Abstract
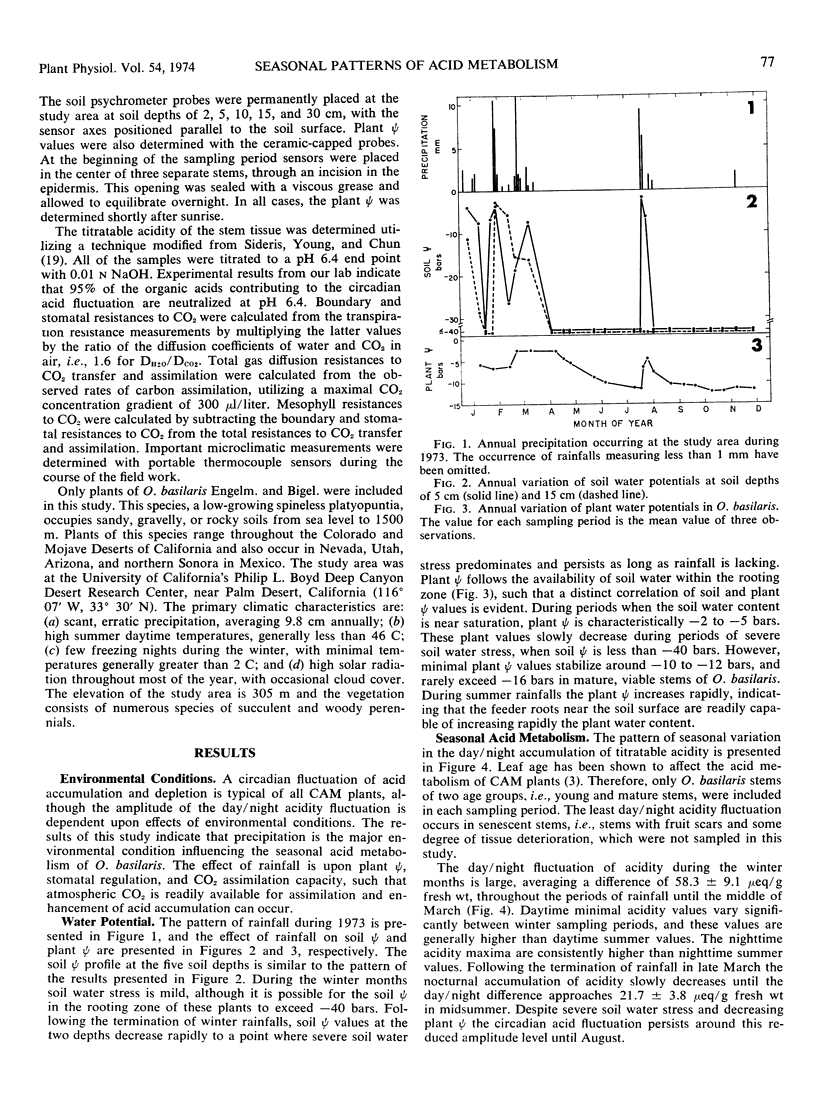
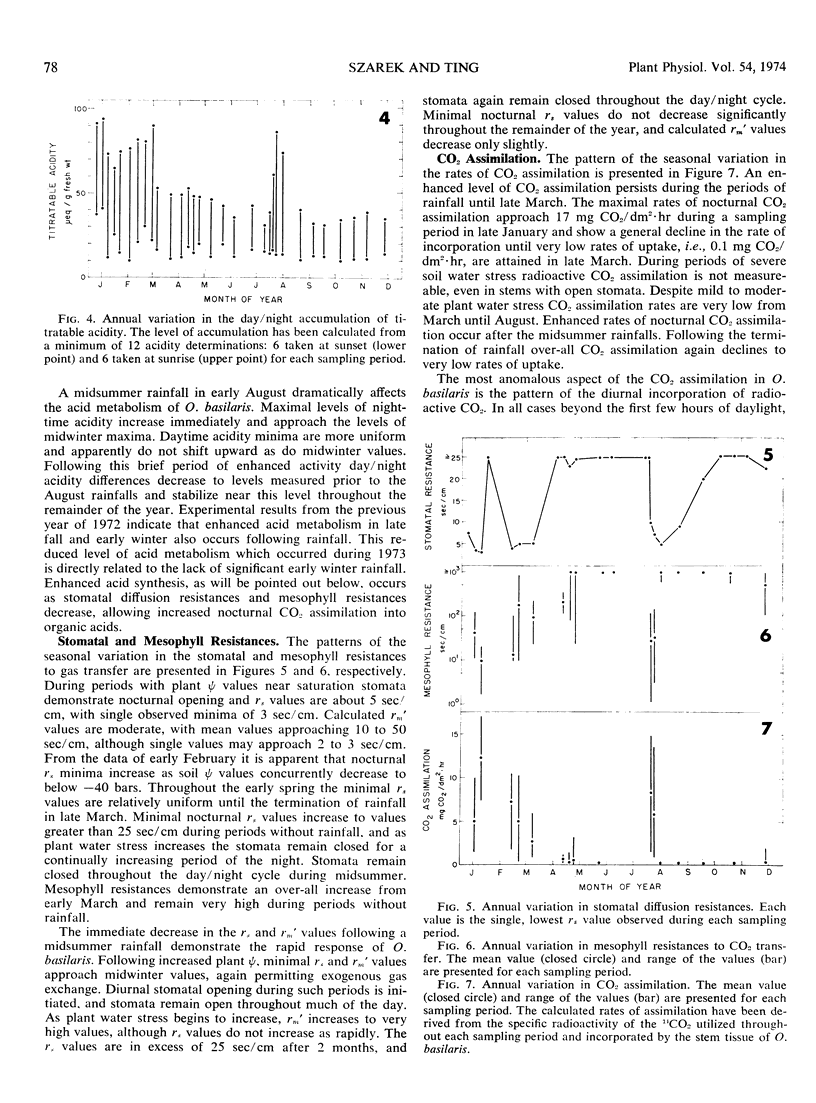
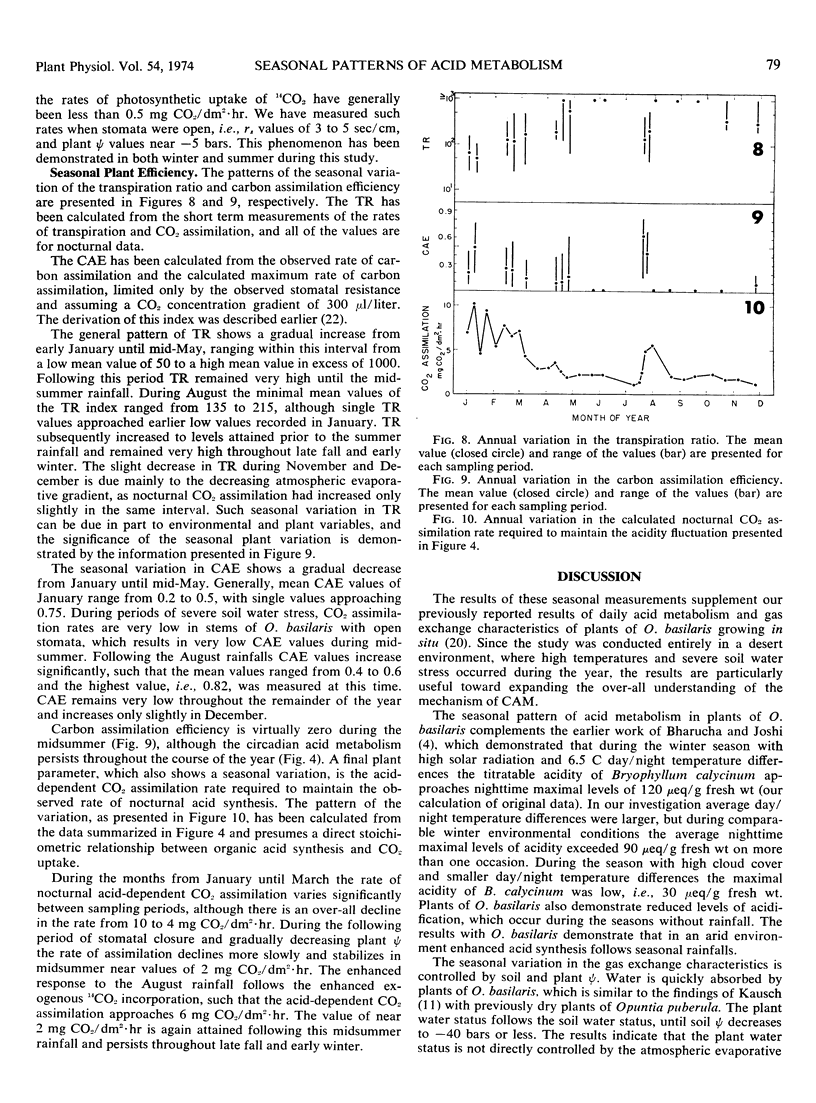
Acid metabolism and gas exchange studies were conducted in situ on the cactus Opuntia basilaris Engelm. and Bigel. A pattern of significant seasonal variation was evident. The pattern was controlled by rainfall, which significantly influenced plant water potentials, total gas transfer resistances, and nocturnal organic acid synthesis. In winter and early spring, when plant water stress was mild, stomatal and mesophyll resistances remained low, permitting enhanced nocturnal assimilation of 14CO2. The day/night accumulation of acidity was large during these seasons. In summer and fall, plant water stress was moderate, although soil water stress was severe. The nocturnal assimilation of 14CO2 was very low during these seasons, even in stems with open stomata, indicating large mesophyll resistances restricting exogenous gas incorporation. The day/night accumulation of acidity was reduced, and a low level of acid metabolism persisted throughout this period. The rapid response to a midsummer rainfall emphasizes the importance of plant water potential as a parameter controlling over-all metabolic activity. The seasonal variations of acid metabolism and gas exchange significantly influenced the efficiency of water use and carbon dioxide assimilation. Periods of maximal efficiency followed rainfall throughout the course of the year.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkman O., Pearcy R. W., Harrison A. T., Mooney H. Photosynthetic adaptation to high temperatures: a field study in death valley, california. Science. 1972 Feb 18;175(4023):786–789. doi: 10.1126/science.175.4023.786. [DOI] [PubMed] [Google Scholar]
- Ekern P. C. Evapotranspiration of Pineapple in Hawaii. Plant Physiol. 1965 Jul;40(4):736–739. doi: 10.1104/pp.40.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVERIO V. T., DENHAM C., DAVIDSON J. D. Oxygen flask combustion in determination of C-14 and H3 in biological materials. Anal Biochem. 1962 Aug;4:188–189. doi: 10.1016/0003-2697(62)90035-0. [DOI] [PubMed] [Google Scholar]
- Sideris C. P., Young H. Y., Chun H. H. DIURNAL CHANGES AND GROWTH RATES AS ASSOCIATED WITH ASCORBIC ACID, TITRATABLE ACIDITY, CARBOHYDRATE AND NITROGENOUS FRACTIONS IN THE LEAVES OF ANANAS COMOSUS (L.) MERR. Plant Physiol. 1948 Jan;23(1):38–69. doi: 10.1104/pp.23.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarek S. R., Johnson H. B., Ting I. P. Drought Adaptation in Opuntia basilaris: Significance of Recycling Carbon through Crassulacean Acid Metabolism. Plant Physiol. 1973 Dec;52(6):539–541. doi: 10.1104/pp.52.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bavel C. H., Nakayama F. S., Ehrler W. L. Measuring Transpiration Resistance of Leaves. Plant Physiol. 1965 May;40(3):535–540. doi: 10.1104/pp.40.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]


