Abstract
Background:
Older adults with dementia are at high risk for drug-related adverse outcomes. While much is known about potentially inappropriate medication use in older adults, its prevalence and characteristics among those with dementia are not as well elucidated. We conducted a literature review to examine the prevalence of potentially inappropriate medication use among home-dwelling older adults with dementia. Our secondary aim was to determine the most frequently implicated medications and factors associated with potentially inappropriate medication use.
Methods:
MEDLINE, EMBASE, CINAHL, and International Pharmaceutical Abstracts were searched between 1946 and 2014 for articles that referenced potentially inappropriate medication use and types of dementia. One reviewer screened all titles and abstracts from the initial search and full-text articles after the initial screen for eligibility, then 2 reviewers independently abstracted data from included studies.
Results:
Searches yielded 81 articles, of which 7 met inclusion criteria. Prevalence of potentially inappropriate medication use varied from 15% to 46.8%. No single drug or drug class was reported consistently across all studies as the most frequent potentially inappropriate medication, but anticholinergics and benzodiazepines, drugs that affect cognition, were among the most common medications or pharmacological classes listed.
Discussion:
Older adults with dementia may be particularly vulnerable to potentially inappropriate medications because of cognitive impairment from their condition and the greater likelihood of experiencing adverse events from medications. Given this population’s greater susceptibility to adverse events, more intense medication and patient monitoring may be warranted, especially among those taking anticholinergics and benzodiazepines, as these drugs can contribute to cognitive impairment.
Knowledge into Practice.
The prevalence of potentially inappropriate medications among older adults with cognitive impairment and/or dementia ranges from 15% to 47%, depending on the population assessed and the tool used to detect such medications.
Proactive recognition and management of potentially inappropriate medications in older adults with dementia is warranted, especially use of benzodiazepines and drugs with anticholinergic properties, as these agents are frequently implicated.
Review indications for all potentially inappropriate agents in this population, but in particular benzodiazepines and benzodiazepine analogues and agents with anticholinergic properties, such as over-the-counter antihistamines, tricyclic antidepressants and urinary antispasmodics.
Consider nonpharmacological therapy or safer alternatives, if available, to these agents. For benzodiazepines and benzodiazepine analogues, consider tapering or deprescribing.
Mise En Pratique Des Connaissances.
La prévalence des médicaments potentiellement inappropriés chez les personnes âgées souffrant de troubles cognitifs ou de démence varie de 15 à 47 % selon la population à l’étude et les outils servant à détecter ce type de médicaments.
Il est nécessaire de reconnaître et de prendre en charge de manière proactive les médicaments potentiellement inappropriés chez les personnes âgées qui souffrent de démence, spécialement quand il s’agit de benzodiazépines et de médicaments aux propriétés anticholinergiques, car ces agents sont souvent impliqués.
Il faut revoir les indications pour tous les agents potentiellement inappropriés pour cette population, en particulier les benzodiazépines et les composés analogues ainsi que les agents aux propriétés anticholinergiques comme les antihistaminiques, les antidépresseurs et les antipsychotiques urinaires qui sont en vente libre.
Si possible, envisager un traitement non pharmacologique ou des solutions plus sécuritaires à la place de ces agents. Ainsi, pour les benzodiazépines et les composés analogues, il est possible de diminuer la prise de ces médicaments ou de ne plus les prescrire.
Introduction
An estimated 747,000 Canadians had Alzheimer’s disease and other dementias in 2011, a figure expected to nearly double to 1.4 million by 2031.1 The impact of dementia on individuals, their families and society is substantial. In Canada, the cost of medical care and lost earnings because of dementia totals $33 billion annually.1 In addition to these more tangible costs, family caregivers spend an estimated 444 million unpaid hours per year looking after someone with cognitive impairment, including dementia.1
The prevalence of chronic conditions and the number of medications taken to treat them increase with age. Drug-use data from the Canadian Institute for Health Information revealed that among Canadians between 65 and 74 years of age, 20% had submitted a claim for 10 or more drugs.2 For those between 75 and 84 years of age, the corresponding figure was 31.9% and among those 85 years or older, it was 39.3%.2 Older adults are not only more vulnerable to the adverse effects of drugs because of physiological changes related to aging3 but also more likely to be hospitalized from adverse effects, the risk of which increases with polypharmacy and use of potentially inappropriate medications.4-7
Mark H. Beers,8 the geriatrician who conducted the seminal research on drug interactions in older adults, defined potentially inappropriate medications as “those medications that pose greater risks than they provide in therapeutic value or those medications for which a safer alternative is available.” Laroche and colleagues9 similarly defined potentially inappropriate medications as those “with an unfavourable benefit/risk ratio when safer or equally effective alternatives are available.” Several criteria have been developed to guide prescribing for the elderly.8,10-15 These tools help clinicians identify medications that may be inappropriate for all older adults, but they are especially relevant for older adults with cognitive impairment and dementia. Such individuals may be at greater risk for adverse effects from potentially inappropriate medications, given the impact of anticholinergic drugs and benzodiazepines on cognition, the effect of progressive cognitive dysfunction on medication management and adherence, and the potential for even greater drug-disease and drug-drug interactions with medications frequently prescribed for persons with dementia.16-22
Accordingly, we conducted a literature review to examine the prevalence of potentially inappropriate medication use among community-dwelling elderly persons with dementia. Our secondary objectives were to determine the most frequently implicated medications and factors associated with use of potentially inappropriate medications in this population.
Methods
Data sources and search strategy
A single reviewer (K.S.) searched the MEDLINE (Ovid), EMBASE, CINAHL, and International Pharmaceutical Abstracts databases using the Boolean terms AND and OR with the following key terms: inappropriate medication,* potentially inappropriate prescription medication, potentially inappropriate medication, unnecessary medications, suboptimal prescribing, inappropriate prescribing AND dementia, Parkinson disease, Alzheimer’s disease, Pick disease of the brain (the EMBASE search included the following terms: AND home-dwelling and community;* in addition, Pick disease of the brain was included as Pick presenile dementia). All databases were searched for records published between 1946 to the third week of February 2014 and limited to those in English. Bibliographies of eligible studies were searched for additional relevant studies. Search results were entered into a Microsoft Excel spreadsheet. One reviewer (K.S.) screened all titles and abstracts from the initial search as well as full-text articles after the initial screen for eligibility. Two reviewers (K.S. and T.P.) independently abstracted data from included studies. Discrepancies in data abstraction were resolved by discussion to reach consensus.
Study selection
Studies were included if participants were ambulatory home-dwelling adults, 65 years of age or older. While our literature review focused predominantly on inappropriate medication use among older persons with dementia, studies often combined dementia and cognitive impairment, so our review included older adults with both dementia and mild to moderate cognitive impairment. The intervention examined in this review was inappropriate medication use. No limits on study methodology were applied. Outcomes were abstracted from review articles (including systematic reviews, meta-analyses, scoping reviews, literature reviews and qualitative systematic reviews) and from primary studies (randomized case-control trials, cohort studies, prospective and retrospective studies and qualitative analyses). Studies were excluded if they were published only as conference abstracts, editorials and commentaries, letters to the editor or discussion papers; investigated the use of potentially inappropriate medications in populations outside ambulatory home-dwelling older adults; or focused on specific drugs or on inappropriate interventions.
Data extraction
The following data were abstracted: study design, location and time of study, demographics (mean age, percentage female participants), tool used to identify potentially inappropriate medications, reported rates of potentially inappropriate medication use, drugs or pharmacological classes most frequently implicated and factors associated with increased use of potentially inappropriate medications.
Outcomes
We did not adopt a definition for what constitutes a control group; however, most articles compared medication use in older adults with dementia to medication use in older adults without dementia. The outcomes sought were prevalence rates of potentially inappropriate medication use, associated medications and univariate and multivariate factors associated with increased potentially inappropriate medication use.
Results
Literature review
Our search yielded 81 articles. After applying exclusion criteria, 7 articles were included in our review (Figure 1).
Figure 1.
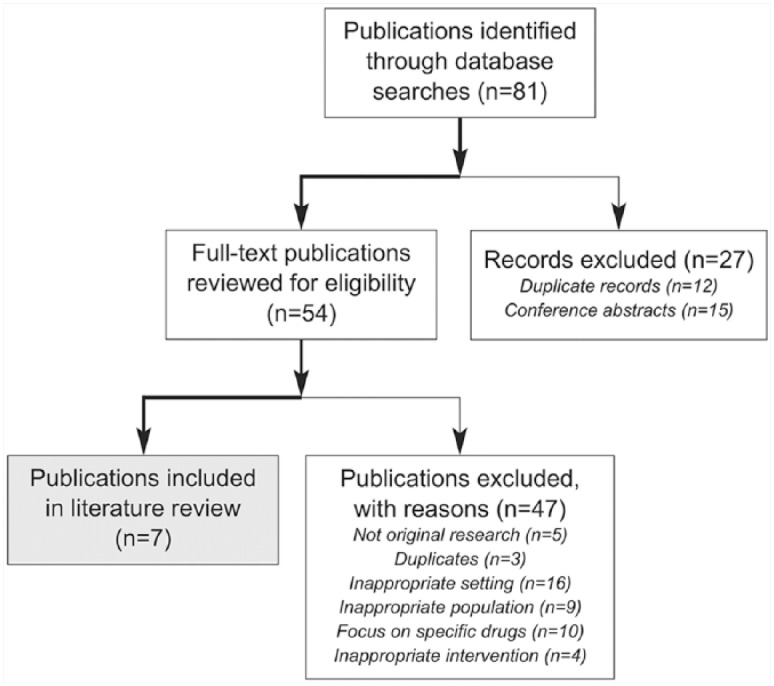
Literature review and study selection flow chart
Summary of included studies
Of the 7 articles, 2 were prospective cohort studies,23,27 1 was a longitudinal cohort study,24 3 were retrospective studies25,26,28 and 1 was a secondary analysis of prospective data.29 Sample sizes ranged from 34223 to 131,808,28 and the mean age of study participants ranged from 77 years25,26 to 80.9 years.23 See Table 1 for a summary of these articles by study design, geographic location and time of study, patient population, sample size, mean age of participants with and without dementia and percentage of female participants.
Table 1.
Included studies: Methodology and participant characteristics
| Study | Design, location and time of study | Patient population | Sample size | Mean age (years) | Gender (% female) |
|---|---|---|---|---|---|
| Fiss et al., 201323 | Prospective cohort; Germany | Elderly patients in primary care with home-based assessment; subset had home-based medication reviews | Total, N = 342 Suspected dementia, n = 111 No signs of dementia, n = 231 |
80.9 82.7 with dementia 80 without dementia |
31.7 with dementia 69.3 without dementia |
| Koyama et al., 201324 | Longitudinal cohort; United States; enrolled 1986-1988; followed for 10 years; study of osteoporotic fractures; follow-up at 6 years and 10 years for medication reviews | Community-dwelling women 75 years of age and older | Total, N = 1484 | 78 | 100 |
| Lau et al., 201025 | Retrospective cohort; United States; September 2005–September 2007; National Alzheimer’s Coordinating Center, Uniform Data Set | Community-dwelling seniors 65 years of age and older | Total, N = 4518 (4087
analyzed) Dementia (+), n = 2665 (2467 analyzed) Dementia (–), n = 1853 (1620 analyzed) |
77 total sample 77.7 with dementia 76 without dementia |
59 |
| Lau et al., 201126 | Retrospective cohort, United States; September 2005–September 2009; National Alzheimer’s Coordinating Center, Uniform Data Set | Community-dwelling seniors 65 years or older with dementia | Dementia (+), N = 1994 | 77 | 49.2 |
| Montastruc et al., 201327 | Prospective cohort; France, enrolled 2000-2002; followed for 4 years | Community-dwelling patients with mild to moderate Alzheimer’s disease | 684 | 77.9 | 71.1 |
| Pugh et al., 201128 | Retrospective cross-sectional database; United States; 2004-2006; Healthcare Effectiveness Data and Information Data Set; outpatient and pharmacy data | Community-dwelling veterans | Total, N = 131,808 with
dementia With drug-disease interaction, n = 26,650 Without drug-disease interaction, n = 105,158 |
78.5 with drug-disease interaction 79.5 without drug-disease interaction |
2.9 with drug interaction 2.1 without drug interaction |
| Thorpe et al., 201229 | Secondary analysis of prospective data study; United States; 1996-2001; Resource for Enhancing Alzheimer’s Caregiver Health study | Family caregivers and dementia patients | Total, N = 566 Dementia patients, n = 187 Caregivers, n = 379 |
79.5 with dementia 74.2 caregivers |
Not reported |
Tools used to detect potentially inappropriate medications
Four tools (Table 2 and Appendix 1, available in the online version of the article) were used to detect and calculate the prevalence of potentially inappropriate medication use across studies in this review.
2003 Beers criteria
Four studies used the 2003 Beers criteria to assess potentially inappropriate medication use.24-26,29 However, because of data limitations, the 2 studies by Lau and colleagues25,26 limited potentially inappropriate medications to 44 agents and drug classes of the Beers medications that generally should be avoided in all elderly patients regardless of indications or health conditions, including dementia (Table 2).
Table 2.
Criteria and tools used to identify potentially inappropriate medication use among participants
| Study | Tool |
|---|---|
| Fiss et al., 201323 | PRISCUS list: 1. Generally inappropriate in elderly patients; 2. Inappropriate drug-disease combinations |
| Koyama et al., 201324 | 2003 Beers criteria (Beers criteria: 1. Medications that should be avoided in all older adult patients; 2. Medications at a specified dose that should not be exceeded; 3. Medications that should be avoided in specific comorbid conditions) |
| Lau et al., 201025 | 2003 Beers criteria; however, because of data limitations, able to assess only 1 of 3 Beers criteria (Criterion 1: Medications that should be avoided in all older adult patients) |
| Lau et al., 201126 | 2003 Beers criteria; however, because of data limitations, able to assess only 1 of 3 Beers criteria (Criterion 1: Medications that should be avoided in all older adult patients) |
| Montastruc et al., 201327 | 1. Laroche list 2. Plus the addition of a list of atropinic drugs developed through expert consensus* 3. Comparison to 2003 Beers criteria |
| Pugh et al., 201128 | Lindblad classification of 28 drug-disease interactions, of which 3 were measured in 3 groups of patients: those with dementia, those who have fallen and those with chronic renal failure |
| Thorpe et al., 201229 | 2003 Beers criteria |
List developed by including all atropinic drugs in the Laroche list, La Revue Prescrire list and the Anticholinergic Cognitive Burden Scale.
Developed in 1991 by researchers at the University of California, the Beers criteria were the first set of standards that sought to identify inappropriate medication use in elderly nursing home populations.30 Since their creation, Beers criteria have been updated twice by their original author, in 19978 and again in 2003.10 The original list of potentially inappropriate medications was developed to protect the frailest elderly.17 The list was expanded in 1997 to apply to all older persons and to consider 15 specific medical conditions (i.e., heart failure, diabetes, hypertension, chronic obstructive pulmonary disease, asthma, ulcers, seizures or epilepsy, peripheral vascular disease, blood-clotting disorders, benign prostatic hyperplasia, incontinence, constipation, syncope or falls, arrhythmias and insomnia) in the use of medications. The 2003 update used a modified Delphi approach to reevaluate the 1997 criteria to reflect new products and literature, assign or reevaluate relative rating of severity for each medication and identify new conditions for consideration.10 The 2003 Beers criteria includes 48 medications or classes of medications that apply to any individual over the age of 65, as well as drugs or categories of drugs that are inappropriate for persons with any of 20 medical conditions (i.e., heart failure, hypertension, gastric or duodenal ulcers, seizures or epilepsy, blood-clotting disorders, bladder outflow obstruction, stress incontinence, arrhythmias, insomnia, Parkinson’s disease, cognitive impairment, depression, anorexia and malnutrition, syncope or falls, seizure disorder, syndrome of inappropriate diuretic hormone/hyponatremia, obesity, chronic obstructive pulmonary disease and chronic constipation).4 In 2012 and again in 2015, the American Geriatrics Society updated the Beers criteria using a modified Delphi method and systematic review approach. The 2012 update includes 53 medications or medication classes that are divided into 3 categories: 1) criteria for potentially inappropriate medication use in older adults, 2) criteria for potentially inappropriate medication use in older adults because of drug-disease or drug-syndrome interactions that may exacerbate the disease or syndrome and 3) medications to be used with caution in older adults.11
In the 2015 update, American Geriatric Society expert panelists added 2 new categories: 1) drugs for which dose adjustment is required based on kidney function and 2) select drug-drug interactions that are associated with harms in older adults.12 In this most recent update, expert panelists removed 1 class of agents (i.e., antiarrhythmic drugs as a first-line treatment for atrial fibrillation) and 3 specific drugs (trimethobenzamide, mesoridazine and chloral hydrate) as those that are independent of diagnoses or condition.12 They also removed the entire criterion of chronic constipation and lower urinary tract treated by inhaled anticholinergic drugs when taking into consideration disease and syndrome interactions.12
Panelists added proton pump inhibitors, desmopressin and meclizine as drugs that are independent of diagnoses or condition and also added opioids (when considering falls and fractures), armodafinil and modafinil (when considering insomnia), eszopiclone and zaleplon (when considering dementia and cognitive impairment) and antipsychotics (when considering delirium).12 The tables listing medications to avoid now also include the rationale, recommendation, quality of evidence and strength of the recommendation.12
PRISCUS list, Laroche list and Lindblad classification
Three of the reviewed studies relied on country- or condition-specific tools to identify potentially inappropriate medications (Table 2). Fiss et al.23 used the PRISCUS list of potentially inappropriate medications developed for the German market, which contains 37 drugs from 18 substance classes.31 Montastruc et al.27 used the Laroche list, which was developed for use in France.9 This list is composed of 34 criteria, which fall into 1 of 3 categories: 1) drugs with unfavourable benefit-to-risk ratio, 2) drugs with questionable efficacy and 3) drugs with both unfavourable benefit-to-risk ratio and questionable efficacy.9 Pugh and colleagues28 based their inappropriate drug use on a subset of measures, developed by Lindblad and colleagues,32 that measured 28 drug-disease interactions involving 14 diseases or conditions. Their study did not indicate how many measures were used, but they state that the “NCQA [National Committee on Quality Assurance] expert panel reached consensus on a subset of drug–disease interactions that could be readily measured using administrative data and that were potentially associated with adverse outcomes.”28
Potentially inappropriate medications by tool or criteria
Drugs identified as potentially inappropriate medications based on the criteria or tool used to identify them are listed in Appendix 1.
Prevalence of potentially inappropriate medication use
The prevalence of potentially inappropriate medication use among individuals 65 years of age or older with a diagnosis or suspicion of dementia ranged from 15% to 46.8% (Table 3).
Table 3.
Prevalence of use of potentially inappropriate medications in different studies
| Study | Total population | Potentially inappropriate medication prevalence | Potentially inappropriate medication criteria/tool |
|---|---|---|---|
| Fiss et al., 201323 | 342 111 (with dementia) 231 (without dementia) |
Not reported 27.0% 29.0% |
PRISCUS list |
| Koyama et al., 201324 | 1484 (total sample) 260 (dementia at 10 years’ follow-up) 354 (mild cognitive impairment at 10 years’ follow-up) 870 (cognitively normal at 10 years’ follow-up) |
24.3% (baseline); 27.3% (at 6 years’ follow-up); 23.9% (at
10 years’ follow-up) 26.1% (baseline); 33.5% (10 years’ follow-up) 26.1% (baseline); 24.4% (10 years’ follow-up) 23.0% (baseline); 20.6% (10 years’ follow-up) |
2003 Beers criteria |
| Lau et al., 201025 | 4087 (total sample) 2467 (with dementia) 1620 (without dementia) |
Not reported 15% 20% |
1 of 3 2003 Beers criteria |
| Lau et al., 201126 | 1994 (total sample, all with dementia) | 16.2% | 1 of 3 2003 Beers criteria |
| Montastruc et al., 201327 | 684 (total sample, all with dementia) | 25.3% | 2003 Beers criteria |
| Montastruc et al., 201327 | 684 | 46.8% | Laroche list |
| Pugh et al., 201128 | 305,041 (total sample) 131,808 (with dementia) |
15.2% 20.2% |
Lindblad |
| Thorpe et al., 201229 | 566 (total sample) 187 (with dementia) 379 (caregivers (without dementia)) |
Not reported 33.0% 39% |
2003 Beers criteria |
Drugs and pharmacological classes implicated in potentially inappropriate medication use
The proportions of drugs and pharmacological agents implicated as potentially inappropriate medications as well as proportions of study participants on specific potentially inappropriate medications are provided in Tables 4 and 5. Of patients with a suspicion of dementia, the greatest proportion were taking amitriptyline (14.8%) and diazepam (11.1%).23 Among patients diagnosed with mild to moderate dementia, the greatest proportion were taking benzodiazepines or benzodiazepine analogues (8.5%).27 Koyama et al.24 reported that 15.2% of their study population were using anticholinergic agents and 8.6% were using benzodiazepines; data on use of potentially inappropriate medications by cognitive status of participants were not provided. Lau et al.25 reported that of all subjects, including those with and without dementia, that were using potentially inappropriate medications, the highest proportion were using estrogens (22%) followed by muscle relaxants or antispasmodics (14%), fluoxetine (13%), short-acting nifedipine (11%) and doxazosin (7%). Using the total number of potentially inappropriate medications as a denominator, Thorpe et al.29 reported antihistamines with anticholinergic effects (11.8%), oral estrogens (11.6%), muscle relaxants or antispasmodics (9.4%), fluoxetine (8.0%) and short-acting nifedipine (6.6%) as the most commonly used potentially inappropriate medications in their sample of dementia patients (Table 5).
Table 4.
Proportion of participants on specific potentially inappropriate medications by study
| From Fiss et al.23 Pharmacological class |
Proportion (%) of patients with no suspicion of dementia | Proportion (%) of patients with suspicion of dementia |
|---|---|---|
| Antidepressants | ||
| Amitriptyline | 16.7 | 14.8 |
| Trimipramine | 5.6 | 3.7 |
| Doxepine | 5.6 | 3.7 |
| Maprotiline | 5.6 | 0 |
| Fluoxetine | 0 | 3.7 |
| Antihistamines | ||
| Diphenhydramine | 5.6 | 3.7 |
| Dimehydrinate | 3.7 | 3.7 |
| Dimetindene | 1.9 | 5.6 |
| Antipsychotics | ||
| Haloperidol | 0 | 7.4 |
| Levomepromazine | 1.9 | 0 |
| Thioridazine | 0 | 3.7 |
| Benzodiazepines and analogues | ||
| Alprazolam | 0 | 3.7 |
| Bromazapam | 3.7 | 3.7 |
| Brotizolam | 1.9 | 0 |
| Diazepam | 7.4 | 11.1 |
| Medazepam | 1.9 | 0 |
| Nitrazepam | 3.7 | 0 |
| Temazepam | 1.9 | 0 |
| Zopiclone | 1.9 | 3.7 |
| Zolpidem | 13 | 0 |
| Peripheral vasodilators | ||
| Pentoxifylline | 7.4 | 3.7 |
| Naftidrofuryl | 5.6 | 7.4 |
| Urinary antispasmodics | ||
| Solifenacin | 5.6 | 3.7 |
| Tolterodine | 0 | 7.4 |
| Oxybutynin | 0 | 3.7 |
| From Koyama et al.24,*Pharmacological class | Proportion (%) of total population (separation by cognition status not provided) | |
| Anticholinergic drugs | 15.2 | |
| Antispasmodics | 8.0 | |
| Barbiturates | 0.5 | |
| Benzodiazepines | 8.6 | |
| Central nervous system stimulants | 0.7 | |
| Muscle relaxants | 0.9 | |
| Sedative hypnotics | 3.8 | |
| From Monstastruc et al.27Pharmacological class | Proportion (%) of patients with dementia | |
| Analgesics | ||
| Indomethacin | 0.1 | |
| Association with at least 2 nonsteroidal anti-inflammatory drugs | 0.3 | |
| Antibiotic | ||
| Nitrofurantoin | 0.1 | |
| Antidepressants | ||
| Imipramine | 0.7 | |
| Antihistamines | ||
| H1 antihistamines | 2.2 | |
| Antihypertensives | ||
| Centrally acting antihypertensives | 3.1 | |
| Short-acting calcium channel blockers | 2.9 | |
| Antiarrhythmics | ||
| Disopyramide | 0.3 | |
| Antiplatelets | ||
| Ticlodipine | 0.6 | |
| Dipyridamole | 1 | |
| Antipsychotics | ||
| Phenothiazine neuroleptics | 0.6 | |
| Atropinic neuroleptics | 1.2 | |
| Benzodiazepines and benzodiazepine analogues | 8.5 | |
| Muscle relaxants/antispasmodics | ||
| Atropinic | 1.5 | |
| Muscle relaxants, nonatropinic | 0.1 | |
| Atropinic antispasmodic, gastrointestinal | 0.4 | |
| Other drugs with atropinic properties (antiemetics, antidrowsiness, nasal decongestants, cough suppressants) | 1.7 | |
| Cerebral vasodilators (dihydroergotamine, vincamine, ginkgo biloba, piribedil) | 24.1 | |
| From Lau et al., 201025Pharmacological class | Proportion (%) of all potentially inappropriate medication cases; data on 5 most common provided, similar in both those with and without dementia | |
| Antidepressants | ||
| Fluoxetine | 13 | |
| Antihypertensives | ||
| Nifedipine (immediate release) | 11 | |
| Doxazosin | 7 | |
| Estrogen | ||
| Oral estrogens | 22 | |
| Muscle relaxants/antispasmodics | 14 |
Drugs implicated in 75% of all reported potentially inappropriate medications included oxybutynin, tolterodine, lorazepam, alprazolam, paroxetine, temazepam, zolpidem and meclizine.
Table 5.
Frequency of specific drugs and/or pharmacological classes of all potentially inappropriate medications or cases
| From Thorpe et al.29 Pharmacological class | Proportion (%) of all potentially inappropriate medications |
|---|---|
| Analgesics | |
| Nonsteroidal anti-inflammatory drugs | 5.6 |
| Antidepressants | |
| Amitriptyline | 5.6 |
| Fluoxetine | 8 |
| Antihypertensives | |
| Nifedipine (immediate release) | 6.6 |
| Doxazosin | 4.6 |
| Antihistamines with anticholinergic effect | 11.8 |
| Antiplatelet drugs | |
| Ticlopidine | 3.8 |
| Antipsychotics | |
| Thioridazine | 4 |
| Estrogens | |
| Oral estrogens | 11.6 |
| Muscle relaxants or antispasmodics | 9.4 |
Factors associated with potentially inappropriate medication use
Six studies conducted bivariate and/or multivariate analyses to determine the strength of association between participant characteristics and potentially inappropriate medication use. Most studies examined the impact of gender, age, race or ethnicity, the presence of preexisting or comorbid health conditions and number of medications used. These are presented below.
Age, gender and race
In Lau et al.’s 2010 study,25 the mean age of dementia patients with and without potentially inappropriate medication use did not differ significantly. Similarly, in Montastruc et al.’s study,27 the frequency of potentially inappropriate medication use did not differ significantly among age groups between 50 and 75 years, between 76 and 85 years and in those >85 years (p = 0.848).
Five studies investigated the association of gender and potentially inappropriate medication use. Among patients with dementia or a suspicion of dementia, being female was associated with an increased risk of potentially inappropriate medication use (odds ratio [OR] = 10.362, 95% confidence interval [95% CI] = 1.280-83.875 in Fiss et al.’s study23; OR =1.4, 95% CI = 1.0-2.0 in Montastruc et al.’s study27; and OR = 1.43, 95% CI = 1.3-1.57 in Pugh et al.’s study28), while being male was associated with decreased risk (OR = 0.65, 95% CI = 0.53–0.80 in Lau et al.’s 2010 study25 and OR = 0.48, 95% CI = 0.3-0.79 in Thorpe et al.’s study29).
Race/ethnicity was not a risk factor for potentially inappropriate medication use among patients with dementia in Lau et al.’s 2010 study,25 but Koyama et al.,24 Pugh et al.,28 and Thorpe et al.29 found significant associations between race/ethnicity and potentially inappropriate medication use in their study populations. Potentially inappropriate medication use was associated significantly with being Caucasian (p = 0.03) in Koyama et al.’s study,24 and being Hispanic was associated with slightly higher odds of drug-disease interactions among patients with dementia (OR = 1.07, 95% CI = 1.01-1.13) in Pugh et al.’s study.28 Pugh et al.28 also found that African Americans had a lower odds ratio for drug-disease interactions (OR = 0.88, 95% CI = 0.83-0.92). Thorpe et al.29 found that having a Hispanic caregiver was associated with a higher odds ratio of potentially inappropriate medication use among patients with dementia (OR = 2.60, 95% CI = 1.04-6.52).
Medical conditions
Medical conditions were associated with both increased and decreased risk of potentially inappropriate medication use. Fiss et al.23 calculated an OR of 4.2 (95% CI = 1.1-16.01) for potentially inappropriate medication use among patients with musculoskeletal system disease in their sample of patients with a suspicion of dementia. Koyama et al.24 found significantly higher geriatric depression scale scores (p < 0.001), poorer sleep quality (p < 0.001) and increased anxiety scores (p < 0.001) among users of potentially inappropriate medications. Koyama et al.24 also found that potentially inappropriate medication use was significantly associated with urinary incontinence, osteoarthritis, myocardial infarction and chronic pulmonary obstructive disease (p values for all conditions were 0.02 or lower). Lau et al.25 found that among patients with dementia who were determined to be using potentially inappropriate medications, a significantly higher proportion had hypertension (62% v. 52%, p < 0.001) and incontinence (30% v. 24%, p < 0.05) and higher scores on the Neuropsychiatric Inventory Questionnaire for depression and anxiety (1.2 v. 1.3, p < 0.05) than among those who were not using potentially inappropriate medications. However, Lau et al.’s25 group found no significant difference between those who were using potentially inappropriate medications and those who were not among patients with hypercholesterolemia (49% v. 49%), cardiovascular disease (17% v. 18%), thyroid disease (16% v. 13%), diabetes (14% v. 11%) and cerebrovascular disease (6.4% v. 5.5%).
Medication use
The 5 studies that investigated the association between the number of medications taken and potentially inappropriate medication use found statistically significant relationships. Fiss et al.23 found that taking fewer than 5 drugs regularly was strongly associated with lower risk (OR = 0.06, 95% CI = 0.006-0.55) of potentially inappropriate medication use among patients with a suspicion of dementia. In Lau et al.’s 2010 study,25 patients with dementia taking at least 1 potentially inappropriate medication took more medications on average than those who were not taking any potentially inappropriate medications (mean = 6.4 ± 2.8 v. 4.7 ± 2.5; p < 0.001). Similarly, in Montastruc et al.’s study,27 polypharmacy, which was defined as taking 5 or more medications, was associated with greater potentially inappropriate medication use among patients with dementia (OR = 3.8, 95% CI = 2.8-5.2; p = 0.001) in a bivariate analysis. This significant association remained after a multivariate analysis was conducted (OR = 3.6, 95% CI = 2.6-4.5).27 In Pugh et al.’s study,28 the number of unique medications was identified as a risk factor for drug-disease interactions among dementia patients (OR = 1.13, 95% CI = 1.12-1.13), while in Thorpe et al.’s study,29 the number of medications was significantly associated with the use of potentially inappropriate medications. Specifically, when compared with patients taking 0 to 3 medications, patients taking 4 to 8 medications had a greater odds of using potentially inappropriate medications (OR = 3.3, 95% CI = 2.17-5.03). The OR more than doubled among patients taking 9 or more medications (OR = 7.60, 95% CI = 4.57-12.62).29
Discussion
Our systematic literature review examined the prevalence of potentially inappropriate medications among several populations of ambulatory, home-dwelling older adults with dementia. Our review indicates that use of potentially inappropriate medications is highly prevalent, with as many as almost 1 of every 2 older adults with dementia taking medications with the potential for significant adverse outcomes. This suggests that pharmacists should be vigilant in their assessment of their patients with dementia.
A precise prevalence figure for potentially inappropriate medication use is not possible to determine because of methodological differences across studies (see Table 2 and Appendix 1). While some variability can be expected because of differences in the criteria the various tools employ—for example, in drug-disease interactions and dosing limits for certain drugs—the ability to apply criteria consistently across studies was also not possible because of the availability of certain drugs in different countries (Appendix 1). The variability in the prevalence of potentially inappropriate medications based on the tool used to detect it is illustrated dramatically in Montastruc et al.’s study27 of older adults with Alzheimer’s disease in France. To compare their results with those of U.S. studies, this team also calculated prevalence rates using 2003 Beers criteria (Table 3). Starting with the Laroche list, Montastruc’s team27 calculated the prevalence of potentially inappropriate medication use as 46.8%, but when using Beers criteria, the prevalence dropped to 25.3%, cutting the figure almost in half. Among the 5 studies that used the 2003 Beers criteria,24-27,29 the prevalence of potentially inappropriate medication use ranged from 16.2%26 to 33%.29 However, of these studies, the 2 conducted by Lau and colleagues assessed only 1 of 3 Beers criteria (i.e., agents and drug classes that generally should be avoided in all elderly patients regardless of health conditions and indications) because of data limitations, which may explain the lower prevalence of potentially inappropriate medication use among their population of persons with dementia.25,26 Of the other 3 studies that used 2003 Beers criteria, the 2 conducted in the United States reported rates of 26.1%24 and 33%,29 while the study conducted in France found a rate of 25.3%.27 These findings suggest that when using 2003 Beers criteria, about one-quarter to one-third of persons with dementia or at risk of dementia may be taking potentially inappropriate medications.
While the specific drugs listed as potentially inappropriate varied within pharmacological classes based on the tools used to detect them (Appendix 1), we identified 2 pharmacological classes—agents with anticholinergic properties and benzodiazepines and their analogues—as being consistently found to be prevalent potentially inappropriate medications among patients with dementia. Prevalence of anticholinergic agents as a drug class was available only in Koyama et al.’s study (15.2%),24 but other researchers had noted agents with anticholinergic properties as frequent contributors of potentially inappropriate medications; for example, tricyclic antidepressants (0.7%-14.8%),23,27 antihistamines (2.2%-7.4%)23,27 and urinary antispasmodics (3.7%-7.4%).23 Among patients with dementia, potentially inappropriate benzodiazepine and benzodiazepine analogue use ranged from 3.7% to 11.1%.23,27 These 2 classes of medications can be problematic for all elderly patients but likely pose a greater risk to patients with dementia. Not only do agents with anticholinergic properties and benzodiazepine/benzodiazepine analogues affect physical function and balance, resulting in falls, but these agents also increase the risk of delirium; decrease immediate recall and verbal fluency; impair visuospatial and visuomotor abilities, motor coordination, psychomotor speed, speed of information processing and concentration; and decrease scores on cognitive tests such as the Mini-Mental Status Exam.33-36 Such adverse effects are concerning in any older adult population but particularly among those with dementia.
Multivariate analyses identified female gender and quantity of medication use as significant risk factors for potentially inappropriate medication use. Five studies found female gender to be a risk factor for use of potentially inappropriate medications in patients with or at risk of dementia.23,25,27-29 In Lau et al.’s 2010 study,25 this may have been driven by the use of estrogens among women. Indeed, when the authors analyzed the data based on a model in which they removed estrogen use, no significant differences were found between men and women in potentially inappropriate medication use.25 However, other studies reported a statistically significant relationship between being female and using potentially inappropriate medications.23,27-29 One explanation for this finding may be in the higher proportion of women presenting with psychosomatic complaints of sleeplessness, nervousness and depression,37 which may be related to a higher prevalence of insomnia, depression and anxiety among women.38-40 All of these medical conditions are treated primarily with psychotropic medications such as antidepressants and benzodiazepines.
Study limitations
Limiting studies to those of ambulatory older adults excluded 1 article that developed and validated an inappropriate prescribing detection tool in a population of acutely hospitalized patients. Known as the Improving Prescribing in Elderly Tool, or IPET, this brief screening tool was developed by Canadian researchers and validated in a Canadian population.14 Given that the effectiveness of tools designed to identify potentially inappropriate medications may be affected by availability of medications and prescribing practices in the jurisdiction where they are used, it is imperative to design, validate and use location-specific tools. A tool such as IPET would likely be of most relevance in Canada, but it would need to be validated for use in ambulatory home-dwelling older adult populations to have broader applicability.
Our literature review identified studies that used tools developed several years ago. For example, the studies that used Beers criteria relied on the 2003 iteration. Since then, the Beers criteria have been updated twice—in 201211 and most recently in 2015.12 Consequently, the findings of our review may be limited in the current prescribing climate, particularly the climate in Canada, as Beers criteria were developed for use in the United States. As well, our review was not designed to investigate the impact of interventions to decrease use of inappropriate medications on health or health service utilization outcomes.
While we did not evaluate the robustness of the different tools used to detect potentially inappropriate medication use, the Beers criteria appear to be the most comprehensive. However, these criteria undergo periodic updates and in the process have become progressively more detailed and complex. This likely limits their applicability in busy practitioner environments such as a family physician’s office or a community pharmacy. Furthermore, application of the tool in its entirety in a community pharmacy may not be possible across Canada because of the limited availability of information on indications for use, laboratory and imaging investigations. Collaborative health care environments in which physicians and pharmacists work in the same location and treat the same roster of patients may increase the feasibility of applying such time-intensive tools and, indeed, ensure that recommendations are carried out. Until such collaborative practice is widespread, the best solution may be use of a simplified tool to evaluate the appropriateness of medications in elderly patients. Given that benzodiazepines and anticholinergic agents are frequently prescribed in older adult populations, a starting point may be use of a tool designed to alert the clinician and linking it to a deprescribing guideline to reduce use of benzodiazepines and anticholinergic medications. An example of such a tool is the evidence-based deprescribing algorithm for benzodiazepine receptor agonists, a freely available resource developed by Canadian researchers and clinicians to help reduce or eliminate benzodiazepines safely.19,21 Other research teams have developed guidance on the process of such deprescribing in general.20,22
We did not assess the quality of reviewed studies. The Effective Public Health Practice Project’s quality assessment tool for quantitative studies, for example, can be used to assess the internal validity of studies, as well as their strengths and limitations.41 Such an assessment could help to assign weight to study findings. As well, our initial database searches excluded studies published in languages other than English, which may have introduced potential bias.
Conclusion
This literature review provides strong evidence that potentially inappropriate medication use among ambulatory community-dwelling older adults with dementia is of clinical significance. Proactive recognition and management of inappropriate medications in this population may be warranted, especially the use of benzodiazepines and agents with anticholinergic properties. As the older adult population with dementia is particularly vulnerable because of cognitive impairment as well as more likely to experience adverse events from these medications, greater caution and monitoring are warranted. ■
Supplementary Material
Acknowledgments
The authors thank Deval Mehta for assisting with the creation of Appendix 1 and Joe Petrik for copy-editing.
Footnotes
Author Contributions:K. Slonim conducted the literature search and analysis and contributed to the initial draft of the manuscript. T. Patel assisted with developing and finalizing the research question, data collection, analysis, interpretation, and drafting and editing the manuscript. L. Lee assisted with developing and finalizing the research question and editing the manuscript. All authors approved the final version of the manuscript.
Declaration of Conflicting Interests:The authors declare no conflicts of interest with respect to research, authorship and publication of this review.
Funding:This review was conducted in response to an applied health research question submitted by Alzheimer Society Ontario to the Innovations Strengthening Primary Healthcare through Research (INSPIRE–PHC) Program and supported by a grant from the Government of Ontario (Ministry grant 06547). The views expressed in this article are those of the authors and do not necessarily reflect those of the Government of Ontario.
References
- 1.Alzheimer Society Canada. Dementia numbers in Canada. Available: www.alzheimer.ca/en/About-dementia/What-is-dementia/Dementia-numbers (accessed Oct. 27, 2016).
- 2. Canadian Institute for Health Information. Drug use among seniors on pubic drug programs in Canada 2012. Available: https://secure.cihi.ca/free_products/Drug_Use_in_Seniors_on_Public_Drug_Programs_EN_web_Oct.pdf (accessed Oct. 27, 2016).
- 3. Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 2003;57(1):6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fick DM, Mion LC, Beers MH, et al. Health outcomes associated with potentially inappropriate medication use in older adults. Res Nurs Health 2008;31(1):42-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jano E, Aparasu RR. Healthcare outcomes associated with Beers’ criteria: a systematic review. Ann Pharmacother 2007;41(3):438-47. [DOI] [PubMed] [Google Scholar]
- 6. Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events and potential adverse drug interactions in the elderly patients presenting to an emergency department. Ann Emerg Med 2001;38(6):666-71. [DOI] [PubMed] [Google Scholar]
- 7. Reich O, Rosemann T, Rapold R, et al. Potentially inappropriate medication use in older patients in Swiss managed care plans: prevalence, determinants and association with hospitalization. PLoS One 2014;9(8):e105425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly: an update. Arch Intern Med 1997;157(14):1531-6. [PubMed] [Google Scholar]
- 9. Laroche ML, Charmes JP, Nouaille Y, et al. Is inappropriate medication use a major cause of adverse drug reactions in the elderly? Br J Clin Pharmacol 2007;63(2):177-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med 2003;163(22):2716-24. [DOI] [PubMed] [Google Scholar]
- 11. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012;60(4):616-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63(11):2227-46. [DOI] [PubMed] [Google Scholar]
- 13. Gallagher R, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Persons Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008;46(2):72-83. [DOI] [PubMed] [Google Scholar]
- 14. Naugler CT, Brymer C, Stolee P, et al. Development and validation of an improving prescribing in the elderly tool. Can J Clin Pharamcol 2000;7(2):103-7. [PubMed] [Google Scholar]
- 15. Opondo D, Eslami S, Visscher S, et al. Inappropriateness of medication prescriptions to elderly patients in the primary care setting: a systematic review. PLoS One 2012;7(8):e43617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ganjavi H, Herrmann N, Rochon PA, et al. Adverse drug events in cognitively impaired elderly patients. Dement Geriatr Cogn Disord 2007;23(6):395-400. [DOI] [PubMed] [Google Scholar]
- 17. Arlt S, Lindner R, Rösler A, et al. Adherence to medication in patients with dementia: predictors and strategies for improvement. Drugs Aging 2008;25(12):1033-47. [DOI] [PubMed] [Google Scholar]
- 18. Hanlon JT, Aspinall SL, Handler SM, et al. Potentially suboptimal prescribing for older veteran nursing home patients with dementia. Ann Pharmacother 2015;49(1):20-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pottie K, Thompson W, Davies S, et al. Evidence-based clinical practice guideline for deprescribing benzodiazepine receptor agonists. 2016. Available: www.open-pharmacy-research.ca/evidence-based-deprescribing-algorithm-for-benzodiazepines (accessed Oct. 27, 2016). [PMC free article] [PubMed]
- 20. Farrell B, Eisener-Parsche P, Dalton D. Turning over the rocks: role of anticholinergics and benzodiazepines in cognitive decline and falls. Can Fam Physician 2014;60(4):345-50. [PMC free article] [PubMed] [Google Scholar]
- 21.Deprescribing.org. Optimizing medication use. Available: http://deprescribing.org (accessed Oct. 27, 2016).
- 22. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015;175(5):827-34. [DOI] [PubMed] [Google Scholar]
- 23. Fiss T, Thyrian JR, Fendrich K, et al. Cognitive impairment in primary ambulatory health care: pharmacotherapy and the use of potentially inappropriate medications. Int J Geriatr Psychiatry 2013;28(2):173-81. [DOI] [PubMed] [Google Scholar]
- 24. Koyama A, Steinman M, Ensrud K, et al. Ten-year trajectory of potentially inappropriate medications in very old women: importance of cognitive status. J Am Geriatr Soc 2013;61(2):258-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lau DT, Mercaldo ND, Harris AT, et al. Polypharmacy and potentially inappropriate medication use among community-dwelling elders with dementia. Alzheimer Dis Assoc Disord 2010;24(1):56-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lau DT, Mercaldo ND, Shega JW, et al. Functional decline associated with polypharmacy and potentially inappropriate medications in community-dwelling older adults with dementia. Am J Alzheimers Dis Other Demen 2011;28(8):606-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montastruc F, Gardette V, Cantet C, et al. Potentially inappropriate medication use among patients with Alzheimer disease in the REAL.FR cohort: be aware of atropinic and benzodiazepine drugs! Eur J Clin Pharmacol 2013;69(8):1589-97. [DOI] [PubMed] [Google Scholar]
- 28. Pugh MJ, Starner CI, Amuan ME, et al. Exposure to potentially harmful drug-disease interactions in older community-dwelling vetrans based on the Healthcare Effectiveness Data and Information Set quality measure: who is at risk? J Am Geriatr Soc 2011;59(9):1673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thorpe JM, Thorpe CT, Kennelty KA, et al. The impact of family caregivers on potentially inappropriate medication use in noninstitutionalized older adults with dementia. Am J Geriatr Pharmacother 2012;10(4):230-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991;151(9):1825-32. [PubMed] [Google Scholar]
- 31. Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: the PRISCUS List. Dtsch Arztebl Int 2010;107(31-32):543-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindblad CI, Hanlon JT, Gross CR, et al. Clinically important drug-disease interactions and their prevalence in older adults. Clin Ther 2006;28(8):1133-43. [DOI] [PubMed] [Google Scholar]
- 33. Madhusoodanan S, Bogunovic OJ. Safety of benzodiazepines in the geriatric population. Expert Opin Drug Saf 2004;3(5):485-93. [DOI] [PubMed] [Google Scholar]
- 34. Stewart SA. The effects of benzodiazepines on cognition. J Clin Psychiatry 2005;66(Suppl. 2):9-13. [PubMed] [Google Scholar]
- 35. Cardwell K, Hughes CM, Ryan C. The association between anticholinergic medication burden and health related outcomes in the ‘oldest old’: a systematic review of the literature. Drugs Aging 2015;32(10):835-48. [DOI] [PubMed] [Google Scholar]
- 36. Fox C, Smith T, Maidment I, et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age Ageing 2014;43(5):604-15. [DOI] [PubMed] [Google Scholar]
- 37. Obermeyer CM, Schulein M, Hardon A, et al. Gender and medication use: an exploratory, multi-site study. Women Health 2004;39(4):57-73. [DOI] [PubMed] [Google Scholar]
- 38. Mackenzie CS, Reynolds K, Chou KL, et al. Prevalence and correlates of generalized anxiety disorder in a national sample of older adults. Am J Geriatr Psychiatry 2011;19(4):305-15. [DOI] [PubMed] [Google Scholar]
- 39. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 2002;6(2):97-111. [DOI] [PubMed] [Google Scholar]
- 40. Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 2003;58(3):249-65. [DOI] [PubMed] [Google Scholar]
- 41. Thomas BH, Ciliska D, Dobbins M, et al. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004;1(3):176-84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


