Abstract
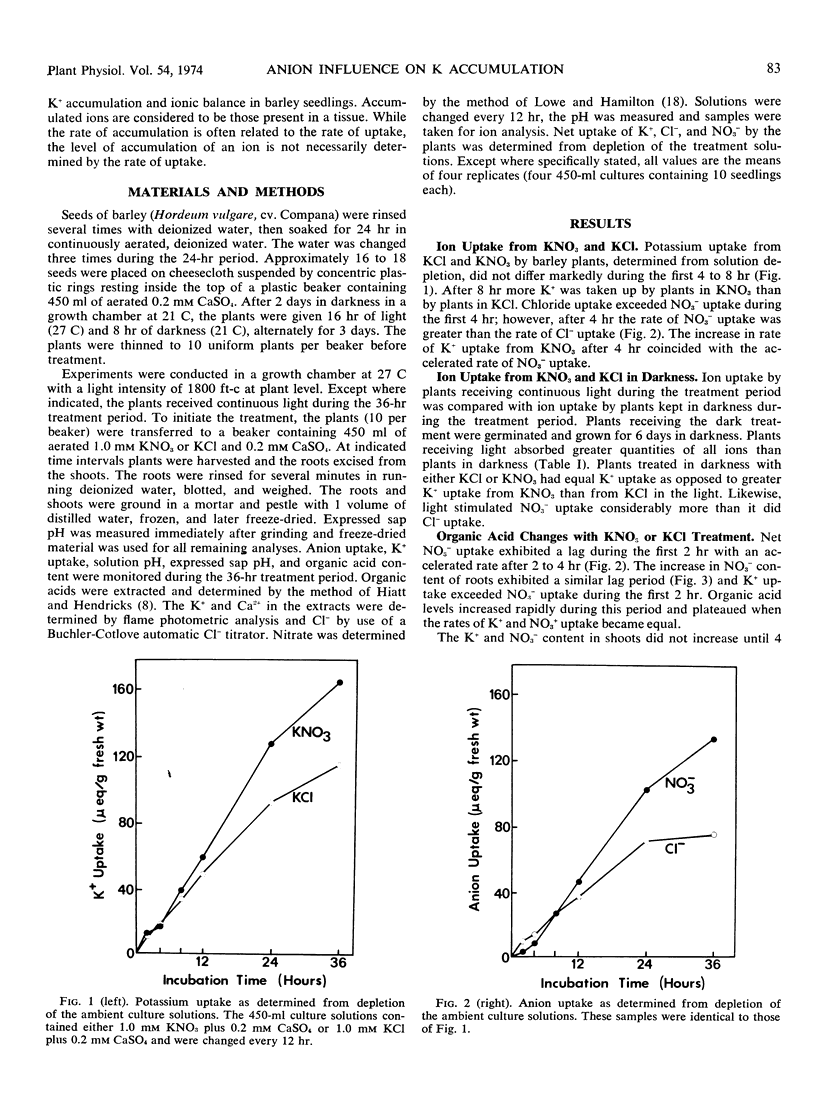
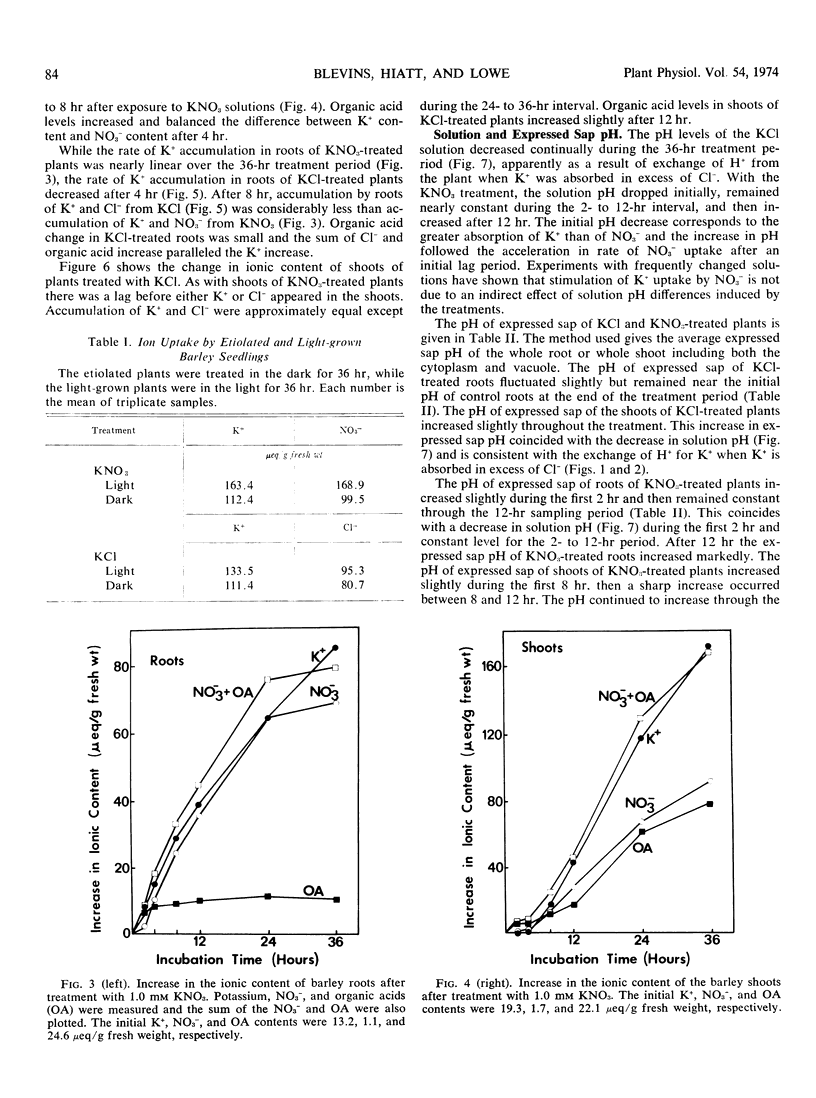
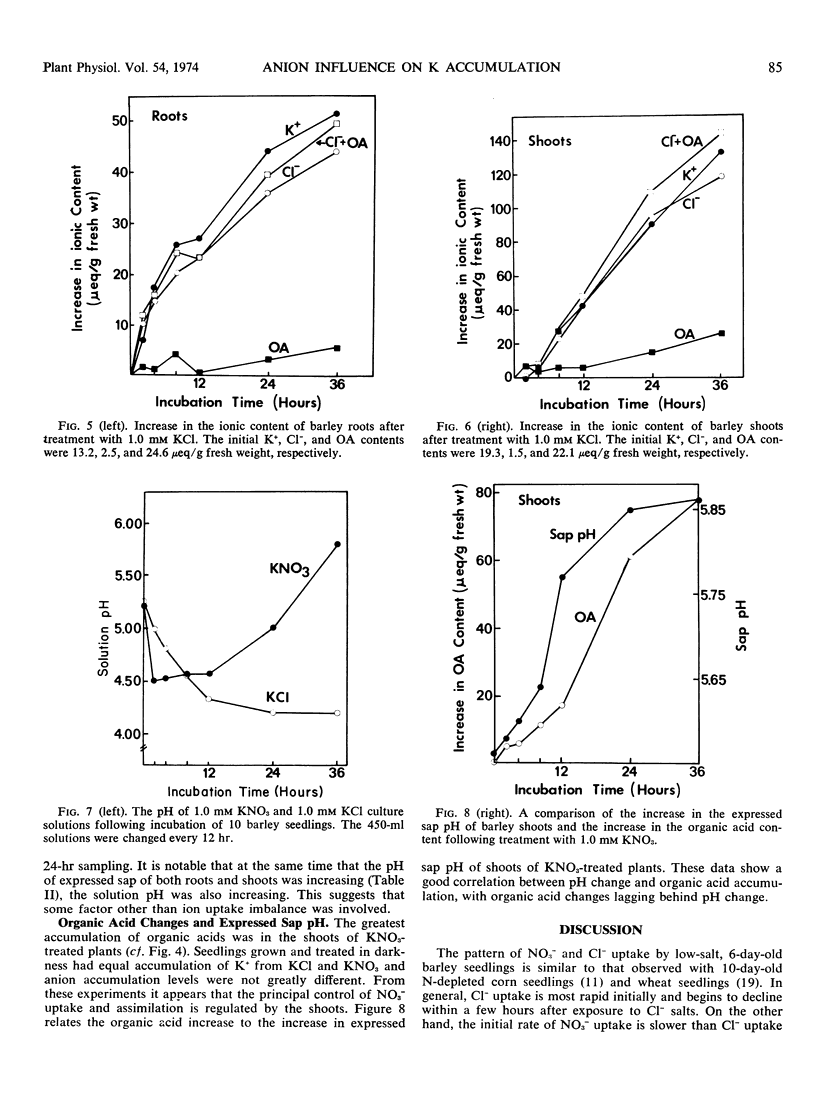
The influence of NO3− uptake and reduction on ionic balance in barley seedlings (Hordeum vulgare, cv. Compana) was studied. KNO3 and KCl treatment solutions were used for comparison of cation and anion uptake. The rate of Cl− uptake was more rapid than the rate of NO3− uptake during the first 2 to 4 hours of treatment. There was an acceleration in rate of NO3− uptake after 4 hours resulting in a sustained rate of NO3− uptake which exceeded the rate of Cl− uptake. The initial (2 to 4 hours) rate of K+ uptake appeared to be independent of the rate of anion uptake. After 4 hours the rate of K+ uptake was greater with the KNO3 treatment than with the KCl treatment, and the solution pH, cell sap pH, and organic acid levels with KNO3 increased, relative to those with the KCl treatment. When absorption experiments were conducted in darkness, K+ uptake from KNO3 did not exceed K+ uptake from KCl. We suggest that the greater uptake and accumulation of K+ in NO3−-treated plants resulted from (a) a more rapid, sustained uptake and transport of NO3− providing a mobile counteranion for K+ transport, and (b) the synthesis of organic acids in response to NO3− reduction increasing the capacity for K+ accumulation by providing a source of nondiffusible organic anions.
Full text
PDF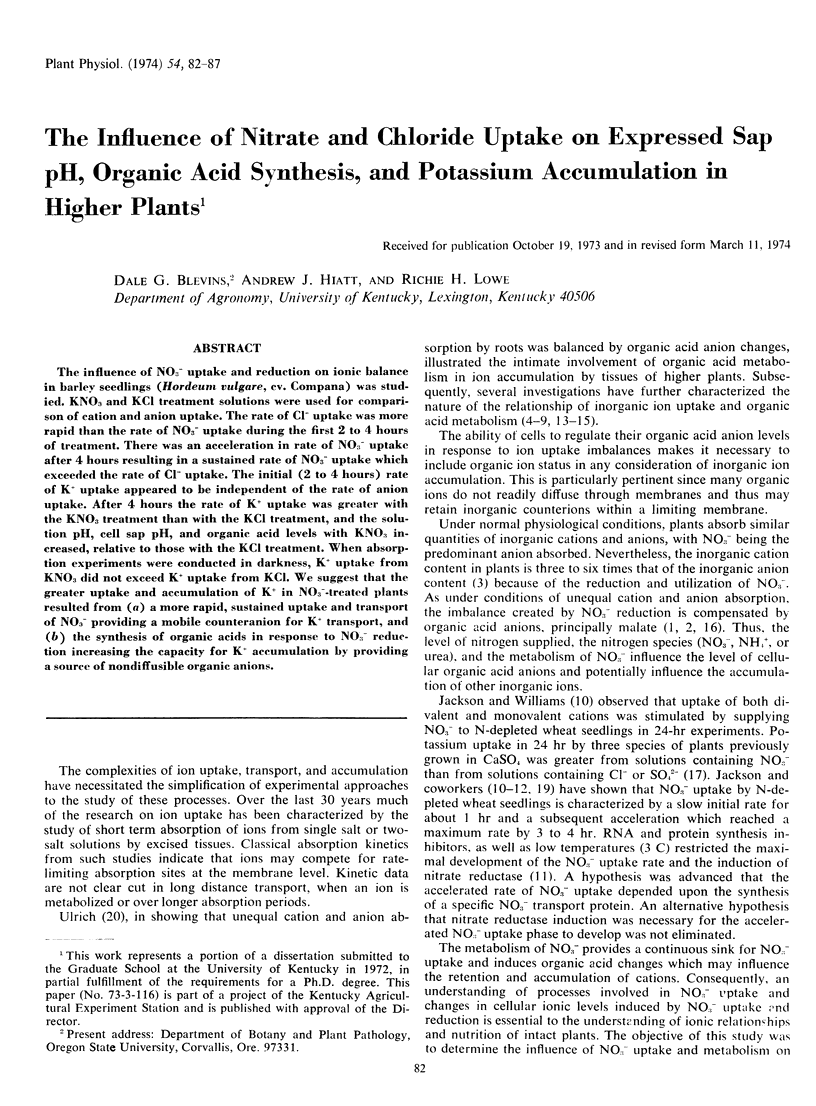


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hiatt A. J. Electrostatic association and donnan phenomena as mechanisms of ion accumulation. Plant Physiol. 1968 Jun;43(6):893–901. doi: 10.1104/pp.43.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt A. J. Relationship of Cell Sap pH to Organic Acid Change During Ion Uptake. Plant Physiol. 1967 Feb;42(2):294–298. doi: 10.1104/pp.42.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson W. A., Flesher D., Hageman R. H. Nitrate Uptake by Dark-grown Corn Seedlings: Some Characteristics of Apparent Induction. Plant Physiol. 1973 Jan;51(1):120–127. doi: 10.1104/pp.51.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L. Carbon Dioxide Fixation and Ion Absorption in Barley Roots. Plant Physiol. 1955 May;30(3):264–269. doi: 10.1104/pp.30.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L., Ordin L. Organic Acid Metabolism and Ion Absorption in Roots. Plant Physiol. 1954 Jan;29(1):70–75. doi: 10.1104/pp.29.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby B., Laties G. G. Bicarbonate Fixation and Malate Compartmentation in Relation to Salt-induced Stoichiometric Synthesis of Organic Acid. Plant Physiol. 1971 Apr;47(4):525–531. doi: 10.1104/pp.47.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby E. A., Mengel K. Ionic balance in different tissues of the tomato plant in relation to nitrate, urea, or ammonium nutrition. Plant Physiol. 1967 Jan;42(1):6–14. doi: 10.1104/pp.42.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]


