Osteoarthritis (OA) is the most common form of arthritis, affecting more than 1 in 8 Canadians.1 With an aging population, the prevalence of OA in Canada is rising. From 2010 to 2031, the prevalence of OA is projected to increase from 13.8% to 18.6%, with the total direct costs projected to increase from $2.9 billion to $7.6 billion, an almost 2.6-fold increase.2 OA has traditionally been thought to be a progressive disease of the synovial joints that is due to daily “wear and tear” from excessive and repetitive force on joint cartilage. While this is partially true, OA is now believed to be a systemic disorder due to an imbalance between joint destruction and repair.3-6 The result is a breakdown of cartilage and bone, leading to symptoms of pain, stiffness and functional disability.
The joints most commonly affected by OA (in order of prevalence) are the hands, knees, hips and spine. OA is more prevalent with age, affecting nearly half the population older than 70.3 In addition to advancing age, female gender is a significant risk factor. Other risk factors include obesity, quadriceps muscle weakness, family history, joint injury and joint overuse or injury (e.g., participation in certain sports).3,7
Evidence for pharmacist care in OA
Pharmacists are frontline, accessible health care professionals who see patients 5 times more frequently than family physicians.8 Community pharmacists have proven they can address gaps in OA patient care. Many people with OA are not diagnosed and are not referred for treatment. Pharmacists are ideally placed to screen for OA and make treatment recommendations, as they are often consulted about the choice of over-the-counter (OTC) analgesics to manage OA pain. Research conducted in Canadian pharmacies has shown that pharmacists can effectively screen for OA. Community pharmacists in Edmonton and Vancouver, using a simple screening questionnaire (<10 minutes to complete), identified >80% of patients with knee pain who had undiagnosed knee OA.9 Within 6 months of receiving their diagnosis, >90% of participants had visited their family physician to discuss their OA, >50% took either a prescription or nonprescription analgesic and a significant number (p < 0.001 for all) of patients saw improvements in their pain, function and daily activity scores.10 Canadian pharmacists have also effectively launched multidisciplinary interventions to improve health outcomes for patients with undiagnosed knee OA.11 Patients who reported knee pain at community pharmacies in Vancouver were effectively screened for OA and assigned to either “usual care” (i.e., provided with a pamphlet on knee OA created by the Arthritis Society) or “intervention care” (i.e., one-on-one consultation where the pharmacist offered arthritis education, medication reviews and referral to a physiotherapist-guided exercise program). Outcomes from the pharmacist-patient consultation were recorded and faxed to the patient’s primary care physician. Compared to “usual care,” significantly more patients in the intervention group noted improvements in their quality of care and significant improvements in global, pain and functional scales. This evidence, combined with the expanding role of the pharmacist across the country, ideally positions pharmacists to improve the care of patients with OA.
The following recommendations are made with input from a number of OA treatment guideline documents, as applicable to pharmacists.12-16
Box 1. Levels of evidence.
We have chosen to follow the Osteoarthritis Research Society International (OARSI) criteria for summarizing the strength of the available literature for each recommendation.12 Meta-analyses, systematic reviews (SRs) and randomized control trials (RCTs) are considered the highest level of evidence. The quality and level of evidence are described by OARSI as follows:
Level/type of evidence: The highest level of evidence available at this time (e.g., meta-analysis or most current RCT).
Quality of evidence: Meta-analyses and SRs were assigned a quality rating of “good,” “fair” or “poor” according to the Assessment of Multiple Systematic Reviews Tool (AMSTAR). The Cochrane Risk of Bias Assessment Method was used to rate RCTs. In instances where no new evidence existed, we used the quality of evidence rating as assigned by OARSI. Where new evidence existed, our quality ratings were assigned based on AMSTAR total score: 8 to 11 was considered “good,” 5 to 7 was considered “fair,” and 0 to 4 was considered “poor.”
What should a pharmacist do? Recommendations for pharmacist interventions
- 1. Screen for OA
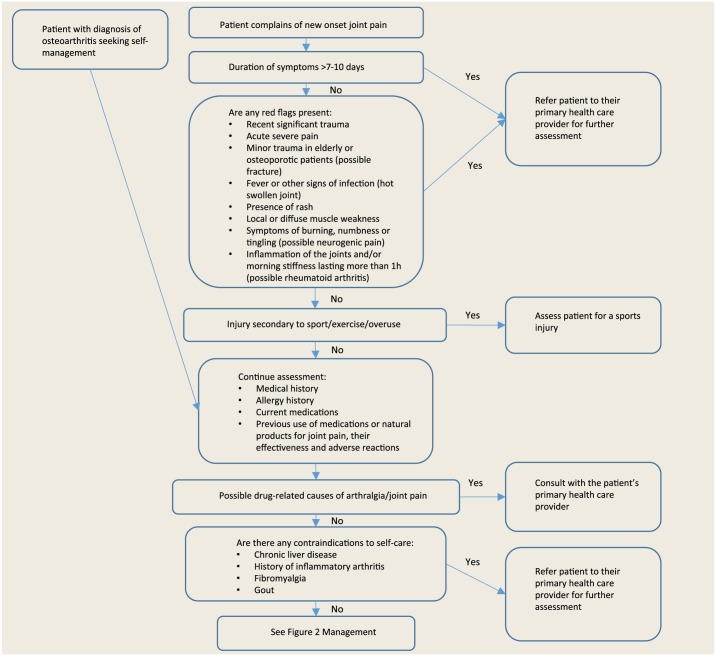
- Figure 1 illustrates the approach a pharmacist should take when assessing a patient presenting for self-management with either new-onset joint pain or a diagnosis of OA.
- Patients who do not meet these criteria may be referred by their primary care providers for imaging to rule out other serious joint conditions.
- OA typically presents as a deep aching pain in a joint. There may be morning joint stiffness, typically lasting <30 minutes. Joints may also exhibit crepitus, enlargement, deformity and limited range of motion. Patients may present with a local inflammatory response, resulting in a tender, red, swollen and hot joint.
Figure 1.
Patient assessment
Adapted with permission from the Compendium of Therapeutics for Minor Ailments.3
For patients who screen positive for OA:
- 2. Provide arthritis education
- Provide counselling on the signs and symptoms of OA.
- Provide OA information handouts/pamphlets (www.arthritis.ca or http://rheuminfo.com/).
- 3. Provide a medication review and treatment recommendations in accordance with current OA guidelines
- Conduct reviews of the patient’s prescription and OTC medications.
- Counsel patients on risks, benefits and appropriate use of medications to achieve maximum therapeutic benefit.
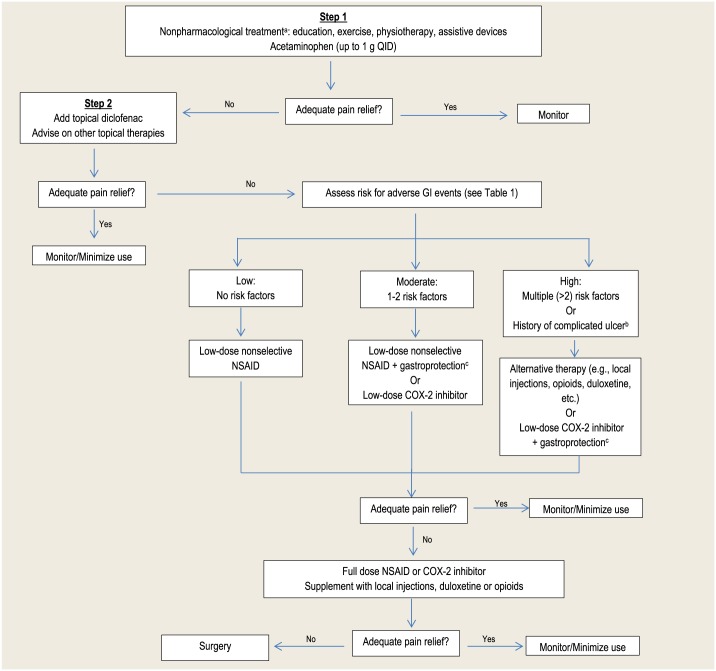
- See Figure 2 for a stepwise treatment algorithm.
- 4. Refer to allied health professionals as appropriate
- See Box 2: Recommendations for nonpharmacological therapy.
- Patients may benefit from referral to physiotherapists, dietitians and/or occupational therapists.
Figure 2.
Treatment of osteoarthritis
Adapted with permission from the Compendium of Therapeutics for Minor Ailments.3
aRefer to appropriate health care professional.
bComplications of peptic ulcer disease include bleeding, perforation, penetration and gastric outlet obstruction.
cGastroprotection: either misoprostol or proton pump inhibitors (e.g., omeprazole) are the recommended options. Misoprostol is generally not well tolerated.
Principles of therapy
The goal of OA therapy is to relieve symptoms of pain, improve joint function and mobility and improve quality of life while minimizing medication-related adverse events.
Pharmacists can educate patients and caregivers to help them understand their condition and work with both the patients and their primary health care provider to choose an appropriate therapy. Current nonpharmacological and pharmacological treatment options for OA provide symptom relief, but they are not curative and do not slow progression of OA.3 Choice of treatment is typically based on risk versus benefit, cost and patient preference. Pharmacological treatment options should always be used in combination with nonpharmacological options (Figure 2). Generally therapies should be tried for at least 1 to 2 weeks to allow for adequate assessment of effectiveness.3
Nonpharmacological treatment options
Box 2. Recommendations for nonpharmacological therapy.
All patients should be referred to patient education programs, such as those offered by the Arthritis Society across Canada. (Level of Evidence: systematic reviews [SRs] and meta-analyses [MAs] of randomized controlled trials [RCTs]. Quality of Evidence: Good.)
All patients, having been cleared for exercise by their primary care physicians, should be counselled to participate in both strength training and aerobic exercise, either land or water based or both, under the guidance of a physiotherapist. (Level of Evidence: SRs and MAs of RCTs. Quality of Evidence: Good.)
Patients with a body mass index (BMI) <25 kg/m2 should be referred to a dietitian for weight management. (Level of Evidence: SRs and MAs of RCTs. Quality of Evidence: Good.)
Consider referring patients with hip, knee or wrist osteoarthritis (OA) to an occupational therapist for splints, braces, taping, corrective footwear or walking aids. (Level of Evidence: Expert consensus. Quality of Evidence: Insufficient evidence.)
Patients interested in acupuncture should be referred to a licensed acupuncturist. (Level of Evidence: SRs and MAs of RCTs. Quality of Evidence: Good.)
Patients interested in transcutaneous electrical nerve stimulation should be referred to a physiotherapist. (Level of Evidence: SRs and MAs of RCTs. Quality of Evidence: Good.)
May consider recommending hot or cold local therapy for those with knee OA. (Level of Evidence: SRs and MAs of RCTs. Quality of Evidence: Good.)
Do not recommend balneotherapy (treatment through bathing), ultrasound or electrotherapy for patients with OA. (Level of Evidence: SRs and MAs of RCTs. Quality of Evidence: Fair.)
Nonpharmacologic therapies are important in OA and are supported by varying levels of evidence for safety and efficacy. These modalities should always be tried first or initiated at the same time as drug therapy.3,12-14 The role of the pharmacist in the nonpharmacological treatment of OA is mainly to recognize the need for referral to other health care professionals and ensure the patient has access to appropriate specialized care.
Nonpharmacological therapies with the most evidence for benefit and the greatest consensus from guidelines include patient education for self-management,17-24 land- and water-based exercise25-31 and weight loss.32,33 Supports and braces34,35 may also offer pain relief and improved function, while those with knee OA may benefit from corrective footwear.35-39 Patients who wish to incorporate these therapies into their OA management can be referred to a dietitian, physiotherapist or occupational therapist as appropriate. Those with knee OA may also benefit from the use of assistive walking devices such as canes39,40 or walkers or transcutaneous electrical nerve stimulation (TENS) devices.41-43 Corrective footwear can reduce pain and medication use in knee OA by redistributing the balance of weight-bearing pressures in the foot.43,44 Use of TENS has been shown to reduce pain and stiffness.41,42 Acupuncture,45 heat, cold46 and massage may also reduce pain and stiffness in those with knee OA. Nonpharmacological therapies with limited evidence are not recommended; these include balneotherapy (bathing in mineral springs),47-50 ultrasound51,52 and electrotherapy.53,54
Pharmacological treatment options
Natural health products
Box 3. Recommendations for natural health products.
Glucosamine or chondroitin supplementation should not be recommended for osteoarthritis. (Level of Evidence: systematic reviews [SRs] and meta-analyses [MAs] of randomized controlled trials [RCTs]. Quality of Evidence: Good.)
For patients who wish to supplement with glucosamine and chondroitin despite the lack of evidence for efficacy, recommend discontinuation if there is no improvement in symptoms after a 3-month trial. (Level of Evidence: Expert consensus. Quality of Evidence: No available trials.)
Other natural products should not be recommended until such a time as high-quality trials demonstrate clinical efficacy. (Level of Evidence: Expert consensus. Quality of Evidence: Insufficient evidence.)
Due to a lack of evidence for efficacy, published guidelines are in agreement regarding therapy with the 2 most popular natural health products for osteoarthritis, and all recommend against the use of glucosamine or chondroitin for OA.12-14 These agents are generally considered safe, with few drug interactions, although glucosamine and chondroitin can potentiate the anticoagulant effects of warfarin.55 Taking glucosamine/chondroitin together with warfarin should be avoided if possible; otherwise, patients will initially require more frequent monitoring of their international normalized ratio (INR) and should be counselled on signs of increased warfarin effects (e.g., bruising, bleed). Patients wishing to try them may do so, although we encourage setting a date to reassess efficacy. Other natural products that patients may request include S-adenosyl-L-methionine, methylsulfonylmethane, Indian frankincense, avocado soybean unsaponifiables, shark cartilage and rose hip, although there is little high-quality evidence for their efficacy in OA.12-14
Topical therapy
Box 4. Recommendations for topical pharmacological therapy.
Consider capsaicin or zucapsaicin for the treatment of pain associated with hand or knee osteoarthritis (OA). (Level of Evidence: systematic reviews [SRs] of randomized controlled trials [RCTs]. Quality of Evidence: Good.)
For mild to moderate knee OA pain, consider a trial of topical diclofenac sodium prior to initiation of oral nonsteroidal anti-inflammatory drug (NSAID) therapy. (Level of Evidence: SRs and meta-analyses [MAs] of RCTs. Quality of Evidence: Good.)
Initial NSAID therapy should be topical rather than oral in patients ≥75 years old. (Level of Evidence: Expert consensus. Quality of Evidence: No available trials.)
Consider topical therapies as an adjunct to oral agents when pain relief is not adequate. (Level of Evidence: Expert consensus. Quality of Evidence: No available trials.)
Capsaicin
Capsaicin depletes substance P, thus reducing the pain the patient experiences, and has been found to be both moderately effective in reducing pain up to 20 weeks and generally is well tolerated.56 Capsaicin does not have an official indication for OA. Zucapsaicin is the cis isomer of capsaicin and is indicated in those with knee OA pain not controlled by either oral nonsteroidal anti-inflammatory drugs (NSAIDs) or cyclooxygenase-2 (COX-2) inhibitors alone. Either capsaicin or zucapsaicin may be considered an adjunct to oral therapy in knee or hand OA.3,57-60 The maximum therapeutic effect may not be seen until 4 weeks with regular administration (3-4 times daily).3
Topical NSAIDs
NSAIDs are available topically in multiple dosage forms, including both a gel and a topical solution. All OA guidelines recommend the use of topical NSAIDs for the treatment of OA pain of either the hand or knee, although no recommendations are made regarding hip OA.12-14 The only official indication for topical NSAIDs in OA is for diclofenac sodium solution for knee OA.61 The maximum therapeutic effect may not be seen until 2 weeks of regular administration (3-4 times daily).3
Acetaminophen
Box 5. Recommendations for acetaminophen therapy.
Acetaminophen (up to 4 g/day) is the initial analgesic of choice for osteoarthritis (OA) treatment in patients without relevant hepatic comorbidities. (Level of Evidence: systematic reviews and metaanalyses of randomized controlled trials. Quality of Evidence: Good.)
Maximum therapeutic doses should be used for 2 weeks to assess the efficacy of acetaminophen. (Level of Evidence: Expert consensus. Quality of Evidence: No available trials.)
Acetaminophen continues to be recommended as the initial drug of choice for treatment of OA.12-16 The rationale for using acetaminophen is largely based on the fact that acetaminophen is effective, relatively safe, well tolerated and easily accessible.3 Evidence suggests that short-term use of acetaminophen has a low to moderate effect on OA pain.12,62,63 Newer evidence suggests that acetaminophen may be less effective and has greater risks than previously thought.3,12,64,65 The U.S. Food and Drug Administration has recommended that all products containing acetaminophen be limited to 325 mg per dosage unit,22 while Health Canada is assessing whether similar changes be made in Canada.23 Patients taking acetaminophen should be counselled not to exceed the daily maximum dose and to consider acetaminophen from all sources (i.e., OTC and prescription products).
Typically, studies use the maximum daily dose of acetaminophen (1 gram 4 times daily) for a short period of time (median 6 weeks).63 Maximum therapeutic doses should be used for 2 weeks to assess efficacy, then use the lowest effective dose. Risk of hepatotoxicity may be increased in the elderly and those with excessive alcohol intake (>3 drinks per day) or liver disease. These are not contraindications to therapy, but some experts recommend dosage reductions (elderly: maximum dose 3200 mg/day; alcohol use/preexisting hepatotoxicity: maximum dose 2600 mg/day).66
NSAIDs
Box 6. Recommendations for nonsteroidal anti-inflammatory drug (NSAID) therapy.
Oral NSAIDs should be used for patients who have had an inadequate response to an adequate trial of acetaminophen. (Level of Evidence: systematic reviews [SRs] and meta-analyses [MAs] of randomized controlled trials [RCTs]. Quality of Evidence: Good.)
Prior to starting oral NSAID therapy, patients must be thoroughly assessed for gastrointestinal (GI), cardiovascular and renal complications. (Level of Evidence: Expert consensus. Quality of Evidence: No available trials.)
For patients at moderate risk of GI events, use either a nonselective NSAID plus a proton pump inhibitor (PPI) or a cyclooxygenase–2 (COX–2) selective agent (i.e., celecoxib). (Level of Evidence: SRs and MAs of RCTs. Quality of Evidence: Good.)
For patients at high risk of GI events, avoid using oral NSAIDs and instead use alternative agents. (Level of Evidence: Expert consensus. Quality of Evidence: No available trials.)
For patients at high risk of GI events, if an oral NSAID is chosen despite the risks, use a COX-2 selective agent (i.e., celecoxib) plus a PPI. (Level of Evidence: SRs and MAs of RCTs. Quality of Evidence: Good.)
NSAIDs should be used with caution in patients with cardiovascular risk factors. If an oral NSAID is to be used, naproxen is the agent of choice. (Level of Evidence: SRs and MAs of RCTs. Quality of Evidence: Good.)
NSAIDs are contraindicated and should be avoided in patients with a creatinine clearance (CrCl) <30 mL/min. Use in patients with preexisting renal impairment (CrCl <60 mL/min) should be avoided if possible. (Evidence: Expert consensus. Quality of Evidence: No available trials)
Due to the risk of serious adverse effects, NSAIDs are generally reserved for treatment after failure of acetaminophen in the management of OA pain.12-16 Although acetaminophen is considered a safer option, NSAIDs are often preferred by patients due to improved pain relief.3,67,68 Generally, it is reasonable to consider initiating topical NSAID therapy before starting therapy with oral NSAIDs, particularly in elderly patients.69 Prior to starting NSAID therapy, patients must be assessed for their risk of cardiovascular, gastrointestinal and renal complications (see Table 1).
Table 1.
Risk factors for the development of serious adverse events with NSAID therapy
| Cardiovascular complications | GI complications | Renal complications | |
|---|---|---|---|
| Age | >65 years | >65 years | >65 years |
| Medical history | Heart failure | Alcoholic liver disease | Heart failure |
| CV disease | Helicobacter pylori infection | Dehydration | |
| Diabetes | History of uncomplicated ulcer* | Hypertension | |
| Hypertension | Rheumatoid arthritis | Preexisting renal disease | |
| Myocardial infarction | Upper GI bleeding | ||
| Stroke | |||
| Rheumatoid arthritis | |||
| Concomitant medications |
Regular use of multiple NSAIDs Antithrombotic (e.g., daily low- dose ASA, warfarin) Oral glucocorticoids |
ACE inhibitors ARBs Direct renin inhibitors Diuretics |
Adapted with permission from the Compendium of Therapeutics for Minor Ailments.3
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; CV, cardiovascular; GI, gastrointestinal; NSAID, nonsteroidal anti-inflammatory drug.
Patients with history of complicated ulcer are automatically considered high risk.
All NSAIDs demonstrate an increased risk of thromboembolic events (e.g., myocardial infarction [MI] or stroke).3,61 This increase in risk, while concerning, is much smaller when compared to traditional risk factors such as high blood pressure and smoking. Studies suggest that the risk of cardiovascular events is highest with diclofenac, celecoxib and high-dose ibuprofen. Naproxen has been found to have the lowest risk of cardiovascular events.70-73 Health Canada advises that NSAIDs be used with caution in patients with cardiovascular risk factors and recommends that they be used at the lowest effective dose for the shortest period of time.74 If treatment with an NSAID is essential, patients at increased risk of cardiovascular complications (see Table 1) should be treated with naproxen (and with low-dose acetylsalicylic acid [ASA], if indicated for cardiovascular protection).12 All NSAIDs can increase blood pressure and worsen preexisting hypertension; therefore, baseline and periodic monitoring of blood pressure is necessary. When starting NSAIDs in patients on antihypertensive therapy, reevaluate the patients’ blood pressure in 1 week to determine whether dosage changes are needed to either the NSAID or the antihypertensive.3
NSAIDs can irritate the stomach (bloating, nausea, heartburn, etc.), so patients should be encouraged to take them with food. While dyspepsia has been reported in up to 60% of patients, the actual incidence is likely close to 5% to 10%.75 Minor heartburn can be managed with either antacids or histamine-2 receptor antagonists (H2RAs). Serious GI complications (e.g., perforated ulcers, hemorrhage) are estimated to occur at an incidence of less than 1% per year.76 Celecoxib is as effective as the nonselective NSAIDs and is associated with a lower incidence of gastrointestinal ulcers.77 However, this reduced risk is modest and may not extend past 6 months of therapy. Prior to starting therapy, identify patients at risk of gastrointestinal complications and take preventative measures (Table 1 and Figure 2). Either misoprostol or proton pump inhibitors (e.g., omeprazole) are the recommended options for preventing serious GI complications in patients at risk of NSAID-induced ulcer.76–78 However, misoprostol is generally not well tolerated, the most common adverse effects being abdominal pain and diarrhea. H2RAs and antacids do not provide protection against serious NSAID-induced GI complications and should not be recommended for this indication.
All NSAIDs increase the risk of impaired renal function and can cause acute kidney injury, especially when added to antihypertensives like diuretics, angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers.79,80 NSAIDs should be avoided in patients with severe renal impairment (creatinine clearance [CrCl] <30 mL/min), and their prolonged use is generally not recommended in those with mild to moderate renal impairment.61 Patients who take medications that affect renal function or those who have underlying renal dysfunction should have their renal function reevaluated 7 to 10 days after starting NSAID therapy and periodically thereafter.
Opioids and tramadol
Box 7. Recommendations for opioids and tramadol.
Avoid the use of transdermal opioids. (Level of Evidence: systematic reviews [SRs] and meta-analyses [MAs] of randomized controlled trials [RCTs]. Quality of Evidence: Good.)
Reserve oral opioids as a last-line option, particularly for short-term pain control for those awaiting surgical intervention. (Level of Evidence: SRs and MAs of RCTs. Quality of Evidence: Good.)
Reserve tramadol as a last-line option. (Level of Evidence: SRs and MAs of RCTs. Quality of Evidence: Good.)
Opioids: transdermal
Given the limited ability to titrate doses using transdermal opioid formulations and the paucity of evidence for the use of opioids in general, these formulations are not ideal for the treatment of OA pain and should be avoided.81
Opioids: oral
The evidence is conflicting when it comes to the use of opioids to treat pain associated with OA.12-14 While studies have found small statistically significant improvements in pain with codeine over placebo, patients taking opioids were 4 times more likely to withdraw due to adverse effects.12 The evidence for opioid analgesia in OA is poor and there remains significant concern about toxicity.14 We recommend reserving opioids as a last-line option, particularly for short-term pain control for those awaiting surgical intervention.
Tramadol
Tramadol has demonstrated limited efficacy in the treatment of OA.12 Guidelines conditionally recommend the use of tramadol for hand, knee and hip OA, especially in those 75 years of age or older.13 The combination of tramadol and acetaminophen may pose additional risks for acetaminophen toxicity in those patients taking other acetaminophen products or at otherwise increased risk of toxicity. We recommend reserving tramadol as a last-line option, as the beneficial effects are often outweighed by its increased risk of adverse events.
Duloxetine
Box 8. Recommendations for duloxetine.
Duloxetine may be used as monotherapy or in combination with nonsteroidal anti-inflammatory drugs (NSAIDs) for osteoarthritis of the knee for patients with contraindications to or treatment failure of NSAID therapy. (Level of Evidence: systematic reviews and metaanalyses of randomized controlled trials. Quality of Evidence: Fair.)
Currently available evidence for use of duloxetine in OA is limited and restricted to OA of the knee. A 13-week RCT in knee OA showed positive effects of duloxetine on both pain (number needed to treat [NNT] = 6) and physical function (NNT = 9) with no increase in serious adverse events but significantly more treatment-emergent adverse effects compared to placebo.82 A second RCT found that the addition of duloxetine to optimized NSAID therapy provided significantly greater pain reduction and functional improvement compared to placebo.83 A 2012 systematic review comparing duloxetine to placebo found duloxetine to be efficacious and tolerable for chronic pain associated with OA. Again it was noted that a higher number of patients in the duloxetine group withdrew due to adverse effects.12
Localized/injectable therapy
Box 9. Recommendations for injectable therapy.
For short-term relief of knee osteoarthritis unresponsive to acetaminophen or nonsteroidal anti-inflammatory drugs, consider referral for intra-articular corticosteroids. For longer duration of pain relief, alternative treatment options should be considered. (Level of Evidence: systematic reviews and metaanalyses of randomized controlled trials. Quality of Evidence: Good.)
Intra-articular (IA) corticosteroids may be used to manage acute pain and localized inflammation associated with OA.13-16 Guidelines recommend the use of IA corticosteroids for OA pain that is unresponsive to acetaminophen and NSAIDs. The majority of evidence to support the use of this treatment is found in OA of the knee. Studies have found clinically significant short-term decreases in knee pain 2 to 3 weeks postinjection. For a longer duration of pain relief, other treatment options should be considered.12 There is little evidence to support the use of IA corticosteroids in the hand and hip.13 Rheumatologists, using radiographic guidance, where appropriate, may provide injections to these joints for certain patients. As a general rule, the number of injections is limited to 3 or 4 for a single joint per year.
The role of hyaluronic acid injections in the management of OA is uncertain.12,13 Meta-analyses investigating the efficacy and safety of these agents have found inconsistent and conflicting results.12 These products are usually reserved for patients who have failed other therapies. The costs of hyaluronan products are high ($200-$400 per treatment course), and they are not routinely covered by insurance plans. Currently, there is insufficient evidence to support treatment recommendations for IA hyaluronan.
All IA therapies should be administered by a trained health care professional.
Oral steroids
Oral steroids are not routinely used or recommended for management of OA.12,13
Monitoring
Details on monitoring for efficacy and safety are provided in Tables 2 and 3.
Table 2.
Efficacy
| Parameter | Timeframe | Comments |
|---|---|---|
| Pain relief •Prior to starting therapy, it is important to establish an acceptable level of pain control and function with the patient •This may be accomplished with visual analog scales or individual measures that quantify pain (e.g., ability to walk, exercise, do gardening, etc.) |
Patients: Assess daily Pharmacist: Call on days 3, 7 and 14 |
•If pain control is optimal: •Continue current therapy and find lowest effective dose. •If there has been no improvement after 14 days: •Switch to alternative agent •Refer for further assessment if an adequate trial of 2 agents has been unsuccessful •If pain is improving but not optimized: •Ensure optimal therapy (maximum therapeutic dose, regular dosing, etc.) •Consider addition of appropriate adjunctive agent (e.g., topical analgesic, etc.) |
Table 3.
Safety
| Parameter | Timeframe | Comments |
|---|---|---|
| Medication-specific side effects | Patients: Assess daily Pharmacist: Call on days 3, 7 and 14 |
•General •Taking medications with food may help reduce stomach upset. •Counsel patients to avoid exceeding the maximum daily doses of medications. •Patients should consider acetaminophen from all over-the-counter sources. •Laboratory investigations: •Acetaminophen → baseline liver function tests should be performed in patients at high risk of hepatotoxicity. •NSAIDs → Baseline SCr and CrCl should be assessed in patients with preexisting renal disease and those taking medications that affect kidney function (e.g., diuretics, ACEIs). Renal function should be reassessed within 7 to 10 days. •Drug interactions: •Both acetaminophen and NSAIDs have been noted to increase INR in warfarin-treated patients. •Monitor the INR more frequently (i.e., starting at day 3) during the initial period of analgesic therapy and counsel patient to watch for signs of bleeding. •NSAID specific: •Hypertension •Blood pressure should be measured at baseline and then every 2 to 4 weeks for 1 to 2 months. •Those with uncontrolled hypertension or taking antihypertensives require more frequent monitoring (i.e., every 1-2 weeks for 1-2 months). •Avoid concurrent use of more than 1 NSAID product when possible; the only exception is for patients taking low-dose aspirin for cardiovascular protection. |
ACEI, angiotensin-converting enzyme inhibitor; CrCl, creatinine clearance; INR, international normalized ratio; NSAID, nonsteroidal anti-inflammatory drug; SCr, serum creatinine.
Conclusion
For a number of patients with OA, the combination of nonpharmacological measures with optimal acetaminophen or NSAID therapy provides adequate pain relief and functional improvement. OA is a common chronic condition in Canada, with dramatic increases in incidence on the horizon as our population ages. Pharmacists have the resources, the skills and the opportunity to become leaders in OA care and improve the lives of those affected by the condition. We believe these guidelines will be a valuable resource to pharmacists, as they highlight the most relevant aspects of OA management to help them care for their patients.■
Footnotes
Author Contributions:J. Kielly and E. Davis oversaw the manuscript preparation and drafted and edited the final manuscript. C. Marra reviewed and edited the final manuscript.
Declaration of Conflicting Interests:The authors declare no conflict of interest with this submitted work.
References
- 1.The Arthritis Society. Available: www.arthritis.ca (accessed Mar. 24, 2016).
- 2. Sharif B, Kopec J, Bansback N, et al. Projecting the direct cost burden of osteoarthritis in Canada using a microsimulation model. Osteoarthritis Cartilage 2015;23(10):1654-63. [DOI] [PubMed] [Google Scholar]
- 3. Grindrod K, Kielly J, Marra C. In: Jovaisas B, ed. Compendium of Therapeutics for Minor Ailments. Ottawa (ON): Canadian Pharmacists Association; c2016. Available: www.e-therapeutics.ca (accessed Apr. 22, 2016).
- 4. Pritzker KP. Pathology of osteoarthritis. In: Brandt KD, Doherty M, Lohmander LS. eds. Osteoarthritis. New York: Oxford University Press; 2000. p. 49-58. [Google Scholar]
- 5. Aspden RM. Osteoarthritis: a problem of growth not decay? Rheumatology (Oxford) 2008;47:1452-60. [DOI] [PubMed] [Google Scholar]
- 6. Lane NE, Brandt K, Hawker G, et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage 2011;19(5):478-82. [DOI] [PubMed] [Google Scholar]
- 7. Kielly J, Marra CA; The Arthritis Society. Arthritis medications: A reference guide. Available: https://arthritis.ca/manage-arthritis/educational-resources-tools/printed-publications/arthritis-medications-a-reference-guide (accessed Mar. 24, 2016).
- 8. Shiu JR, Simpson SH, Johnson JA, et al. Quantifying opportunities to affect diabetes management in the community. Can Pharm J (Ott) 2006;139:37-8. [Google Scholar]
- 9. Marra CA, Cibere J, Tsuyuki RT, et al. Improving osteoarthritis detection in the community: pharmacist identification of new diagnostically confirmed osteoarthritis. Arthritis Care Res 2007;57:1238-44. [DOI] [PubMed] [Google Scholar]
- 10. Grindrod KA, Marra CA, Colley L, et al. After patients are diagnosed with knee osteoarthritis, what do they do? Arthritis Care Res 2010;62:510-5. [DOI] [PubMed] [Google Scholar]
- 11. Marra CA, Cibere J, Grubisic M, et al. Pharmacist-initiated trial in osteoarthritis: a multidisciplinary intervention for knee osteoarthritis. Arthritis Care Res 2012;64:1837-45. [DOI] [PubMed] [Google Scholar]
- 12. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014:22:363-88. [DOI] [PubMed] [Google Scholar]
- 13. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip and knee. Arthritis Care Res 2012;64:465-74. [DOI] [PubMed] [Google Scholar]
- 14. NICE Guidance. Osteoarthritis: care and management. National Institute for Health and Care Excellence (NICE) Available: https://www.nice.org.uk/guidance/cg177 (accessed Apr. 14, 2016). [PubMed] [Google Scholar]
- 15. Zhang W, Doherty M, Arden N, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2005;64:669-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Du S, Yuan C, Xiao X, Chu J, Qiu Y, Qian H. Self-management programs for chronic musculoskeletal pain conditions: a systematic review and meta-analysis. Patient Educ Couns 2011;85(3):e299-310. [DOI] [PubMed] [Google Scholar]
- 18. Chondosh J, Morton SC, Mojica W, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med 2005;143(6):427-38. [DOI] [PubMed] [Google Scholar]
- 19. Ravaud P, Flipo RM, Boutron I, et al. ARTIST (osteoarthritis intervention standardized) study of standardised consultation versus usual care for patients with osteoarthritis of the knee in primary care in France: pragmatic randomised controlled trial. BMJ 2009;338:b421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hurley MV, Walsh NE, Mitchell H, Nicholas J, Patel A. Long-term outcomes and costs of an integrated rehabilitation program for chronic knee pain: a pragmatic, cluster randomized, controlled trial. Arthritis Care Res 2012;64(2):238-47. [DOI] [PubMed] [Google Scholar]
- 21. Hansson EE, Jonsson-Lundgren M, Ronnheden AM, Sorensson E, Bjarnung A, Dahlberg LE. Effect of an education programme for patients with osteoarthritis in primary care—a randomized controlled trial. BMC Musculoskelet Disord 2010;11:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allen KD, Oddone EZ, Coffman CJ, Datta SK, Juntilla KA, Lindquist JH, et al. Telephone-based self-management of osteoarthritis: a randomized trial. Ann Intern Med 2010;153(9):570-9. [DOI] [PubMed] [Google Scholar]
- 23. Ackerman IN, Buchbinder R, Osborne RH. Challenges in evaluating an Arthritis Self-Management Program for people with hip and knee osteoarthritis in real-world clinical settings. J Rheumatol 2012;39(5):1047-55. [DOI] [PubMed] [Google Scholar]
- 24. Warsi A, LaValley MP, Wang PS, et al. Arthritis self-management education programs: a meta-analysis of the effect on pain and disability. Arthritis Rheum 2003;48:2207-13. [DOI] [PubMed] [Google Scholar]
- 25. Jansen MJ, Viechtbauer W, Lenssen AF, Hendriks EJ, de Bie RA. Strength training alone, exercise therapy alone, and exercise therapy with passive manual mobilisation each reduce pain and disability in people with knee osteoarthritis: a systematic review. J Physiother 2011;57(1):11-20. [DOI] [PubMed] [Google Scholar]
- 26. Iversen MD. Rehabilitation interventions for pain and disability in osteoarthritis: a review of interventions including exercise, manual techniques, and assistive devices. Orthop Nurs 2012;31(2):103-8. [DOI] [PubMed] [Google Scholar]
- 27. Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Does land-based exercise reduce pain and disability associated with hip osteoarthritis? A meta-analysis of randomized controlled trials. Osteoarthritis Cartilage 2010;18(5):613-20. [DOI] [PubMed] [Google Scholar]
- 28. Fransen M, McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev 2008;(4):CD004376. [DOI] [PubMed] [Google Scholar]
- 29. Bannuru RR, Abariga S, Wang C. How effective is tai chi mind-body therapy for knee osteoarthritis? A systematic review and meta-analysis. Osteoarthritis Research Society International World Congress; 2012 Apr 26e29; Barcelona, Spain. Osteoarthritis Cartilage 2012;20(Suppl 1):S281e. [Google Scholar]
- 30. Kang JW, Lee MS, Posadzki P, Ernst E. T’ai chi for the treatment of osteoarthritis: a systematic review and meta-analysis. BMJ Open 2011;1(1):e000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bartels EM, Lund H, Hagen KB, Dagfinrud H, Christensen R, Danneskiold-Samsoe B. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev 2007;(4):CD005523. [DOI] [PubMed] [Google Scholar]
- 32. Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis 2007;66(4):433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum 2004;50:1501-10. [DOI] [PubMed] [Google Scholar]
- 34. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage 2006;14:777-83. [DOI] [PubMed] [Google Scholar]
- 35. Brouwer RW, Jakma TS, Verhagen AP, et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev 2005;(1):CD004020. [DOI] [PubMed] [Google Scholar]
- 36. Raja K, Dewan N. Efficacy of knee braces and foot orthoses in conservative management of knee osteoarthritis: a systematic review. Am J Phys Med Rehabil 2011;90(3):247-62. [DOI] [PubMed] [Google Scholar]
- 37. Bennell KL, Bowles KA, Payne C, et al. Lateral wedge insoles for medial knee osteoarthritis: 12 month randomised controlled trial. BMJ 2011;342:d2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Raaij TM, Reijman M, Brouwer RW, Bierma-Zeinstra SM, Verhaar JA. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin Orthop Relat Res 2010;468(7):1926-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Erhart JC, Mundermann A, Elspas B, Giori NJ, Andriacchi TP. Changes in knee adduction moment, pain, and functionality with a variable-stiffness walking shoe after 6 months. J Orthop Res 2010;28(7):873-9. [DOI] [PubMed] [Google Scholar]
- 40. Jones A, Silva PG, Silva AC, et al. Impact of cane use on pain, function, general health and energy expenditure during gait in patients with knee osteoarthritis: a randomised controlled trial. Ann Rheum Dis 2012;71(2):172e9. [DOI] [PubMed] [Google Scholar]
- 41. Rutjes AW, Nuesch E, Sterchi R, et al. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database Syst Rev 2009;(4):CD002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Atamaz FC, Durmaz B, Baydar M, et al. Comparison of the efficacy of transcutaneous electrical nerve stimulation, interferential currents, and shortwave diathermy in knee osteoarthritis: a double-blind, randomized, controlled, multicenter study. Arch Phys Med Rehabil 2012;93(5):748-56. [DOI] [PubMed] [Google Scholar]
- 43. Chen LX, Zhou Z-R, Li YL, et al. Transcutaneous electrical nerve stimulation in patients with knee osteoarthritis. Clin J Pain 2016;32:146-54. [DOI] [PubMed] [Google Scholar]
- 44. Raja K, Dewan N. Efficacy of knee braces and foot orthoses in conservative management of knee osteoarthritis: a systematic review. Am J Phys Med Rehabil 2011;90(3):247e62. [DOI] [PubMed] [Google Scholar]
- 45. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev 2010;(1):CD001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brosseau L, Yonge KA, Welch V, et al. Thermotherapy for treatment of osteoarthritis. Cochrane Database Syst Rev 2003;(4):CD004522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Falagas ME, Zarkadoulia E, Rafailidis PI. The therapeutic effect of balneotherapy: evaluation of the evidence from randomised controlled trials. Int J Clin Pract 2009;63(7):1068-84. [DOI] [PubMed] [Google Scholar]
- 48. Harzy T, Ghani N, Akasbi N, Bono W, Nejjari C. Short- and long-term therapeutic effects of thermal mineral waters in knee osteoarthritis: a systematic review of randomized controlled trials. Clin Rheumatol 2009;28(5):501-7. [DOI] [PubMed] [Google Scholar]
- 49. Sherman G, Zeller L, Avriel A, Friger M, Harari M, Sukenik S. Intermittent balneotherapy at the Dead Sea area for patients with knee osteoarthritis. Isr Med Assoc J 2009;11(2):88-93. [PubMed] [Google Scholar]
- 50. Forestier R, Desfour H, Tessier JM, et al. Spa therapy in the treatment of knee osteoarthritis: a large randomised multicentre trial. Ann Rheum Dis 2010;69(4):660e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rutjes AW, Nuesch E, Sterchi R, Juni P. Therapeutic ultrasound or osteoarthritis of the knee or hip. Cochrane Database Syst Rev 2010;(1):CD003132. [DOI] [PubMed] [Google Scholar]
- 52. Loyola-Sanchez A, Richardson J, MacIntyre NJ. Efficacy of ultrasound therapy for the management of knee osteoarthritis: a systematic review with meta-analysis. Osteoarthritis Cartilage 2010;18(9):1117-26. [DOI] [PubMed] [Google Scholar]
- 53. Giggins O, Fullen B, Coughlan G. Neuromuscular electrical stimulation in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Clin Rehabil 2012;26(10):867-81. [DOI] [PubMed] [Google Scholar]
- 54. Yilmaz OO, Senocak O, Sahin E, et al. Efficacy of EMG-biofeedback in knee osteoarthritis. Rheumatol Int 2010;30(7):887-92. [DOI] [PubMed] [Google Scholar]
- 55. Lexicomp Online. Lexi-Interact. Hudson (OH): Lexi-Comp, Inc.; 2016. [Google Scholar]
- 56. Laslett LL, Jones G. Capsaicin for osteoarthritis pain. Prog Drug Res 2014;68:277-91. [DOI] [PubMed] [Google Scholar]
- 57. Compendium of Pharmaceutical and Specialties. Capsaicin derivatives monograph. Ottawa (ON): Canadian Pharmacists Association; c2015. Available: www.e-therapeutics.ca (accessed Aug. 3, 2016).
- 58. Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ 2004;328(7446):991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kosuwon W, Sirichatiwapee W, Wisanuyotin T, Jeeravipoolvarn P, Laupattarakasem W. Efficacy of symptomatic control of knee osteoarthritis with 0.0125% of capsaicin versus placebo. J Med Assoc Thai 2010;93(10):1188-95. [PubMed] [Google Scholar]
- 60. Chou R, McDonagh MS, Nakamoto E, Griffin J. Analgesics for osteoarthritis: an update of the 2006 comparative effectiveness review. Rockville (MD): Agency for Healthcare Research and Quality; 2011. [PubMed] [Google Scholar]
- 61. Compendium of Pharmaceutical and Specialties. NSAID monograph. Ottawa (ON): Canadian Pharmacists Association; c2015 Available: http://www.e-therapeutics.ca (accessed Aug. 3, 2016). [Google Scholar]
- 62. Bannuru RR, McAlindon TE. Reassessing the role of acetaminophen in osteoarthritis: systematic review and meta-analysis. Osteoarthritis Research Society International World Congress; 2010 Sep 23-26; Brussels, Belgium. Osteoarthritis Cartilage 2010;18(Suppl 2):S250. [Google Scholar]
- 63. Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev 2006;(1):CD004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 2015;162(1):46-54. [DOI] [PubMed] [Google Scholar]
- 65. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ 2015;350:h1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Regier L. Chronic non-cancer pain (CNCP): overview of drugs used in treatment. RxFiles drug comparison charts. 10th ed. Saskatoon (SK): Saskatoon Health Region; 2016. p. 95-96. Available: www.RxFiles.ca (accessed Jul. 6, 2016). [Google Scholar]
- 67. Pincus T, Swearingen C, Cummins P, et al. Preference for nonsteroidal antiinflammatory drugs versus acetaminophen and concomitant use of both types of drugs in patients with osteoarthritis. J Rheumatol 2000;27:1020-7. [PubMed] [Google Scholar]
- 68. Wolfe F, Zhao S, Lane N. Preference for nonsteroidal antiinflammatory drugs over acetaminophen by rheumatic disease patients: a survey of 1,799 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Arthritis Rheum 2000;43:378-85. [DOI] [PubMed] [Google Scholar]
- 69. Kean WF, Kean CA, Hogan MG. Osteoarthritis. In: Jovaisas B, ed. Compendium of therapeutic choices. Ottawa (ON): Canadian Pharmacists Association; c2016 Available: www.e-therapeutics.ca (accessed Apr. 2, 2016). [Google Scholar]
- 70. Chou R, Helfand M, Peterson K, et al. Comparative effectiveness and safety of analgesics for osteoarthritis. Rockville (MD): Agency for Health Care Research and Quality; 2006. Comparative Effectiveness Reviews, No. 4. [PubMed] [Google Scholar]
- 71. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 2011;342:c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med 2011;8(9):e1001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Coxib and Traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382(9894):769-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Health Canada. Basic product monograph information for nonsteroidal anti-inflammatory drugs (NSAIDs). Available: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/nsaid-ains/nsaids_ains-eng.php#product (accessed Aug. 18, 2016).
- 75. Ofman JJ, Maclean CH, Straus WL, et al. Meta-analysis of dyspepsia and nonsteroidal antiinflammatory drugs. Arthritis Rheum 2003;49:508-18. [DOI] [PubMed] [Google Scholar]
- 76. Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med 1999;340:1888-99. [DOI] [PubMed] [Google Scholar]
- 77. Tannenbaum H, Bombardier C, Davis P, et al. An evidence-based approach to prescribing nonsteroidal antiinflammatory drugs. Third Canadian Consensus Conference J Rheumatol 2006;33:140-57. [PubMed] [Google Scholar]
- 78. Lanza FL, Chan FKL, Quigley EMM; Practice Parameters Committee of the ACG. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol 2009;104:728-38. [DOI] [PubMed] [Google Scholar]
- 79. Ungprasert P, Cheungpasitporn W, Crowson CS, Matteson EL. Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: a systematic review and meta-analysis of observational studies. Eur J Int Med 2015;26:285-91. [DOI] [PubMed] [Google Scholar]
- 80. Rahman S, Malcoun A. Nonsteroidal anti-inflammatory drugs, cyclooxygenase-2 and the kidneys. Prim Care Clin Office Pract 2014;41:803-21. [DOI] [PubMed] [Google Scholar]
- 81. Nuesch E, Rutjes AW, Husni E, Welch V, Juni P. Oral or trans dermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev 2009;(4):CD003115. [DOI] [PubMed] [Google Scholar]
- 82. Chappell AS, Desaiah D, Liu-Seifert H, et al. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Pract 2011;11:33-41. [DOI] [PubMed] [Google Scholar]
- 83. Frakes EP, Risser RC, Ball TD, Hochber MC, Wohlreich MM. Duloxetine added to oral nonsteroidal anti-inflammatory drugs for treatment of knee pain due to osteoarthritis: results of a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin 2011;27:2361-72. [DOI] [PubMed] [Google Scholar]