Abstract
The aim of this work was to prepare coenzyme Q10 (CoQ10) long-circulating liposomes, and establish the quality standard to determine the content and entrapment efficiency. CoQ10 long-circulating liposomes were prepared by the film dispersion method, HPLC assay for the determination of CoQ10 was developed. Free drugs and liposomes were separated using the protamine aggregation method and entrapment efficiency was determined. The liposomes were homogeneous and the mean diameter was 166.0 nm, Zeta potential was −22.2 mV. The content and entrapment efficiency of CoQ10 were 98.2% and 93.2% for three batches of liposomes, respectively. The lyophilized form of liposomes prepared by freeze-drying showed stable quality characteristics during storage. The formulation and preparative method can be used to prepare CoQ10 long-circulating liposomes with high entrapment efficiency and high quality, the determination method of drug content and entrapment efficiency were effective and rapid and can be used for quality evaluation of liposomes.
Keywords: Coenzyme Q10, Long-circulating liposomes, Protamine aggregation method, Quality evaluation, Freeze-drying
1. Introduction
Coenzyme Q10 (CoQ10) is one of fat-soluble ubiquinone compounds, acts as a hydrogen carrier in the cell respiratory chain, it can activate cell metabolism and decrease the oxidative damage from peroxidation and free-radical-induced reaction to the cell membrane in vivo (Salomon and Buchholz, 2000, Portakal and Inal-Erden, 1999). Thus it has been used to reduce physical fatigue, anti-oxidation and improve immunity (Mohammadi-Bardbori et al., 2015, Barbiroli et al., 1999). It was reported that CoQ10 was more effective than vitamin B and E in inhibiting lipid peroxidation in the skin and improving the antisenility effect. Nowadays, CoQ10 is widely used as functional food, drug and health supplements, clinically, it is also used as an adjuvant agent in the treatment of cardiovascular disease, scorbutus, diabetes, gastric ulcers, necrotizing periodontitis, viral hepatitis and cancer (Yang et al., 2015, Villalba et al., 2010).
However, while used as a therapeutic agent with the dosage form such as tablets, capsules, injections, CoQ10 is unstable and difficult to store for a long term due to its large molecular weight, poor water solubility, extreme hydrophobicity, instability to light and thermolability, resulting in the low bioavailability in vivo after oral administration (Yamada et al., 2015, Shao et al., 2015, Butt et al., 2015) Therefore, how to improve the stability and bioavailability with new pharmaceutical technologies has become the hot topic. New formulations that have been developed included the solid dispersion system, cyclodextrin inclusion compound, nanoparticles and microcapsules (Zhou et al., 2014, Onoue et al., 2012, Beg et al., 2010). Preparation of nano-liposomes with long-circulating materials, would help to improve the stability, prolong circulation times and increase the bioavailability of CoQ10. In the present study, new CoQ10 long-circulating liposomes were prepared and developed as freeze-dried preparations, and evaluated in detail by particle size and Zeta potential measurement, content and EE determination, to provide an elementary experiment reference for the in vitro and in vivo release properties.
2. Materials and methods
2.1. Chemicals and reagents
CoQ10 was purchased from Aladdin Industrial Corporation, soybean phospholipid (SP) was purchased from Shanghai Taiwei Pharmaceutical Co., Ltd, DSPE-PEG (2000), DPPG and cholesterol were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Protamine Sulfate injection was provided by Shanghai No.1 Biochemical Pharmaceutical Co., Ltd, vitamin E (VE) was purchased from Guangzhou rekon Food Chemicals Co., Ltd. Methanol and n-hexane were chromatographically pure and purchased from Tianjin Shield Specialty Chemical Ltd. Co. and Tianjin Kemiou Chemical Reagent Co., Ltd., respectively. Trichloromethane was purchased from Luoyang Haohua Chemical Reagent Co. Ltd. All other reagents were of analytical grade.
2.2. Preparation of liposomes
Dry-film dispersion method (DF) was used to prepare liposomes (Yang et al., 2012). Briefly, CoQ10, SP 98, DPPG, DSPE-PEG(2000) and cholesterol were dissolved in 10 ml chloroform and the solution was evaporated for about 15 min at 25 °C. After the dried film was formed, N2 gas was used to remove the residuary solvent. The dried film was hydrated with deionized water. After sufficient hydration, the film was suspended by vortexing. The liposomes were then sonicated. Finally, the liposome suspension was further disrupted by using an ultrasonic probe. Resulting liposomes were sterilized by extruding through a 0.22 μm sterile filter. Liposomes were prepared the day before the experiment, stored overnight at 4 °C.
2.3. Particle size and Zeta potential measurement
For the determination process, about 200 μl of each sample was taken and dispersed in deionized water to a final volume of 3 ml, their particle size and Zeta potential were analyzed using Laser particle analyzer (Malvern Zetasizer 3000HS, Malvern, UK) (Lu et al., 2012). Volume-weighted Gaussian size distribution was fit to autocorrelation functions and particle size values were obtained.
2.4. Assay method for CoQ10
2.4.1. Preparation of stock and working solutions
The stock solution of CoQ10 was prepared in methanol at the concentration of 300 μg mL−1 and stored in brown volumetric flasks at 4 °C before use. Standard solution of CoQ10 was prepared by diluting stock solutions of the analyte with methanol. Working solution was prepared by taking 1 mL of liposomes to volumetric flasks and demulsificated in methanol.
2.4.2. High performance liquid chromatography (HPLC) condition
The chromatography separation was performed with a Diamonsil™C18 Column (200 mm × 4.6 mm, 5 μm), the flow rate was 1.5 mL·min−1, with a column temperature of 30 °C, the mobile phase was methanol-n-hexane (4:1), the drug was detected at 275 nm for determining the content of CoQ10.
2.4.3. Specificity
The reference solution of CoQ10, demulsification solution of blank liposomes, reference solution mixed with blank liposomes and demulsification solution of long-circulating liposomes were taken and injected for HPLC analysis, respectively.
2.4.4. Linearity
The CoQ10 testing solutions of different concentrations were prepared. The calibration curve samples were assayed in triplicate, using concentration (C) as abscissa (X) and peak area as ordinates (Y).
2.4.5. Accuracy
Precision was assessed by determining the replicate QC samples on the same day (intra-day precision) and three consecutive days (inter-day precision).
2.4.6. Recovery
Proper amounts of blank liposomes were mixed precisely with different volumes of stock solution, after filtering through a 0.45-μm sterile membrane, filtrate was collected for determination. Recoveries were calculated by comparing the mean concentration obtained from the tested solutions with that of the neat standard samples.
2.4.7. Determination of CoQ10
The CoQ10 standard solution and working solution were respectively taken for HPLC analysis, to quantify CoQ10 by integrating the peak area using the external standard method.
2.5. Encapsulation efficiencies (EE) determination
2.5.1. Effect of protamine dosage on separation of liposomes
Protamine aggregation method was developed to separate free drugs and liposomes. Proper amounts of protamine sulfate injection (10 mg/ml) were taken and diluted by 2, 4, 8, 16, 32, 64, 128 and 256 fold, which signed as 0 (no diluted), 1, 2, 3, 4, 5, 6, 7, and 8 dilutions, respectively. 100 μl liposomes and 100 μl dilution 0 were taken and mixed, placed for about 3 min, centrifugated with the relative centrifugal force of 350g for 15 min after diluting with 5 ml purified water precisely. The supernatant was selected to measure absorbance by using an ultraviolet spectrophotometer at 540 nm, signed as A1. Another 100 μl of liposomes was mixed with purified water directly, centrifugated and the supernatant was collected to determine turbidity, signed as A0, the clarity was calculated according to the formula: (A0 − A1)/A0 × 100%. By the same method, the clarities of supernatant from 1 to 8 dilutions by separate liposomes were determined.
2.5.2. Effect of protamine dosage on EE of CoQ10
100 μl liposomes and 100 μl protamine sulfate were taken and mixed; the free drugs and liposomes were separated as described above. The supernatant was collected for HPLC analysis. The chromatography separation was performed with a Diamonsil™C18 Column (200 mm × 4.6 mm, 5 μm), the flow rate was 1.5 ml min−1, with column temperature of 30 °C, the mobile phase was methanol-n-hexane (4:1), the drug was detected at 275 nm for determining the content of CoQ10. By the same method, EE of CoQ10 from the series of dilutions by separating liposomes were determined.
2.5.3. Determination of recovery of CoQ10
Different concentrations of CoQ10 solutions (80%, 100% and 120%) were prepared with methanol. Proper amounts of blank liposomes were mixed with CoQ10 solutions, 100 μl of mixture was taken for separation with protamine sulfate and centrifugation. The supernatant was collected for HPLC analysis to determine the recovery.
2.5.4. EE determination
A quantity of 100 μl liposomes were taken precisely and mixed with 100 μl protamine sulfate, after centrifugation the supernatant was collected for HPLC analysis to determine the amounts of free drug (W1). Meanwhile, another 100 μl of liposomes was taken and dissolved by 4 ml methanol; samples were taken to determine the total amount of lomustine (W0). EE was calculated according to the formula: EE = (1 − W1/W0) × 100%.
2.6. Freeze-drying for coenzyme Q10 long-circulating liposomes
After vortexing and sonicating, liposomes were placed at −20 °C for about 3 h for freezing. At 40 min before the freezing was finished, the refrigerator was opened to reduce the temperature. To press the vacuum gauge switch until the pro-freezing was over, samples were taken on a dry baking sheet. Freeze-drying was started as the degree of vacuum was decreased below 1000 Pa by opening the vacuum pump. After 24 h, the lyophilized samples were unpacked. Before the experiment, deionized water was used to disperse the freeze-dried preparation to re-form the liposome suspension by shaking (Kuu et al., 2005, Safi et al., 2015a).
Non freeze-dried and freeze-dried preparations were stored at 4 °C and −20 °C, respectively, the content and EE of CoQ10 were determined in 0 day, 1 month and 3 months after storage.
3. Results
3.1. Particle size and Zeta potential measurement
Results of particle size and Zeta potential determinations were displayed in
3.2. Development of HPLC assay method of CoQ10
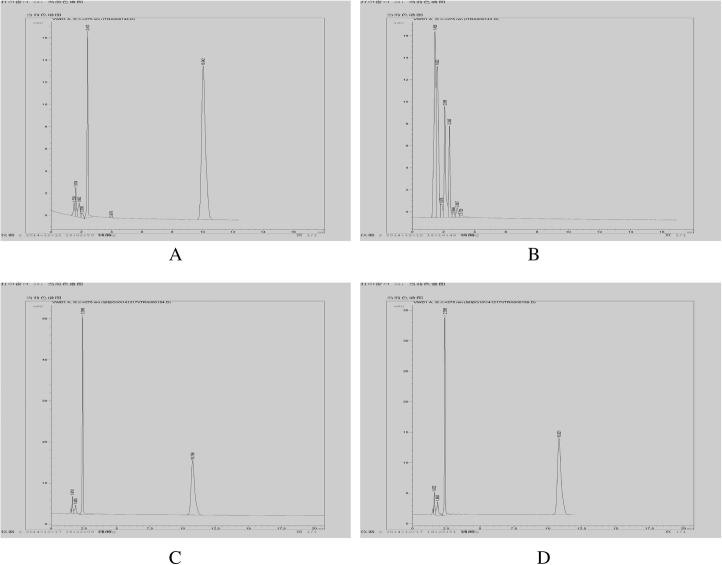
HPLC chromatograms of CoQ10 are shown in Fig 1, it was indicated that the retention time (RT) of CoQ10 was about 11 min, lipid and other pharmaceutical necessities were eluted within 4 min, without interfering with the determination.
Figure 1.
Chromatograms of CoQ10 (A), blank liposome (B), reference solution + blank liposomes (C), and long-circulating liposomes (D).
Further, the calibration curve of CoQ10 was calculated as: A = 9.385 × C + 30.303 (r = 0.9992), indicating the good linear relationship from 5.0 to 150.0 μg·ml−1. The recovery was 99.5%, 99.7% and 96.3%, with RSD of 0.64%, 0.90% and 0.54% (n = 3). The RSD of intra-day and inter-day precisions were 1.20% and 7.30% (n = 3), respectively.
3.3. Development of protamine aggregation method
3.3.1. Effect of protamine dosage on separation of liposomes
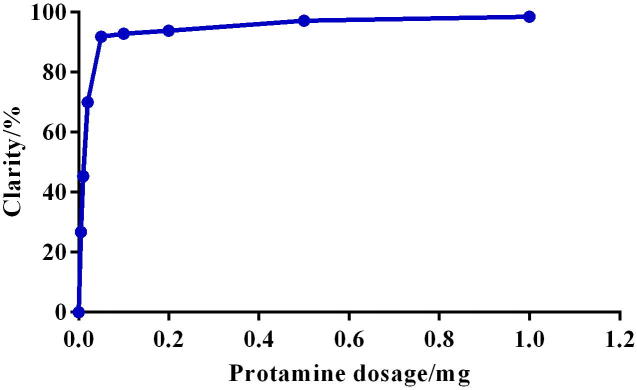
The clarities determination result is shown in Fig 2, we can conclude that free drugs and liposomes are well separated (Clarities were higher than 95%) by using very low amounts of protamine sulfate (0.5 mg).
Figure 2.
The effect of protamine dosage on separation.
3.3.2. Effect of protamine dosage on EE
Results indicated that while protamine dosage was between 0.01 and 1.0 mg, EE of CoQ10 determined were 82–90%, thus there were little effects of protamine dosage on the EE of CoQ10 (Table 1).
Table 1.
Particle size and Zeta potential measurement results of liposomes.
| Batch | Particle size (nm) | Polydispersity index (PDI) | Zeta potential (mV) |
|---|---|---|---|
| 1 | 164.4 | 0.291 | −21.0 |
| 2 | 171.9 | 0.192 | −21.8 |
| 3 | 161.7 | 0.202 | −23.7 |
3.3.3. Determination of recovery
Through HPLC analysis and calculation, recovery of CoQ10 was 100.4%, 104.5% and 99.6%, with RSD of 4.31%, 4.14% and 4.59% (n = 3).
3.3.4. Characterization of liposomes
The determination results of liposomes are shown in Table 2.
Table 2.
Sample determination results of liposomes (n = 5).
| Batch | Content (%) | RSD (%) | EE (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 96.7 | 2.36 | 94.0 | 4.63 |
| 2 | 102.3 | 1.87 | 94.2 | 6.74 |
| 3 | 95.6 | 4.15 | 91.4 | 2.54 |
3.4. Stability investigation for freeze-dried preparations
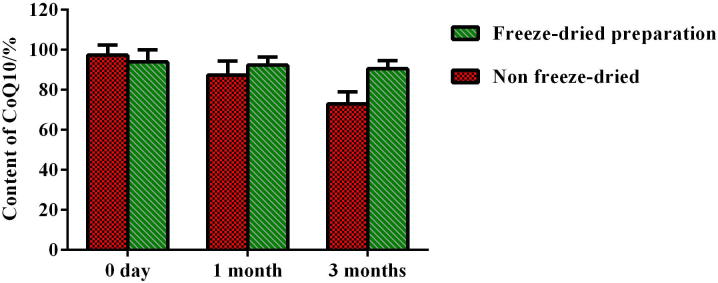
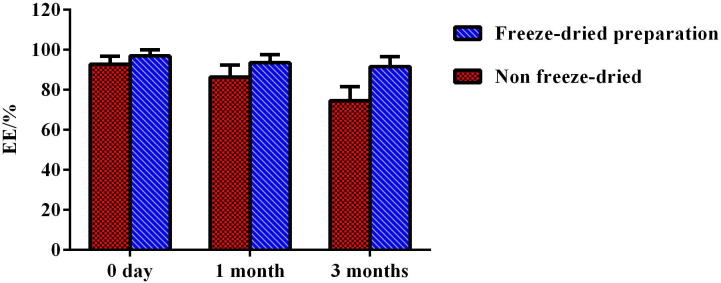
The content and EE determination results of CoQ10 in liposomal preparations during storage are shown in Figure 3, Figure 4. Result indicated that while storing at 4 °C, both content and EE of CoQ10 in liposomes dropped markedly (the content and EE were decreased by 24.3% and 18.1%, respectively). However, freeze-dried preparations remained substantially unchanged for these two parameters at −20 °C within 3 months, exhibiting good stability and a rational and effective freeze-drying process.
Figure 3.
Content determination results of liposomal non freeze-dried and freeze-dried preparations during storage at 4 °C (n = 3).
Figure 4.
EE determination results of liposomal non freeze-dried and freeze-dried preparations during storage at −20 °C (n = 3).
4. Discussion
EE is one of the most important evaluation parameters for liposomes (Ma et al., 2007, Ewert et al., 2006, Liu et al., 2016) Protamine sulfate is one kind of polycation macromolecule and is composed of basic amino acids. After mixing with liposomes protamine sulfate can adsorb to their surface through electrostatic interaction, thus the density of liposomes would be increased and may be separated effectively with free drugs by a lower centrifugal force. For advantages of quickness, simple operation and high efficiency, furthermore, the separation is based on electrostatic attraction and independent of the drugs enveloped in liposomes, thus this method is applicable for EE determination of most of the drugs.
In this study, several EE measurement methods including high speed centrifugation, equilibrium dialysis and Sephadex filtration, had been applied to separate liposomes (Wasungu and Hoekstra, 2006, Safi et al., 2015b) However, due to the poor water solubility of CoQ10, the methods mentioned above cannot be used to separate free drugs and liposomes effectively. Through large experimental validation and comparison with other methods, protamine aggregation method was selected to separate liposomes, which could determine EE accurately.
As for the demulsifiers, we tried to use methanol, alcohol, chloroform, methanol–chloroform (1:1, v/v), and alcohol–chloroform (1:1, v/v). The results suggested that while using an equal volume of the above organic solvents, liposomes can be well demulsificated by all these solvents except chloroform, and methanol was better than alcohol. Thus methanol was finally selected as a demulsifier so as to simplify the operation process.
Instability was the key disadvantage for liposomal formulations, lipid-based vectors would be prone to aggregate and lead to form clustered complexes with larger dimension and higher turbidity (Yang et al., 2013) Further, EE was decreased resulting from the continuing leakage of encapsulated chemicals from lipid layers. As one of the health care products and medicines, how to improve the stability during storage for preparations of CoQ10 was especially important. Through freeze-drying, liposomal structures would become loose and porous, their solid state tends to recover activity rapidly by rehydration. The physical and chemical properties and physiological activities of drugs can remain the same during this process. In the present study, the lyophilized form of liposomes prepared by freeze-drying showed stable quality characteristics during storage and was conducive to long-term storage.
Footnotes
Peer review under responsibility of King Saud University.
References
- Barbiroli B., Iotti S., Lodi R. Improved brain and muscle mitochondrial respiration with CoQ. An in vivo study by 31P-MR spectroscopy in patients with mitochondrial cytopathies. BioFactors. 1999;9:253–260. doi: 10.1002/biof.5520090221. (Oxford, England) [DOI] [PubMed] [Google Scholar]
- Beg S., Javed S., Kohli K. Bioavailability enhancement of coenzyme Q10: an extensive review of patents. Recent Pat. Drug Delivery Formulation. 2010;4:245–255. doi: 10.2174/187221110793237565. [DOI] [PubMed] [Google Scholar]
- Butt M.A., Ahmad M., Fatima A., Sultana S., Zafar M., Yaseen G., Ashraf M.A., Shinwari Z.K., Kayani S. Ethnomedicinal uses of plants for the treatment of snake and scorpion bite in Northern Pakistan. J. Ethnopharmacol. 2015;2015:1–14. doi: 10.1016/j.jep.2015.03.045. [DOI] [PubMed] [Google Scholar]
- Ewert K.K., Evans H.M., Bouxsein N.F., Safinya C.R. Dendritic cationic lipids with highly charged headgroups for efficient gene delivery. Bioconjugate Chem. 2006;17:877–888. doi: 10.1021/bc050310c. [DOI] [PubMed] [Google Scholar]
- Kuu W.Y., Hardwick L.M., Akers M.J. Correlation of laboratory and production freeze drying cycles. Int. J. Pharm. 2005;302:56–67. doi: 10.1016/j.ijpharm.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Liu X., Tian J., Bai Q., Ashraf M.A., Sarfraz M., Zhao B. The effect and action mechanism of resveratrol on the vascular endothelial cell by high glucose treatment. Saudi J. Biol. Sci. 2016;23:S16–S21. doi: 10.1016/j.sjbs.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Ding N., Yang C., Huang L., Liu J., Xiang G. Preparation and in vitro evaluation of a folate-linked liposomal curcumin formulation. J. Liposome Res. 2012;22:110–119. doi: 10.3109/08982104.2011.627514. [DOI] [PubMed] [Google Scholar]
- Ma B., Zhang S., Jiang H., Zhao B., Lv H. Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J. Controlled Release. 2007;123:184–194. doi: 10.1016/j.jconrel.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Mohammadi-Bardbori A., Najibi A., Amirzadegan N., Gharibi R., Dashti A., Omidi M., Saeedi A., Ghafarian-Bahreman A., Niknahad H. Coenzyme Q10 remarkably improves the bio-energetic function of rat liver mitochondria treated with statins. Eur. J. Pharmacol. 2015;762:270–274. doi: 10.1016/j.ejphar.2015.05.041. [DOI] [PubMed] [Google Scholar]
- Onoue S., Uchida A., Kuriyama K., Nakamura T., Seto Y., Kato M., Hatanaka J., Tanaka T., Miyoshi H., Yamada S. Novel solid self-emulsifying drug delivery system of coenzyme Q(1)(0) with improved photochemical and pharmacokinetic behaviors. Eur. J. Pharma. Sci. 2012;46:492–499. doi: 10.1016/j.ejps.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Portakal O., Inal-Erden M. Effects of pentoxifylline and coenzyme Q10 in hepatic ischemia/reperfusion injury. Clin. Biochem. 1999;32:461–466. doi: 10.1016/s0009-9120(99)00041-7. [DOI] [PubMed] [Google Scholar]
- Safi S.S., Qvist R., Chinna K., Ashraf M.A., Paramasivam D., Ismail I.K. Gene expression profiling of the peripheral blood mononuclear cells of offspring of one type 2 diabetic parent. Int. J. Diabetes Dev. Countries. 2015;2015:1–8. [Google Scholar]
- Safi S.S., Batumalaie K., Mansor M., Chinna K., Mohan S., Karimian H., Qvist R., Ashraf M.A., Siok Yan G.O. Glutamine treatment attenuates hyperglycemia-induced mitochondrial stress and apoptosis in umbilical vein endothelial cells. Clinics. 2015;70:8. doi: 10.6061/clinics/2015(08)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M., Buchholz F. Effects of temperature on the respiration rates and the kinetics of citrate synthase in two species of Idotea (Isopoda, Crustacea), comparative biochemistry and physiology. Part B, Biochem. Mol. Biol. 2000;125:71–81. doi: 10.1016/s0305-0491(99)00158-3. [DOI] [PubMed] [Google Scholar]
- Shao L., Yang L., Han H.K. TPGS-chitosome as an effective oral delivery system for improving the bioavailability of Coenzyme Q10. Eur. J. Pharm. Biopharm. 2015;89:339–346. doi: 10.1016/j.ejpb.2014.12.026. [DOI] [PubMed] [Google Scholar]
- Villalba J.M., Parrado C., Santos-Gonzalez M., Alcain F.J. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin. Invest. Drugs. 2010;19:535–554. doi: 10.1517/13543781003727495. [DOI] [PubMed] [Google Scholar]
- Wasungu L., Hoekstra D. Cationic lipids, lipoplexes and intracellular delivery of genes. J. Controlled Release. 2006;116:255–264. doi: 10.1016/j.jconrel.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Nakamura K., Abe J., Hyodo M., Haga S., Ozaki M., Harashima H. Mitochondrial delivery of coenzyme Qvia systemic administration using a MITO-Porter prevents ischemia/reperfusion injury in the mouse liver. J. Controlled Release. 2015;213:86–95. doi: 10.1016/j.jconrel.2015.06.037. [DOI] [PubMed] [Google Scholar]
- Yang S., Chen J., Zhao D., Han D., Chen X. Comparative study on preparative methods of DC-Chol/DOPE liposomes and formulation optimization by determining encapsulation efficiency. Int. J. Pharm. 2012;434:155–160. doi: 10.1016/j.ijpharm.2012.05.041. [DOI] [PubMed] [Google Scholar]
- Yang Y.K., Wang L.O., Chen L., Yao X.P., Yang K.Q., Gao L.G., Zhou X.L. Coenzyme Q10 treatment of cardiovascular disorders of ageing including heart failure, hypertension and endothelial dysfunction. Clin. Chim. Acta; Int. J. Clin. Chem. 2015;450:83–89. doi: 10.1016/j.cca.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Yang S.Y., Zheng Y., Chen J.Y., Zhang Q.Y., Zhao D., Han D.E., Chen X.J. Comprehensive study of cationic liposomes composed of DC-Chol and cholesterol with different mole ratios for gene transfection, colloids and surfaces. B, Biointerfaces. 2013;101:6–13. doi: 10.1016/j.colsurfb.2012.05.032. [DOI] [PubMed] [Google Scholar]
- Zhou H., Liu G., Zhang J., Sun N., Duan M., Yan Z., Xia Q. Novel lipid-free nanoformulation for improving oral bioavailability of coenzyme Q10. Biomed Res. Int. 2014;2014:793879. doi: 10.1155/2014/793879. [DOI] [PMC free article] [PubMed] [Google Scholar]