Abstract
Diabetes mellitus is a major leading cause of end-stage renal failure, characterized by kidney inflammation and glomerular dysfunction, in worldwide. Kidney inflammation is associated to modifications in the expression levels of pro-inflammatory molecules, such as nuclear factor-κB (NFκB) and adhesion molecules, such as E-cadherin, leading to glomerular dysfunction. However, the relationships between these two processes in human diabetic nephropathy remain an open question. Since Psammomys obesus is an ideal animal model to study diabetes mellitus temporal evolution, we have used this model to study the correlation between kidney structural changes and modification on the expression levels of NFκB and E-cadherin over time. We have demonstrated that, after induction of diabetes metillus with a high energy diet (HED), P. obesus develops the characteristic symptoms of human disease. In detail, at the third month nuclear factor NFκB is expressed in the kidney of diabetic P. obesus and structural renal changes, such as mesangial expansion or interstitial fibrosis, are detectable; at 6 months, thickening of glomerular basement membrane, glomerular sclerosis, and tubular atrophy occurs; at 9 months, symptoms of the final stages of the disease, such as down expression of E-cadherin, happens. As a result of these observations we proposed that NFκB activation and E-cadherin down-expression are interlinked on diabetic kidney disease (DKD).
Abbreviations: DN, diabetic nephropathy; ECM, extra cellular matrix; HED, high energy diet; ND, natural diet; NFκB, nuclear factor kappa B
Keywords: Diabetic nephropathy, Psammomys obesus, Nuclear factor kB (NFκB), E-cadherin, Inflammation, High energy diet
1. Introduction
Diabetes is an important problem of public health (Centers for Disease Control and Prevention, 2007) characterized by levels of plasma glucose concentration consequence of the defect of insulin secretion or insulin action or both (The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, 2003). The latest stage of this disease usually evolves into diabetic nephropathy (DN) leading to end-stage renal insufficiency worldwide (Lozano et al., 2012). It is characterized by inflammation and specific renal morphological alterations, such as, mesangial proliferation, capillary basement membrane thickening, interstitial fibrosis, and tubular atrophy (Lane et al., 1993). Hyperglycemia, induces overexpression of the nuclear factor kappa B (NFκB) on several organs Chen et al. (2001) and, among them, kidney (Guijarro and Egido, 2001; Xiao et al., 2001). NFκB, which is implicated in tumoral processes (Disis, 2010; Basseres and Baldwin, 2006), is a proinflammatory factor and plays an important role in the expression of other proinflammatory genes including cytokines, chemokines, and adhesion molecules such as cadherin genes (Kuphal et al., 2004). Indeed, recent studies have suggested that NF-κB activation reduces E-cadherin expression (Kuphal et al., 2004). E-cadherin is a transmembrane glycoprotein localized on the surfaces of epithelial cells in regions of cell–cell contact known as adherent-junctions (Gumbiner, 1996) playing a key role in the maintenance of normal structure and function in normal epithelial tissues. Thus, down expression of E-cadherin has been correlated with tumor cell and metastasis (Vleminckx et al., 1991). However, the precise relationships between NFκB and E-cadherin expression in human diabetic nephropathy and tumors are not known. Therefore, study of kidney structural changes associated with alterations on the expression levels of NFκB and E-cadherin over time can help in the prognosis of the evolution from diabetic nephropathy toward cancer. Our hypothesis is that DN, manifested as modifications on kidney structure, are related with anti-correlated changes on NFκB and E-cadherin expression. To validate it, we have performed histological analysis and NFκB and E-cadherin immuno-labeling on Psammomys obesus, which has been proposed as an ideal animal model to study diabetes mellitus and its complications (Kalman et al., 1993, Kalman et al., 1996, Scherzer et al., 2011). We have confirmed that, after induction of diabetes metillus with a high energy diet (HED), P. obesus develops the same characteristic of human DN. This nephropathy is associated, at early stages with an inflammatory state and an overexpression of NFκB and, at final stages, with a down expression of E-cadherin. Therefore we proposed that NFκB activation and E-cadherin down-expression are interlinked on diabetic nephropathy.
2. Materials and methods
2.1. Animals and experimental protocol
2.1.1. Animals
In the present study, we have used 27 mature P. obesus Cretzschmar (Morsy et al. 1996), of both sexes, aged from 3 to 5 months (90–150 g weigh), trapped in the semi-desert region of Beni-Abbes-Abadla south-west of Algiers (wilaya of Béchar 30° 7′ North latitude and 2° 10′ West longitude). P. obesus were housed and adapted to laboratory conditions (25 °C, 70% hygrometry, and a 12-h light–dark cycle). Upon their arrival in the laboratory, the animals were numbered, weighed, and transferred to separate suitable cages. After a two-week acclimation period, during which they were raised on a natural diet (20 kcal/day) and were fed only halophilic plants rich in water and mineral salts (Salsolafoetida), they were randomly distributed into two groups:
-
–
9 animals, divided into 3 subgroups (n = 3), were raised with natural diet (50 g/day/animal, equivalent to 20 kcal/day).
-
–
18 animals, divided into 3 subgroups (n = 6), fed a standard laboratory high energy diet (32 kcal/day/animal) and salty water (0.9% NaCl) ad libitum.
2.1.2. Biochemical analysis
Both groups were followed up for 3, 6 and 9 months, blood glucose and weight were measured every month. Blood was collected from retro orbital venous plexus in EDTA tubes. Plasma glucose measurements were done with the enzymatic method using test kits from BioSystems. During all experimental procedures animals were maintained in an Animal Research Facility according with institutional guidelines for animal’s care (Directive 2010/63/EU).
2.1.3. Histological study
Kidneys from animals fed with natural diet or with HED were resected, immersed in 10% formaldehyde for 48 h and then were paraffin embedded. Sections of 5 μm, obtained on a microtome, were stained with hematoxylin–eosin, Trichrome’s Masson or Periodic acid–Schiff. They were observed using a light microscope (Motic SFC-18, Hong Kong) for general histologic analysis.
2.1.4. Immunohistochemical staining
2.1.4.1. E-cadherin labeling
Kidneys were labeled with E-cadherin with purified mouse monoclonal antibody (BD Transduction Laboratories purified mouse anti-E-cadherin, n°610182, BD Biosciences). Sections 5 μm thick of the selected paraffin blocks were dewaxed in xylene for 20 min, rehydrated in graded alcohol, permeabilized in PBS/0.25% Tween ® 20 and incubated in 3% hydrogen peroxide/PBS for 10 min to block endogenous peroxidase activity. The antigens were retrieved by boiling for 20 min in citrate buffer (tri-sodium dehydrate, MERCK Millipore) pH 6.0, using a microwave, followed by cooling to room temperature for 20 min. After washing in phosphate buffered saline, different sections were incubated with mouse monoclonal anti-E-cadherin diluted in blocking solution (5% Normal Goat Serum in 3% BSA/PBT).
Sections were washed in phosphate buffered saline before incubation with Anti-Mouse biotinylated secondary antibody (BA9200, anti-mouse biotinylated, Vector Laboratories) for 25 min at room temperature. The sections were then washed in phosphate buffered saline, incubated with avidin/biotin technology system allowing more accessibility for binding to a biotinylated target (Vectastain Elite ABC Peroxydase kits universal, Vector, PK-6100) for 30 min at room temperature, washed and revealed with 3,30-diaminobenzidine peroxydase substrate (kit DAB Vector, SK-Vector Laboratories) solution to yield an insoluble brown deposit.
2.1.4.2. NFκB immunolabeling
Antibody against NF-κB p50 (Abcam, Ab32360) was associated with the secondary donkey anti-rabbit antibody conjugated with an Alexa Fluor 594 from Life Technologies (A21207 Thermo Fisher).
The sections 5 μm thick were dewaxed in xylene for 20 min, rehydrated in graded alcohol and permeabilized by incubation in PBS/0.25% Tween ® 20. Heat mediated antigen retrieval was performed using citrate buffer pH 6.0 and sections were incubated with the primary rabbit anti-NF-κB p50 antibody diluted in blocking solution at 1:200. Slides were washed three times with PBS and incubated for 25 min with secondary donkey anti-rabbit antibody conjugated with Alexa Fluor 594 in a dark wet chamber. After final washing, the slides were cover slipped. To determine localization of NF-κB, the nuclei were counter-stained with Fluoro shield DAPI histology mounting medium (Sigma–Aldrich).
Pictures were taken with a digital camera unit (Canon Powershot A640, Japan) connected to a fluorescent microscope (Zeiss Axioskop 2, Germany).
2.2. Statistical analysis
All results were expressed as mean ± standard deviation. Statistical differences were assessed using (ANOVA) followed by post hoc Tukey honest significant difference test. Difference is considered statistically significant for p values < 0.05.
3. Results
3.1. Body weight and blood glucose evolution
We have observed that the body weight in P. obesus fed with natural diet remains stable during the nine months of the study whereas in the case of rats nourished with high energy diet the body weight significantly increases since the third month (Fig. 1A). This weight gain is preceded by a significant increment in the levels of blood glucose, starting at the second month, (Fig. 1B) in animals fed with HED while in animals following ND this level is constant all along the experience. The plasma glucose concentration was raised to 2 g/l, at the third month of our study, which is higher to the glycemic level required to consider P. obesus as diabetic animals, level that has been established as 1.4 g/l–8.3 mmol/l (Leibowitz et al., 2001).
Figure 1.
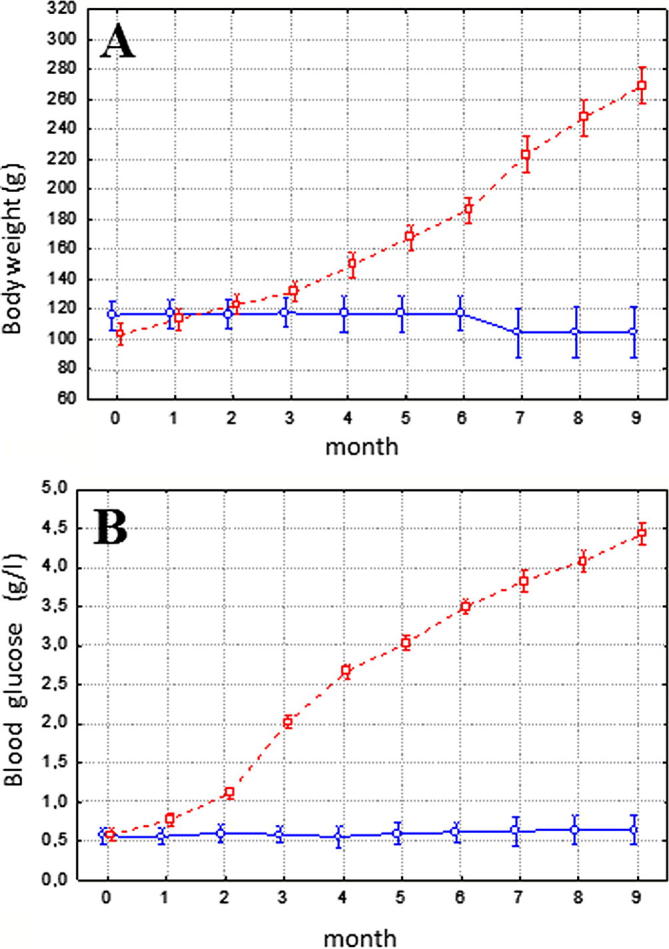
Body weight (A) and blood glucose evolution (B) in P. obesus (n = 27). Although animals (n = 9) that received natural halophilic plants remained stable throughout the entire period (continuous line), 18 animals filled on high energy diets (discontinuous line) showed an important increase in body weight and blood glucose concentration. Data are expressed as mean ± standard deviation.
3.2. Histological results
The histological analysis performed on animals fed with ND and HED depict clear differences on kidney observable since the third month of HED. A mesangial expansion occurs at the third month on HED rats leading to the shrinkage of the capsular space of the Bowman’s capsule (Figs. 2B and 3B). This expansion is associated with an increment on the number of vessels (Fig. 4B) and interstitial fibrosis (Fig. 4B). Mesangial atrophy becomes apparent at the six month (Fig. 2D and C) and it is associated with glomerulus sclerosis (Fig. 2C) and inflammation (Fig. 3C). This mesangial expansion decreases at the nine month, leading to a glomerular atrophy (Fig. 2D), and an increment of the inflammation is observable (Figs. 2D, 3D and F). Simultaneously, hyaline deposits appear (Fig. 3E and F) and the inner layer of arterioles(intima) growths (Fig. 3E). These phenomena are accompanied by an interstitial fenestration (Fig. 3F).
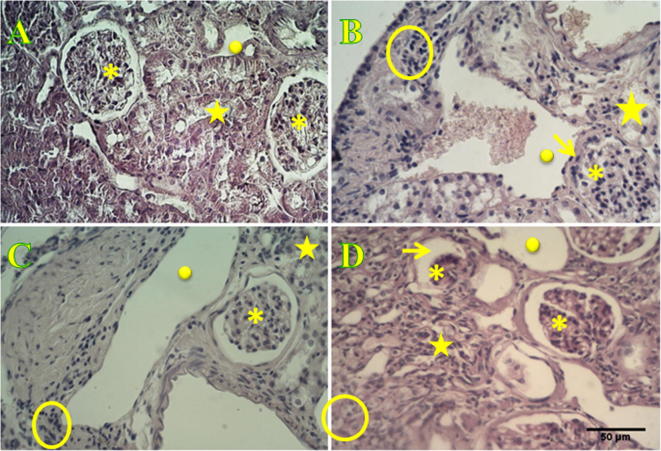
Figure 2.
Histological characterization of P. obesus kidney using hematoxylin and eosin staining. Histological section of non-diabetic P. obesus kidney, fed with ND, evidence normal glomerulus encircled by a Bowman’s space and blood capillary have standard diameters. Absence of cell accumulation at the interstitial space denotes that inflammatory process does not occur. Proximal and distal tubes are well organized. Sections P. obesus kidney observed in 3, 6 and 9 months after animals were fed with an HED (B, C and D respectively shown an increment on the blood capillary diameters and blood flow, and incipient inflammation which is evidenced by cell accumulation at the interstitium appearing between tubules (focal interstitial inflammation) leading to their disorganization and denaturation. Glomerular hypertrophy characterized by the absence of the Bowman’s space is observed at the 3rd month and evolves toward atrophy at the 6th month leading to the total degeneration of the glomerulus at the 9th month. Asterisks correspond to glomerulus, filled-circles to blood capillary, empty-circles to the interstitium between tubules, stars to proximal and distal tubes and arrows points to the Bowman’s space. Scale bar is 50 μm.
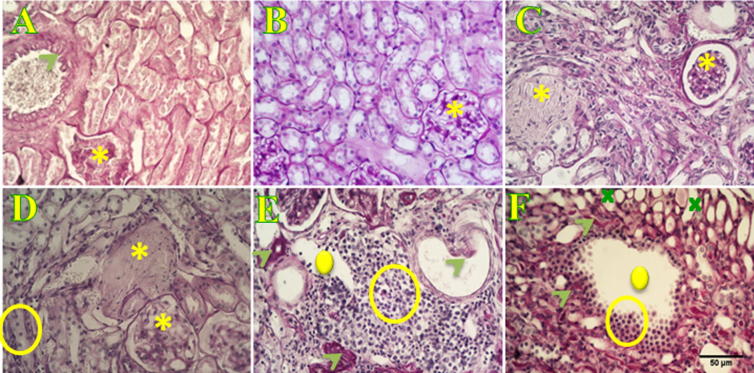
Figure 3.
Histological characterization of P. obesus kidney using PAS staining. Normal kidney (A) of animals following a ND, as well as kidney of animals fed with an HED for three months (B) do not show any symptoms of atherosclerosis, glomerulosclerosis, glomerular endothelial interstitial fenestration and focal interstitial inflammation. On the contrary, animals fed with an HED after 6 (C and D) and 9 months (E and F) depict global glomerular sclerosis (asterisk in C and D). Atherosclerosis, evidenced by the presence of hyaline deposits (arrow-heads in E) leading to arterial intima thickening (arrow in E), and glomerular and interstitial fenestration (cross) occurs at nine months after initiating a high energy diet (F). Scale bar is 50 μm.
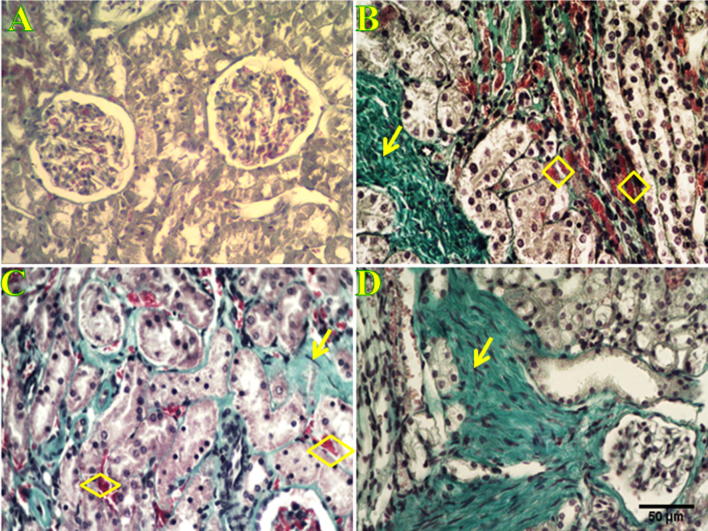
Figure 4.
Histological characterization of P. obesus kidney using Masson’s Trichrom staining. Histological section stained with Masson’s Trichrom in normal kidney (A) of P. obesus fed with a ND does not show any deposit of extracellular matrix evidencing the absence of interstitium fibrosis. On the contrary, sections from kidney of P. obesus fed with an HED for 3, 6 and 9 months (B, C and D respectively) show interstitial fibrosis, characterized by a loss of renal parenchyma and the occurrence of collagen fibers accumulation (arrows), and an increment of the number of vessels (rhombus). Scale bar is 50 μm.
3.3. NFκB and E-cadherin inmuno-labeling
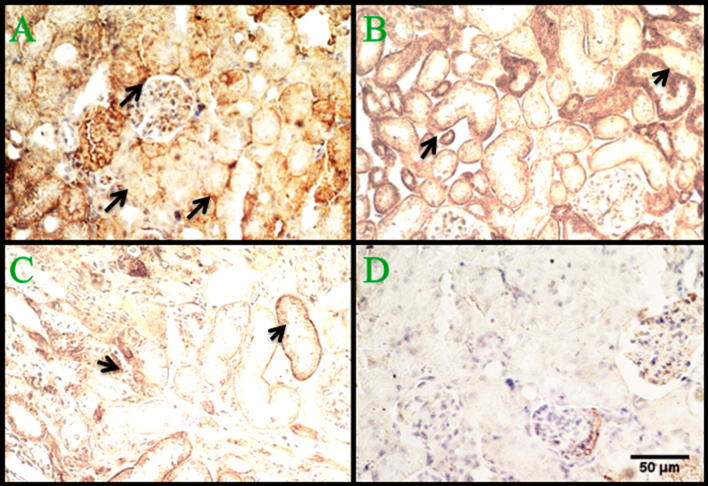
We have observed that in P. obesus fed with ND the nuclear factor kB is not over-expressed (Fig. 5A), whereas its expression increases in animals following an HED, becoming detectable by inmuno-labeling since three months (Fig. 5B–D). On the contrary, E-cadherin is high-expressed in the kidney of P. obesus fed with ND (Fig. 6A) and in animals following an HED until the third month (Fig. 6B) but its expression levels decrease at the sixth month (Fig. 6C) becoming almost undetectable at 9 months (Fig. 6D) after starting the HED.
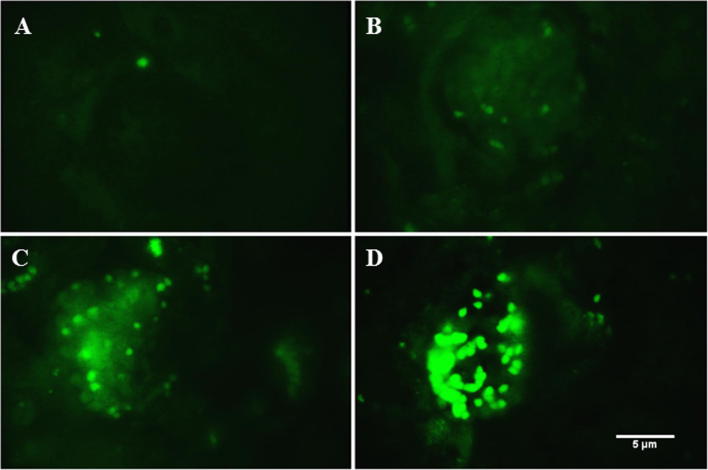
Figure 5.
Immuno-labeling of NFκB within P. obesus kidney. The nuclear factor kB is not expressed on control kidney (A) of animals been fed by ND, whereas it is induced at the first 3 months (B) after starting the HED. NFκB expression levels increase at later stages of diabetic nephropathy as observed at 6 (C) and 9 (D) months. Scale bar is 5 μm.
Figure 6.
Immuno-histological labeled sections of E-cadherin within P. obesus kidney. E-chadherin (depicted by arrows) normal expression can be observed at the level of renal parenchyma structures, in normal kidney of P. obesus fed with ND (A) and in kidney of animals after three months of starting a high energy diet (B), whereas its expression is reduced at 6 (C) and 9 (D) months. Scale bar 50 μm.
4. Discussion
Despite that it is known that NFκB and E-cadherin are implicated on diabetes mellitus prognosis, the relationships between the expressions of these two proteins are not well described in human diabetic nephropathy. We have studied the possible correlation existing between the expressions of both proteins by using the animal model P. obesus which it is known to reproduce all the characteristics of human diabetes (Kalderon et al., 1986). Our work shows that the sand rat P. obesus, maintained on high energy diet, develops hyperglycemia and obesity at three months after HED initiation. This is in agreement with previous works (Kahn, 2000; Donath et al., 1999) showing that, when the gerbil P. obesus is fed a HED, the animals rapidly develop hyperglycemia associated with hyperinsulinemia leading to absolute insulin deficiency. We also show that after the same period of time, P. obesus shows kidney pathologic lesions. This can be explained by an adaptation of new glucose metabolism leading to a remodeling of kidney parenchyma, associated with a decrease on matrix-degrading metalloproteinase activity (Kanwar et al., 2011), and characterized by the accumulation of collagen and fibronectin at the extracellular matrix (ECM) (Wang et al., 2011). It has also been described that ECM is accumulated in the glomeruli, tubule-interstitial regions and in kidney arteriolar, leading to glomerular hypertrophy, interstitial fibrosis and atherosclerosis (Scherzer et al., 2000). We observe, in P. obesus kidney, the occurrence of these phenomena in the third month following HED. Moreover, our histological study shows vacuolar changes associated with glycogen deposition at the nine month from HED. Since glycogenic vacuolization of cells cytoplasm at the renal tubules is related with the initiation of Armanni–Ebstein lesions (Lau et al., 2013) we suggest that, like humans, P. obesus develops this lesion at the end-stage of diabetic nephropathy. In addition, vascular hypertrophy observed in this study, characterized by thickening of renal arterial wall, is similar to what has been observed in humans whom arteriosclerosis leads to hypertension and ischemic nephropathy as a consequence of the increase of angiotensin II blood levels (Remuzzi et al., 1998). We observe that these structural changes of renal arteries are already prominent before glomerular alterations and advanced kidney disorders as previously described (Sato and Yoshinaga, 1987).
Taking together our results, and considering that diabetic nephropathy in humans became observable 10 years after diabetes mellitus initiation (Melmed et al., 2011) P. obesus can reproduce its symptoms in only nine months, this gerbil becomes the ideal model to study kidney pathologies and consequences of diabetes. Among them inflammatory processes are one of the first results of hyperglycemia. Indeed, hyper caloric diet and diabetes are the origin of inflammation and lifestyle diseases such as obesity, insulin resistance, coronary heart disease, and atherosclerosis (Smith et al., 2009).This inflammation process involves the expression of pro-inflammatory molecules, such as nuclear factor-kB (Medzhitov and Janeway, 2000), which together orchestrate the activation of other genes including those of proteins implicated on cell adhesion (Kuphal et al., 2004), such as E-cadherin (Chua et al., 2007) Our results demonstrate that higher expression of NF-κB in glomerulus and interstitium in P. obesus is correlated, at end-stage of DN, with an increment on the macrophage infiltrations. This is in accordance with the relationship between NF-κB expressions, tubule-interstitial injury and interstitial infiltration of macrophages in human diabetic nephropathy (Sanz et al., 2010). Associated to these observations we also demonstrate that expression of the intercellular adhesion protein E-cadherin is reduced at the end-stage of DN. Therefore, our results show that the anti-correlation on the expression levels of NFκB and E-cadherin, already demonstrated biochemically (Kim et al., 2000), leads to injuries in the kidney which are associated with diabetes nephropathies. Since down expression of E-cadherin is associated with tumor progression and metastasis (Jeanes et al., 2008) and over-expression of NF-κB is related with the increment of the expression levers of pro-metastasis proteins (Nozaki et al., 2005) we suggest the use of NF-κB and E-cadherin proteins as markers to predict possible diabetic nephropathy evolution toward cancer.
5. Conclusions
We have demonstrated that HED modifies P. obesus homeostasis leading to a diabetic process evidenced by lesions on different kidney structures which are associated with changes in the expression levels of NFκB and E-cadherin. Since the modification on the expression levels of these two proteins is associated with tumor processes and metastasis when studied independently, we propose that taken together they can be used as prognosis markers of the evolution from diabetic nephropathy toward cancer.
Acknowledgments
The authors would like to express their sincere appreciation to the Histology facility of the Institut Curie and to PICT-IBiSA Institut Curie Orsay for facilitate sample preparation and provide access to equipment. We also want to thank Dr. Florent Poyer for his advice on immune-labelling.
Footnotes
Peer review under responsibility of King Saud University.
References
- Basseres D.S., Baldwin A.S. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . US Department of Health and Human Services, Centers for Disease Control and Prevention; Alanta, GA: 2007. National Diabetes Fact Sheet. General Information and National Estimates on Diabetes in the United States. [Google Scholar]
- Chen H., Carlson E.C., Pellet L., Moritz J.T., Epstein P.N. Overexpression of metallothionein in pancreatic beta-cells reduces streptozotocin-induced DNA damage and diabetes. Diabetes. 2001;50:2040–2046. doi: 10.2337/diabetes.50.9.2040. [DOI] [PubMed] [Google Scholar]
- Chua H.L., Bhat-Nakshatri P., Clare S.E., Morimiya A., Badve S., Nakshatri H. NFk represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007 doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- Directive 2010/63/EU of the European Parliament and of the Council of 22/07/2010 on the Protection of Animals Used For Scientific Purposes. Official Journal of the EU 20.10.2010, L276, 33–79.
- Disis M.L. Immune regulation of cancer. J. Clin. Oncol. 2010;28:4531–4538. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath M.Y., Gross D.J., Cerasi E., Kaiser N. Hyperglycemia-induced-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes. 1999;48:738–744. doi: 10.2337/diabetes.48.4.738. [DOI] [PubMed] [Google Scholar]
- Guijarro C., Egido J. Transcription factorkappa B (NF-kappa B) and renal disease. Kidney Int. 2001;59:415–424. doi: 10.1046/j.1523-1755.2001.059002415.x. [DOI] [PubMed] [Google Scholar]
- Gumbiner B.M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Jeanes A., Gottardi C.J., Yap A.S. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S.E. The importance of the beta-cell in the pathogenesis of type 2 diabetes mellitus. Am. J. Med. 2000;108(6):2S–8S. doi: 10.1016/s0002-9343(00)00336-3. [DOI] [PubMed] [Google Scholar]
- Kalderon B., Gutman A., Levy E., Shafrir E., Adler J.H. Characterization of stages in development of obesity-diabetes syndrome in sand rat (Psammomys obesus) Diabetes. 1986;35(6):717–724. doi: 10.2337/diab.35.6.717. [DOI] [PubMed] [Google Scholar]
- Kalman R., Ziv E., Adler J.H., Lazarovici G., Bar-On H. The efficiency of the sand rat metabolism is responsible for the development of obesity and diabetes. J. Basic Clin. Physiol. Pharmacol. 1993;4:57–68. doi: 10.1515/JBCPP.1993.4.1-2.57. [DOI] [PubMed] [Google Scholar]
- Kalman R., Lazarovici G., Bar-On H., Ziv E. The sand rat (Psammomys obesus): morphologic, physiologic, and biochemical characteristics of a model for type-II diabetes mellitus. Contemp. Topics Lab. Anim. Sci. 1996;35:67–70. [PubMed] [Google Scholar]
- Kanwar Y.S., Sun L., Xie P., Liu F.Y., Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.W., Sovak M.A., Zanieski G., Nonet G., Romieu-Mourez R., Lau A.W. Activationof NF-kappaB/Rel occurs early during neoplastic transformation of mammary cells. Carcinogenesis. 2000;21:871–879. doi: 10.1093/carcin/21.5.871. [DOI] [PubMed] [Google Scholar]
- Kuphal S., Poser I., Jobin C., Hellerbrand C., Bosserhoff A.K. Loss of E- cadherin leads to upregulation of activity in malignant melanoma. Oncogene. 2004;23:8509–8519. doi: 10.1038/sj.onc.1207831. [DOI] [PubMed] [Google Scholar]
- Lane P.H., Steffes M.W., Fioretto P., Mauer S.M. Renal interstitial expansion in insulin-dependent diabetes mellitus. Kidney Int. 1993;43:661–667. doi: 10.1038/ki.1993.95. [DOI] [PubMed] [Google Scholar]
- Lau X., Zhang Y., Kelly D.J. Attenuation of Armanni–Ebstein lesions in a rat model of diabetes by a new anti-fibrotic, anti-inflammatory agent, FT011. Diabetologia. 2013;56:675–679. doi: 10.1007/s00125-012-2805-9. [DOI] [PubMed] [Google Scholar]
- Leibowitz G., Yuli M., Donath M.Y., Nesher R., Melloul D., Cerasi E., Gross D.J., Kaiser N. Beta-cell glucotoxicity in the Psammomys obesus model of type 2 diabetes. Diabetes. 2001;50(1):113–117. doi: 10.2337/diabetes.50.2007.s113. [DOI] [PubMed] [Google Scholar]
- Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;80:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C., Jr. Innate immunity. N. Engl. J. Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Melmed S., Kenneth S., Polonsky P. vol. 12. Sanders Company; 2011. pp. 937–2061. (Williams Textbook of Endocrinology). [Google Scholar]
- Morsy T.A., Sabry A.H., Rifaat M.M., Wahba M.M. Psammomys obesus Cretzschmar, 1828 and zoonotic cutaneous leishmaniasis in Sinai Peninsula. Egypt. J. Egypt. Soc. Parasitol. 1996:375–381. [PubMed] [Google Scholar]
- Nozaki K., Tanaka H., Ikehara Y., Cao X., Nakanishi H., Azuma T., Yamazaki S., Yamaoka Y., Shimizu N., Mafune K., Kaminishi M., Tatematsu M. Helicobacter pylori-dependent NF-kappa B activation in newly established Mongolian gerbil gastric cancer cell lines. Cancer Sci. 2005;96:170–175. doi: 10.1111/j.1349-7006.2005.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remuzzi A., Fassi A., Sangalli F. Prevention of renal injury in diabetic MWF rats by angiotensin II antagonism. ExpNephrol. 1998;6:28–38. doi: 10.1159/000020502. [DOI] [PubMed] [Google Scholar]
- Sanz A.B., Dolores M., Sanchez-Nin A., Ramos M., Moreno J.A., Santamaria B., Ruiz-Ortega M., Egido J., Ortiz A. NFkB in renal inflammation. J. Am. Soc. Nephrol. 2010;21:1254–1262. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- Sato T., Yoshinaga K. Sclerosis of the renal artery and hyalinization of the renal glomeruli in diabetics. Tohoku J. Exp. Med. 1987;153:327–330. doi: 10.1620/tjem.153.327. [DOI] [PubMed] [Google Scholar]
- Scherzer P., Nachlieli I., Ziv E., Bar-On H., Popovtzer M.M. Effect of variations in food intake on renal sodium pump activity and its gene expression in Psammomys kidney. Am. J. Physiol. 2000;279:F1124–F1131. doi: 10.1152/ajprenal.2000.279.6.F1124. [DOI] [PubMed] [Google Scholar]
- Scherzer P., Katalan S., Got G., Pizov G., Londono I., Gal-Moscovici A., Popovtzer M.M., Ziv E., Bendayan M. Psammomys obesus, a particularly important animal model for the study of the human diabetic nephropathy. Anat. Cell Biol. 2011;44:176–185. doi: 10.5115/acb.2011.44.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B.J., Lightfoot S.A., Lerner K.D., Denson D.L., Morgan D.L., Hanas J.S. Induction of cardiovascular pathology in a novel model of low- grade chronic inflammation. Cardiovasc. Pathol. 2009;18:1–10. doi: 10.1016/j.carpath.2007.07.011. [DOI] [PubMed] [Google Scholar]
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee in the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(2) doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- Vleminckx K., Vakaet L., Mareel M., Fiers W., VanRoy F. Geneticmanipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- Wang Q., Usinger W., Nichols B. Cooperative interaction of CTGF and TGF-b in animal models of fibrotic disease. Fibrogenesis Tissue Repair. 2011;4:4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G., Harhaj E.W., Sun S.C. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]