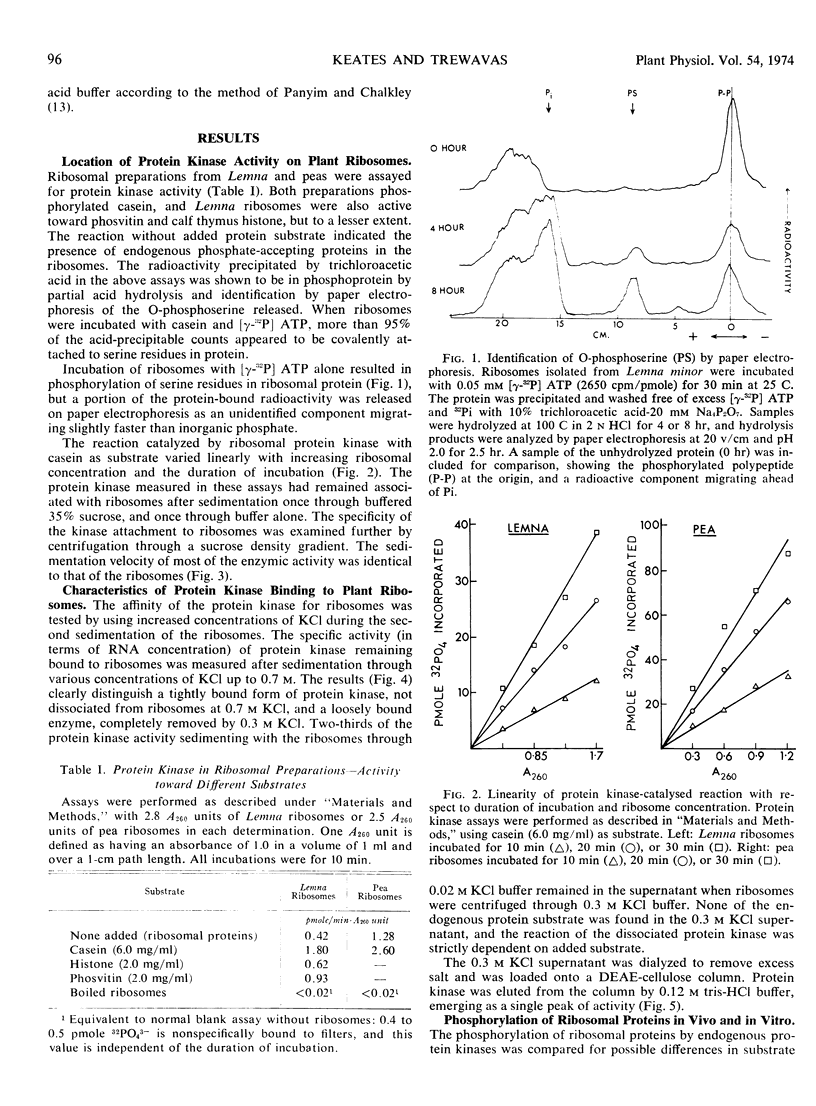
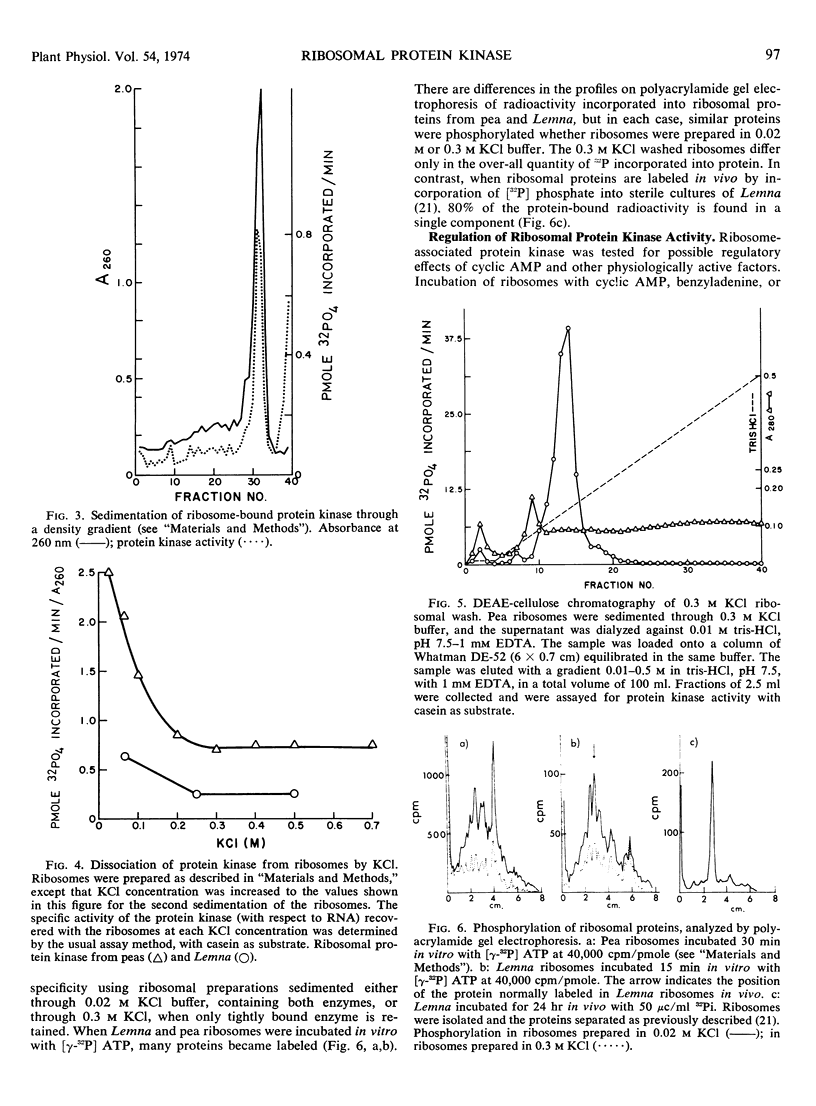
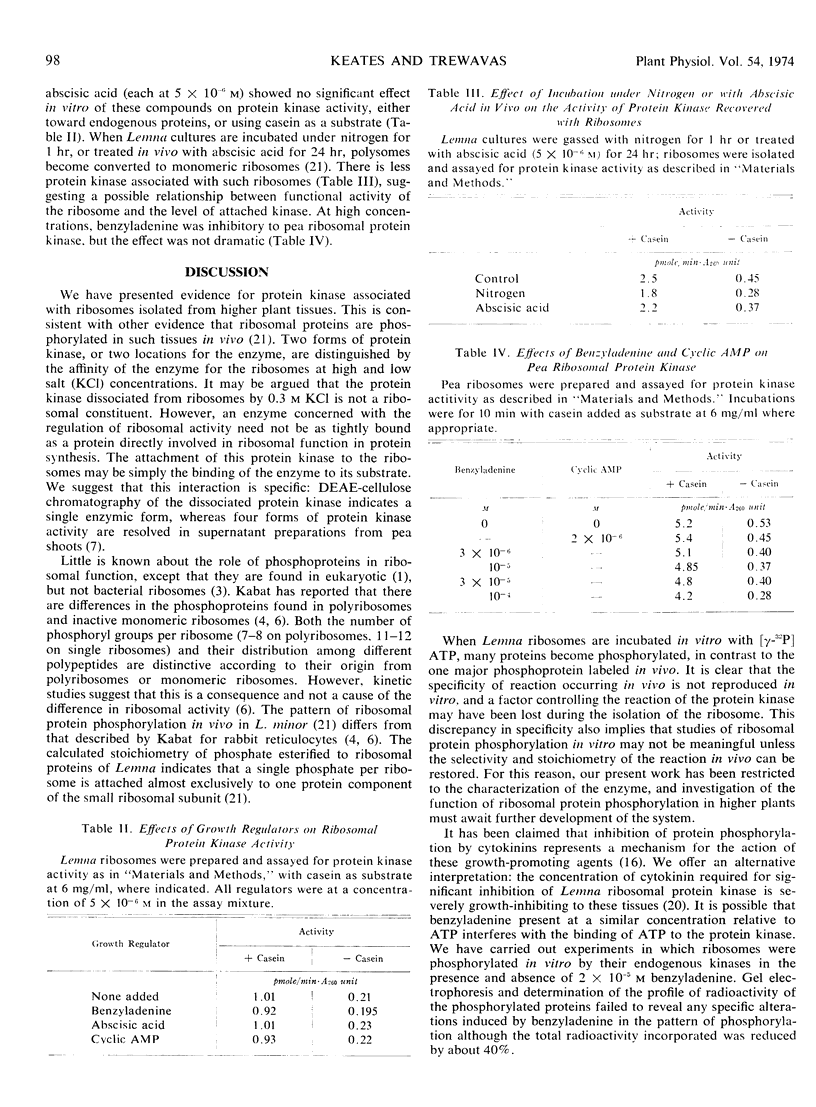
Abstract
Protein kinase (ATP:protein phosphokinase) has been found associated with ribosomes prepared from 5-day-old etiolated pea shoots and sterile cultures of Lemna minor. The enzyme catalyzes the phosphorylation of ribosomal proteins in vitro and may be involved in the formation of phosphoproteins in the ribosomes in vivo. Protein kinase sediments with ribosomes during sucrose density gradient centrifugation through a buffer containing 0.02 m KCl. Seventy per cent of the enzymic activity dissociates from the ribosomes in 0.3 m KCl, but the remaining protein kinase remains firmly bound in 0.7 m KCl. Cyclic AMP does not modify the activity of these protein kinases in vitro. Benzyladenine inhibits the protein kinase, but only at high concentrations of this cytokinin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitte L., Kabat D. Phosphorylation of ribosomal proteins in sarcoma 180 tumor cells. J Biol Chem. 1972 Sep 10;247(17):5345–5350. [PubMed] [Google Scholar]
- Fontana J. A., Picciano D., Lovenberg W. The identification and characterization of a cyclic AMP dependent protein kinase on rabbit reticulocyte ribosomes. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1225–1232. doi: 10.1016/0006-291x(72)90599-2. [DOI] [PubMed] [Google Scholar]
- Gordon J. Determination of an upper limit to the phosphorus content of polypeptide chain elongation factor and ribosomal proteins in Escherichia coli. Biochem Biophys Res Commun. 1971 Aug 6;44(3):579–586. doi: 10.1016/s0006-291x(71)80122-5. [DOI] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. A cell-free system with ribosomal protein kinase activity. Biochemistry. 1971 Jan 19;10(2):197–203. doi: 10.1021/bi00778a001. [DOI] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. Characterization and regulatory aspects. Biochemistry. 1970 Oct 13;9(21):4160–4175. doi: 10.1021/bi00823a019. [DOI] [PubMed] [Google Scholar]
- Kabat D. Turnover of phosphoryl groups in reticulocyte ribosomal phosphoproteins. J Biol Chem. 1972 Sep 10;247(17):5338–5344. [PubMed] [Google Scholar]
- Keates R. A. Cyclic nucleotide-independent protein kinase from pea shoots. Biochem Biophys Res Commun. 1973 Sep 18;54(2):655–661. doi: 10.1016/0006-291x(73)91473-3. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. An adenosine 3',5'-monophosphate-dependent protein kinase from Escherichia coli. J Biol Chem. 1969 Jun 25;244(12):3417–3419. [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake R. S., Salzman N. P. Occurrence and properties of a chromatin-associated F1-histone phosphokinase in mitotic Chinese hamster cells. Biochemistry. 1972 Dec 5;11(25):4817–4826. doi: 10.1021/bi00775a027. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ M., LIPMANN F. Reversible phosphate transfer between yolk phosphoprotein and adenosine triphosphate. J Biol Chem. 1960 Apr;235:1043–1050. [PubMed] [Google Scholar]
- Ralph R. K., McCombs P. J., Tener G., Wojcik S. J. Evidence for modification of protein phosphorylation by cytokinins. Biochem J. 1972 Dec;130(4):901–911. doi: 10.1042/bj1300901a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Cyclic adenosine 3',5'-monophosphate-dependent protein kinase of human erythrocyte membranes. J Biol Chem. 1972 Oct 10;247(19):6135–6139. [PubMed] [Google Scholar]
- Sy J., Richter D. Separation of a cyclic 3',5'-adenosine monophosphate binding protein from yeast. Biochemistry. 1972 Jul 18;11(15):2784–2787. doi: 10.1021/bi00765a008. [DOI] [PubMed] [Google Scholar]
- Trewavas A. The Phosphorylation of Ribosomal Protein in Lemna minor. Plant Physiol. 1973 Apr;51(4):760–767. doi: 10.1104/pp.51.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller M., Rodnight R. Protein kinase activity in membrane preparations from ox brain. Stimulation of intrinsic activity by adenosine 3':5'-cyclic monophosphate. Biochem J. 1973 Mar;132(3):483–492. doi: 10.1042/bj1320483. [DOI] [PMC free article] [PubMed] [Google Scholar]


