Abstract
Current study was aimed to investigate the effect of dihydromyricetin on hydrogen peroxide induced oxidative stress in the osteosarcoma cells. MTT assay showed that hydrogen peroxide treatment at a concentration of 100 μM caused a significant (p < 0.005) reduction in the viability of MG63 cells. However, reduction in cell viability caused by 100 μM concentration of hydrogen peroxide was completely prevented on incubation with 30 μM dose of dihydromyricetin. Treatment with 100 μM concentration of hydrogen peroxide for 24 h led to condensation of chromatin material, rounding of cell shape and detachment of cells. The results from flow cytometry using annexin V-FITC and PI double staining showed apoptosis induction in 47.84 ± 5.21% cells on treatment with 100 μM concentration of hydrogen peroxide compared to 2.32 ± 0.54% in controlcells. The apoptotic alterations in MG63 cell morphology were prevented significantly on pre-treatment with 30 μM doses of dihydromyricetin for 48 h. Annexin V-FITC and PI staining showed reduction of hydrogen peroxide induced apoptotic cell percentage to 3.07 ± 0.86% on pre-treatment of MG63 cells with 30 μM dose of dihydromyricetin. Western blot analysis showed a significant increase in the activation of caspase-3 and -9 on treatment of MG63 cells for 24 h with 100 μM concentration of hydrogen peroxide. The expression level of Bcl-2 was decreased significantly by 100 μM concentration of hydrogen peroxide in MG63 cells. However, pre-treatment of MG63 cells with 30 μM dose of dihydromyricetin for 48 h significantly prevented hydrogen peroxide induced increase in caspase-3 and -9 levels and reduction in Bcl-2 level. Thus dihydromyricetin prevents hydrogen peroxide induced reduction in viability and induction of apoptosis in MG63 cells through down-regulation of caspase activation and up-regulation of Bcl-2 levels.
Keywords: Osteosarcoma, Caspase activation, Peroxide, Apoptosis
1. Introduction
Osteoporosis a disease of skeletal system causing degradation of bone strengthening tissues and subsequently bones become susceptible to fracture (Wactawski-Wende, 2001). The process of formation of bones by osteoblasts is retarded during postmenopausal phase leading to weakening of the bones (Isomura et al., 2004, Arslan et al., 2006). The factors responsible for osteoporosis include generation of reactive oxygen species including nitric oxide, peroxide ion, etc. in the cells and their subsequent transfer into the blood stream (Basu et al., 2001, Forrest et al., 2006, Maggio et al., 2003). In normal bones homeostasis is maintained through generation of peroxides by osteoclasts and production of oxidant quenchers like glutathione peroxidase by the osteoblasts (Dreher et al., 1988, Yang et al., 2001). In the rat model of osteoporosis increased level of hydrogen peroxide causing lipid peroxidation and reduced level of anti-oxidants has been observed (Muthusami et al., 2005). Thus molecules having anti-oxidant activity have better scope for the treatment of osteoporosis through targeting expression of reactive oxygen species.
Flavonoids are the class of natural products possessing structural features which enable these molecules to act as potential antioxidant agents (Antonisamy et al., 2015, Balamurugan, 2015, Rathi et al., 2015, Nandhini and Stella Bai, 2015, Kalaiselvi et al., 2016, Neelamkavil and Thoppil, 2016, Valsan and Raphael, 2016). Dihydromyricetin is a member of flavonoid family obtained by the phytochemical investigation of Ampelopsis grossedentata (Chen et al., 2007). Biological screening of dihydromyricetin revealed its broad spectrum of activities such as anti-oxidant, radical scavenging activity, inhibition of inflammation and antithrombotic activity (Zhong et al., 2002, Xu et al., 2008, Noorudheen and Chandrasekharan, 2016, Santhosh et al., 2016, Sreeshma et al., 2016, Puthur, 2016, Serasanambati and Chilakapati, 2016). Dihydromyricetin inhibits the per-oxidation of membrane lipids and thus prevents death of normal cells (Shen et al., 2012). In addition, it also exhibits hepato-protective activity in the animals. The current study was aimed to investigate the effect of dihydromyricetin on hydrogen peroxide induced oxidative stress in the osteosarcoma cells. It was observed that dihydromyricetin prevents hydrogen peroxide induced reduction in viability and induction of apoptosis in MG63 cells through down-regulation of caspase activation and up-regulation of Bcl-2 levels.
2. Materials and methods
2.1. Reagents and chemicals
Hydrogen peroxide was obtained from Sigma–Aldrich (St. Louis, MO, USA). A 0.1 μM stock solution of dihydromyricetin was prepared in methanol and then stored at a temperature of −10 °C prior to use.
2.2. Cell lines and culture
MG63 cells purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) were used in this study. The cell cultures were grown in RPMI-1640 medium containing 10% (v/v) fetal bovine serum (FBS). The medium also contained l-glutamine (1%, v/v), penicillin (100 U/ml) and streptomycin (100 μg/ml) antibiotics. The cell cultures were cultured in an incubator at 37 °C temperature in a humidified atmosphere of air: CO2 in the ratio of 95:5%.
2.3. Induction of oxidative stress by hydrogen peroxide
The cells distributed in 100-mm dishes at a density of 3 × 107 cells per dish were allowed to attain confluence. After attaining confluence cells were pre-treated with various doses of dihydromyricetin for 48 h or with dimethyl sulfoxide as control. Following incubation, the cells were treated with hydrogen peroxide to induce oxidative stress.
2.4. Cell viability assay
Viability and growth of MG63 cells was analysed using MTT cell proliferation kit (Roche Applied Science). The cells were putinto 96-well microplates at 2.5 × 106 cells per well density. After culture for 24 h cells were subjected to pre-treatment with various concentrations of dihydromyricetin for 48 h or with dimethyl sulfoxide as control. After incubation, the cells were treated with hydrogen peroxide to induce oxidative stress in an incubator at 37 °C under 5% CO2atmosphere. The cells were then treated for 4 h with 10 μl solution of MTT under same conditions. The medium was removed and 150 μl solution of DMSO was added to each of the well for dissolution of formazan crystals. The plates were stirred in shaker for 15 min. The absorbance measurements were made for each plate three times independently at 470-nm using a microplate reader (3550-UV, Bio-Rad, USA).
2.5. Apoptosis analysis
Apoptosis induction in MG63 cells was analysed and measured using the Annexin V-FITC Apoptosis Detection kit (BD Bioscience, San Jose, CA, USA). The cells at a density of 3 × 106 cells per well in 6-well plates were incubated with various concentrations of dihydromyricetin for 48 h or with dimethyl sulfoxide as control. Then the cells were treated with hydrogen peroxide to induce oxidative stress in an incubator at 37 °C under 5% CO2 atmosphere. The cells then washed three times with ice-cold PBS and subsequently treated with 100 μl binding buffer. Incubation of the cells for 20 min was then performed with 3 μl Annexin V-FITC (BD Bioscience) and 10 μl propidium iodide (PI; BD Bioscience). Apoptosis analysis was performed using flow cytometry (Becton–Dickinson, San Jose, CA, USA).
2.6. Western blot analysis
The expression level of proteins involved in apoptosis in MG63 cells was detected using western blot assay. The cells after 24 h of culture were subjected to pre-treatment with various concentrations of dihydromyricetin for 48 h or with dimethyl sulfoxide as control. Following incubation, the cells were treated with hydrogen peroxide to induce oxidative stress in an incubator at 37 °C under 5% CO2 atmosphere. The cells were lysed on treatment with ice-cold lysis buffer for 45 min. The cell lysates were subjected to centrifugation at 4 °C temperature for 20 min at 12000×g to collect the supernatant. The supernatants were detected using bicinchoninic assay for analysis of protein concentration. The proteins were separated on SDS-PAGE and subsequently transferred to polyvinylidene difluoride membranes blocked with 5% non-fat milk in Tris-buffered saline and Tween 20 (TBST).The membrane incubation was performed with polyclonalrabbit primary antibodies against caspase-3, -9 and Bcl-2 (Chemicon, Temecula, CA, USA) for overnight. The membrane washing with TBST was followed by 1 h incubation with horseradish peroxidase-conjugated secondary antibodies. Analysis of the bands was performed using an enhanced chemi luminescence blotting detection system (Fluor Chem E; Protein simple, Santa Clara, CA, USA).
2.7. Statistical analysis
For the statistical analysis of data MedCalc version 10.0 program (Frank Schoonjans, University of Gent, Belgium) was used. In addition, Mann–Whitney U-test, analysis of variance, and the Kruskal–Wallis tests were also employed. p-value < 0.05 was taken to indicate a statistically significant result.
3. Results
3.1. Hydrogen peroxide treatment causes inhibition of viability in MG63 cells
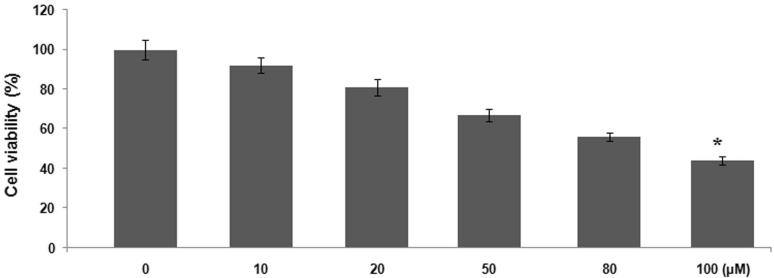
Hydrogen peroxide treatment at a concentration of 100 μM caused a significant (p < 0.005) reduction in the viability of MG63 cells compared to the control. The cells were incubated for 24 h with 10, 20, 50, 80 and 100 μM concentration of hydrogen peroxide and then analysed by MTT assay. The viability of MG63 cells was reduced to 18.65% on incubation with hydrogen peroxide at 100 μM concentration after 24 h (Fig. 1).
Figure 1.
Hydrogen peroxide treatment exhibits inhibitory effect on viability of MG63 cells. The cells were incubated with 10, 20, 50, 80 and 100 μM concentration of hydrogen peroxide for 24 h and then analysed by MTT assay. *p<0.05 vs control cultures.
3.2. Dihydromyricetin prevents hydrogen peroxide induced reduction in cell viability
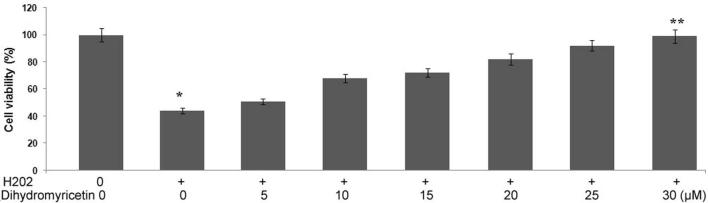
However, pre-treatment ofMG63 cells with dihydromyricetin for 48 prevented hydrogen peroxide induced reduction in cell viability.MG63 cells were incubated with 5, 10, 15, 20, 25 and 30 μM doses of dihydromyricetin before treatment with hydrogen peroxide. The reduction in cell viability caused by 100 μM concentration of hydrogen peroxide was completely prevented on incubation with 30 μM dose of dihydromyricetin (Fig. 2)
Figure 2.
Dihydromyricetin treatment prevents hydrogen peroxide induced inhibition of MG63 cell viability. The cells were pre-treated with 5, 10, 15, 20, 25 and 30 μM doses of dihydromyricetin before treatment with hydrogen peroxide. Cell viability was then analysed using MTT assay. *p<0.05 vs control cultures.
3.3. Hydrogen peroxide treatment causes induction of apoptotic changes in MG63 cells
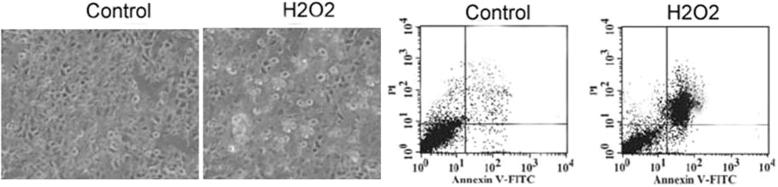
MG63 cells were treated with various concentrations of hydrogen peroxide for analysis of induction of apoptotic changes. Treatment with 100 μM concentration of hydrogen peroxide for 24 h led to condensation of chromatin material, rounding of cell shape and detachment of cells (Fig. 3). The results from flow cytometry using annexin V-FITC and PI double staining showed apoptosis induction in 47.84 ± 5.21% cells on treatment with 100 μM concentration of hydrogen peroxide compared to 2.32 ± 0.54% in control cells.
Figure 3.
Effect of hydrogen peroxide on MG63 cell morphology. The cells were treated with various concentrations of hydrogen peroxide for 24 h and then analysed for alterations in morphology. Flow cytometry was also performed to examine the induction of apoptosis in MG63 cells by treatment with hydrogen peroxide.
3.4. Dihydromyricetin prevents hydrogen peroxide induced apoptotic alterations in MG63 cells
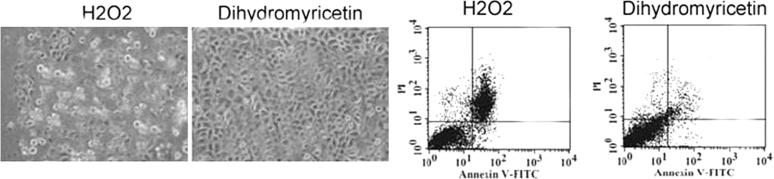
The cells were incubated with 5, 10, 15, 20, 25 and 30 μM doses of dihydromyricetin before treatment with 100 μM concentration of hydrogen peroxide. The apoptotic alterations in MG63 cell morphology were prevented significantly on pre-treatment with 30 μM doses of dihydromyricetin for 48 h (Fig. 4). Annexin V-FITC and PI staining showed reduction of hydrogen peroxide induced apoptotic cell percentage to 3.07 ± 0.86% on pre-treatment of MG63 cells with 30 μM doses of dihydromyricetin (Fig. 4).
Figure 4.
Dihydromyricetin prevents hydrogen peroxide induced alterations in MG63 cell morphology. The cells were pre-treated with various concentrations of dihydromyricetin for 48 h and then treated with hydrogen peroxide for 24 h.
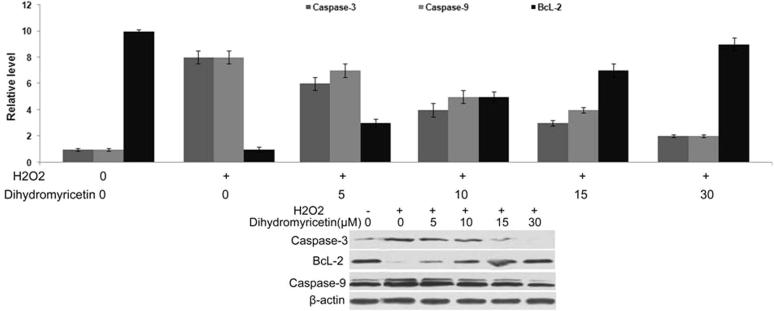
3.5. Hydrogen peroxide treatment promotes caspase-3 and -9 expression and inhibits Bcl-2 expression
Western blot as analysis showed a significant increase in the activation of caspase-3 and -9 on treatment of MG63 cells for 24 h with 100 μM concentration of hydrogen peroxide (Fig. 5). The expression level of Bcl-2 was decreased significantly by 100 μM concentration of hydrogen peroxide in MG63 cells. However, pre-treatment of MG63 cells with 30 μM dose of dihydromyricetin for 48 h significantly prevented hydrogen peroxide induced increase in caspase-3 and -9 levels and reduction in Bcl-2 level (Fig. 5).
Figure 5.
Dihydromyricetin pre-treatment inhibits hydrogen peroxide induced increase in expression of caspases and down-regulation of Bcl-2 in MG63 cells. The cells were pre-treated with dihydromyricetin for 48 h or with DMSO as control and then with hydrogen peroxide. The expression of caspases and Bcl-2 was then analysed using western blot assay. The expression of β-actin was taken as internal control.
4. Discussion
Osteoporosis formation is contributed mainly by the generation of reactive oxygen species in the osteocytes leading to bone degeneration (Basu et al., 2001). The increased production of reactive oxygen species has been found to suppress the rate of osteoclast proliferation and induce oxidative stress (Fraser et al., 1996, Mody et al., 2001). Dihydromyricetin exhibits broad spectrum of activities such as anti-oxidant, radical scavenging activity and inhibition of inflammation (Xu et al., 2008). Thus involvement of reactive oxygen species in osteoporosis and anti-oxidant property of dihydromyricetin prompted us to perform the present study. Our results from the current study showed that oxidative stress by hydrogen peroxide treatment inhibited the viability of MG63 cells significantly in consistence with the earlier reports. However, when MG63 cells were pre-treated with dihydromyricetin hydrogen peroxide induced reduction in cell viability was inhibited. Thus dihydromyricetin pre-treatment prevented the oxidative stress induced reduction in cell viability caused by hydrogen peroxide. Apoptosis is the programmed death of cells which maintains the homeostasis and removes unwanted cells from the body. There are two pathways through which cells undergo apoptosis such as mitochondrial and extrinsic pathway (Lorenzo and Susin, 2007). Our results showed that MG63 cells on treatment with hydrogen peroxide suffered condensation of chromatin material, rounding of cell shape and detachment. Annexin V-FITC and PI double staining showed apoptosis induction in higher proportion of cells on treatment with hydrogen peroxide compared to control cells. The apoptotic alterations in MG63 cell morphology were prevented significantly on pre-treatment with dihydromyricetin for 48 h. A marked reduction in hydrogen peroxide induced apoptotic cell percentage was observed on pre-treatment of MG63 cells with dihydromyricetin. Caspase protease activation plays an important role in the induction of cell apoptosis through various pathways prevailing in cells (Pacova et al., 2009). The most important caspase involved in the apoptosis induction through mitochondrial pathway is the caspase-9 (Desagher and Martinou, 2000). Activation of caspase-9 leads to the mitochondrial release of cytochrome c which in turn results in the activation of protease activation factor 1. Induction of apoptosis and viability of the cells is regulated by the expression of Bcl-2 through maintenance of cytochrome c release from the mitochondria (Reed, 1997). The current study revealed a significant increase in the activation of caspase-3 and -9 in MG63 cells under hydrogen peroxide induced oxidative stress. The oxidative stress by hydrogen peroxide also led to suppression of Bcl-2 level in MG63 cells. On the other hand, pre-treatment of MG63 cells with dihydromyricetin significantly prevented hydrogen peroxide induced increase in caspase-3 and -9 levels and reduction in Bcl-2 level. Therefore, dihydromyricetin prevents oxidative stress induced apoptosis in MG63 cells by targeting the activation levels of caspases and Bcl-2.
In summary dihydromyricetin prevents hydrogen peroxide induced reduction in viability and induction of apoptosis in MG63 cells through down-regulation of caspase activation and up-regulation of Bcl-2 levels. Thus dihydromyricetin can be used for the treatment of oxidative stress induced osteoporosis.
Acknowledgment
This study was supported by Natural Science Foundation of Liaoning Province (project No: 201602448).
Footnotes
Peer review under responsibility of King Saud University.
References
- Antonisamy P., Duraipandiyan V., Ignacimuthu S., Kim J.-H. Anti-diarrhoeal activity of friedelin isolated from Azima tetracantha lam. in wistar rats. South Indian J. Biol. Sci. 2015;1:34–37. [Google Scholar]
- Arslan H., Yalin S., Erdogan C., Bagis S., Aksit S.C. Effect of free radicals and antioxidants on postmenopausal osteoporosis. Asian J. Chem. 2006;18:1091–1096. [Google Scholar]
- Balamurugan R. Smilax chinensis Linn. (Liliaceae) root attenuates insulin resistance and ameliorate obesity in high diet induced obese rat. South Indian J. Biol. Sci. 2015;1:47–51. [Google Scholar]
- Basu S., Michaelsson K., OIofsson H. Association between oxidative stress and bone mineral density. Biochem. Biophys. Res. Commun. 2001;288:275–279. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- Chen Y.Q., Ni D.J., Cheng Q. Study on the hypolipidemic effect of flavones and dihydromyricetin from Tengcha. J. Tea Sci. 2007;3:221–225. [Google Scholar]
- Desagher S., Martinou J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- Dreher I., Schutze N., Baur A. Selenoproteins are expressed in fetal human osteoblast-like cells. Biochem. Biophys. Res. Commun. 1988;245:101–107. doi: 10.1006/bbrc.1998.8393. [DOI] [PubMed] [Google Scholar]
- Forrest C.M., Mackay G.M., Oxford L. Kynurenine pathway metabolism in patients with osteoporosis after 2 years of drug treatment. Clin. Exp. Pharmacol. Physiol. 2006;33:1078–1087. doi: 10.1111/j.1440-1681.2006.04490.x. [DOI] [PubMed] [Google Scholar]
- Fraser J.H., Helfrich M.H., Wallace H.M., Ralston S.H. Hydrogen peroxide, but not superoxide, stimulates hone resorption in mouse calvariae. Bone. 1996;19:223–226. doi: 10.1016/8756-3282(96)00177-9. [DOI] [PubMed] [Google Scholar]
- Isomura H., Fujie K., Shibata K. Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology. 2004;197:93–100. doi: 10.1016/j.tox.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Kalaiselvi V., Binu T.V., Radha S.R. Preliminary phytochemical analysis of the various leaf extracts of Mimusops elengi L. South Indian J. Biol. Sci. 2016;2:24–29. [Google Scholar]
- Lorenzo H.K., Susin S.A. Therapeutic potential of AIF-mediated caspase-independent programmed cell death. Drug Resist. Updat. 2007;10:235–255. doi: 10.1016/j.drup.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Maggio D., Barabani M., Pierandrei M. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J. Clin. Endocrinol. Metab. 2003;88:1523–1527. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- Mody N., Parhami F., Saraflian T.A., Demer L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- Muthusami S., Ramachandran I., Muthusamy B. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin. Chim. Acta. 2005;360:81–86. doi: 10.1016/j.cccn.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Nandhini V.S., Stella Bai G.V. In-vitro Phytopharmacological effect and cardio protective activity of Rauvolfia tetraphylla L. South Indian J. Biol. Sci. 2015;1:97–102. [Google Scholar]
- Neelamkavil S.V., Thoppil J.E. Evaluation of the anticancer potential of the traditional medicinal herb Isodon coetsa. South Indian J. Biol. Sci. 2016;2:41–45. [Google Scholar]
- Noorudheen N., Chandrasekharan D.K. Effect of ethanolic extract of Phyllanthus emblica on captan induced oxidative stress in vivo. South Indian J. Biol. Sci. 2016;2:95–102. [Google Scholar]
- Pacova H., Astl J., Martinek J. The pathogenesis of chronic inflammation and malignant transformation in the human upper airways: the role of β-defensins, eNOS, cell proliferation and apoptosis. Histol. Histopathol. 2009;24:815–820. doi: 10.14670/HH-24.815. [DOI] [PubMed] [Google Scholar]
- Puthur J.T. Antioxidants and cellular antioxidation mechanism in plants. South Indian J. Biol. Sci. 2016;2:14–17. [Google Scholar]
- Rathi M.A., Meenakshi P., Gopalakrishnan V.K. Hepatoprotective activity of ethanolic extract of alysicarpus vaginalis against nitrobenzene-induced hepatic damage in rats. South Indian J. Biol. Sci. 2015;1:60–65. [Google Scholar]
- Reed J.C. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- Santhosh S.K., Venugopal A., Radhakrishnan M.C. Study on the phytochemical, antibacterial and antioxidant activities of Simarouba glauca. South Indian J. Biol. Sci. 2016;2:119–124. [Google Scholar]
- Serasanambati M., Chilakapati S.R. Function of nuclear factor kappa B (NF-kB) in human diseases-a review. South Indian J. Biol. Sci. 2016;2:368–387. [Google Scholar]
- Shen Y., Lindemeyer A.K., Gonzalez C. Dihydromyricetin as a novel anti-alcohol intoxication medication. J. Neurosci. 2012;32:390–401. doi: 10.1523/JNEUROSCI.4639-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreeshma P.S., Raphael K.R., Baby A.A. Pharmacognostic studies of leaves of Naravelia zeylanica (Linn) DC. South Indian J. Biol. Sci. 2016;2:179–182. [Google Scholar]
- Valsan A.l., Raphael K.R. Pharmacognostic profile of Averrhoa bilimbi Linn. leaves. South Indian J. Biol. Sci. 2016;2:75–80. [Google Scholar]
- Wactawski-Wende J. Periodontal diseases and osteoporosis: association and mechanisms. Ann. Periodontol. 2001;6:197–208. doi: 10.1902/annals.2001.6.1.197. [DOI] [PubMed] [Google Scholar]
- Xu J.J., Yao M.J., Wu M.C. Study on biological efficacy of dihydromyricetin. Food Sci. 2008;29:622–625. [Google Scholar]
- Yang S., Madyastha P., Bingel S. A new superoxide generating oxidase in murine osteoclasts. J. Biol. Chem. 2001;276:5452–5458. doi: 10.1074/jbc.M001004200. [DOI] [PubMed] [Google Scholar]
- Zhong Z.X., Zhou G.F., Chen X.F., Qin J.P. Experimental study on the protective effect of dihydromyricetin from Guangxi Ampelopsis grossepentata on liver. China J. Traditional Med. Sci. Technol. 2002;9:155–156. [Google Scholar]