stinson WW, Dussault RG. MR imaging of the normal shoulder: variants and pitfalls. Radiology. 1992;184:519–524. [DOI] [PubMed] [Google Scholar]
- 31. Kelly BT, Dunn WR, Ramirez L, Warren RF. Traumatic rupture of the coracoacromial ligament. Arthroscopy. 2005;21:763. [DOI] [PubMed] [Google Scholar]
- 32. Kesmezacar H, Akgun I, Ogut T, Gokay S, Uzun I. The coracoacromial ligament: the morphology and relation to rotator cuff pathology. J Shoulder Elbow Surg. 2008;17:182–188. [DOI] [PubMed] [Google Scholar]
- 33. Kijima H, Minagawa H, Saijo Y, et al. Degenerated coracoacromial ligament in shoulders with rotator cuff tears shows higher elastic modulus: measurement with scanning acoustic microscopy. J Orthop Sci. 2009;14:62–67. [DOI] [PubMed] [Google Scholar]
- 34. Kopuz C, Baris S, Yildirim M, Gulman B. Anatomic variations of the coracoacromial ligament in neonatal cadavers: a neonatal cadaver study. J Pediatr Orthop B. 2002;11:350–354. [DOI] [PubMed] [Google Scholar]
- 35. Lee TQ, Black AD, Tibone JE, McMahon PJ. Release of the coracoacromial ligament can lead to glenohumeral laxity: a biomechanical study. J Shoulder Elbow Surg. 2001;10:68–72. [DOI] [PubMed] [Google Scholar]
- 36. McGinley JC, Agrawal S, Biswal S. Rotator cuff tears: association with acromion angulation on MRI. Clin Imaging. 2012;36:791–796. [DOI] [PubMed] [Google Scholar]
- 37. Michener LA, McClure PW, Karduna AR. Anatomical and biomechanical mechanisms of subacromial impingement syndrome. Clin Biomech (Bristol, Avon). 2003;18:369–379. [DOI] [PubMed] [Google Scholar]
- 38. Milz S, Jakob J, Büttner A, Tischer T, Putz R, Benjamin M. The structure of the coracoacromial ligament: fibrocartilage differentiation does not necessarily mean pathology. Scand J Med Sci Sports. 2008;18:16–22. [DOI] [PubMed] [Google Scholar]
- 39. Moorman CT, Warren RF, Deng XH, Wickiewicz TL, Torzilli PA. Role of coracoacromial ligament and related structures in glenohumeral stability: a cadaveric study. J Surg Orthop Adv. 2012;21:210–217. [DOI] [PubMed] [Google Scholar]
- 40. Moorman CT, 3rd, Hussain SS, Warren RF, Deng XH, Wickiewicz TL, Torzilli PA. Anatomy of the coracoacromial veil. J Surg Orthop Adv. 2008;17:69–73. [PubMed] [Google Scholar]
- 41. Morisawa Y. Morphological study of mechanoreceptors on the coracoacromial ligament. J Orthop Sci. 1998;3:102–110. [DOI] [PubMed] [Google Scholar]
- 42. Muraki T, Yamamoto N, Zhao KD, et al. Effect of posteroinferior capsule tightness on contact pressure and area beneath the coracoacromial arch during pitching motion. Am J Sports Med. 2010;38:600–607. [DOI] [PubMed] [Google Scholar]
- 43. Muraki T, Yamamoto N, Zhao KD, et al. Effects of posterior capsule tightness on subacromial contact behavior during shoulder motions. J Shoulder Elbow Surg. 2012;21:1160–1167. [DOI] [PubMed] [Google Scholar]
- 44. Neer CS., 2nd Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54:41–50. [PubMed] [Google Scholar]
- 45. Ogata S, Uhthoff HK. Acromial enthesopathy and rotator cuff tear. A radiologic and histologic postmortem investigation of the coracoacromial arch. Clin Orthop Relat Res. 1990;254:39–48. [PubMed] [Google Scholar]
- 46. Panni AS, Milano G, Lucania L, Fabbriciani C, Logroscino CA. Histological analysis of the coracoacromial arch: correlation between age-related changes and rotator cuff tears. Arthroscopy. 1996;12:531–540. [DOI] [PubMed] [Google Scholar]
- 47. Pieper HG, Radas CB, Krahl H, Blank M. Anatomic variation of the coracoacromial ligament: a macroscopic and microscopic cadaveric study. J Shoulder Elbow Surg. 1997;6:291–296. [DOI] [PubMed] [Google Scholar]
- 48. Putz R, Liebermann J, Reichelt A. The function of the coracoacromial ligament. Acta Anat (Basel). 1988;131:140–145. [PubMed] [Google Scholar]
- 49. Reichmister JP, Reeder JD, McCarthy E. Ossification of the coracoacromial ligament: association with rotator cuff pathology of the shoulder. Md Med J. 1996;45:849–852. [PubMed] [Google Scholar]
- 50. Rudez J, Zanetti M. Normal anatomy, variants and pitfalls on shoulder MRI. Eur J Radiol. 2008;68:25–35. [DOI] [PubMed] [Google Scholar]
- 51. Sarkar K, Taine W, Uhthoff HK. The ultrastructure of the coracoacromial ligament in patients with chronic impingement syndrome. Clin Orthop Relat Res. 1990;254:49–54. [PubMed] [Google Scholar]
- 52. Shaffer B, Evans B, Ferrero G. Release and reattachment of the coracoacromial ligament: a cadaveric study. J Shoulder Elbow Surg. 1997;6:297–305. [DOI] [PubMed] [Google Scholar]
- 53. Soslowsky LJ, An CH, Johnston SP, Carpenter JE. Geometric and mechanical properties of the coracoacromial ligament and their relationship to rotator cuff disease. Clin Orthop Relat Res. 1994;304:10–17. [PubMed] [Google Scholar]
- 54. Su WR, Budoff JE, Luo ZP. The effect of coracoacromial ligament excision and acromioplasty on superior and anterosuperior glenohumeral stability. Arthroscopy. 2009;25:13–18. [DOI] [PubMed] [Google Scholar]
- 55. Tillmann B, Tichy P. Functional anatomy of the shoulder. Unfallchirurg. 1986;89:389–397. [PubMed] [Google Scholar]
- 56. Totterman SM, Miller RJ, Meyers SP. Basic anatomy of the shoulder by magnetic resonance imaging. Top Magn Reson Imaging. 1994;6:86–93. [PubMed] [Google Scholar]
- 57. Uhthoff HK, Hammond DI, Sarkar K, Hooper GJ, Papoff WJ. The role of the coracoacromial ligament in the impingement syndrome. A clinical, radiological and histological study. Int Orthop. 1988;12:97–104. [DOI] [PubMed] [Google Scholar]
- 58. Wang J, Huang F. A biomechanical study on coracoacromial ligament as anterosuperior restraint of shoulder joint. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:49–51. [PubMed] [Google Scholar]
- 59. Wang YC, Wang HK, Chen WS, Wang TG. Dynamic visualization of the coracoacromial ligament by ultrasound. Ultrasound Med Biol. 2009;35:1242–1248. [DOI] [PubMed] [Google Scholar]
- 60. Wellmann M, Petersen W, Zantop T, Schanz S, Raschke MJ, Hurschler C. Effect of coracoacromial ligament resection on glenohumeral stability under active muscle loading in an in vitro model. Arthroscopy. 2008;24:1258–1264. [DOI] [PubMed] [Google Scholar]
- 61. Werner SL, Gill TJ, Murray TA, Cook TD, Hawkins RJ. Relationships between throwing mechanics and shoulder distraction in professional baseball pitchers. Am J Sports Med. 2001;29:354–358. [DOI] [PubMed] [Google Scholar]
- 62. Williams A, Calvert P, Bayley I. The bifurcate coracoacromial ligament: an arthroscopic variant. Arthroscopy. 1997;13:233–234. [DOI] [PubMed] [Google Scholar]
- 63. Wu CH, Chang KV, Su PH, Kuo WH, Chen WS, Wang TG. Dynamic ultrasonography to evaluate coracoacromial ligament displacement during motion in shoulders with supraspinatus tendon tears. J Orthop Res. 2012;30:1430–1434. [DOI] [PubMed] [Google Scholar]
- 64. Wu CH, Wang YC, Wang HK, Chen WS, Wang TG. Evaluating displacement of the coracoacromial ligament in painful shoulders of overhead athletes through dynamic ultrasonographic examination. Arch Phys Med Rehabil. 2010;91:278–282. [DOI] [PubMed] [Google Scholar]
- 65. Wuelker N, Plitz W, Roetman B. Biomechanical data concerning the shoulder impingement syndrome. Clin Orthop Relat Res. 1994;303:242–249. [PubMed] [Google Scholar]
- 66. Yamamoto N, Muraki T, Sperling JW, et al. Contact between the coracoacromial arch and the rotator cuff tendons in nonpathologic situations: a cadaveric study. J Shoulder Elbow Surg. 2010;19:681–687. [DOI] [PubMed] [Google Scholar]