Abstract
Background:
More than half of all heart disease and stroke are attributable to hypertension, which is associated with approximately 10% of direct medical costs globally. Clinical trial evidence has demonstrated that the benefits of pharmacist intervention, including education, consultation and/or prescribing, can help to reduce blood pressure; a recent Canadian trial found an 18.3 mmHg reduction in systolic blood pressure associated with pharmacist care and prescribing. The objective of this study was to evaluate the economic impact of such an intervention in a Canadian setting.
Methods:
A Markov cost-effectiveness model was developed to extrapolate potential differences in long-term cardiovascular and renal disease outcomes, using Framingham risk equations and other published risk equations. A range of values for systolic blood pressure reduction was considered (7.6-18.3 mmHg) to reflect the range of potential interventions and available evidence. The model incorporated health outcomes, costs and quality of life to estimate an overall incremental cost-effectiveness ratio. Costs considered included direct medical costs as well as the costs associated with implementing the pharmacist intervention strategy.
Results:
For a systolic blood pressure reduction of 18.3 mmHg, the estimated impact is 0.21 fewer cardiovascular events per person and, discounted at 5% per year, 0.3 additional life-years, 0.4 additional quality-adjusted life-years and $6,364 cost savings over a lifetime. Thus, the intervention is economically dominant, being both more effective and cost-saving relative to usual care.
Discussion:
Across a range of one-way and probabilistic sensitivity analyses of key parameters and assumptions, pharmacist intervention remained both effective and cost-saving.
Conclusion:
Comprehensive pharmacist care of hypertension, including patient education and prescribing, has the potential to offer both health benefits and cost savings to Canadians and, as such, has important public health implications.
Knowledge into Practice.
Pharmacist intervention (either partial or full) is an effective management strategy for hypertension.
Pharmacists are ideally placed to fill in the care gap for the 35% to 65% of hypertensive patients who are inadequately controlled.
Full management (prescription, education and consultation) of hypertension by pharmacists is a dominant (saves money and improves outcomes) strategy.
Partial management by pharmacists improves outcomes at a cost generally thought to be cost-effective.
Given the compelling economic argument for pharmacist management of hypertension, pharmacists and policy-makers have a societal duty to implement this type of care.
Mise En Pratique Des Connaissances.
Dans le cas de l’hypertension, l’intervention des pharmaciens (partielle ou complète) constitue une stratégie de prise en charge efficace.
Les pharmaciens sont les mieux placés pour combler les lacunes dans le traitement des 35 à 65 % de patients qui souffrent d’une hypertension artérielle mal contrôlée.
La prise en charge complète de l’hypertension (ordonnances, éducation et consultations) par les pharmaciens est une stratégie dominante (permet de faire des économies et améliore les résultats).
La prise en charge partielle par les pharmaciens améliore les résultats de façon rentable en général.
Compte tenu des arguments économiques qui appuient la prise en charge de l’hypertension par les pharmaciens, il leur appartient de mettre en œuvre ce type de traitement dans notre société.
Introduction
Hypertension is the single most important risk factor for premature morbidity and mortality worldwide.1 Indeed, it is estimated that 1.13 billion people (about 24% prevalence) have hypertension,2 and this is responsible for about 7.5 million deaths per year.3 Furthermore, the treatment and control of hypertension is poor, with more than 40% of patients with hypertension being uncontrolled,4 indicating a considerable care gap that requires new thinking to address. Canada does fare better than most, with a prevalence rate of 23% of adults having hypertension, and of these, one-third not adequately controlled.5 However, because hypertension is a major risk factor for cardiovascular disease (CVD), renal disease and death, there is considerable interest in reducing this care gap to prevent significant morbidity and mortality.6-8
Pharmacists are ideally placed, highly accessible health care providers who have shown that they can effectively contribute to solving this care gap in hypertension management. Santschi et al.9 recently conducted a systematic review and meta-analysis of 39 randomized controlled trials assessing the effect of pharmacist interventions on blood pressure management. These interventions were largely patient education and counselling, feedback to physicians about management (including drug-related problems, recommendations for changing pharmacotherapy and development of care plan) and medication management (including monitoring with adjustment of change in medication). This review found that, compared with usual care, pharmacist interventions were significantly better at lowering both systolic and diastolic blood pressure, with an average systolic blood pressure reduction of 7.6 mmHg.9
More recently, a patient-level randomized controlled trial by Tsuyuki et al.10 evaluated the impact of pharmacist prescribing on blood pressure control of community-dwelling patients. The intervention group received from their pharmacist an assessment of blood pressure and CVD risk, education on hypertension, prescribing of antihypertensive medications, laboratory monitoring and monthly visits for 6 months. The control group received some educational material, blood pressure measurements and usual care from their pharmacist and physician. Of the 248 patients enrolled, those randomized to the intervention arm experienced a statistically and clinically significant reduction in systolic blood pressure of 18.3 mmHg.
Although we have robust randomized controlled evidence of the benefits of pharmacist management of hypertension, there is no quantitative evidence to suggest that it is good value for the scarce health care dollars that would need to be allocated to its provision. As such, we embarked on a study to extrapolate the observed benefits in trials of pharmacist intervention in blood pressure control, in order to project potential clinical and cost-effectiveness of pharmacist interventions over a longer time horizon.
Methods
Model structure
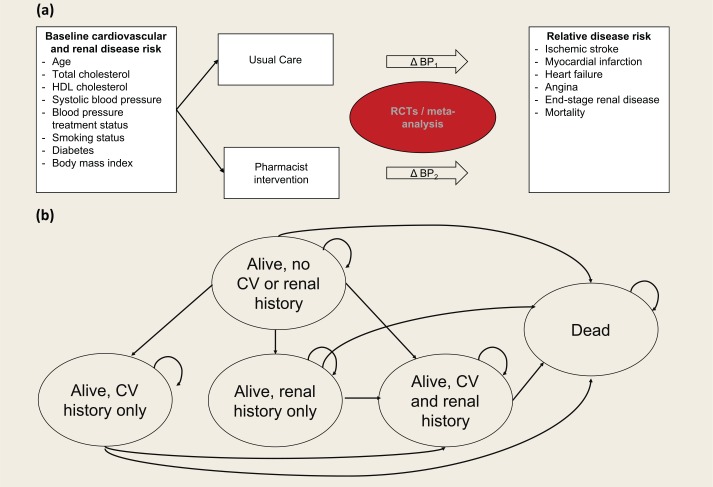
The model was structured as a 5-state Markov model, with health states defined by history of CVD and/or end-stage renal disease (ESRD) and death (Figure 1). Within the model, baseline risk for CVD and ESRD was defined based on patient clinical and demographic characteristics, and this risk was modified in the pharmacist intervention arm resulting from changes in systolic blood pressure. CVD and ESRD outcomes were tracked over time, along with corresponding survival, health-related quality of life (HRQoL) and direct medical costs. Specific CVD outcomes considered were myocardial infarction (MI), stroke, heart failure (HF) and angina. The modelled time horizon was 30 years in the base case, with 5% annual discounting applied to costs and outcomes. The analysis was done from a third-party payer perspective.
Figure 1.
(a) Overall approach to model structure. (b) Markov health states
Impact of pharmacist care
The impact of the pharmacist care was characterized by reduction in systolic blood pressure in individuals with hypertension. The base case value was 18.3 mmHg, as observed in the clinical trial conducted by Tsuyuki et al.,10 reflecting the 6-month outcome observed for an intervention including consultation, medication review and prescribing (referred to as the “full-scope pharmacist intervention,” as it refers to the full-scope of pharmacist practice). A second value of 7.6 mmHg was also considered, based on the systematic literature review and meta-analysis conducted by Santschi et al.,9 which included a range of interventions, most of which did not incorporate pharmacist prescribing (referred to as the “partial-scope pharmacist intervention”).
Within the model, the usual-care arm was assumed to stay consistent with baseline, with no change in blood pressure, reflecting the fact that usual care would be characterized by consistent care, without any additional intervention; in the absence of intervention, no blood pressure reduction would be expected. The assumption of no change represents an average outcome, reflecting individual patients experiencing both increases and decreases over time, respectively, but no evidence of a consistent trend in the absence of further intervention. Note that in the Tsuyuki et al. trial, the “control” arm did receive a modified intervention, so is not a true reflection of outcomes under actual usual care, and these results were thus not felt to be an appropriate description of actual usual care with no intervention. Because of the importance of this assumption, results are presented graphically across a range of plausible values for systolic blood pressure reduction, from 5 to 20 mmHg.
Health outcomes over time
Baseline risk of disease is based on clinical and demographic characteristics observed in the clinical trial conducted by Tsuyuki et al.10 (Table 1). Thirty-year Framingham risk equations were used to generate long-term CVD probabilities for baseline characteristics, including calibration factors to differentiate the risk for coronary heart disease (CHD), stroke and HF.11,12 The absolute difference in the risk score for “hard” CVD outcomes (excluding angina) and all outcomes (including angina) was calculated to extrapolate the risk of angina, while the difference between risk of CHD and risk of angina was calculated to extrapolate the risk of MI. Risk calculators made available by Framingham investigators13 were used to calculate CVD risk scores annually for 30 years for each treatment arm, based on baseline risk factors. The difference between the usual care and intervention groups was based on the relationship between systolic blood pressure and major CVD events reported by the Blood Pressure Lowering (BPL) Treatment Trialists’ Collaboration.14 The plot describing this relationship was digitized, and a simple linear regression model was fit, which found a decrease of 0.026 in the relative risk of CVD for every mmHg decrease in systolic blood pressure, relative to no change. The resulting relative risk of CVD for the pharmacist intervention group relative to the usual care group was 0.50 for the full-scope pharmacist intervention and 0.77 for the partial-scope pharmacist intervention.
Table 1.
Assumed patient characteristics for pharmacist hypertension intervention, based on observed population in Tsuyuki et al.10 clinical trial
| Characteristic | Value |
|---|---|
| Age (years) | 63.5 |
| Sex (% male) | 48.8 |
| Systolic blood pressure (mmHg) | 149.5 |
| Diastolic blood pressure (mmHg) | 83.7 |
| Treatment for hypertension (%) | 77.8 |
| Smoking (%) | 16.5 |
| Diabetes mellitus (%) | 44.0 |
| Body mass index | 32.0 |
Risk of ESRD was calculated based on incident rates reported in a historical cohort study conducted in the United States.15 Within this study, the incidence of ESRD is reported based on the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure categories: normal, prehypertension, hypertension stage 1 and hypertension stage 2.16 To interpolate the relationship between systolic blood pressure on a continuous scale, a simple linear regression model was fit between the midpoint systolic blood pressure for each category and resulting ESRD incidence per 100,000 person-years. The rate of ESRD per 100,000 person-years was then converted to an annual risk, assuming an exponential function. The resulting annual probability of ESRD was 0.000194 for the usual-care arm, 0.000150 for the full-scope pharmacist intervention and 0.000191 for the partial-scope pharmacist intervention. The respective treatment arm–specific annual probabilities of disease were applied each year.
For the pharmacist intervention arm, the difference in systolic blood pressure associated with the intervention was used to vary long-term risk projections for CVD and ESRD. All risk factors besides systolic blood pressure were held constant. Additional details describing the methods undertaken to model CVD and ESRD are provided in the supplementary appendix in the online version of the article.
Canadian life tables were used to estimate age- and sex-specific mortality over time. A hazard ratio for mortality of 1.71 was applied after experiencing CVD, to reflect increased mortality in this population.17
HRQoL
A published catalogue of EQ-5D utility values was used to quantify HRQoL for health states of interest, under the assumption that utility values derived for a U.S. population would be relevant to Canada.18 Resulting utilities were 0.694 for stroke, 0.725 for MI, 0.636 for HF, 0.709 for angina and 0.708 for ESRD (Table 2). In addition, a utility decrement of 0.00029 per year was applied to all years accrued older than age 70 years (e.g., for individuals surviving to age 75, a quality-adjusted life year [QALY] decrement of 0.00029 × 5 = 0.00145).18
Table 2.
Markov model parameters and distributions
| Parameter | Value | Probabilistic | Source |
|---|---|---|---|
| Base case | |||
| Reduction in systolic blood pressure | −18.3 mmHg | Normal (–18.3, 1.2) Normal (–7.6, 0.69) |
Tsuyuki et al.10
Santschi et al.9 |
| Relative risk of cardiovascular disease in intervention group | 0.50 | Normal (0.50, 0.02) | BPL Treatment Trialists19 |
| Relative risk of renal disease in intervention group | 0.77 | Ratio: normal (2.6, 0.30)/normal (1.6, 0.25) | Hsu et al.15 |
| Hazard ratio for mortality after cardiovascular disease | 1.7 | Lognormal (0.538, 0.075) | Pocock et al.17 |
| Cost of pharmacist intervention | Assumption | ||
| Year 1 | $200.00 | ||
| Year 2 | $75.00 | ||
| Year 3+ | $50.00 | ||
| Cost of stroke | |||
| Year 1 | $79,925 | Gamma (197.02, 405.66) | Mittman et al.,20 Sorensen et al.21 |
| Year 2+ | $12,126 | Gamma (25, 485.03) | |
| Cost per year of heart failure | $13,240 | Gamma (25, 529.6) | Bentkover et al.22 |
| Cost per year of angina | $3,764 | Gamma (37.42, 100.58) | McGillion et al.23 |
| Cost of myocardial infarction | Coyle et al.24 | ||
| Year 1 | $11,511 | Gamma (25, 460.46) | |
| Year 2+ | $3,367 | Gamma (25, 134.68) | |
| Cost per year of end-stage renal disease | $66,837 | Gamma (25, 2673.46) | Manns et al.25 |
| Cost of background medical costs | $6,105 | Canadian Institutes for Health Information26 | |
| Utility | Sullivan et al.18 | ||
| General population | 0.867 | ||
| After stroke | 0.694 | Beta (7090, 3126) | |
| After heart failure | 0.636 | Beta (480, 275) | |
| After angina | 0.709 | Beta (4843, 1988) | |
| After myocardial infarction | 0.725 | Beta (61446, 23307) | |
| Post end-stage renal disease | 0.708 | Beta (1248, 515) | |
| Disutility per year after age 70 | 0.00029 | ||
| One-way sensitivity analyses | |||
| Framingham 30-year risk equations for blood pressure impact | Pencina et al.12 | ||
| Blood pressure reduction based on partial intervention | Santschi et al.9 | ||
| Age-specific background cost estimates | Canadian Institutes for Health Information26 | ||
| Reduced time horizon (5 years, 10 years) | Assumption | ||
| Cost of pharmacist intervention doubled and training costs added | Assumption | ||
| Reduction in background medical costs for intervention group | Assumption | ||
| Reduced efficacy of pharmacist intervention over time (effect decayed after 3 years, effect decayed after 10 years) | Assumption | ||
| “Optimistic” scenario regarding cost of full-scope intervention | Assumption | ||
Costs
The cost of the pharmacist intervention was based on assumptions derived from investigator familiarity with implementing such programs in a clinical trial setting. It was assumed that individuals would be seen 6 times in the first year and quarterly thereafter, reflecting a protocol of monthly visits until 2 consecutive visits with controlled measures, followed by quarterly visits. The unit cost of the first consultation of each year is $125 CAD and $25 for subsequent consultations, reflecting the current fee schedule in Alberta.27 While no net difference in number of blood pressure medications was observed in clinical trial,28 a conservative assumption was made in the base case that medication costs would increase by $30/month as a result of the intervention. It was also assumed that there would be no difference in other background medical costs; this is a conservative assumption given that the intervention group would likely have physician visits for medication management offset by the additional pharmacist consultations. All aspects of the intervention program are within the current core competencies of Canadian pharmacists, and if any additional training is required, it would likely be funded by the pharmacy rather than a third-party payer. As such, no training costs are included in the base case.
Costs of CVD and ESRD were based on a review of the published peer-reviewed literature, restricted to Canadian studies (Table 2). The Canadian Health and Personal Care component of the Consumer Price Index was used to inflate values to 2015 $CAD values.29
Background noncardiovascular medical costs were assumed to be $6,105 per person per year, as reported by the Canadian Institutes for Health Information as the overall Canadian average.26 The overall average was used in the base case, rather than age-specific values, because age-specific values in older individuals are expected to be composed of a substantial proportion of cardiovascular-related costs, and if these were explicitly incorporated into the model, double-counting of costs would occur.
Sensitivity analysis
In addition to the base case and the key sensitivity analysis of difference in systolic blood pressure being based on partial vs full intervention, several other one-way sensitivity analyses were conducted, listed in Table 2. A sensitivity analysis was conducted in which Framingham risk equations were used to account for the impact of systolic blood pressure on CVD risk as an alternative to the BPL equations. Reduced time horizons of 5 and 10 years were considered. The model also included an option to dampen the effects of the intervention over time. In the base case, it was assumed that the observed trial results would be sustained, while sensitivity analyses were tested in which 1) benefits of the intervention decayed by 50% after 3 years and were 100% decayed (i.e., equivalent efficacy of the 2 arms) after 10 years and 2) benefits of the intervention decayed by 100% after 3 years. In these decayed benefit scenarios, it was assumed that once the benefit had entirely stopped, the costs of the intervention would stop also, as it would be discontinued if no longer effective.
Several sensitivity analyses were also conducted regarding cost implications of the intervention. In the base case analysis, it was assumed that there would be no difference in background medical costs across the 2 arms. In sensitivity analysis, a decrease of $100 per year in background medical costs was considered for the pharmacist intervention group, to reflect the potential for reduced general practitioner visits resulting from pharmacist contact. In sensitivity analysis regarding the costs of the intervention itself, training costs for practitioners were considered and other costs related to the intervention were doubled. At a cost of $1,000 per trainee per day to fund the session and a half-day training program, prorated over an assumed 15 patients per pharmacist, the resulting cost per participating individual was $33.33. In the base case, a common crude annual background medical cost was applied to individuals of all ages, and age-specific values were applied in sensitivity analysis. An optimistic sensitivity analysis was included, in which it was assumed that after the first year, only 2 follow-ups per year would be needed (compared with quarterly in the base case) and, consistent with observed trial results, that no increase in medications and corresponding increase in medication costs would be observed in the base case.
A probabilistic sensitivity analysis was also conducted, in which ranges of plausible uncertainty were considered for all relevant parameters and varied simultaneously, to assess the impact on economic and health outcomes. Separate probabilistic sensitivity analysis outcomes were generated for the full-scope pharmacist intervention and partial-scope pharmacist intervention, respectively. Parameter values used in the probabilistic sensitivity analysis are listed in Table 2.
Finally, a range of potential systolic blood pressure decreases resulting from the intervention, from 5 to 20 mmHg, were assessed and key outcomes generated and assessed graphically. In addition to the full- and partial-scope pharmacist intervention values, key outcomes were reported across a range of systolic blood pressure reductions from 5 to 20 mmHg.
Results
Modeled population characteristics, based on the trial population in the RxACTION10 clinical trial, are reported in Table 1. The average age was 63.5 years, and approximately half (49%) were male. The mean systolic blood pressure was 149.5, with 78% already being treated for hypertension.
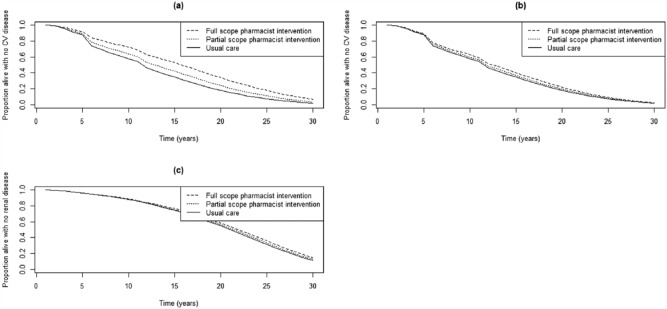
CVD and ESRD outcomes for the base case scenario and key sensitivity analyses over time are shown in Figure 2. The “full-scope pharmacist intervention” refers to the 18.3 mmHg difference relative to usual care reported by Tsuyuki et al.,10 while the “partial-scope pharmacist intervention” represents the 7.6 mmHg relative to usual care reported by Santschi et al.9 The base case impact of blood pressure reduction on CVD, based on the relationship reported by the BPL Treatment Trialists’ Collaboration, is shown in Figure 2a, while the sensitivity analysis using only Framingham equations is shown in Figure 2b. In all analyses, rates of CVD and ESRD are lowest for the full-scope pharmacist intervention and highest for usual care. The differences between the pharmacist intervention and usual care are less pronounced for the partial-scope pharmacist intervention or when Framingham equations are used to measure the impact of blood pressure reduction.
Figure 2.
Time until onset of (a) cardiovascular disease with blood pressure impact based on Blood Pressure Lowering Treatment Trialists’ risk equations, (b) cardiovascular disease with blood pressure impact based on Framingham risk equations and (c) end-stage renal disease
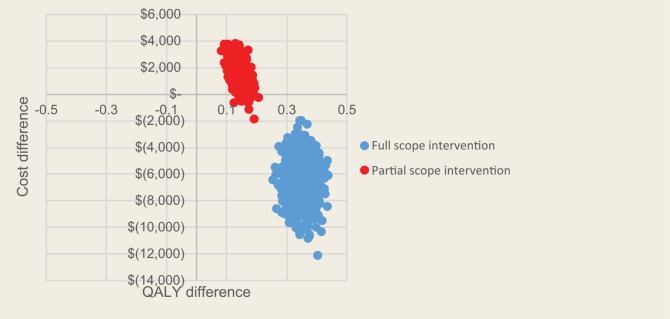
For base case settings for the full-scope pharmacist intervention, the 30-year risk of a cardiovascular event is reduced from 0.61 to 0.41, that is, a reduction of 2 cardiovascular events for every 10 people receiving the intervention (Table 3). Discounted at 5% per year, the full-scope pharmacist intervention is associated with 0.3 additional life-years and 0.4 additional QALYs relative to usual care. The reduction in costs associated with CVD and ESRD were found to more than offset the cost of the intervention itself, resulting in a discounted cost savings of $6,365 over 30 years for an individual in the full intervention group relative to usual care. The intervention was associated with increased (discounted) costs of $7,145 related to the intervention itself and related increases to medication costs and $1,584 associated with background medical costs. This was offset by a reduction of $14,002 in CVD costs and $1,092 in CKD costs. Thus, the intervention was found to be dominant (i.e., less costly and more effective) than usual care. In probabilistic sensitivity analyses, 100% of iterations remained in the dominant quadrant of the cost-effectiveness plane for the full-scope pharmacist intervention (Figure 3). For the partial-scope pharmacist intervention, 98% of iterations were in the quadrant of the plane corresponding to improved health outcomes and increased costs, and 100% of these were within a cost-effectiveness threshold of $40,000 per QALY.
Table 3.
Results of Markov model of full-scope pharmacist intervention in hypertension management, base case and sensitivity analyses
| Usual care | Full-scope pharmacist intervention (18.3 mmHg reduction in systolic bold pressure) | Difference | |
|---|---|---|---|
| Base case | |||
| Cardiovascular events | 0.61 | 0.40 | −0.21 |
| End-stage renal disease events | 0.0039 | 0.0031 | −0.0008 |
| Life-years | |||
| Discounted | 12.4 | 12.7 | 0.3 |
| Undiscounted | 20.0 | 20.7 | 0.8 |
| Quality-adjusted life-years (QALYs) | |||
| Discounted | 10.4 | 10.8 | 0.3 |
| Undiscounted | 16.5 | 17.4 | 0.9 |
| Costs | |||
| Discounted | $140,641 | $134,277 | −$6,365 |
| Undiscounted | $261,444 | $252,582 | −$8,862 |
| Category-specific costs (discounted) | |||
| Intervention costs | $0 | $7,145 | $7,145 |
| Background medical costs | $75,764 | $77,348 | $1,584 |
| Total cardiovascular disease | $36,134 | $22,133 | −$14,002 |
| Stroke | $18,723 | $11,471 | −$7,251 |
| Myocardial infarction | $8,260 | $5,059 | −$3,201 |
| Angina | $2,166 | $1,326 | −$840 |
| Heart failure | $6,985 | $4,276 | −$2,709 |
| Chronic kidney disease | $28,743 | $27,651 | −$1,092 |
| Incremental cost-effectiveness per QALY | |||
| Discounted | Intervention dominates | ||
| Undiscounted | Intervention dominates | ||
| Incremental cost-effectiveness: one-way sensitivity analyses (per QALY, discounted) | |||
| Framingham risk equations for blood pressure impact | $28,688 | ||
| Blood pressure reduction based on partial-scope intervention | $12,612 | ||
| Age-specific background cost estimates | Intervention dominates | ||
| 10-year time horizon | Intervention clinically equivalent | ||
| 5-year time horizon | Intervention clinically equivalent | ||
| Doubled cost of pharmacist intervention plus $33.33 per patient training costs incorporated | Intervention dominates | ||
| Reduced background annual medical costs in intervention group | Intervention dominates | ||
| Efficacy reduced: 50% after year 3, 100% after year 10 | Intervention dominates | ||
| Efficacy reduced: 100% after year 3 | Intervention clinically equivalent | ||
| “Optimistic” scenario regarding cost of full-scope intervention | Intervention dominates | ||
Figure 3.
Cost-effectiveness plane scatterplot for base case model parameters for both full-scope and partial-scope pharmacist interventions
Across the majority of one-way sensitivity analyses, the pharmacist intervention remained more effective than the status quo (Table 3). However, when a shorter (5- or 10-year) time horizon was used, or the intervention was assumed to have zero benefits after 3 years, the interventions were clinically equivalent as characterized by QALYs, suggesting that more time is required to realize a reduction in clinical events and corresponding increase in QALYs. With respect to incremental costs, in approximately half of sensitivity analyses, the pharmacist intervention remained less costly than the status quo. When Framingham risk equations were used to characterize the impact of blood pressure on CVD risk, or when the partial-scope pharmacist intervention was modelled rather than the full intervention, the intervention was associated with increased medical costs, with incremental cost-effectiveness ratios ranging from approximately $12,000 to $29,000. Additional clinical and cost outcomes for the one-way sensitivity analyses are included in the supplementary appendix in the online version of the article. Across sensitivity analyses, the life-years gained associated with the pharmacist intervention (discounted) ranged from 0.0 to 0.3, while QALYs gained ranged from 0.0 to 0.3. Incremental discounted costs associated with the intervention ranged from a cost savings of $11,509 to an increase of $1,730. Thus, while the magnitude of clinical and cost benefits associated with the intervention varied across analyses, the overall interpretation of the cost-effectiveness (and potentially economic dominance) of the intervention was consistent.
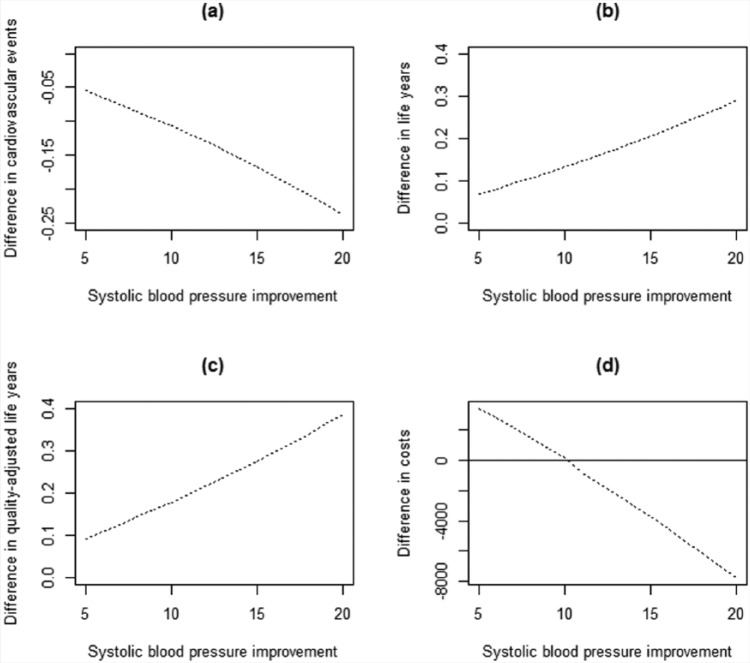
When the decrease in systolic blood pressure ranged from 5 to 20 mmHg, resulting outcomes varied in an approximately linear manner (Figure 4). For incremental life-years, QALYs and cardiovascular events, while larger blood pressure decreases were associated with larger improvements, the intervention was consistently associated with improved outcomes for the full range of values tested. For incremental costs, the costs of the intervention were greater than medical cost offsets for blood pressure reductions less than approximately 10 mmHg; for larger reductions, the intervention shifted to being cost-saving overall.
Figure 4.
Relationship between reduction in systolic blood pressure associated with a pharmacist intervention and estimated incremental difference between arms in (a) cardiovascular events, (b) discounted life-years, (c) discounted quality-adjusted life-years and (d) discounted direct medical costs
Discussion
This is the first study to examine the cost-effectiveness of pharmacists providing advanced scope of practice for management (prescription, education, consultation) of hypertension compared with usual care. In the base case of a 30-year time horizon, pharmacist management of hypertension was an economically dominant strategy when compared with usual care, that is, saving money and improving health outcomes, with an estimated discounted cost savings of more than $6,000 per individual. Across a number of strategies and sensitivity analyses, results either remained dominant or, under increasingly conservative assumptions about the efficacy of the strategy, continued to show that the intervention would provide good value for money, well within standard cost-effectiveness thresholds. In a sensitivity analysis in which it was assumed that the intervention would require 2 visits per year after year 1 and that there would not be a net increase in medication costs—an assumption that is supported by empirical trial data10—the estimated discounted cost savings increased to more than $11,000 per individual. These results reflect the relatively low costs of the program, particularly relative to the costs of treating CVD or ESRD. In the base case, based on extrapolation of observed trial results, an approximately 20% reduction in CVD incidence over 30 years was predicted. This finding has important public health implications, as pharmacist-based strategies could be used to contribute to filling the hypertension care gap in a cost-effective manner. Indeed, the infrastructure for these services is already present; what is now needed is to expand pharmacists’ scope of practice and to appropriately incentivize pharmacists to provide this care.
In sensitivity analyses in which the modelled time horizon was restricted to 5 or 10 years, or for which the intervention was assumed to be discontinued after 3 years, cost-effectiveness ratios could not be calculated, as the intervention was equivalent to the status quo with respect to QALYs. This highlights the need for the intervention to be maintained over time in order to be both effective and cost-effective. An advantage of pharmacist intervention is patient access to timely care; it is essential that individuals continue to routinely make use of the service in order to achieve beneficial health outcomes. The particular intervention modeled in the base case (“full-scope pharmacist intervention”) was relatively aggressive, including pharmacist prescription in addition to medication review and education and follow-up visits every 1 to 3 months; the observed SBP reduction in the intervention arm of 18.3 mmHg was notably higher than the corresponding SBP reduction of 7.6 mmHg across intervention arms within the meta-analysis conducted by Santschi et al.9 (“partial-scope pharmacist intervention”). Thus, the economic benefits from the full-scope pharmacist intervention likely represent an upper bound of cost savings and health benefits that could be achieved through pharmacist intervention. While less aggressive interventions may not achieve the same level of benefit, the smaller clinical benefits observed within the partial intervention were still associated with improved health outcomes and cost savings, with the magnitude of such benefits being the primary difference.
Other studies have examined the cost-effectiveness of providing more optimal care in hypertension, and results are generally consistent with those reported here. A study by Moran et al.30 evaluated the cost-effectiveness of treating hypertension in U.S. adults according to the 2014 guidelines.31 This well-done modeling study generated similar results to those reported here: the application of the guidelines to U.S. adults between the ages of 35 and 74 years would reduce cardiovascular events (about 56,000 per year in the United States) and lower costs. The authors concluded that pharmacist interventions could be one solution to implementing these guidelines in the population.
Another economic evaluation modelled the results from a cluster-randomized clinical trial (the CAPTION trial) comparing a physician-pharmacist collaboration (either a 9-month or 24-month blood pressure intervention) to usual care.32,33 The intervention in this trial included a medication history, assessment of blood pressure medications, assessment of barriers to blood pressure control (side effects, nonadherence), lifestyle modifications and specific recommendations to the prescribing physician. Pharmacists were embedded directly within physician offices and thus could provide face-to-face consultation. The main results from this trial at 9 months were a reduction of 6.1 mmHg systolic blood pressure, 2.9 mmHg diastolic blood pressure and an incremental improvement of 11% in individuals achieving hypertension control. Costs collected were only those associated with the provider and medications used to manage hypertension, so this was not a full economic evaluation.34 The authors concluded that the costs to lower blood pressure by 1 mmHg were approximately $39 for systolic blood pressure and $82 for diastolic blood pressure. In addition, the cost associated with providing blood pressure control to one individual was $22.55. Not accounting for the costs to manage long-term complications (CVD, stroke, ESRD) was a major limitation of this study, and, as such, the results are likely conservative.
As for any cost-effectiveness model, a key limitation is the assumption required to extrapolate observed data into long-term outcomes, and the overarching strategy for addressing this limitation was to perform extensive probabilistic and deterministic analyses, as well as a series of threshold analyses. In this model, a key source of uncertainty was the assumed long-term CVD reduction based on observed 6-month outcomes in blood pressure reduction. In the base case, the 18.3 mmHg reduction in systolic blood pressure over 6 months translated to a notable decrease in risk of CVD incidence (relative risk of 0.50, based on equations extrapolated from BPL Treatment Trialists’ published results). While this estimate was based on the most relevant high-quality data identified, it is not known whether this long-term effect would be realized in actual clinical practice. However, under alternative assumptions based on other data sources, regarding the blood pressure reduction that would result from the intervention and/or the resulting relative risk of CVD, the intervention remained a cost-effective strategy. Therefore, while the exact parameter values used in the base case of the model and hence the specific numeric results are subject to uncertainty, the overall interpretation of a cost-effective strategy is robust across all plausible scenarios and parameter values.
As accessible front-line health care practitioners, pharmacists are well positioned to intervene in hypertension management. While the magnitude of the impact is dependent on the specific details of the intervention, randomized controlled trials have consistently found pharmacist intervention to be effective at reducing blood pressure in hypertensive patients.9,10 As demonstrated in the economic evaluation presented here, this clinical effectiveness is anticipated to lead to cost savings or cost-effectiveness for third-party health payers, and as a result, implementing such programs represents good value for money. As pharmacist scope of practice is expanded in Canada, reimbursement schedules may be revisited; in particular, there may be a need to incorporate more disaggregated fees across a range of specific interventions. Cost-effectiveness analyses such as the one presented here can help to define the most appropriate fees for services, to ensure that pharmacists are remunerated appropriately for their time and expertise, considering the corresponding income to other components of health services expenditure. With 7.5 million Canadians currently living with hypertension5 and 35% to 65% inadequately controlled,4,5,28 a comprehensive and multidisciplinary approach is required to manage cardiovascular risk—a leading cause of morbidity and mortality in our society. Robust trial data have demonstrated that increased pharmacist intervention is one effective strategy for improving blood pressure control in the community, and this companion economic analysis demonstrates that if individuals adhere to an intervention that makes use of the full scope of pharmacist services, there is potential for substantial economic benefits to complement clinical improvements. ■
Supplementary Material
Footnotes
Author Contributions:C. Marra designed and conceptualized the study, provided guidance into model design and interpretation and contributed to the drafting and revision of the manuscript. K. Johnston led the development of the model and contributed to the drafting and revision of the manuscript. V. Santschi provided guidance on the interpretation of results in the context of related research and contributed to the drafting and revision of the manuscript. R. T. Tsuyuki led the conduct of the trial that was the key data source of the model, provided guidance on the interpretation of results in the context of related research and contributed to the drafting and revision of the manuscript. All authors approved the final version of this article.
Declaration of Conflicting Interests:The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding:The authors received no financial support for the research, authorship and/or publication of this article. The research was sponsored by the Canadian Pharmacists’ Association.
References
- 1. Ezzati M, Lopez AD, Rodgers A, et al. Selected major risk factors and global and regional burden of disease. Lancet 2002;360(9343):1347-60. [DOI] [PubMed] [Google Scholar]
- 2. Zhou B, Bentham J, Di Cesare M, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017;389(10064):37-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. WHO global atlas of cardiovascular disease. Available: www.who.int/cardiovascular_diseases/en/ (accessed Mar. 2, 2017).
- 4. Bakris G, Sarafidis P, Agarwal R, Ruilope L. Review of blood pressure control rates and outcomes. J Am Soc Hypertens 2014;8(2):127-41. [DOI] [PubMed] [Google Scholar]
- 5. Padwal RS, Bienek A, McAlister FA, Campbell NR. Epidemiology of hypertension in Canada: an update. Can J Cardiol 2016;32(5):687-94. [DOI] [PubMed] [Google Scholar]
- 6. Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA 1996;275(20):1571-6. [PubMed] [Google Scholar]
- 7. Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention and treatment of hypertension. Can J Cardiol 2016;32(5):569-88. [DOI] [PubMed] [Google Scholar]
- 8. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364(9438):937-52. [DOI] [PubMed] [Google Scholar]
- 9. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc 2014;3(2):e000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsuyuki RT, Houle SK, Charrois TL, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation 2015;132(2):93-100. [DOI] [PubMed] [Google Scholar]
- 11. D’Agostino RB, Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117(6):743-53. [DOI] [PubMed] [Google Scholar]
- 12. Pencina MJ, D’Agostino RB, Sr., Larson MG, et al. Predicting the 30-year risk of cardiovascular disease: the Framingham Heart Study. Circulation 2009;119(24):3078-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Framingham Heart Study. A project of the National Heart, Lung and Blood Institute and Boston University. Available: www.framinghamheartstudy.org (accessed Mar. 2, 2017).
- 14. Blood Pressure Lowering Treatment Trialists’ Collaboration, Ying A, Arima H, et al. Effects of blood pressure lowering on cardiovascular risk according to baseline body-mass index: a meta-analysis of randomised trials. Lancet 2015;385(9971):867-74. [DOI] [PubMed] [Google Scholar]
- 15. Hsu CY, McCulloch CE, Darbinian J, et al. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 2005;165(8):923-8. [DOI] [PubMed] [Google Scholar]
- 16. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Hypertension 2003;42(6):1206-52. [DOI] [PubMed] [Google Scholar]
- 17. Pocock SJ, McCormack V, Gueyffier F, et al. A score for predicting risk of death from cardiovascular disease in adults with raised blood pressure, based on individual patient data from randomised controlled trials. BMJ 2001;323(7304):75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sullivan PW, Lawrence WF, Ghushchyan V. A national catalog of preference-based scores for chronic conditions in the United States. Med Care 2005;43(7):736-49. [DOI] [PubMed] [Google Scholar]
- 19. Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet 2014;384(9943):591-8. [DOI] [PubMed] [Google Scholar]
- 20. Mittmann N, Seung SJ, Hill MD, et al. Impact of disability status on ischemic stroke costs in Canada in the first year. Can J Neurol Sci 2012;39(6):793-800. [DOI] [PubMed] [Google Scholar]
- 21. Sorensen SV, Kansal AR, Connolly S, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation: a Canadian payer perspective. Thromb Haemost 2011;105(5):908-19. [DOI] [PubMed] [Google Scholar]
- 22. Bentkover JD, Stewart EJ, Ignaszewski A, et al. New technologies and potential cost savings related to morbidity and mortality reduction in class III/IV heart failure patients in Canada. Int J Cardiol 2003;88(1):33-41. [DOI] [PubMed] [Google Scholar]
- 23. McGillion MH, Croxford R, Watt-Watson J, et al. Cost of illness for chronic stable angina patients enrolled in a self-management education trial. Can J Cardiol 2008;24(10):759-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coyle D, Coyle K, Cameron C, et al. Cost-effectiveness of new oral anticoagulants compared with warfarin in preventing stroke and other cardiovascular events in patients with atrial fibrillation. Value Health 2013;16(4):498-506. [DOI] [PubMed] [Google Scholar]
- 25. Manns BJ, Mendelssohn DC, Taub KJ. The economics of end-stage renal disease care in Canada: incentives and impact on delivery of care. Int J Health Care Finance Econ 2007;7(2-3):149-69. [DOI] [PubMed] [Google Scholar]
- 26. Canadian Institute of Health Information. National health expenditure trends. Table E. 1.16.2. Available: https://secure.cihi.ca/free_products/nhex_trends_narrative_report_2015_en.pdf (accessed Mar. 2, 2017).
- 27. Canadian Pharmacists Association. 2016 fees and claims data for government-sponsored pharmacist services, by province. 2016. Available: www.cfpnet.ca/bank/document_en/84-2015-provincial-chart.pdf (accessed Mar. 2, 2017).
- 28. Tsuyuki RT, Al Hamarneh YN, Jones CA, Hemmelgarn BR. The effectiveness of pharmacist interventions on cardiovascular risk: the multicenter randomized controlled RxEACH Trial. J Am Coll Cardiol 2016;67(24):2846-54. [DOI] [PubMed] [Google Scholar]
- 29. Statistics Canada. Table 326-0021 Consumer Price Index. 2002. Available: http://www5.statcan.gc.ca/cansim/a26?id=3260021 (accessed Mar. 2, 2017).
- 30. Moran AE, Odden MC, Thanataveerat A, et al. Cost-effectiveness of hypertension therapy according to 2014 guidelines. N Engl J Med 2015;372(5):447-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311(5):507-20. [DOI] [PubMed] [Google Scholar]
- 32. Carter BL, Coffey CS, Ardery G, et al. A cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes 2015;8(3):235-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Polgreen LA, Han J, Carter BL, et al. Cost effectiveness of a physician-pharmacist collaboration intervention to improve blood pressure control. Hypertension 2015;66(6):1145-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Drummond M, Sculpher M, Torrance G, et al. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford (UK): Oxford University Press; 2005. [Google Scholar]
- 35. Leenen FH, Dumais J, McInnis NH, et al. Results of the Ontario survey on the prevalence and control of hypertension. CMAJ 2008;178(11):1441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.