Abstract
Background:
Meniscal root tears cause menisci and their insertions to inadequately distribute loads and potentially leave underlying articular cartilage unprotected. Untreated meniscal root tears are becoming increasingly recognized to induce joint degradation; however, little information is known about anterior meniscal root tears and how they affect joint tissue.
Purpose:
To observe the early degenerative changes within the synovial fluid, menisci, tibial articular cartilage, and subchondral bone after arthroscopic creation of untreated anterior meniscal root tears.
Study Design:
Controlled laboratory study.
Methods:
Anterolateral meniscal root tears were created in 1 knee joint of 5 adult Flemish Giant rabbits, and anteromedial meniscal root tears were created in 4 additional rabbits. The contralateral limbs were used as nonoperated controls. The animals were euthanized at 8 weeks postoperatively; synovial fluid was aspirated, and tissue samples of menisci and tibial articular cartilage were collected and processed for multiple analyses to detect signs of early degeneration.
Results:
Significant changes were found within the synovial fluid, meniscal tissue, and tibial subchondral bone of the knees with anterior meniscal root tears when compared with controls. There were no significant changes identified in the tibial articular cartilage when comparing the tear groups with controls.
Conclusion:
This study demonstrated early degenerative changes within the synovial fluid, menisci, and tibial subchondral bone when leaving anterior meniscal root tears untreated for 8 weeks. The results suggest that meniscal tissue presents measurable, degenerative changes prior to changes within the articular cartilage after anterior meniscal root tears. Anterior destabilization of the meniscus arthroscopically may lead to measurable degenerative changes and be useful for future in vivo natural history and animal repair studies.
Clinical Relevance:
The present study is the first to investigate various tissue changes after anterior meniscal root tears of both the medial and lateral menisci. The results from this study suggest that degenerative changes occur within the synovial fluid, meniscus, and tibial subchondral bone prior to any measurable changes to the tibial articular cartilage. Further studies should expand on this study to evaluate how these components continue to progress when left untreated for long periods.
Keywords: meniscus, anterior meniscus roots, meniscal root tear, osteoarthritis, animal model
Menisci are crescent-shaped, fibrocartilaginous wedges that play an important role in complex knee mechanics and function.3,39 The menisci are primarily responsible for distributing loads through the tibiofemoral joint, joint stabilization, and congruency.1,20,27,30,49,55 The circumferential collagen fibers in the meniscus body continue into the anterior and posterior root insertional ligaments that attach to the tibial plateau.5,7,18,22,45 Continuity of the circumferential fibers between the meniscus body and its insertions enables proper fixation into bone and facilitates the distribution of axial tibiofemoral stresses to circumferential hoop stresses.13,50
Meniscal root tears (MRTs) are complete radial tears or avulsion injuries of the meniscal root insertions from the tibial plateau and are a subset of injuries that cause the meniscus to inadequately distribute loads and protect the underlying articular cartilage.26,32,48 Untreated MRTs are becoming increasingly recognized to induce articular cartilage degradation over time2,24; therefore, proper understanding of injury and progression of degeneration is essential.
Previously, studies on meniscal release, or destabilization of the medial meniscus (DMM), have been used to induce and analyze degeneration of knee joint tissues in murine, lapine, canine, and ovine models over time.10,11,21–23,29,36 These models all demonstrate measurable degeneration within the knee joint tissues. Despite the wide use of these models for osteoarthritis research, however, the authors are not aware of any studies assessing the degeneration of several tissues within the knee joint after release of the anterior insertions for both the lateral and medial menisci. Cadavers have also been used to investigate MRTs; however, these studies primarily focus on changes in knee biomechanics to the posteromedial and posterolateral meniscal insertions.2,6,34
Although tears of the anterior meniscal root insertions may be less common than posterior tears, a recent study reported that iatrogenic injury occurs at the anterior insertions of the lateral and medial menisci while reaming tibial tunnels for anterior cruciate ligament (ACL) reconstruction.56 Currently, literature on anterior MRTs is limited to reports of case studies and anatomic analysis of the relationship between the ACL and anterior meniscal root insertions.15,31,33,40,54 Since the anterior meniscal insertions are susceptible to damage during ACL reconstructions, increasing the risk for MRTs, it is important to understand how these injuries affect the joint tissues. Additionally, since clinical samples of articular cartilage and menisci are usually salvaged from advanced stages of osteoarthritis after total knee reconstructions, animal models are commonly used to experimentally induce injury and assess early degeneration.
Therefore, the purpose of this study was to measure characteristics of early degeneration in the rabbit knee after untreated anterior MRTs for major sites of earliest discernible joint involvement seen in osteoarthritis.38 The amount and type of inflammatory cells present in the synovial fluid, the compressive material properties of the menisci and tibial articular cartilage, the subchondral bone morphology of the tibial plateau, the coverage and content of glycosaminoglycans (GAGs) of menisci and tibial articular cartilage, the total content of intact DNA, and the relative gene expression of matrix-degrading enzymes were measured after anterolateral MRTs (ALMRTs) and anteromedial MRTs (AMMRTs). It was hypothesized that if anterior MRTs of either the medial or lateral menisci were left untreated after injury, early osteoarthritic change would occur within the joint tissues.
Methods
Institutional Animal Care and Use Committee (IACUC) approval was obtained prior to performing the study. Nine skeletally mature Flemish Giant rabbits (5.2 ± 0.2 kg) were housed in individual cages and given 3 weeks to acclimate to the housing facility. During housing, animals were monitored daily for health status, and no adverse events were observed. Before surgery, the animals were given 0.2 mg/kg butorphanol, 0.05 mg acepromazine, and 0.005 mg glycopyrrolate and then anesthetized with 5% isoflurane. A small-joint arthroscope was used to confirm the absence of preexisting arthritis or meniscal injury. Under arthroscopic visualization, a scalpel was used to create ALMRTs in 1 knee joint of 5 rabbits and AMMRTs were created in the remaining 4. After sectioning with a scalpel, the meniscal roots were probed to verify they were completely sectioned off their root attachments. A top view of the dissected proximal tibia with the menisci intact is presented for visualization of the rabbit knee anatomy in Figure 1. To minimize the effects of subjective bias, the animals were randomly assigned to an MRT group and surgeries were alternated between left and right limbs. The contralateral knees were left intact with no sham incision and used as nonoperative controls. The animals were monitored postoperatively and returned to normal activity in individual cages before euthanasia 8 weeks postsurgery.
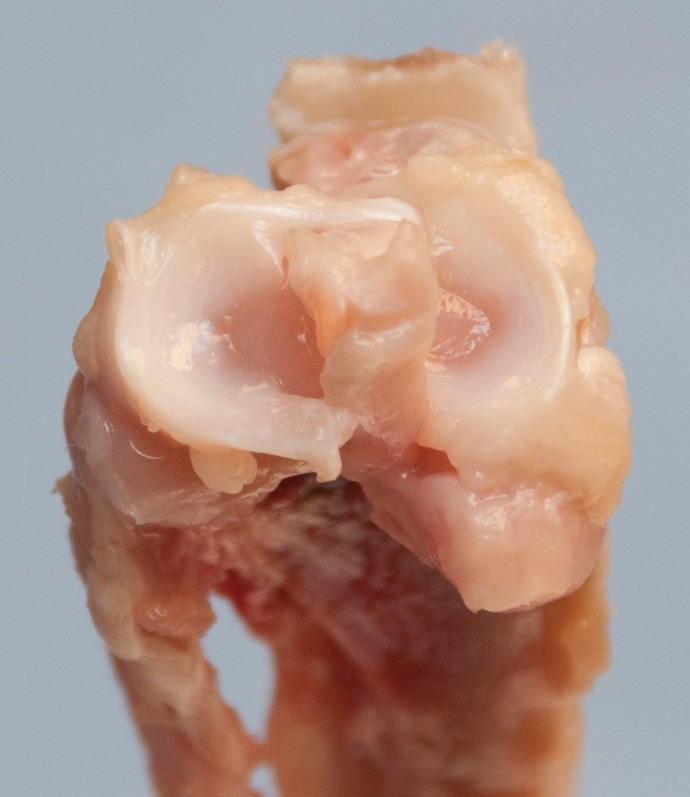
Figure 1.
A dissected, proximal tibia with menisci intact for visualization of the rabbit knee anatomy from a left, control limb.
Cytologic Joint Evaluation
Immediately after euthanasia, 1.5 mL of sterile saline was injected into both injured and control knee joints just medial to the patellar tendon. The knee was flexed and extended 3 to 5 times, and diluted synovial fluid was reaspirated from the joint using the initial delivery syringe by a veterinary technician. The volume acquired from each joint was recorded to determine whether joint effusion was present. After collection, approximately 20 µL of joint fluid was placed onto a microscope slide and spread using a separate slide to make a standard “push smear” for cytologic evaluation. Duplicate smears for each joint were blinded and viewed by a board-certified veterinary clinical pathologist. Joint fluid was then subjectively interpreted and objectively scored for overall cellularity, individual cell types observed, evidence of synovial hyperplasia, and presence of osteoclasts (Table 1).
TABLE 1.
Objective Scoring Scheme for Grading Cytologic Findings Present in Synovial Fluid Smearsa
| Cytology Score Grading Scheme | ||||
|---|---|---|---|---|
| Measure | Score | |||
| 0 | 1 | 2 | 3 | |
| Number of cells observed | Normal | Mild increase | Moderate increase | Marked increase |
| Cell types observed | >95% large mononuclear cells present | >95% large mononuclear cells present but activated cells seen | Mixed inflammation | Neutrophilic inflammation |
| Synovial hyperplasia (aggregates of spindle-shaped fibroblasts) | Absent | Present | ||
| Osteoclast (evidence for bone remodeling) | Absent | Present | ||
aThis system, which allows increments from 0 to 8, provides an interpretation of inflammation, synovial hyperplasia, and bone remodeling that may occur in a joint.
Mechanical Testing
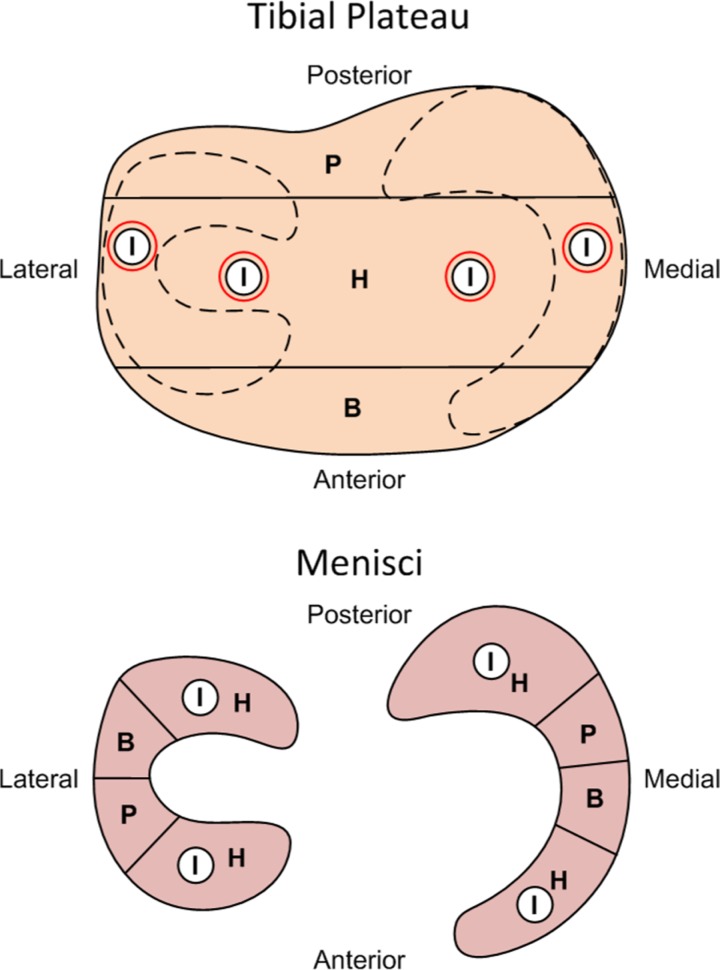
Indentation-relaxation testing was performed on menisci and tibial cartilage similar to previous studies within 12 hours of sacrifice and dissection.16,17,35,52 The menisci and proximal tibias were wrapped separately in gauze, saturated with 1× phosphate-buffered saline, and stored at 4°C until ready to test. All tissues were hydrated in a 1× phosphate-buffered saline bath at room temperature during testing. Prior to mechanical testing, the menisci were each transected into anterior and posterior halves. Indentation-relaxation tests were then conducted in the middle of each anterior and posterior meniscus half (Figure 2). A spherical, steel indenter with a 1.59 mm diameter was used to indent to a depth of 0.2 mm at 0.2 mm/s for all samples and held for 900 s to reach equilibrium. Indentation-relaxation tests were conducted at 4 locations on the articular cartilage of the tibial plateau to account for locations normally covered and uncovered by the menisci, similar to a previous study (Figure 2).16 Thickness measurements of the articular cartilage indentation sites were estimated by inserting a needle at a location adjacent to each indentation site and observing when the force changed due to contact with calcified cartilage, similar to previous studies.16,46 The distance between the initial force due to the needle first contacting the cartilage surface and the force peak observed when the needle displaced through the articular cartilage and contacted the calcified cartilage was used as the thickness measurement. The spherical indenter was then pressed into the cartilage to a depth of 20% of its estimated thickness at 20% strain/s and held for 180 seconds to reach equilibrium. After a 1200-second rest time, the indenter was replaced with the needle and the actual thickness of the articular cartilage at the indentation site was determined.16 Hertzian contact was assumed between the tissue (elastic half-space) and the steel, spherical indenter (rigid sphere), similar to previous studies.16,17,35,46 The instantaneous and equilibrium elastic moduli were then calculated to determine the compressive elasticity immediately after compression before interstitial fluid dissipated and again once fluid dissipation reached equilibrium, respectively. Poisson ratios for menisci and articular cartilage in rabbits have been estimated in previous studies.16,17,46,52 Based on these studies, the Poisson ratio was assigned to be 0.01 for menisci and 0.3 for the articular cartilage. The elastic modulus and Poisson ratio of the indenter were 210 GPa and 0.3, respectively.16,17
Figure 2.
Tissue locations allocated for different analyses on the menisci and tibial plateaus including: indentation-relaxation (I), histology (H), biochemical assays (B), and quantitative reverse transcription polymerase chain reaction (qRT-PCR) (P).
After mechanical testing, all tissue was divided and stored separately for additional analysis (Figure 2). Menisci were sectioned to store anterior and posterior samples in 10% formalin to fix the tissue for histological analysis. The central portions of each menisci were cut to store half in RNAlater RNA Stabilization Reagent (QIAGEN) to immediately stabilize tissue RNA for gene expression analysis and the rest in Allprotect Tissue Reagent (QIAGEN) to stabilize DNA, RNA, and protein in tissue samples for quantitative biochemical assays. Cylindrical, full-depth samples of the articular cartilage using a 5-mm biopsy punch were taken from the anterior and posterior locations of the tibial plateau, with half of the tissue stored in RNAlater and the rest stored in Allprotect. The remainder of the proximal tibias were fixed in 10% formalin. All samples in RNAlater and Allprotect were refrigerated for 24 hours to allow the reagents to penetrate the tissues and then stored at −80°C until analyzed.
Bone Morphology Analysis
Proximal tibias were scanned via a micro–computed tomography machine (Scanco Medical AG) to evaluate subchondral bone changes.44 Briefly, 4 spatially distributed, cylindrical volumes of interest (VOIs) were identified for each tibia based on anatomical markers and corresponding with the mechanical indentation testing sites of the articular cartilage (Figure 2). Trabecular VOIs had a diameter of 2.2 mm and height of 3.7 mm and were taken immediately under the subchondral bone plate to analyze the trabeculae within the subarticular spongiosa.37 Subchondral bone plate VOIs had a diameter of 2.2 mm with varying heights because of anatomic differences. The following variables were measured within the analyzed VOIs: trabecular material bone mineral density, trabecular bone volume fraction, trabecular number, trabecular thickness, trabecular spacing, subchondral bone mineral density, and subchondral bone volume fraction.
Histological Analysis
Fixed menisci were embedded in optimum cutting temperature medium (Pelco), flash frozen using liquid nitrogen, and sectioned into 6-μm slices. The fixed proximal tibias were decalcified with 10% formic acid and cut to evaluate central, coronal sections of the tibial plateaus (Figure 2). The tibial plateaus were then embedded in paraffin and sectioned into 6-μm slices. All sections were stained using hematoxylin, safranin-O, and fast green before being imaged using a light microscope (Olympus) and camera setup (QImaging).47
Imaged meniscal and tibial articular cartilage sections were analyzed similarly to previous studies.16,17,43 Briefly, the meniscal sections were analyzed quantitatively by measuring the percentage of red-stained GAG covering the entire meniscal section using Image J software (National Institutes of Health) with the FIJI package. The GAG stain intensity of the menisci was then blindly evaluated by 4 individuals using a previously used grading scale: no staining = 0, slight staining = 1, moderate staining = 2, and strong staining = 3. Articular cartilage and subchondral bone sections were graded histologically using a modified Mankin scale in 3 categories (Table 2).16 Qualitative grades of the menisci and articular cartilage for each specimen and region were averaged across all graders.
TABLE 2.
Modified Mankin Grading Scale Used to Assess Histological Staining of the Tibial Articular Cartilage
| Measure | Score | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Glycosaminoglycan staining | Uniform | Loss of staining in superficial zone <50% of length of plateau | Loss of staining in superficial zone >50% of length of plateau | Loss of staining in upper 2/3 <50% of length of plateau | Loss of staining in upper 2/3 >50% of length of plateau | Loss of staining in full depth of cartilage <50% of length of plateau | Loss of staining in full depth of cartilage >50% of length of plateau |
| Fissures | Absent | Surface fibrillation <50% of length of plateau | Surface fibrillation >50% of length of plateau | 1-2 midzone fissures | 3-5 midzone fissures | Full depth fissures or 5+ midzone fissures | Large segments of cartilage eroded with full depth fissures |
| Tidemark integrity | Normal | Disrupted | |||||
Biochemical Analysis
The menisci and articular cartilage samples stored in Allprotect Tissue Reagent were used to assess the biochemical content between the anterior MRT groups and controls. Samples were removed from the −80°C freezer, thawed, and all immediately digested with 200 µL of papain (125 μg/mL) in 0.1 M sodium acetate, 5 mM l-cysteine–HCl, 0.05 mM ethylenediamine tetra-acetic acid, pH 6 (Sigma-Aldrich) constantly agitated at 60°C for 18 hours. DNA content of each sample was quantified using a PicoGreen double-stranded DNA quantification kit (Molecular Probes). Proteoglycan content was estimated by quantifying the amount of sulfated GAGs using a dimethylmethylene blue assay with a shark chondroitin sulfate standard.14 Each constituent was normalized to the tissue wet weight and the GAG was normalized to the DNA content for analysis.
Quantitative Reverse Transcription Polymerase Chain Reaction
Tissue samples stored in RNAlater were used to assess the relative gene expression of various proteins between the anterior MRT and control groups using quantitative reverse transcription polymerase chain reaction (qRT-PCR). Expressions of known catabolic proteins within articular cartilage and meniscal tissue, matrix metalloproteinase (MMP)-1, MMP-9, MMP-13, and aggrecanase-2 (ADAM-TS5), were targeted for gene analysis similar to previous studies.8,9,19,53 Furthermore, tissue inhibitor of metalloproteinase-1 (TIMP-1) was assessed to measure the expression of catabolic protein inhibition within the tissues.8,9
Thawed menisci and articular cartilage samples were pulverized and homogenized in Trizol (Invitrogen) on ice using a tissue homogenizer to extract RNA. Homogenized samples were then allowed to incubate at room temperature for 20 minutes in Trizol followed by centrifugation at 12,000g for 12 minutes at 4°C. Supernatants were transferred to a new microcentrifuge tube, mixed with 200 µL of chloroform, incubated at room temperature for 3 minutes, and then centrifuged at 12,000g for 15 minutes at 4°C. The upper aqueous phase was transferred to a new microcentrifuge tube and mixed with 500 µL of isopropanol. Samples were incubated at room temperature for 10 minutes followed by centrifugation at 12,000g for 10 minutes at 4°C to pellet the RNA. The RNA fraction was rinsed with 70% ethanol and resuspended with RNase-free water (QIAGEN). qRT-PCR was then carried out through a single-step process using an iTaq Universal SYBR Green One-Step kit (Bio-Rad Laboratories) as per manufacturer’s instructions with the primers listed in Table 3 (Integrated DNA Technologies). To determine relative expression of the target genes, 5 ng of RNA were used per sample and assessed in duplicate. Quantification cycle (Cq) values were determined, and duplicates were averaged for the targeted genes of interest and the reference gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Expression values were presented relative to GAPDH, and each gene was adjusted using the respective PCR amplification efficiencies.
TABLE 3.
Specific Primer Sequences, Product Sizes, and References for Targeted Genes and Reference Gene for qRT-PCR Analysisa
| Gene of Interest | Primer Direction | Primer Sequence | Product Size, bp | Reference Sequence |
|---|---|---|---|---|
| MMP-1 | Forward | 5′-CCA AAG TCT CCA AGG GTC AA-3′ | 83 | NM_001171139.1 |
| Reverse | 5′-CTG TCC TTC AGG TCC ATC AAA-3′ | |||
| MMP-9 | Forward | 5′-TGC GAG TTT CCG TTC ATC TT-3′ | 117 | NM_001082203.1 |
| Reverse | 5′-GTA GAG CTT GTC CTT GTC GTA G-3′ | |||
| MMP-13 | Forward | 5′-GGG ATT CCC AAG AGA GGT TAA-3′ | 100 | NM_001082037.1 |
| Reverse | 5′-TCA TAG CTC CAG ACT TGG TTT C-3′ | |||
| TIMP-1 | Forward | 5′-ACT CCC ACA AAT CCC AGA A-3′ | 98 | NM_001082232.2 |
| Reverse | 5′-GGA ACC ACG AAA CTG CAA-3′ | |||
| ADAM-TS5 | Forward | 5′-GTC ATC CAT CCT CAC CAG TAT C-3′ | 116 | AF317415 |
| Reverse | 5′-TCG TGG TAC ATC TAG CAA ACA G-3′ | |||
| GAPDH | Forward | 5′-GGT CGG AGT GAA CGG ATT T-3′ | 114 | NM_001082253.1 |
| Reverse | 5′-TGT AGT GGA GGT CAA TGA ATG G-3′ |
aADAM-TS5, aggrecanase-2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MMP, matrix metalloproteinase; qRT-PCR, quantitative reverse transcription polymerase chain reaction; TIMP-1, tissue inhibitor of metalloproteinase-1.
Statistical Analysis
Synovial fluid volumes were confirmed for Gaussian distribution by the D’Agostino-Pearson omnibus normality test; therefore, paired t tests were used to determine differences between knees with anterior MRTs and control knees. Objective scorings of synovial fluid and histological staining were not normally distributed; thus, data were analyzed using the Wilcoxon matched-pairs signed rank test. The remainder of analysis methods utilized 2-sample, equal variance Student t tests to determine differences between the anterior MRT groups and controls. An a priori power analysis established that with 9 total specimens, our study was powered to detect differences with power of ≥80% for all statistical tests except the objective scoring. The study was powered to detect differences with power of ≥70% for the objective scoring of the synovial fluid and histological staining. Significant differences were set at P < .05 for all tests performed.
Results
After surgery, the rabbits favored the contralateral limb for the initial 1 to 2 days but showed no further signs of gait irregularity prior to euthanasia. All meniscal insertions were inspected during dissection and remained free-floating. Little to no macroscopic differences of the menisci and articular cartilage were observed between the injured and control knees during dissection. Inflammation of the synovial membrane surrounding the knee was observed for all anterior MRT knees when compared with the contralateral macroscopically.
Cytology Evaluation
A significant increase (P = .001) in synovial fluid volume was aspirated from the joints with surgically induced MRTs, suggesting an effusive process was present. Synovial fluid volume aspirated from the control and injured knees was 297 ± 112 and 1050 ± 125 µL, respectively. Compared with control knees, injured knees demonstrated a significantly higher (P = .004) objective cytology score. Cytology scores were interpreted from the synovial fluid of each joint using the scoring scheme in Table 1, and medians with interquartile ranges were calculated between groups. The cytology score was 0 with an interquartile range of (0, 0) and 3 with an interquartile range of (2, 3) for control and injured knees, respectively. Synovial fluid from control knees did not demonstrate cytologic abnormalities. Joint fluid collected from injured knees, however, had mild to moderate increases in activated large mononuclear cells interpreted as nonsuppurative inflammation. Neither synovial hyperplasia nor osteoclasts were observed.
Mechanics
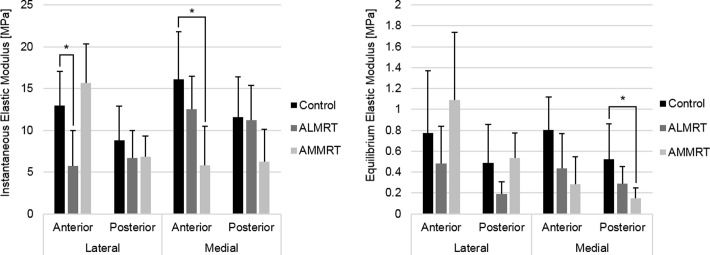
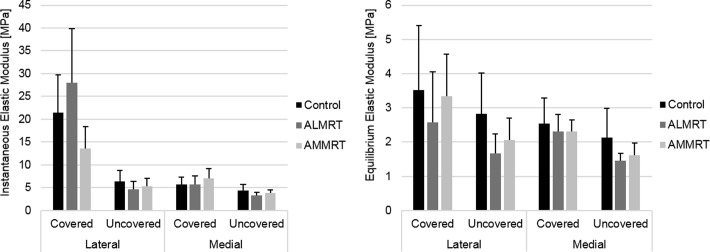
Indentation-relaxation testing of the menisci resulted in decreases of both the instantaneous and equilibrium elastic moduli in the injured limbs compared with control limbs. The instantaneous (P = .01) and equilibrium (P = .02) elastic moduli of the anteromedial region significantly decreased when comparing the AMMRT group with the control group (Figure 3). Similarly, the instantaneous elastic modulus of the anterolateral region in the ALMRT group significantly decreased when compared with the control group (P = .009) (Figure 3A). Although not significant, the mean equilibrium elastic modulus decreased for both the anterior and posterior regions of the lateral meniscus after the ALMRT (Figure 3B). In the analysis of the tibial articular cartilage, significant changes were not present in either the instantaneous or equilibrium elastic moduli for either MRT group when compared with the control group (Figure 4).
Figure 3.
Instantaneous and equilibrium elastic moduli of meniscus regions (mean with standard deviation). *Statistically significant (P < .05). ALMRT, anterolateral meniscal root tear; AMMRT, anteromedial meniscal root tear.
Figure 4.
Instantaneous and equilibrium elastic moduli of tibial articular cartilage regions (mean with standard deviation). *Statistically significant (P < .05). ALMRT, anterolateral meniscal root tear; AMMRT, anteromedial meniscal root tear.
Bone Morphology
Significant decreases in subchondral bone integrity were present after anterior MRTs, primarily within the trabecular structure of the subarticular spongiosa region (Table 4). When an anterior MRT was left untreated, the region uncovered by the menisci within the hemijoint opposite the MRT demonstrated a significant decrease in the mean number of trabeculae and a significant increase in the average spacing between trabeculae. For example, the number of trabeculae decreased and spacing between trabeculae increased for the lateral uncovered region after the AMMRT. Additionally, the mean thickness of the trabeculae significantly decreased in the medial covered region after the AMMRT (P = .04). No significant changes were seen in trabecular thickness for any regions after ALMRT. There were no significant changes within the trabecular or subchondral bone volume fraction for all regions when compared between MRT groups and controls. Similarly, there were also no significant changes to the mineral content within the trabecular or subchondral bone plate tissue in any regions. Volume-rendering examples for the trabecular and subchondral bone VOIs in control and both anterior MRT limbs are shown in Figure 5.
TABLE 4.
Subchondral Bone Morphology Measurement Variables for Meniscal Root Tear and Control Groupsa
| Trabecular Thickness, mm | Trabecular Spacing, mm | Trabecular Number, 1/mm | Trabecular Bone Volume Fraction, mm3/mm3 | Trabecular Bone Mineral Density, mg HA/mm3 | Subchondral Bone Volume Fraction, mm3/mm3 | Subchondral Bone Mineral Density, mg HA/mm3 | |||
|---|---|---|---|---|---|---|---|---|---|
| Control | Lateral | Covered | 0.24 (0.03) | 0.33 (0.06) | 2.88 (0.49) | 0.56 (0.07) | 873 (22) | 0.92 (0.07) | 905 (14) |
| Uncovered | 0.24 (0.04) | 0.50 (0.14) | 2.15 (0.50) | 0.41 (0.04) | 889 (26) | 0.90 (0.06) | 952 (13) | ||
| Medial | Covered | 0.28 (0.04) | 0.52 (0.12) | 2.10 (0.44) | 0.46 (0.09) | 865 (26) | 0.91 (0.04) | 897 (29) | |
| Uncovered | 0.25 (0.06) | 0.68 (0.22) | 1.67 (0.51) | 0.34 (0.12) | 871 (34) | 0.89 (0.08) | 934 (25) | ||
| ALMRT | Lateral | Covered | 0.26 (0.05) | 0.34 (0.07) | 2.65 (0.32) | 0.57 (0.08) | 884 (18) | 0.95 (0.02) | 920 (9) |
| Uncovered | 0.29 (0.06) | 0.43 (0.04) | 2.28 (0.15) | 0.47 (0.05) | 920 (26) | 0.96 (0.01) | 957 (4) | ||
| Medial | Covered | 0.29 (0.05) | 0.57 (0.11) | 1.94 (0.25) | 0.44 (0.08) | 885 (26) | 0.92 (0.04) | 926 (15) | |
| Uncovered | 0.27 (0.06) | 0.96 (0.11) | 1.15 (0.09) | 0.27 (0.05) | 902 (27) | 0.91 (0.04) | 946 (25) | ||
| AMMRT | Lateral | Covered | 0.26 (0.03) | 0.56 (0.32) | 2.14 (0.78) | 0.50 (0.11) | 868 (17) | 0.95 (0.03) | 901 (20) |
| Uncovered | 0.21 (0.03) | 0.78 (0.32) | 1.48 (0.48) | 0.32 (0.12) | 871 (21) | 0.87 (0.04) | 938 (11) | ||
| Medial | Covered | 0.23 (0.03) | 0.39 (0.32) | 2.50 (0.24) | 0.50 (0.05) | 868 (18) | 0.93 (0.03) | 910 (32) | |
| Uncovered | 0.22 (0.02) | 0.74 (0.25) | 1.50 (0.43) | 0.31 (0.11) | 871 (24) | 0.85 (0.07) | 913 (30) |
aValues are presented as mean (SD). Values in boldface indicate statistical significance (P < .05 when compared with the control group). ALMRT, anterolateral meniscal root tear; AMMRT, anteromedial meniscal root tear; HA, hydroxyapatite.
Figure 5.
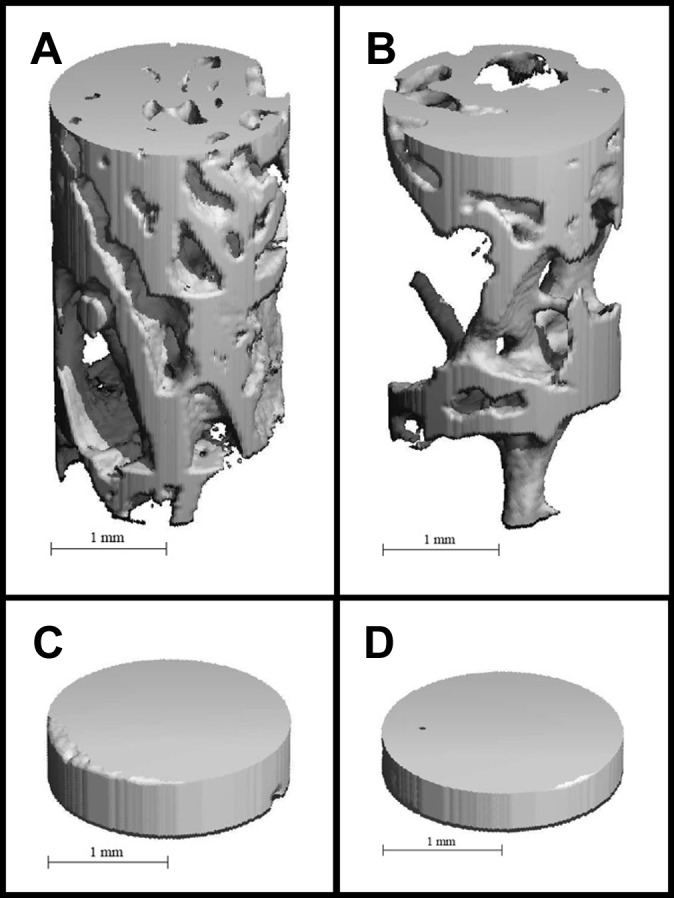
Volume renderings of trabecular and subchondral bone volumes of interest (VOIs) in control and anterior meniscal root tear limbs. Examples are provided for trabecular bone VOI from (A) the lateral uncovered region in the control limb, (B) trabecular bone VOI from the lateral uncovered region in the anteromedial meniscal root tear limb, (C) subchondral bone VOI from the medial uncovered region in the control limb, and (D) subchondral bone VOI from the medial uncovered region in the anterolateral meniscal root tear limb.
Histology
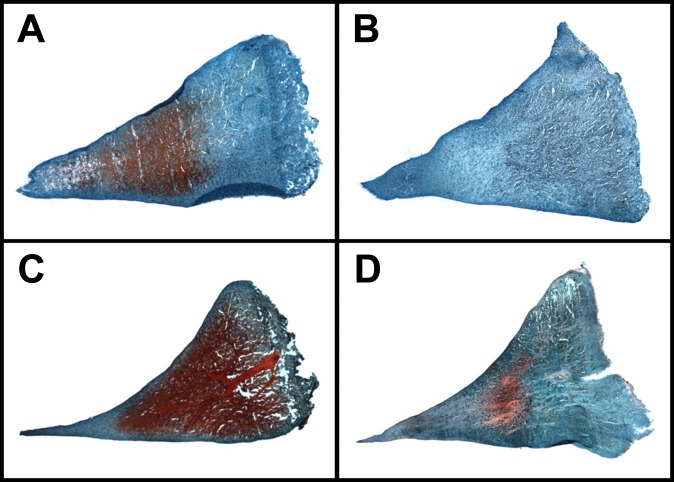
There was a positive correlation between the quantitative percentages of meniscal GAG coverage and the qualitative GAG stain intensity scores (R 2 = 0.84). A significant decrease was found in the mean percentage of GAG coverage in the PM region of the meniscus after the AMMRT (P = .04) (Figure 6). Similar results were obtained from GAG stain intensity grading, where the only significant decrease in GAG was in the PM region after the AMMRT (P = .01) (Table 5). Additionally, although not significant, average GAG decreased in the anterior regions after the transection of that meniscal root, with a greater decrease in the anteromedial region after the AMMRT. Examples of GAG staining in meniscal regions for control, AMMRT, and ALMRT limbs are shown in Figure 7.
Figure 6.
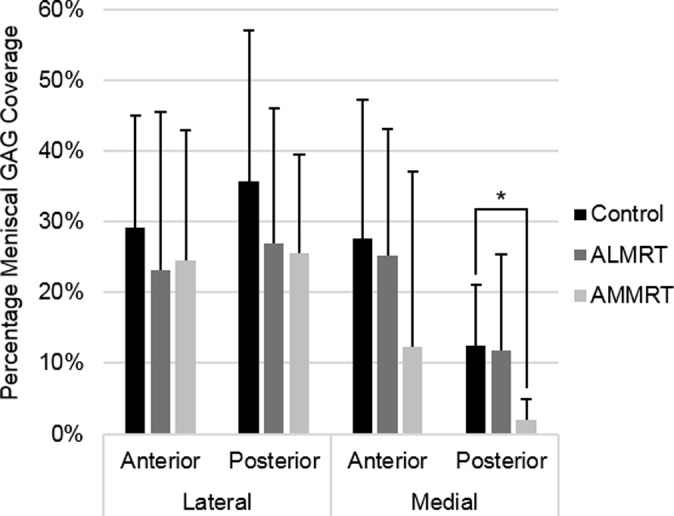
Histological staining results of meniscal glycosaminoglycan (GAG) coverage (mean with standard deviation). *Statistically significant (P < .05). ALMRT, anterolateral meniscal root tear; AMMRT, anteromedial meniscal root tear.
TABLE 5.
Histological Results of Meniscal GAG Stain Intensity for Meniscal Root Tear and Control Groupsa
| GAG Stain Intensity (0-3) | |||
|---|---|---|---|
| Control | Lateral | Covered | 2 (2, 3) |
| Uncovered | 2 (2, 3) | ||
| Medial | Covered | 2 (1.25, 3) | |
| Uncovered | 2 (1,2) | ||
| ALMRT | Lateral | Covered | 1.5 (1, 2.75) |
| Uncovered | 2 (1, 3) | ||
| Medial | Covered | 3 (2, 3) | |
| Uncovered | 1 (0.25, 1) | ||
| AMMRT | Lateral | Covered | 2 (1, 3) |
| Uncovered | 2 (1, 2) | ||
| Medial | Covered | 0 (0, 2.25) | |
| Uncovered | 0 (0, 0.75) |
aValues are presented as median (interquartile range). Values in boldface indicate statistical significance (P < .05 when compared with the control group). ALMRT, anterolateral meniscal root tear; AMMRT, anteromedial meniscal root tear; GAG, glycosaminoglycan.
Figure 7.
Glycosaminoglycan staining in anterior meniscal regions for control and both anterior meniscal root tear limbs. Examples provided are (A) the posterior region of the medial meniscus in a control limb, (B) the posterior region of the medial meniscus in an anteromedial meniscal root tear limb, (C) the anterior region of the lateral meniscus in a control limb, and (D) the anterior region of the lateral meniscus in an anterolateral meniscal root tear limb.
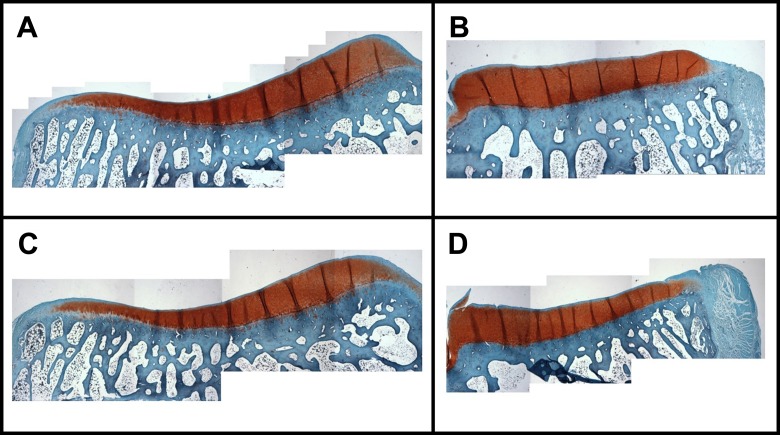
Qualitative Mankin scores suggest little to no change in the tibial articular cartilage after untreated anterior MRTs (Table 6). There were no significant changes to the scores for GAG stain intensity or fissures within the articular cartilage, and tidemark integrity appeared unchanged when the MRTs were left untreated for 8 weeks. Examples of GAG staining in tibial articular cartilage for control and anterior MRT limbs are shown in Figure 8.
TABLE 6.
Average Mankin Scores for Tibial Articular Cartilage Analysis for Meniscal Root Tear and Control Groupsa
| GAG Stain Intensity (0-6) | Fissures (0-6) | Tidemark Integrity (0-1) | ||
|---|---|---|---|---|
| Control | Lateral | 1 (0, 1) | 0.75 (0.25, 1) | 0.25 (0, 0.25) |
| Medial | 0.63 (0.44, 1) | 1.5 (0.44, 2.56) | 0 (0, 0.25) | |
| ALMRT | Lateral | 0.75 (0.75, 1) | 0 (0, 0.5) | 0.25 (0, 0.75) |
| Medial | 0.75 (0.75, 0.75) | 1.5 (0.25, 2.75) | 0 (0, 0.25) | |
| AMMRT | Lateral | 1.13 (1, 1.31) | 0.25 (0, 0.63) | 0.25 (0.19, 0.31) |
| Medial | 1 (1, 1.38) | 3.25 (2.56, 3.63) | 0 (0, 0.06) |
aValues are presented as median (interquartile range). ALMRT, anterolateral meniscal root tear; AMMRT, anteromedial meniscal root tear; GAG, glycosaminoglycan.
Figure 8.
Examples of glycosaminoglycan staining in the tibial articular cartilage for a control limb in the (A) lateral and (B) medial hemijoint as well as an anterolateral meniscal root tear limb in the (C) lateral and (D) medial hemijoint.
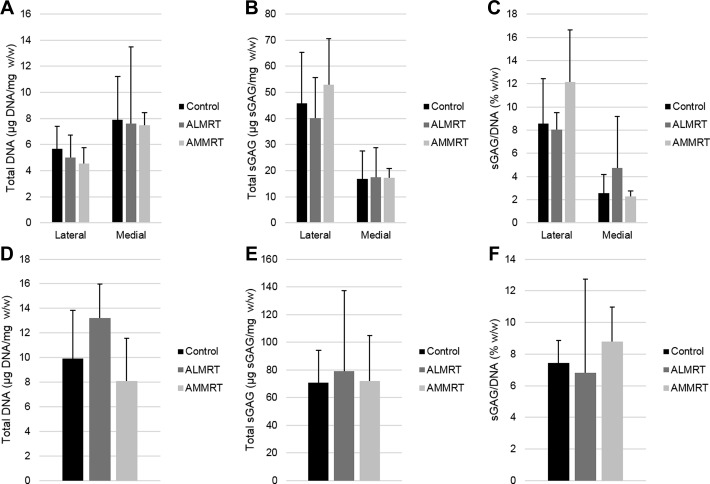
Biochemical Content
There were no significant changes to the meniscus regions in total DNA content normalized to wet weight or total GAG content when normalized to wet weight or DNA content (Figure 9). There were also no significant changes in the total DNA content or GAG content observed in the tibial articular cartilage when comparing the MRT groups to the control group.
Figure 9.
Total DNA content with respect to wet weight, total glycosaminoglycan (GAG) content with regard to wet weight, and total GAG with regard to total DNA content for menisci (A, B, and C, respectively) and tibial articular cartilage (D, E, and F, respectively). ALMRT, anterolateral meniscal root tear; AMMRT, anteromedial meniscal root tear.
Gene Expression
There were no significant differences between the ALMRT and AMMRT meniscus sample groups for any of the targeted genes; they were therefore pooled for comparison with the controls. The results were evaluated to determine relative gene expression changes that occurred after an anterior MRT, regardless of whether the injury was induced in the medial or lateral compartment. For the menisci, the comparison was made between the menisci of the control knees, the menisci in the injured joint that were left intact, and the menisci that were surgically cut to induce an anterior MRT. Since articular cartilage was taken from both compartments for each knee, the control limbs were compared with the limbs with an induced anterior MRT.
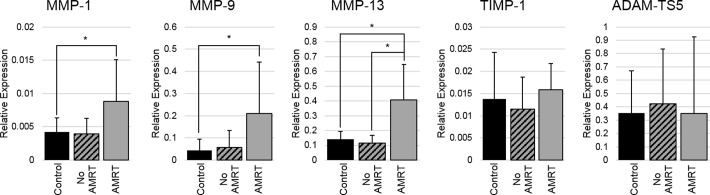
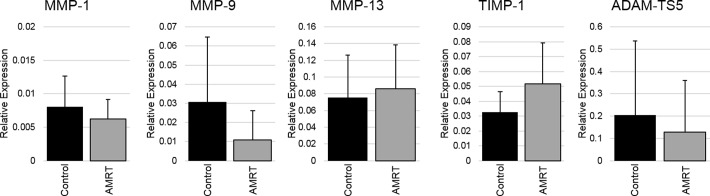
Menisci with surgically induced, anterior MRT showed significant increases in the gene expressions of MMP-1 (P = .03), MMP-9 (P = .03), and MMP-13 (P < .001) when compared with the control meniscal tissue (Figure 10). Additionally, the relative gene expression of MMP-13 was significantly higher in the menisci with the anterior MRT than the menisci within the same joint that were left intact (P = .01). There were no significant changes between any of the menisci groups for TIMP-1 and ADAM-TS5 gene expressions. There were also no significant changes in all targeted gene expressions between the tibial articular cartilage of the control knees and the knees with induced anterior MRTs (Figure 11).
Figure 10.
Relative gene expressions for meniscus samples (mean with standard deviation). Anterior meniscal root tears (AMRT) of medial and lateral insertions were pooled for gene expression analysis. *Statistically significant (P < .05). ADAM-TS5, aggrecanase-2; MMP, matrix metalloproteinase; TIMP-1, tissue inhibitor of metalloproteinase-1.
Figure 11.
Relative gene expressions for tibial articular cartilage samples (mean with standard deviation). Anterior meniscal root tears (AMRT) of medial and lateral insertions were pooled for gene expression analysis. *Statistically significant (P < .05). ADAM-TS5, aggrecanase-2; MMP, matrix metalloproteinase; TIMP-1, tissue inhibitor of metalloproteinase-1.
Discussion
The results of this study demonstrate that knees with surgically induced anterior MRTs left untreated begin to display early degenerative change, particularly within the synovial fluid, menisci, and tibial subchondral bone. The cytologic evaluation of joint inflammation between knees with anterior MRTs and the nonoperated control knees suggest these injuries produce an effusive process in the joint with increases in activated large mononuclear cells. Collectively, these findings are consistent with chronic joint inflammation, suggesting early signs of synovitis possibly contributing to the pathogenesis of osteoarthritis.51
Instantaneous elastic moduli comparisons for the menisci demonstrated significant decreases to the anterior meniscal regions that experience the MRT. This suggests that untreated anterior MRTs decrease the ability for the anterior regions of the injured meniscus to immediately resist compressive loads. Although there have been no previous studies evaluating changes of internal stresses and strains of menisci after MRTs, the separation of the meniscal insertion from the tibial plateau prevents proper load distribution, which may change the mechanical environment the meniscal tissue is exposed to. We theorize that this lack of proper fixation and change in mechanical environment of the region near the torn insertion induces a response to change the structure of the meniscal tissue. Additionally, the equilibrium elastic modulus significantly decreased for the AM meniscal region after AMMRT. These changes may also suggest that decreases in material properties of the menisci are more severe for the medial meniscus after anterior MRTs than the lateral meniscus. This unequal response to anterior MRTs and a greater disturbance in medial meniscal integrity may be a result of the presence of the popliteus tendon helping prevent extrusion and greater meniscal coverage of the lateral tibial condyle.
Calculated elastic moduli values from control samples corresponded well with previous studies using a similar testing procedure analyzing menisci from Giant Flemish rabbits under posttraumatic osteoarthritis conditions.16,17 These studies evaluated meniscal material properties after traumatic impact or surgical injury and showed decreases to both moduli of the lateral and medial menisci when compared with the control groups. Compared with the present study, the equilibrium elastic modulus of the meniscus after an anterior MRT was similar to the menisci after traumatic impact or combined transection of the ACL and menisci. Instantaneous elastic moduli for the menisci after anterior MRTs remained slightly higher compared with menisci in these studies, which may be due to the shorter time period of 8 weeks compared with 12 weeks.
Significant decreases in GAG coverage and stain intensity were found within the PM meniscus region after AMMRT. These results along with the mechanical analysis suggest that both anterior and posterior regions of the meniscus may be negatively affected by untreated anterior MRTs. In a previous study, GAG content measured after release of only the anteromedial meniscal insertion in rabbits demonstrated moderate changes in the subjectively graded scores after 8 weeks.4 However, the subjective scores in this study were analyzed and presented using means and standard deviations even though the Mankin grading scheme is ordinal and may have detected significance when there may not have been any. In other studies, observing significant decreases in meniscal GAG content has been difficult. A previous study evaluating GAG content after surgically inducing bucket-handle tears in a canine model showed that GAG content only significantly decreased after 48 weeks.41 Another study, however, showed significant decreases in GAG content in most meniscal regions after traumatic impact in a lapine model after only 12 weeks.16 These studies suggest that perhaps if MRTs were evaluated after traumatically occurring or left untreated for longer, a greater decrease in meniscal GAG may be apparent. The changes seen in GAG content may also only be temporal as the tissue compensates; therefore, further analysis of the menisci after these injuries over a longer period with multiple time points should be investigated.
There were no significant changes in the compressive elastic moduli or histological parameters of the tibial articular cartilage. The changes in contact mechanics after anterior MRTs are unknown; however, posteromedial MRTs in humans have demonstrated loading conditions similar to total meniscectomies, which are known to lead to degenerative articular cartilage.2 Previous animal studies have demonstrated osteoarthritic change within 3 months of meniscectomy.25 Since the loading conditions after anterior MRTs are unknown, perhaps the anterior MRTs are less severe and may need to be left untreated longer to see results similar to previous meniscectomy animal studies.
Changes to the subchondral bone morphology were present within the hemijoint that did not experience the anterior MRT. These decreases in the subchondral bone integrity may be due to the shift in load distribution along the tibial plateau since the injured meniscus is unable to properly distribute the load. While the biomechanical loading of rabbit knees has not been elucidated, a previous study in human knees evaluating posteromedial MRTs demonstrated these injuries increased lateral translations of the tibia.2 Perhaps this shifting causes abnormal loading patterns, and the trabecular morphology changes are a result of tibial regions that are being loaded much differently than normal. Additionally, another study evaluating the bone baseline trabecular integrity, an indication of number, spacing, and cross-connectivity of trabeculae, suggested these changes may be an early indicator of osteoarthritis progression.28 These findings suggest that anterior MRTs in 1 hemijoint may induce early degenerative change in the form of decreased subchondral bone integrity in the uninjured hemijoint.
Relative gene expressions for catabolic proteins significantly increased in the menisci with an anterior MRT when compared with both the intact menisci and the menisci of control knees. Three of the targeted catabolic genes significantly increased when compared with the control menisci; however, only 1 of those genes significantly increased compared with the intact menisci. This may suggest that there are increases within both the injured and intact menisci of a joint with an anterior MRT, with a greater increase in the catabolic gene expression in the injured menisci. Additionally, there was no difference in TIMP-1 expression between any of the evaluated groups. This may suggest that the tissue is not compensating for the increase in MMP expression with inhibitory proteins. These results may help explain the significant decreases in the compressive material properties of the menisci. With an increase in catabolic proteins degrading the collagen matrix and no change in inhibitory proteins, a disruption in this balance may occur and contribute to tissue breakdown causing a subsequent change in material properties.12 Other MMPs or additional catabolic gene expressions may also have been upregulated; however, the tested genes were limited to a few that were anticipated to change after the surgery and within the time frame of the study. Finally, the lack of gene expression changes within the articular cartilage suggests there are no early pathological changes after these injuries occurring after 8 weeks.
There are limitations of this study that should be considered when reviewing the presented results. The controls used in this study were nonoperative controls; thus, the influence of inflammation on these results is unknown. Despite this, the inflammatory responses present in the synovial fluid after 8 weeks are likely due to the MRTs since inflammatory responses are typically cleared away from an open wound after approximately 2 weeks.57 In the gene expression analysis, for example, the MMP gene expressions measured in the menisci left intact within the operated knee were not significantly different from the nonoperative control, while the surgically cut meniscus expression was upregulated. Thus, the effect of inflammation was predicted to be little or negligible. Another limitation is that only 1 time point of 8 weeks was studied. Multiple time points with longer time periods would allow the tracking of osteoarthritis progression and may reveal changes within the articular cartilage. This study was a short, proof-of-concept study to evaluate results from different analysis methods following untreated MRTs of both the lateral and medial menisci before progressing toward a longer, refined study. The purpose of evaluating changes at 8 weeks in this study was to determine whether the anterior destabilization performed arthroscopically was able to produce measurable, degenerative changes, and a previous study demonstrated this as an appropriate period to detect degradation within rabbit knees.42 Since the present study was able to produce measurable changes, future studies evaluating longer time periods or different repair techniques may confidently produce additional results. Also, the small animal model may not be as useful as a larger animal model for interpreting results for the human knee. Previous studies have utilized similar small animal models and they provide useful information to build on in future studies while keeping the cost low.4,29 Additionally, using the contralateral knees as controls may have skewed results due to altered weightbearing; however, animal gait returned to normal a couple days after surgery, so the impact on the results was likely minimal.
Conclusion
This study demonstrated that early degenerative changes occur within the synovial fluid, menisci, and tibial subchondral bone after MRTs while the tibial articular cartilage appeared mostly unaffected at 8 weeks. The results suggest that changes occur to meniscal tissue prior to the tibial articular cartilage after anterior MRTs. Decreases in the material properties and GAG, and the increasing imbalance of MMP expressions relative to TIMP-1 within the menisci after 8 weeks, suggest that these injuries are capable of diminishing the integrity of the menisci. As this was a single, early time point study, further investigation of longer time points into the progression of these changes in the menisci and the onset of articular cartilage changes after these injuries are warranted. Also, this study showed that anterior destabilization of the meniscus arthroscopically without creating a posterior arthrotomy leads to measurable degenerative changes and may be useful for future in vivo studies of MRTs to expand on the presented results. Clinically, it is important to understand the progression of degenerative changes within the joint tissues after anterior MRTs to properly approach repairs. Therefore, the results of this study provide promising direction for further analysis of these injuries left untreated for longer time points and for establishing a model to evaluate in vivo repairs in future studies.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: R.F.L. receives royalties from Arthrex, Ossur, and Smith & Nephew; is a paid consultant for Arthrex, Ossur, and Smith & Nephew; and receives research support from Arthrex, Smith & Nephew, Ossur, and Linvatec. Funding for this project was provided by the Steadman Philippon Research Institute in Vail, Colorado.
Ethical support for this study was obtained from the Colorado State Research Integrity & Compliance Review Office (animal welfare assurance number: A3572-01).
References
- 1. Ahmed AM, Burke DL. In-vitro measurement of static pressure distribution in synovial joints—part I: tibial surface of the knee. J Biomech Eng. 1983;105:216–225. [DOI] [PubMed] [Google Scholar]
- 2. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus. J Bone Joint Surg Am. 2008;90:1922–1931. [DOI] [PubMed] [Google Scholar]
- 3. Allen AA, Caldwell GL, Fu FH. Anatomy and biomechanics of the meniscus. Oper Tech Orthop. 1995;5:2–9. [Google Scholar]
- 4. Arunakul M, Tochigi Y, Goetz JE, et al. Replication of chronic abnormal cartilage loading by medial meniscus destabilization for modeling osteoarthritis in the rabbit knee in vivo. J Orthop Res. 2013;31:1555–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aspden RM, Yarker YE, Hukins DW. Collagen orientations in the meniscus of the knee joint. J Anat. 1985;140(pt 3):371–380. [PMC free article] [PubMed] [Google Scholar]
- 6. Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intra-articular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14:270–275. [DOI] [PubMed] [Google Scholar]
- 7. Benjamin M, Evans EJ, Rao RD, Findlay JA, Pemberton DJ. Quantitative differences in the histology of the attachment zones of the meniscal horns in the knee joint of man. J Anat. 1991;177:127–134. [PMC free article] [PubMed] [Google Scholar]
- 8. Bluteau G, Conrozier T, Mathieu P, Vignon E, Herbage D, Mallein-Gerin F. Matrix metalloproteinase-1, -3, -13 and aggrecanase-1 and -2 are differentially expressed in experimental osteoarthritis. Biochim Biophys Acta. 2001;1526:147–158. [DOI] [PubMed] [Google Scholar]
- 9. Bluteau G, Gouttenoire J, Conrozier T, et al. Differential gene expression analysis in a rabbit model of osteoarthritis induced by anterior cruciate ligament (ACL) section. Biorheology. 2002;39:247–258. [PubMed] [Google Scholar]
- 10. Brophy RH, Martinez M, Borrelli J, Silva MJ. Effect of combined traumatic impact and radial transection of medial meniscus on knee articular cartilage in a rabbit in vivo model. Arthroscopy. 2012;28:1490–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cake MA, Read RA, Corfield G, et al. Comparison of gait and pathology outcomes of three meniscal procedures for induction of knee osteoarthritis in sheep. Osteoarthritis Cartilage. 2013;21:226–236. [DOI] [PubMed] [Google Scholar]
- 12. Dean DD, Martel-Pelletier J, Pelletier J-P, Howell DS, Woessner JF., Jr Evidence for metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989;84:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B:664–670. [PubMed] [Google Scholar]
- 14. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. [DOI] [PubMed] [Google Scholar]
- 15. Feucht MJ, Minzlaff P, Saier T, Lenich A, Imhoff AB, Hinterwimmer S. Avulsion of the anterior medial meniscus root: case report and surgical technique. Knee Surg Sports Traumatol Arthrosc. 2015;23:146–151. [DOI] [PubMed] [Google Scholar]
- 16. Fischenich KM, Button KD, Coatney GA, et al. Chronic changes in the articular cartilage and meniscus following traumatic impact to the lapine knee. J Biomech. 2014;48:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fischenich KM, Coatney GA, Haverkamp JH, et al. Evaluation of meniscal mechanics and proteoglycan content in a modified anterior cruciate ligament transection model. J Biomech Eng. 2014;136:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res. 1990;252:19–31. [PubMed] [Google Scholar]
- 19. Freemont AJ, Hampson V, Tilman R, Goupille P, Taiwo Y, Hoyland JA. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997;56:542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukubayashi T, Kurosawa H. The contact area and pressure distribution pattern of the knee. A study of normal and osteoarthrotic knee joints. Acta Orthop Scand. 1980;51:871–879. [DOI] [PubMed] [Google Scholar]
- 21. Gao J, Messner K. Natural healing of anterior and posterior attachments of the rabbit meniscus. Clin Orthop Relat Res. 1996;328:276–284. [DOI] [PubMed] [Google Scholar]
- 22. Gao J, Oqvist G, Messner K. The attachments of the rabbit medial meniscus. A morphological investigation using image analysis and immunohistochemistry. J Anat. 1994;185(pt 3):663–667. [PMC free article] [PubMed] [Google Scholar]
- 23. Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. [DOI] [PubMed] [Google Scholar]
- 24. Han SB, Shetty GM, Lee DH, et al. Unfavorable results of partial meniscectomy for complete posterior medial meniscus root tear with early osteoarthritis: a 5- to 8-year follow-up study. Arthroscopy. 2010;26:1326–1332. [DOI] [PubMed] [Google Scholar]
- 25. Hede A, Svalastoga E, Reimann I. Articular cartilage changes following meniscal lesions. Repair and meniscectomy studied in the rabbit knee. Acta Orthop Scand. 1991;62:319–322. [DOI] [PubMed] [Google Scholar]
- 26. Hunter DJ, Zhang YQ, Niu JB, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. [DOI] [PubMed] [Google Scholar]
- 27. Kettelkamp DB, Jacobs AW. Tibiofemoral contact area—determination and implications. J Bone Joint Surg Am. 1972;54:349–356. [PubMed] [Google Scholar]
- 28. Kraus VB, Feng S, Wang S, et al. Subchondral bone trabecular integrity predicts and changes concurrently with radiographic and magnetic resonance imaging–determined knee osteoarthritis progression. Arthritis Rheum. 2013;65:1812–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuroki K, Cook CR, Cook JL. Subchondral bone changes in three different canine models of osteoarthritis. Osteoarthritis Cartilage. 2011;19:1142–1149. [DOI] [PubMed] [Google Scholar]
- 30. Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint: physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res. 1980;149:283–290. [PubMed] [Google Scholar]
- 31. LaPrade CM, Ellman MB, Rasmussen MT, et al. Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med. 2014;42:2386–2392. [DOI] [PubMed] [Google Scholar]
- 32. LaPrade CM, James EW, Cram TR, Feagin JA, Engebretsen L, LaPrade RF. Meniscal root tears: a classification system based on tear morphology. Am J Sports Med. 2015;43:363–369. [DOI] [PubMed] [Google Scholar]
- 33. LaPrade CM, James EW, Engebretsen L, LaPrade RF. Anterior medial meniscal root avulsions due to malposition of the tibial tunnel during anterior cruciate ligament reconstruction: two case reports. Knee Surg Sports Traumatol Arthrosc. 2014;22:1119–1123. [DOI] [PubMed] [Google Scholar]
- 34. LaPrade CM, Jansson KS, Dornan G, Smith SD, Wijdicks CA, LaPrade RF. Altered tibiofemoral contact mechanics due to pull-out suture repairs. J Bone Joint Surg Am. 2014;96:471–479. [DOI] [PubMed] [Google Scholar]
- 35. Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88:1826–1834. [DOI] [PubMed] [Google Scholar]
- 36. Luther JK, Cook CR, Cook JL. Meniscal release in cruciate ligament intact stifles causes lameness and medial compartment cartilage pathology in dogs 12 weeks postoperatively. Vet Surg. 2009;38:520–529. [DOI] [PubMed] [Google Scholar]
- 37. Madry H, van Dijk CN, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18:419–433. [DOI] [PubMed] [Google Scholar]
- 38. McGonagle D, Tan AL, Carey J, Benjamin M. The anatomical basis for a novel classification of osteoarthritis and allied disorders. J Anat. 2010;216:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Messner K, Gao J. The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J Anat. 1998;193:161–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Navarro-Holgado P, Cuevas-Pérez A, Aguayo-Galeote MA, Carpintero-Benítez P. Anterior medial meniscus detachment and anterior cruciate ligament tear. Knee Surg Sports Traumatol Arthrosc. 2007;15:587–590. [DOI] [PubMed] [Google Scholar]
- 41. Nishida M, Higuchi H, Kobayashi Y, Takagishi K. Histological and biochemical changes of experimental meniscus tear in the dog knee. J Orthop Sci. 2005;10:406–413. [DOI] [PubMed] [Google Scholar]
- 42. Papaioannou N, Krallis N, Triantafillopoulos I, Khaldi L, Dontas I, Lyritis G. Optimal timing of research after anterior cruciate ligament resection in rabbits. Contemp Top Lab Anim Sci. 2004;43(6):22–27. [PubMed] [Google Scholar]
- 43. Pauli C, Grogan SP, Patil S, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pauly HM, Larson BE, Coatney GA, et al. Assessment of cortical and trabecular bone changes in two models of post-traumatic osteoarthritis. J Orthop Res. 2015;33:1835–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Petersen W, Tillmann B. Collagenous fibril texture of the human knee joint menisci. Anat Embryol (Berl). 1998;197:317–324. [DOI] [PubMed] [Google Scholar]
- 46. Roemhildt ML, Coughlin KM, Peura GD, Fleming BC, Beynnon BD. Material properties of articular cartilage in the rabbit tibial plateau. J Biomech. 2006;39:2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosenberg L. Chemical basis for the histological rise of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53:69–82. [PubMed] [Google Scholar]
- 48. Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58:1716–1726. [DOI] [PubMed] [Google Scholar]
- 49. Shoemaker SC, Markolf KL. The role of the meniscus in the anterior-posterior stability of the loaded anterior cruciate–deficient knee. Effects of partial versus total excision. J Bone Joint Surg Am. 1986;68:71–79. [PubMed] [Google Scholar]
- 50. Shrive NG, O’Connor JJ, Goodfellow JW. Load-bearing in the knee joint. Clin Orthop Relat Res. 1978;131:279–287. [PubMed] [Google Scholar]
- 51. Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24:365–371. [PubMed] [Google Scholar]
- 52. Sweigart MA, Zhu CF, Burt DM, et al. Intraspecies and interspecies comparison of the compressive properties of the medial meniscus. Ann Biomed Eng. 2004;32:1569–1579. [DOI] [PubMed] [Google Scholar]
- 53. Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–552. [DOI] [PubMed] [Google Scholar]
- 54. Toy JO, Feeley BT, Gulotta LV, Warren RF. Arthroscopic avulsion repair of a pediatric ACL with an anomalous primary insertion into the lateral meniscus. HSS J. 2011;7:190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975;109:184–192. [DOI] [PubMed] [Google Scholar]
- 56. Watson JN, Wilson KJ, LaPrade CM, et al. Iatrogenic injury of the anterior meniscal root attachments following anterior cruciate ligament reconstruction tunnel reaming. Knee Surg Sports Traumatol Arthrosc. 2014;23:2360–2366. [DOI] [PubMed] [Google Scholar]
- 57. Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509–528. [DOI] [PubMed] [Google Scholar]