Abstract
Fig leaf mottle-associated virus-1 (FLMaV-1) is a closterovirus newly identified in fig trees, in the Mecca region, suffering from mosaic disease symptoms and apparently is compromising the fig plantation in the country. In the present study, we demonstrated the efficiency of two in vivo experiments including pre and post treatments using Thuja leaf, ginger roots, Harmal seeds and turmeric rhizome extracts on symptoms expression of rooted cuttings infected with FLMaV-1- and their impact on virus multiplication. Results showed that individual treatments with ginger roots and turmeric rhizomes in pre-grafting experiments and Thuja extract following Harmal seeds in post grafting experiments were efficient against symptom development. In addition, results showed that the total photosynthesis pigments; total soluble intracellular proteins and total phenol contents were higher in infected treated cuttings compared with healthy ones, thus it was taken as evidence on a mutual interaction between these extracts and virus multiplication.
Keywords: Fig leaf mottle-associated virus-1, Ficus carica, Antiviral activity and medical plant extracts
1. Introduction
Family Moraceae contain fig (Ficus carica L.) (Vallejo et al., 2012) which grows in many different environments (Oliveira et al., 2012) and soil types (Vemmos et al., 2013). It is grown throughout the Mediterranean basin, Turkey being the main producer, followed by Algeria, Morocco, Egypt, Spain, Tunisia, Greece and Syria (Anonymous, 2005). Condit and Horne (1933) who described that, the fig crop from California is affected by a mosaic disease (FMD), which is transmitted by grafting and eryophid mite Aceria ficus (Flock and Wallace, 1955). Fig mosaic disease include a wide array of symptoms, such as, discolorations of the leaves including chlorotic mottling, blotching, banding, clearing and feathering of the veins, chlorotic and necrotic ring spots and line patterns following malformations of leaves (Elbeaino et al., 2006). Over time, FMD has been the object of investigations especially intensive in the last few years (Martelli, 2009), and the first molecular information regarding natural virus infection of fig was for two members of the Closteroviridae, Fig leaf mottle-associated virus 1 (FLMaV-1) and Fig leaf mottle-associated virus 2 (FLMaV-2), which were detected in fig trees showing FMD symptoms in Italy and Algeria, respectively (Elbeaino et al., 2006, Elbeaino et al., 2007). Later, the records of fig-infecting viruses rapidly increased and additional viruses joined the list, including fig mild mottle-associated virus (FMMaV), fig mosaic virus (FMV), fig cryptic virus (FCV), fig latent virus 1 (FLV-1) and fig fleck-associated virus (FFkaV) (Elbeaino et al., 2010). Contemporarily, partial or complete nucleotide sequences of other viruses probably belonging to the Partitiviridae (Luteovirus-like) and Caulimoviridae (Badnavirus-like) families were also found in diseased fig plants.
To date, four viruses are reported in Saudi Arabia, i.e. FLMaV-1, FLMaV-2, FMMaV and FMV (Alhudaib, 2012), that were found with different extents of infection.
The plants can be induced to become more resistant to disease through treatment with various biotic (Pathogens, non-pathogens microorganisms), or non-biotic (Plant extracts, synthetic chemicals) agents (Al-Ani et al., 2011a).
Thuja orientalis (Family – Cupressaceae) shrubs are used in various forms of traditional medicines and their active component thujone has great potential against various health problems. It can be used as antioxidant, anticancer, antiviral and anti-inflammatory agents (Al-Ani and Hassan, 2002, Al-Ani et al., 2010, Al-Ani et al., 2011a, Al-ani et al., 2011b, Srivastava et al, 2012).
Zygophyllaceae including Peganum harmala L. is commonly known as “Harmal” (Zargari, 1998, Lamchouri et al., 2002). It has been confirmed that Harmal extract is a rich source of β-carboline alkaloids including harmol, harmine and harmaline. It has quinazoline derivatives: vasicine and vasicinone, too (Abbasipour et al., 2010).
Zingiber officinale Rosc. (Zingiberaceae) is commonly known as ginger and it has been concluded that ginger has a significant anti-inflammatory, (Malhotra and Pal Singh, 2003) effect. Several types of gingers displayed anti-rhinoviral effects. The active ingredients in ginger are thought to reside in its volatile oils. The major active ingredients in ginger oil are the bisabolene, zingiberene, and zingiberol (Connell and Sutherland, 1969, Moghaddasi and Kashani, 2012).
Also, Zingiberaceae including Curcuma longa L. and its curcumin polyphenolic compound have been subjected to a range of antimicrobial investigations due to extensive traditional uses and low side effects. Antimicrobial activities of curcumin and rhizome extract of curcumin against different viruses, bacteria, parasites and fungi have been reported by Moghadamtousi et al. (2013).
Accordingly, the objective of this study was to evaluate the antiviral activity of four extracts from medicinal plants against FLMaV-1-infected plants, in greenhouse conditions. Also, to evaluate the effect of these extracts on the virus activity, percentage of infection and disease severity and their impact on the physiological changes (photosynthesis pigments, total soluble proteins and total phenols) in plants under experimentation.
2. Material and methods
2.1. Virus sources and identification
There was no special permission to collect and visit orchards containing the plant material under experimentation. However, field studies in region Jeddah did not include any endangered or protected species.
Symptomatic leaves showing chlorotic blotches, vein clearing, vein banding, chlorosis, mosaic and chlorotic ring spot were collected from different fig trees in the Mecca region and assayed by RT-PCR for the presence of FLMaV-1, FLMaV-2, FMMaV and FMV. Leaves of RT-PCR positive samples to FLMaV-1 only were collected and the mother fig tree infected by FLMaV-1 only became the source of infected FLMaV-1 buds for healthy potted root cutting and grafting with freshly infected buds, whereas, RT-PCR-negative samples were discarded. Infected buds were used to transmit the causal FLMaV-1 to healthy potted rooted cutting from the local cultivar under glass house conditions at about 22 °C. Grafted seedlings were observed for symptom expression after 3 weeks from inoculation.
2.2. Molecular characterization
2.2.1. Extraction of Total Nucleic Acid (TNA)
TNAs were extracted from 100 mg of leaf veins or cortical scrapings ground in 1 ml grinding buffer and silica-purified according to (Foissac et al., 2001).
2.2.2. cDNA synthesis
10 μl of TNA extracts were mixed with 1 μl of random hexamer primers, (Boehringer Mannheim, GbmH) (0.5 μg/μl), denatured at 95 °C for 5 min and quickly chilled on ice. Reverse-transcription was done for 1 h at 39 °C by adding 4 μl M-MLV buffer 5×, 2 μl of 10 mM DTT, 0.5 μl of 10 mM dNTPs, and 200 units of Moloney Murine Leukaemia virus (M-MLV) reverse transcriptase (Bethesda Research Laboratories, USA) in a final volume of 20 μl.
2.2.3. PCR
The detection of FLMaV-1 in RT-PCR was conducted using specific primer (Reverse primer: FLMaV1-s. 5′-CGTGGCTGATGCAAAGTTTA-3′ and forward primer FLMaV1-a. 5′-GTTAACGCATGCTTCCATGA-3′), whose nucleotides sequences and use-conditions were previously described Elbeaino et al., 2010, Elbeaino et al., 2009a, Elbeaino et al., 2009b, Elbeaino et al., 2009c, Elbeaino et al., 2007, Elbeaino et al., 2006, Mahmoud et al., 2014, Elbeshehy and Elbeaino, 2011. Briefly, 2.5 μl of reverse-transcribed TNA mixture was submitted to amplification with the addition of 2.5 μl of 10 × Taq polymerase buffer (Promega Corporation, USA), with a final concentration of 1.5 mM MgCl2 for 25 μl as a total volume. PCR products were analyze by electrophoresis on 1.2% agarose gel prepared in 1X TBE buffer (Sambrook et al, 1989) examined on a UV trans-illuminator after staining with ethidium bromide.
2.3. Electron microscope
Dips in 2% aqueous uranyl acetate were prepared from symptomatic leaf petioles. For thin sectioning, tissue pieces from veins and mesophyll tissues of the discolored areas of young leaves will be processed according to standard procedures (Martelli et al., 1993, Martelli and Russo, 1984). Thin sections will be stained with lead citrate and viewed with a JOEL-JEA100 CX electron microscope Unit (Research laboratories compound of Cairo University, Faculty of Agriculture, Electron microscopy unit Biotechnology labs). Controls consisted of leaf tissues from a PCR-negative fig seedling processed as above.
2.4. Efficiency evaluation of antiviral extracts on FLMaV-1
Leaves and fruits samples of T. orientalis from Faculty of Agriculture gardens (Suez Canal University, Egypt), fresh roots of ginger, and rhizomes of turmeric from the local market and Harmal seeds from the Mecca region were prepared according to Al-Ani et al. (2010).
2.4.1. Plant materials and treatments
One hundred and eight healthy potted rooted cuttings of fig local cultivars were planted in a combination of sand and clay (1:2 v/v) in plastic pots (30 cm in diameter) in natural and positive conditions suitable for growth. The relative humidity was about 70%. The potted rooted cutting will be kept at 100% water holding capacity. FLMaV-1 that will be used in these experiments was prepared from severely infected fresh buds which give a positive molecular reaction. After 2 months of growth, healthy potted rooted cuttings with a similar size will be selected and divided into six groups of four antiviral extracts from Thuja leaves, ginger roots, Harmal seeds and, rhizomes of turmeric. Each group consists of three replicates (a replicate is three pots, each pot representative of one healthy potted rooted cutting). Namely, the groups were as follows, group 1: control healthy(sprayed with water), group 2: control infected (grafting with infected bud at the same time with the another groups), group 3: (6 gm/L Thuja extract and grafted seedling with infected bud), group 4: (6 gm/L ginger extract and grafted seedling with infected bud), group 5: (6 gm/L Harmal extract and grafted seedling with infected bud) and group 6: (6 gm/L turmeric extract and grafted seedling with infected bud). Groups were treated with two treatments. Treatment 1: healthy potted rooted cuttings were sprayed by antiviral extract and grafted with infected buds 7 days later (pre-grafting experiment). On the other hand, in treatment 2: healthy potted rooted cuttings were sprayed by antiviral extract after 72 h of inoculation (post grafting experiment).
2.4.2. Medicinal plants
2.4.2.1. Preparation of extract
.
2.4.2.2. Plant extracts
Samples from medicine plants were dried for one week in an oven at 45 °C and ground with a grinder (A10 from GMbH, Germany). The powders were sieved and stored in plastic bags at −20 °C until use (Al-Ani et al., 2010).
2.4.2.3. Alcohol extraction
Add 100 g of plant powder to 300 ml of 80% ethyl alcohol in flask of 1000 ml capacity. The mixture was stirred up for 24 h by a magnetic stirrer and filtrated by Whatman No. 2 filter paper. The filtrate was concentrated and stored in a water bath at 42 °C at −20 °C. Solutions at 6 g/L from the concentrated extracts were prepared in distilled water amended with 0.1% Tween-20 (Al-Ani et al., 2010).
2.5. Evaluation of changes in virus infection treated with viruses and antiviral extracts treatment
Every part of the potted rooted cutting was sprayed by antiviral extracts and treated. Half the number of healthy potted rooted cuttings will be sprayed with antiviral extracts before seven days from grafting with infected buds (Deya Eldeen et al., 2010). On the other hand, another half of the healthy potted rooted cuttings were sprayed with antiviral extract after 72 h of grafting. Three weeks after grafting, percentage of infection and disease severity were recorded according to the following scale: 0 = no symptoms; 1 = light mottling and vein clearing; 2 = mild mosaic and vein banding; 3 = severe mosaic and chlorotic blotch, and chlorotic ring spot and 4 = malformation. Disease severity (DS) values were calculated using the following formula according to Yang et al. (1996):
The youngest developed leaves from both healthy and infected fig potted rooted cuttings will be collected after two months from grafting for analysis of changes.
2.5.1. Protein and photosynthesis pigment contents
Total soluble protein contents were identified through Bradford (1976) using standard bovine serum albumin. Total photosynthesis pigments were determined using a spectrophotometer method after extraction with 80% acetone as solvent as described by Chappelle et al. (1992).
2.5.2. Total phenolics content
The use of modified folin–ciocalteu method (William et al., 1965) as shown below. To determine total phenols in different leaf samples. Optical densities of these samples were measured using a Beak man DK-2 spectrophotometer at a wavelength of 650 nm. Concentration of total phenols in the extracts was calculated as mg/gm (D.Wt.) of the extracted tissues using a pyrogallol standard curve.
2.6. Statistical analysis
All experiments were repeated twice with similar results in two time difference to confirm all data from these studies. Data were analyzed using a one-way analysis of variance (ANOVA) and the least significant difference (LSD) test was calculated using CoSTAT software program (CoHort Computer Software, Berkeley, CA, USA). All data presented are the mean values using the LSD test at 5% probability.
3. Results
3.1. FLMaV-1 sources, isolation and propagation
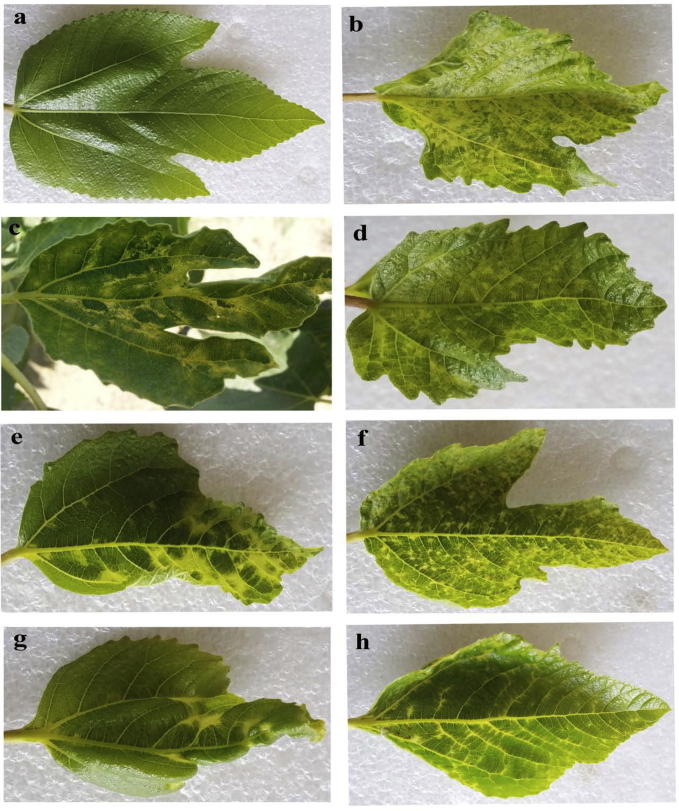
Naturally infected fig trees exhibiting typical symptoms of FLMaV-1 showing a wide array of discoloration symptoms including mosaic, chlorotic mottling, green vein banding, feathering of the veins and chlorotic blotching followed by leaf deformation and blistering in trees grown were collected from Jeddah fields, Saudi Arabia (Fig. 1), were collected and the infected mother fig tree with FLMaV-1 only became the source of infected FLMaV-1 buds for healthy potted rooted cuttings by grafting with freshly infected buds. The developed symptoms were observed on grafted seedlings under glass house conditions.
Figure 1.
Symptoms of FLMaV-1 observed in naturally infected fig trees from Western Saudi Arabia and showing a wide array of discolorations of leaf symptoms, i.e. (a) healthy, (b) mosaic, (c) green vein banding, (d) chlorotic mottling, (e) chlorotic blotching (f) chlorotic ring spots, (g) leaf deformation and blistering and (h) feathering of veins.
3.2. Molecular characterization
Results shown in (Fig. 2) demonstrated that the amplified sequence from total RNA was readily detected in infected samples. A major PCR product of about 352 bp was present in samples bearing FLMaV-1. No specific product occurred for healthy material.
Figure 2.
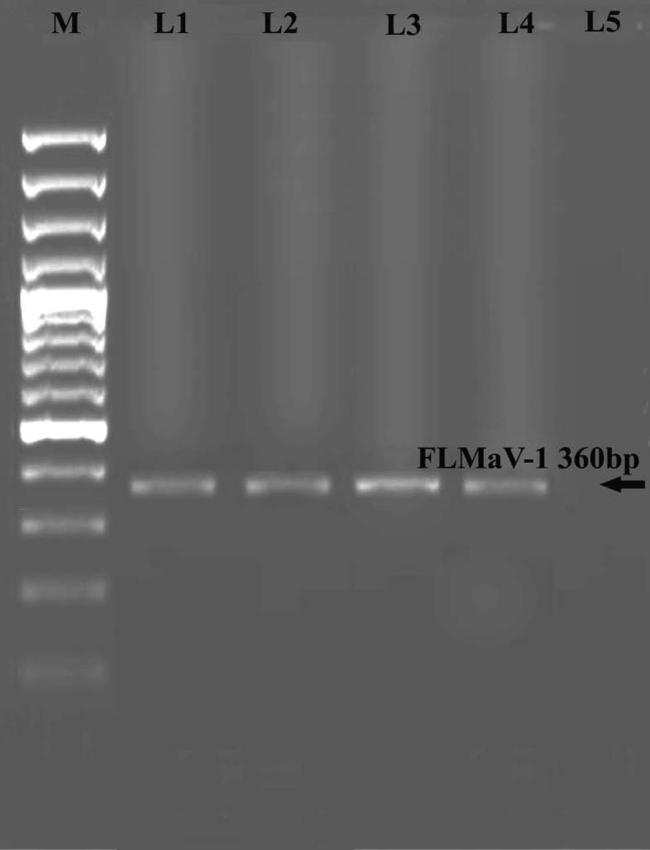
Electropherogram of agarose gel showing PCR amplifications from infected figs. FLMaV-1, (C) FMMaV. Lane M: DNA ladder marker, lanes 1, 2, 3 and 4 represent PCR-positive infected fig plants. Lane 5 represents healthy figs.
3.3. Electron microscope
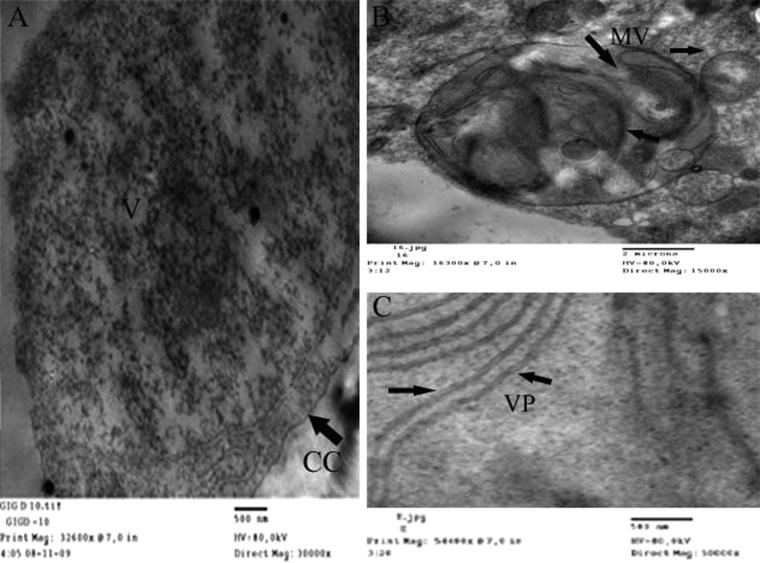
Filamentous viral particles with distinct cross banding and a length of up to 1500 nm, were readily seen in leaf dips from a source of symptomatic leaf, but not in dips from symptomless leaves used as controls. Cytopathological effects similar to those induced by closteroviruses, were observed in phloem parenchyma and companion cells. Similar flexuous particles but no DMBs were seen in phloem elements of the Saudi Arabia Fig leaf samples are present in Fig. 3. RT-PCR assays for the identification of the filamentous particles present in the phloem of Saudi Arabia samples were done using the virus-specific primers designed to detect the HSP70 nucleotide sequence of FLMaV-1.
Figure 3.
Cytopathology of leaf tissues of figs infected with FLMaV-1. (A) Phloem companion cell (CC) with a massive aggregation of virus particles (V). Bar = 500 nm. (B) Clusters of membranous vesicles (MV) in the cytoplasm of a phloem companion cell. Bar = 2 micron. (C) Closterovirus-like particles (VP) in a dip from a symptomatic leaf of F. carica. Bar = 500 nm.
3.4. In vivo screening of antiviral activities of four medicinal plants on FLMaV-1
The effect of four medicine plants on FLMaV-1 infection was designed and evaluated in this current work.
Typical symptoms of FLMaV-1 were observed on grafted potted rooted cuttings compared with non-grafted ones. A higher significant effect of the virus was mentioned in control infected sample compared with other treatments. Results of pre-grafting experiments revealed that spread treatment by ginger roots and rhizomes of turmeric extracts reduce the FLMaV-1 symptoms on grafting potted rooted cuttings. In addition, these extracts not only reduce the virus symptoms, but also, decrease the virus concentration, infection percentage and disease severity of grafted potted rooted cuttings, in comparison with healthy and infected controls. Results in Table 1 recorded that the pre-grafting experiment with ginger root extracts showed the highest activity against FLMaV-1 compared with rhizomes of the turmeric extract. The ginger roots and turmeric rhizome extracts showed a decrease of infection percentage and disease severity (11.11% and 02.78%) and (33.33% and 16.67%), respectively, while Thuja leaves and Harmal seed extracts did not have any significant effect remarkably in this treatment.
Table 1.
The efficiency of four medical plant extracts (6 g/L) of RT-PCR reaction, percentage of infection and diseases severity of infected local fig cultivars under two periods from FLMaV-1 grafting in greenhouse conditions.
| Treatments | Pre-grafting experiment |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | RT-PCR reaction |
Percentage of infection |
Disease severity% |
||||||||||||
| R1 |
R2 |
R3 |
Virus infectivity (I) |
||||||||||||
| r1 | r2 | r3 | r1 | r2 | r3 | r1 | r2 | r3 | R1 | R2 | R3 | (%) | DG | DS% | |
| (Control healthy) | − | − | − | − | − | − | − | − | − | 0/3 | 0/3 | 0/3 | 0.00 | 0 | 00.00 |
| (Control infected) | + | + | + | + | + | + | + | + | + | 3/3 | 3/3 | 3/3 | 100 | 4 | 100 |
| FLMaV-1 and Thuja extract | + | + | + | + | + | + | + | + | + | 3/3 | 3/3 | 3/3 | 100 | 4 | 100 |
| FLMaV-1 and ginger extract | − | − | − | − | − | − | + | − | − | 0/3 | 0/3 | 1/3 | 11.11 | 1 | 2.78 |
| FLMaV-1 and Harmal extract | + | + | + | + | + | + | + | + | + | 3/3 | 2/3 | 3/3 | 100 | 4 | 88.89 |
| FLMaV-1 and turmeric extract | − | + | + | − | − | + | − | − | − | 2/3 | 1/3 | 0/3 | 33.33 | 2 | 16.67 |
| Post-grafting experiment | |||||||||||||||
| (Control healthy) | − | − | − | − | − | − | − | − | − | 0/3 | 0/3 | 0/3 | 0.00 | 0 | 00.00 |
| (Control infected) | + | + | + | + | + | + | + | + | + | 3/3 | 3/3 | 3/3 | 100 | 4 | 100 |
| FLMaV-1 and Thuja extract | − | − | − | − | − | − | − | − | + | 0/3 | 0/3 | 1/3 | 11.11 | 1 | 2.78 |
| FLMaV-1 and ginger extract | + | + | + | + | + | + | + | + | + | 3/3 | 3/3 | 3/3 | 100 | 4 | 100 |
| FLMaV-1 and Harmal extract | − | − | − | − | + | − | − | − | + | 0/3 | 1/3 | 1/3 | 22.22 | 2 | 11.11 |
| FLMaV-1 and turmeric extract | + | + | + | + | + | + | + | + | + | 3/3 | 3/3 | 3/3 | 100 | 4 | 100 |
(DG) = Disease grade. (DS%) = Disease severity percentage. (−) = Positive reaction with RT-PCR. (+) = Negative reaction with RT-PCR.
Also, data in Table 1 showed that the Thuja extract following Harmal seed extract were decreased virus concentration, infection percentage and disease severity in post-grafting experiment compared with other extracts in this treatment.
3.5. Physiological responses of fig seedlings infected with FLMaV-1 and treated with four medical plant extracts
3.5.1. Pre-grafting application
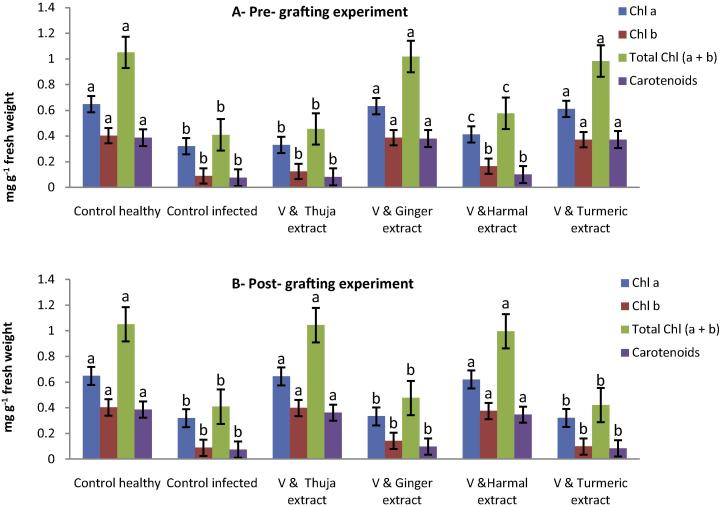
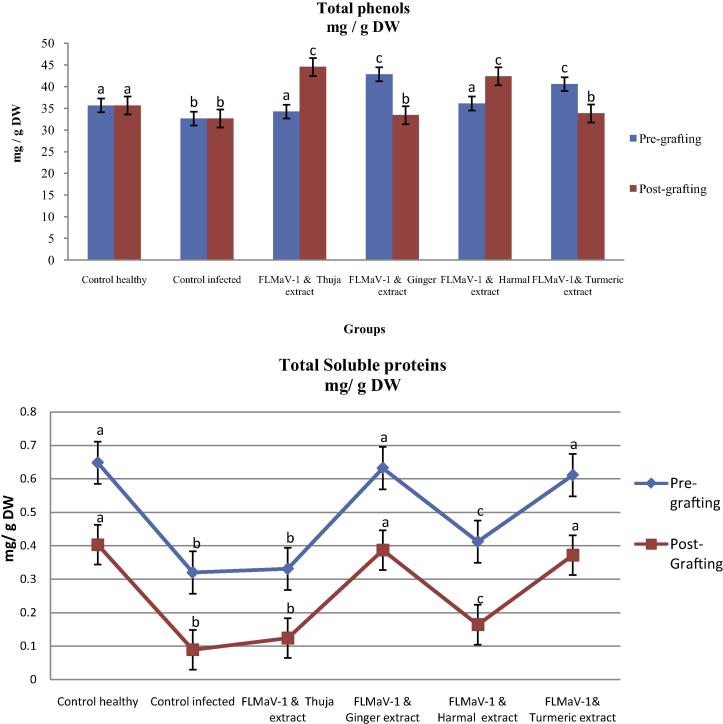
Moreover, results of the physiological parameters of infected and healthy seedlings in pre-grafting experiments in Figs. 4(A) and 5 revealed that the total photosynthesis pigments, total soluble intracellular protein and contents of total phenols were significantly increased in ginger roots and turmeric rhizome extracts which give (0.632, 0.387, 1.019, 0.380, 44.732 and 42.851) and (0.611, 0.372, 0.983, 0.372, 43.884 and 40.561) more than the viral control (0.320, 0.089, 0.409, 0.075, 39.857 and 32.632), respectively.
Figure 4.
The effectiveness of medical plant extracts on the estimation of physiological changes (photosynthesis pigments) of local fig cultivars in the presence of FLMaV-1 under greenhouse conditions.
Figure 5.
The effectiveness of medical plant extracts on the estimation of physiological changes (Total soluble proteins and total phenols) of local fig cultivars in the presence of FLMaV-1 under greenhouse conditions.
3.5.2. Post-grafting application
Data in Figs. 4(B) and 5 showed that the post-grafting treatment of Thuja extract following Harmal seed extract led to an increase in chlorophyll a (0.645 and 0.621), chlorophyll b (0.399 and 0.375), chlorophyll a + b (1.044 and 0.996), carotenoids (0.362 and 0.347), total soluble intracellular protein (45.374 and 43.639) and total phenol contents (44.531 and 41.373), respectively of seedling, respectively, compared to viral control.
4. Discussion
Different patterns of discoloration symptoms including mosaic, chlorotic mottling, green vein banding, feathering of the veins and chlorotic blotching followed by leaf deformation and blistering were observed in leaves of fig grown at Jeddah fields, Saudi Arabia. These results confirm those obtained earlier by several investigators (Kitajima et al., 2003, Elbeaino et al., 2006, Elbeaino et al., 2007, Castellano et al., 2007, Elbeshehy and Elbeaino, 2011, Alhudaib, 2012). This is evidence of infection with FLMaV-1 in the region under study. The cyto-pathological models observed in many studies related that the disease was caused by filamentous virus-like particles that seemed to cluster preferentially next to chloroplasts (Martelli et al., 1993, Caĝlayan et al., 2009). A similar structure was also observed in phloem parenchyma and companion cells in this study. No DMBs were seen in sieve tubes, although several did contain clusters of membranous vesicles and very flexuous, filamentous virus-like particles that often filled the cell lumen. These results agreed with results obtained by Elbeaino et al., 2009a, Elbeaino et al., 2009b, Elbeaino et al., 2009c, Elbeshehy and Elbeaino, 2011, Mahmoud et al., 2014. It was previously reported that FLMaV-1 can be transmitted by vegetative propagation and an A. ficus (Flock and Wallace, 1955).
RT-PCR assays for the identification of the filamentous particles present in the phloem of Saudi Arabia samples were done using the virus-specific primers designed to detect the HSP70 nucleotide sequence of FLMaV-1. A major PCR product of about 352 bp was present in samples-bearing FLMaV-1 (Elbeaino et al., 2006, Elbeaino et al., 2009a, Elbeaino et al., 2009b, Elbeaino et al., 2009c, Elbeshehy and Elbeaino, 2011, Alhudaib, 2012, Mahmoud et al., 2014).
Due to the infectivity of chemicals to control virus disease, research has been focused on this area in searching forproducts that are safe and more effective to supervise the virus disease. Results obtained in this study indicate that plant extracts may be promising in this direction.
In the present study, we demonstrated that the efficiency of two applications of in vivo experiments including pre and post treatments from infected buds grafting using Thuja leaf, ginger roots, Harmal seeds and turmeric rhizomes extracts on potted rooted cutting for reducing the FLMaV-1 infection. These results confirmed by RT-PCR for incidence of FLMaV-1 and the development of disease symptoms, infection percentage and severity of disease were determined.
Also, physiological responses including total photosynthesis pigments, total soluble intracellular protein and total phenol contents of infected and un-infected fig seedlings were studied.
Ginger roots and turmeric rhizome extracts showed a decrease of infection percentage and disease severity when treated before 7 days from grafting, while Thuja leaves and Harmal seed extracts did not have any significant effect remarkably in this treatment. Moreover, results of physiological parameters including photosynthesis pigments, total soluble intracellular protein and contents of total phenols of infected and healthy seedlings in pre-grafting experiments were significantly increased in ginger roots and turmeric rhizome extracts.
Certainly, diverse investigations have been done to increase the antiviral activity of ginger and turmeric, including the synthesis of different chemical derivatives to increase related proteins. The curative action of these products can support this intention. These compounds may act indirectly as a trigger to induce systemic resistance agents in plants against the virus, (Al-ani et al., 2011b, Abdel-Shafi, 2013).
On the other hand, Thuja leaf and Harmal seed extracts were affective on infection percentage and disease severity when treated after 72 h from grafting, while ginger roots and turmeric rhizome extracts did not have any significant affect remarkably in this treatment.
Also, in post-grafting, all physiological parameters were significantly increased in Thuja leaf and Harmal seed extracts. The antiviral activity of the products is connected to their components which may act directly by interaction with virus particles in the early stages of infection and block the liberation of its nucleic acid that lead finally to stop virus multiplication.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (13-130-36-RG). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Amal Y. Aldhebiani, Email: aaldhebiani@kau.edu.sa.
Esam K.F. Elbeshehy, Email: esamelbeshehy@yahoo.com.
Areej A. Baeshen, Email: areejbiology@hotmail.com.
Toufic Elbeaino, Email: elbeaino@iamb.it, touficb@hotmail.com.
References
- Abbasipour H., Mahmoudvand M., Rastegar F., Basij M. Insecticidal activity of Peganum harmala seed extract against the diamondback moth, Plutella xylostella. Bull. Insectology. 2010;63(2):259–263. [Google Scholar]
- Abdel-Shafi S. Preliminary studies on antibacterial and antiviral activities of five medicinal plants. J. Plant Pathol. Microbiol. 2013;4:190. [Google Scholar]
- Al-Ani R.A., Hassan S.A. Effect of henna, thuja, and tamarisk extracts on the multiplication of Tomato yellow leaf curl virus (TYLCV) Jerash J. Res. Stud. 2002;6(2):135–148. (in Arabic) [Google Scholar]
- Al-Ani R.A., Diwan S.N.H., Adhab M.A. Efficiency of Thuja Orientalis and Artimisia campestris extracts to control of Potato leaf roll virus (PLRV) in potato plants. Agric. Biol. J. North Am. 2010;1(4):579–583. [Google Scholar]
- Al-Ani R.A., Mustafa A.A., Kareem A.H. Antiviral activity of Vit-org, 2-nitromethyl phenol and thuja extract against eggplant blister mottled virus (EBMV) Afr. J. Microbiol. Res. 2011;5(21):3555–3558. [Google Scholar]
- Al-ani R.A., Mustafa A.A., Hamad S.A.H., Diwan S.N.H. Tomato yellow leaf curl virus (TYLCV), identification, virus vector relationship, strains characterization and a suggestion for its control with plant extracts in Iraq. Afr. J. Agric. Res. 2011;6(22):5149–5155. [Google Scholar]
- Alhudaib K. Incidence of fig mottle-associated virus and fig mosaic virus in Eastern province of Saudi Arabia. Int. J. Virol. 2012;8:128–132. [Google Scholar]
- Anonymous, 2005. Food and Agriculture Organization of United Nations, Statistical Data (FAOSTAT). Available at: <www.fao.org>. [Last date of consultation: 1.02.2006].
- Bradford M.M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caĝlayan K., Medina V., Gazel M., Serce C.U., Serrano L., Achon A., Soylu S., Calişkano O., Gumuş M. Putative agents of fig mosaic disease in Turkey. Turk. J. Agric. For. 2009;33:469–476. [Google Scholar]
- Castellano M.A., Gattoni G., Minafra A., Conti M., Martelli G.P. Fig mosaic in Mexico and South Africa. J. Plant Pathol. 2007;89:441–443. [Google Scholar]
- Chappelle E.W., Kim M.S., McMurtrey J.E. Ratio analysis of reflectance spectra (RARS): an algorithm for the remote estimation of the concentrations of chlorophyll a, chlorophyll b, and carotenoids in soybean leaves. Remote Sens. Environ. 1992;39:239–247. [Google Scholar]
- Condit I.J., Horne W.T. A mosaic of fig in California. Phytopathology. 1933;23:887–896. [Google Scholar]
- Connell D., Sutherland M. A re-examination of gingerol, shogaol and zingerone, the pungent principles of ginger (Zingiber officinale Roscoe) Aust. J. Chem. 1969;22:1033–1043. [Google Scholar]
- Deya Eldeen M.R., Guoquan Lu., Khalaf A.F., Sabry Y.M. Protective action of salicylic acid against bean yellow mosaic virus infection in vicia faba leaves. J. Plant Physiol. 2010;165:845–857. doi: 10.1016/j.jplph.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Elbeaino T., Digiaro M., De Stradis A., Martelli G.P. Partial characterization of a closterovirus associated with a chlorotic mottling of fig. J. Plant Pathol. 2006;88:187–192. [Google Scholar]
- Elbeaino T., Digiaro M., De Stradis A., Martelli G.P. Identification of a second member of the family Closteroviridae in mosaic-diseased figs. J. Plant Pathol. 2007;89:119–124. [Google Scholar]
- Elbeaino T., Digiaro M., Alabdullah A., De Stradis A., Minafra A., Mielke N., Castellano M.A., Martelli G.P. A multipartite single-stranded negative-sense RNA virus is the putative agent of fig mosaic disease. J. Gen. Virol. 2009;90:1281–1288. doi: 10.1099/vir.0.008649-0. [DOI] [PubMed] [Google Scholar]
- Elbeaino T., Digiaro M., Martelli G.P. Complete nucleotides sequence of four viral RNAs segments of fig mosaic virus. Arch. Virol. 2009;154(11):1719–1727. doi: 10.1007/s00705-009-0509-3. [DOI] [PubMed] [Google Scholar]
- Elbeaino T., Nahdi S., Digiaro M., Alabdullah A., Martelli G.P. Detection of fig leaf mottle-associated virus 1 and fig leaf mottle-associated virus 2 in the Mediterranean region and study on sequence variation of the hsp70 gene. J. Plant Pathol. 2009;91:425–431. [Google Scholar]
- Elbeaino T., Heinoun K., Digiaro M., Martelli G.P. Fig mild mottle-associated virus, a novel closterovirus infecting fig. J. Plant Pathol. 2010;92:165–172. [Google Scholar]
- Elbeshehy E.K.F., Elbeaino T. Viruses infecting figs in Egypt. Phytopathol. Mediterr. 2011;50:327–332. [Google Scholar]
- Flock R.A., Wallace J.M. Transmission of fig mosaic by the eriophyid mite Aceria Ficus. Phytopathology. 1955;45:52–54. [Google Scholar]
- Foissac X., Svanella-Dumas L., Gentit P., Dulucq M.J., Candresse T. Polyvalent detection of fruit tree tricho, capillo and foveavirus by nested RT-PCR using degenerated and inosine containing primers (DOP RT-PCR) Acta Hortic. 2001;550:37–43. [Google Scholar]
- Kitajima E.W., Chagas C.M., Rodrigues J.C.V. Brevipalpus-transmitted plant virus and virus-like diseases: cytopathology and some recent cases. Exp. Appl. Acarol. 2003;30:135–160. doi: 10.1023/b:appa.0000006546.55305.e3. [DOI] [PubMed] [Google Scholar]
- Lamchouri F., Settaf A., Cherrah Y., el Hamidi M., Tigui N., Lyoussi B., Hassar M. Experimental toxicity of Peganum harmala seeds. Ann. Pharm. Fr. 2002;60:123–129. [PubMed] [Google Scholar]
- Mahmoud Sabry Y., Zeidan El Sayed H., Fayez Khalaf A., Rafat Shipat Occurrence of fig mosaic virus in Egypt. J. Agri. Technol. 2014;10(2):439–447. [Google Scholar]
- Malhotra S., Pal Singh Medicinal properties of ginger (Zingiber officinale Rosc.) Indian J. Nat. Prod. Resour. (NPR) 2003;2(6) [Google Scholar]
- Martelli G.P. Fig mosaic and associated viruses. In: Hadidi A., Barba M., Candresse T., Jelkmann W., editors. Virus Diseases of Stone and Pome Fruits. APS Press; St. Paul, MN, USA: 2009. [Google Scholar]
- Martelli G.P., Russo M. Use of thin sectioning for visualization and identification of plant viruses. Methods Virol. 1984;8:143–224. [Google Scholar]
- Martelli G.P., Castellano M.A., Lafortezza R. An ultrastructural study of fig mosaic. Phytopathol. Mediterr. 1993;32:33–43. [Google Scholar]
- Moghadamtousi Z.S., Hajrezaei M., Abdul Kadir H., Zandi K. Loranthus micranthus linn: biological activities and phytochemistry. Evid. Based Complement Altern. Med. 2013;9:273712. doi: 10.1155/2013/273712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddasi M.S., Kashani H.H. Ginger (Zingiber officinale): a review. J. Med. Plants Res. 2012;6(26):4255–4258. [Google Scholar]
- Oliveira A.P., Baptista P., Andrade P.B., Martins F., Pereira J.A., Silva B.M., Valentão P. Characterization of Ficus carica L. cultivars by DNA and secondary metabolite analysis: is genetic diversity reflected in the chemical composition? Food Res. 2012 [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T.A. 2nd ed. Cold Spring Harbor Laboratory Press; New York, USA: 1989. Molecular Cloning: A Laboratory Manual. ISBN-13: 9780879695774, p. 397. [Google Scholar]
- Srivastava P., Kumar P., Singh D.K., Singh V.K. Biological properties of Thuja Orientalis linn. Adv. Life Sci. 2012;2(2):17–20. [Google Scholar]
- Vallejo F., Marín J.G., Tomás-Barberán F.A. Phenolic compound content of fresh and dried figs (Ficus carica L.) Food Chem. 2012;130:485–492. [Google Scholar]
- Vemmos S., Petri E., Stournaras V. Seasonal changes in photosynthetic activity and carbohydrate content in leaves and fruit three fig cultivars (Ficus carica L.) Sci. Hortic. 2013;160:198–207. [Google Scholar]
- William H., Chichilo P.A.C., Reynolds H. tenth ed. ASS. Off Agric. Chem.; Washington, D.C.P.: 1965. Official Methods of Analysis of the Association of Agricultural Chemists. 158. [Google Scholar]
- Yang X., Liangyi K., Tien P. Resistance of tomato infected with Cucumber mosaic virus satellite RNA to potato spindle tuber viroid. Ann. Appl. Biol. 1996;129:543–551. [Google Scholar]
- Zargari A. fourth ed. University of Tehran Publishing; Tehran, Iran: 1998. Medicinal Plants. [Google Scholar]