Abstract
Study Design:
A broad narrative review.
Objectives:
The objective of this article is to provide a technical review of spine stereotactic body radiotherapy (SBRT) planning and delivery, indications for treatment, outcomes, complications, and the challenges of response assessment. The surgical approach to spinal metastases is discussed with an overview of emerging minimally invasive techniques.
Methods:
A comprehensive review of the literature was conducted on the techniques, outcomes, and developments in SBRT and surgery for spinal metastases.
Results:
The optimal management of patients with spinal metastases is complex and requires multidisciplinary assessment from an oncologic team that is familiar with the shifting paradigm as a consequence of evolving techniques in surgery and stereotactic radiation, as well as new developments in systemic agents. The Spinal Instability Neoplastic Score and the epidural spinal cord compression (Bilsky) grading system are useful tools that facilitate communication among oncologic team members and can direct management by providing a baseline assessment of risks prior to therapy. The combined multimodality approach with “separation surgery” followed by postoperative spine SBRT achieves thecal sac decompression, improves tumor control, and avoids complications that may be associated with more extensive surgery.
Conclusion:
Spine SBRT is a highly effective treatment that is capable of delivering ablative doses to the target while sparing the critical organs-at-risk, chiefly the critical neural tissues, within a short and manageable schedule. At the same time, surgery occupies an important role in select patients, particularly with the expanding availability and expertise in minimally invasive techniques. With rapid adoption of spine SBRT in centers outside of the academic setting, it is imperative for the practicing oncologist to understand the relevance and application of these evolving concepts.
Keywords: spinal cord compression, spine metastasis, spine radiosurgery, spine stereotactic body radiotherapy, postoperative radiation
Introduction
Up to 70% of patients with malignancies are found to have skeletal involvement at postmortem examination, with the spine being the most common location.1,2 When left untreated, spinal metastases may cause axial pain, vertebral body fracture, radiculopathy, and the most debilitating complication of metastatic epidural spinal cord compression (MESCC). Within the context of advances in systemic therapy, including targeted therapy and immunotherapy, survival in subgroups of patients with metastatic disease is being prolonged far greater than previously expected.3–7 Coincidentally, advances in image-guided external beam radiation have given rise to stereotactic body radiotherapy (SBRT) that allows for high doses of radiation to be delivered in a few fractions. According to the Canadian Association of Radiation Oncology task force, SBRT has been defined as
the precise delivery of highly conformal and image-guided hypofractionated external beam radiotherapy, delivered in a single or few fraction(s), to an extracranial body target with doses at least biologically equivalent to a radical course when given over a protracted conventionally (1.8-3.0 Gy/fraction) fractionated schedule.”8(p630)
SBRT is effective in providing adequate local disease control in combination with surgery or as a sole treatment in carefully selected cases. Therefore, it has become the treatment of choice when complete local ablation of a metastatic lesion is indicated. This paradigm shift in the management of patients with metastatic disease is best observed in the patient with oligometastatic disease. This distinct intermediate entity between localized disease and widespread malignant dissemination typically allows for no more than 5 sites of metastases, and the data suggests adequate disease control at 2 to 5 years in approximately 20% of these patients when all sites have been treated with effective ablative therapy.9,10
Application of SBRT to the treatment of spinal oncological lesions has been a relatively late advance due to the complex relationship between the vertebral segment and adjacent critical structures such as the spinal cord, esophagus, and bowel. In particular, radiation myelopathy has been a major concern as little was known about spinal cord tolerance with high doses per fraction radiation and the stringent technical requirements required to ensure the highest level of precision.11–14 Ultimately, sufficient clinical experience and guidelines are now available to direct appropriate practice.8,15–18 As a result, spine SBRT is currently being practiced as an alternative to conventional palliative radiation in primary treatment, re-irradiation, and in the postoperative setting, albeit without randomized trials to support practice. Furthermore, we now have a greater understanding of the limitations of spine SBRT, as well as the potential complications and the challenges associated with response determination. This article provides a technical review of spine SBRT planning and delivery, indications for treatment, outcomes with a focus on patterns of failure and the importance of epidural disease, complications including vertebral compression fractures (VCF), and the challenges of response assessment. Moreover, the surgical approach to spinal metastases is discussed with an overview of emerging minimally invasive techniques.
Spine SBRT Planning and Delivery
SBRT has been made possible in recent years due to advances in patient immobilization, target visualization, and delivery methods including image guidance. The challenge lies in creating complex dose distributions due to the inherent irregularity of the vertebral segment and the proximity of the organs-at-risk, which have to be underdosed relative to the target to respect radiation tolerance. Typically, a threshold of less than 5% risk of serious adverse effects is chosen. The first step in planning spine SBRT is immobilizing the patient in a near-rigid body frame for the treatment planning computed tomography (CT) scan. Data has shown that using the BodyFIX (Elekta AB, Stockholm, Sweden) device increases the reproducibility of patient positioning during treatment delivery to within 1.2 mm and 0.9° with 95% confidence.19 An example of a patient immobilized in a BodyFIX device on the treatment couch is illustrated in Figure 1. The importance of bulk patient motion was shown in a recent magnetic resonance imaging (MRI)-based analysis of spinal cord motion.20 The study, which acquired data over a 137-second time frame, found the median physiologic motion of the spinal cord itself to be less than 0.5 mm in all directions. This component of motion was relatively minor compared to the gross patient motion without immobilization, which demonstrated maximal statistically significant displacements of 2.21 mm, 2.87 mm, and 3.90 mm in the anteroposterior, lateral, and superior-inferior planes, respectively. The next step in spine SBRT planning involves acquisition of volumetric thin-slice axial T1 and T2 non–contrast-enhanced MRIs, including the target vertebral segment and at least one vertebral body above and below, which are fused to the treatment planning CT for delineation of the clinical target volume in accordance with the International Spine Research Consortium recommendations. In the event of prior surgery where presence of spinal hardware results in significant ferromagnetic artifacts obscuring the spinal cord, a CT myelogram may be performed just prior to the immobilization step.
Figure 1.
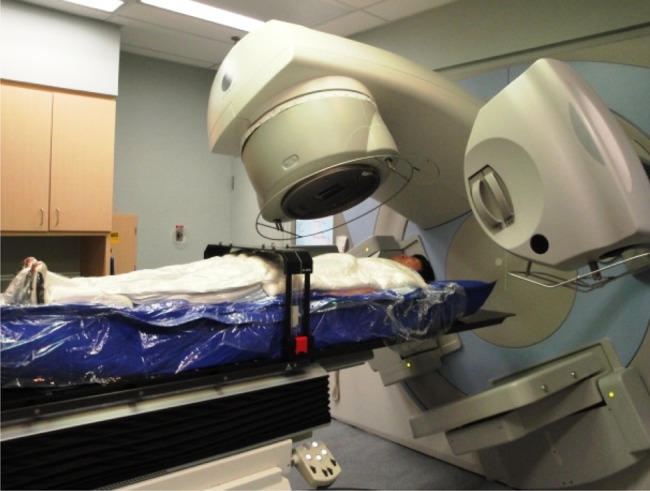
An example of a spine SBRT immobilization device with a patient shown in the BodyFIX system (Elekta AB, Stockholm, Sweden). The device consists of a vacuum cushion, a clear plastic cover sheet, and a dual vacuum pump that maintains a vacuum seal over the top of the patient with the cover sheet molded to the body contours. Precise adjustments of the patient position may be made with the robotic couch, which permits both translational and rotational corrections (6 degrees of freedom).
Appreciation for the approach to target volume delineation is critical in spine SBRT. The tumor is generally defined on CT, and MRI is used to assist in visualizing and delineating relevant anatomy at risk. This approach has been outlined in detail in the consensus guidelines published by International Spine Research Consortium, whereby the target is defined by the clinical target volume, which includes a bony margin around the radiologically visible tumor, and the gross tumor volume, to encompass microscopic subclinical disease. In general, the entire vertebral body is included if any portion of the vertebral body is involved. Inclusion of the pedicle, transverse process, and lamina is recommended if any of these regions are involved. The spinous process should be included within the target volume if the lamina or the spinous process itself is involved. With respect to epidural disease, a 5 mm margin is typically applied within the epidural space respecting the spinal cord and added cranio-caudally. In the postoperative setting, practice may be guided by a recent detailed analysis of patterns of epidural progression following postoperative spine SBRT.21 The authors analyzed 25 spine SBRT cases with preoperative epidural disease with subsequent epidural disease progression after postoperative SBRT. The location of epidural disease in this study was categorized based on dividing the vertebral anatomy into 6 sectors (anterior compartment: vertebral body and anterior epidural space, left and right pedicles and associated epidural space; posterior compartment: left and right transverse processes and laminae and associated epidural space, spinous process, and associated epidural space). It was demonstrated that postoperative MRI epidural disease location alone is insufficient in determining the appropriate target volume for the purposes of SBRT planning, and taking into account the preoperative disease location is critical. An epidural-sparing horseshoe-type clinical target volume may only be appropriate for cases where epidural disease is confined to the anterior aspect on both pre- and postoperative imaging. In other scenarios, a donut-shaped distribution where the entire epidural space is covered circumferentially may be more prudent.
The ideal dose fractionation for spine SBRT in the setting of metastases is at present uncertain. Common practice includes 18 to 24 Gy in 1 fraction, 24 Gy in 2 fractions, 24 to 30 Gy in 3 fractions, 30 Gy in 4 fractions, and 30 to 40 Gy in 5 fractions. Exposing tumors to a dose per fraction of 8 Gy or higher may activate new radiobiological pathways leading to tumor cell death through mechanisms apart from mitotic catastrophe and apoptosis, the dominant forms of cell death in most tumor histologies when irradiated with conventional fractionation (1.8-2 Gy per fraction). Proposed mechanisms of increased cell death in SBRT include radiation-induced tumor-antigen specific immune response, endothelial/vascular injury, or simply increased cell kill secondary to higher delivered dose.22–30 Currently, there are no dose finding randomized trials to confirm the superiority of single-fraction SBRT as compared to multiple-fraction SBRT to make a recommendation. At the University of Toronto, the preferred treatment regimen is 24 Gy in 2 fractions in order to maintain a risk of VCF to approximately 10%. In the scenario of very large tumors or cases that have been heavily irradiated previously, 30 Gy in 4 fractions is typically favored.
Spine SBRT Indications and Approach to Patient Evaluation
The appropriate assessment and management of spinal metastases requires a multidisciplinary approach consisting of radiation oncology, spine surgery, medical oncology, and radiology. Treatment decision-making is a complex process that involve considerations for (1) patient factors including neurological function, presence and severity of pain, age, comorbidities, performance status, estimated life expectancy, and patient preferences; (2) oncologic factors including the tumor histology and molecular characteristics, overall disease burden, and systemic therapeutic options; and (3) treatment-specific factors including the spinal location/level, presence and grade of epidural disease, radiographic appearance, prior surgical or radiation treatment, and degree of spinal instability.
Both the Tomita and Tokuhashi scoring systems were developed for preoperative prognostic evaluation of spinal metastases; however, these systems were devised prior to the availability of spine SBRT and minimally invasive surgical techniques.31,32 Furthermore, as the patient population with spinal metastases is highly heterogeneous, the ability to predict survival with these simple scoring system has been questioned.33,34 More recently, the NOMS (Neurologic, Oncologic, Mechanical Instability, Systemic Disease) decision framework was developed to highlight modern principles specific to spine oncology.35 For the purposes of this review, we have summarized our decision-making framework, which takes into account the relevant literature in Table 1, and this can guide practitioners as to the suitability for spine SBRT.
Table 1.
Approach to Assessment of Suitability for Spine SBRT.
| Factors | Suitable | Cautionary | Unsuitable |
|---|---|---|---|
| Patient | |||
| Performance status | ECOG 0-2 | ECOG ≥3 | |
| Life expectancy | ≥3 months | ||
| Pain | Intractable | ||
| Neurologic | Symptomatic cord compression or cauda equina syndrome | ||
| Oncologic | |||
| Disease burden | Oligometastatic disease | Widespread, rapidly progressive disease | |
| Tumor histology | Histological proof of malignancy | Radiosensitive (eg, myeloma, lymphoma) | |
| Systemic therapy | Systemic therapeutic options available or indolent disease course | ||
| Treatment | |||
| Imaging | ESCC (Bilsky) grade 0-1 | ESCC (Bilsky) grade 2 | ESCC (Bilsky) grade 3 or cauda equina compressions |
| Up to 3 contiguous or noncontiguous levels | >3 contiguous or noncontiguous levels | ||
| Spinal stability | SINS 0-6 | SINS 7-12 | SINS 13-18 |
| Prior radiation | Previous cEBRT to affected level | Previous SBRT to affected level | Previous EBRT to affected level within 90 days or systemic radionuclide within 30 days |
| Positioning | Inability to tolerate near-rigid body immobilization |
Abbreviations: SBRT, stereotactic body radiotherapy; ECOG, Eastern Cooperative Oncology Group; ESCC, epidural spinal cord compression; SINS, Spinal Instability Neoplastic Score; EBRT, external beam radiotherapy; cEBRT, conventional EBRT.
Special Situations
Patients with mechanical instability of the spine should be reviewed with a spinal surgeon for stabilization as SBRT does not address this problem. Identification of these patients may be challenging for many practitioners. Recently, Fisher and colleagues proposed a classification system known as the Spinal Instability Neoplastic Score (SINS) to evaluate the degree of spinal instability in patients with spinal metastatic disease.36–38 This tool has been validated among spinal surgeons, radiation oncologists, and radiologists with respect to reliability. The SINS criteria are summarized in Table 2 and consist of classifying the involved spinal segment according to the location along the spinal axis, the presence of mechanical pain, and radiographic factors including the lesion type (lytic, blastic, mixed), spinal alignment, vertebral body collapse, and involvement of the posterior spinal elements. Each criterion is assigned a score and tallied to a total out of 18. A score of 0 to 6 indicates stability. In contrast, a score higher than 13 demonstrates frank instability and a score between 7 and 12 is in the indeterminate zone where there is potential instability. As SBRT does not address mechanical instability, a surgical consult is recommended for patients with a score ≥7. Moreover, SINS is increasingly being investigated for its predictive capacity for adverse events. Specifically, with respect to post-SBRT VCF, the presence of lytic disease, baseline fracture, and malalignment has been shown to be significant.39 Other recent studies have demonstrated that a higher total score is predictive for increased spinal adverse events following conventional external beam radiation (cEBRT) and for symptomatic VCF after spine SBRT.40,41
Table 2.
The Spinal Instability Neoplastic Score (SINS) criteria36.
| SINS Criterion | Scorea |
|---|---|
| Location | |
| Junctional (occiput-C2, C7-T2, T11-L1, L5-S1) | 3 |
| Mobile spine (C3-6, L2-L4) | 2 |
| Semirigid (T3-T10) | 1 |
| Rigid (S2-S5) | 0 |
| Pain | |
| Mechanical | 3 |
| Occasional and non-mechanical | 1 |
| No pain | 0 |
| Bone lesion type | |
| Lytic | 2 |
| Mixed (lytic and blastic) | 1 |
| Blastic | 0 |
| Radiographic spinal alignment | |
| Subluxation/translation | 4 |
| Kyphosis/scoliosis | 2 |
| Normal alignment | 0 |
| Degree of vertebral body collapse or involvement | |
| >50% collapse | 3 |
| <50% collapse | 2 |
| No collapse but with >50% body involved | 1 |
| None of the above | 0 |
| Posterolateral involvement (fracture or replacement by tumor) of spinal elementsb | |
| Bilateral | 3 |
| Unilateral | 1 |
| None | 0 |
aTotal score: 0 to 6, stability; 7t o 12, indeterminate (possibly impending) instability; 13 to 18, instability.
bFacet, pedicle, or costovertebral joint.
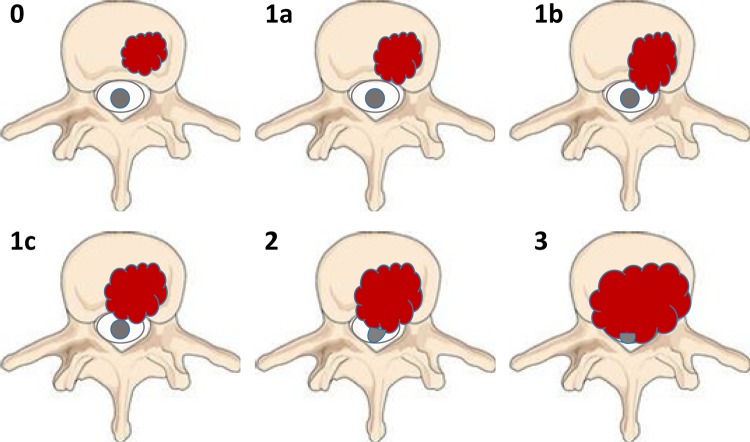
Aside from spinal instability, another group of patients warrant referral to a spine surgeon. Patients with symptomatic high-grade epidural disease should be considered for surgical circumferential decompression. In order to determine the extent of epidural disease, an MRI is required. Based on the imaging characteristics, a useful grading system was proposed by Bilsky and colleagues from the Spine Oncology Study Group. The Bilsky system classifies the degree of epidural spinal cord compression (ESCC) using a 6-point scale.42 The system was modified from an original 4-point scale to further define the varying extent of epidural impingement for the purposes of spine SBRT. In the ESCC scale, grade 0 denotes bone-only disease, grade 1a denotes epidural impingement without deformation of the thecal sac, grade 1b denotes deformation of the thecal sac but without spinal cord abutment, grade 1c denotes deformation of the thecal sac with spinal cord abutment but without cord compression, grade 2 denotes spinal cord compression but with cerebrospinal fluid (CSF) visible around the cord, and grade 3 denotes spinal cord compression without CSF visible around the cord. An illustration of the ESCC scale is shown in Figure 2. Using this classification, high-grade epidural disease (ESCC grade 2 or 3) is optimally managed with surgical decompression prior to SBRT to improve rates of local control while minimizing neurological complications.
Figure 2.
Schematic of the 6-point epidural spinal cord compression (ESCC) grading scale proposed by Bilsky et al.42 The grade represented by each figure is denoted in the upper left hand corner of the figure. Grade of 0 denotes bone-only disease; 1a, epidural impingement, without deformation of the thecal sac; 1b, deformation of the thecal sac, without spinal cord abutment; 1c, deformation of the thecal sac with spinal cord abutment, but without cord compression; 2, spinal cord compression, but with CSF visible around the cord; and 3, spinal cord compression, no CSF visible around the cord.
Spine SBRT Treatment Outcomes
Spine SBRT for De Novo Metastases
Spine SBRT has been used effectively in many reported series in the setting of de novo spinal metastases, that is, in patients who have not received prior surgical intervention or radiotherapy. Given the lack of consensus on an ideal dose fractionation, a variety of regimens have been employed across different series. Local control ranges from 80% to 96% at 1 year, and pain response is generally achieved in the majority of patients although high-quality data is lacking. Table 3 summarizes selected recent representative series in which patients were treated with spine SBRT for de novo metastases. The results suggest superior outcomes with respect to local control when compared to cEBRT, for which reported control rates range from 61% to 86% at 1 year, but can be as low as 46% with mass-type tumors.43–45 This is particularly notable when considering the inclusion of histologies traditionally deemed to be radioresistant such as melanoma, renal cell carcinoma, and sarcoma in SBRT studies. With respect to pain control, multiple systematic reviews have shown the overall pain response rate of conventional radiotherapy to be approximately 60% with a corresponding complete response rate ranging from 0% to 24%.46–48 On the other hand, spine SBRT literature has reported complete response rates as high as 46% to 92%.49–52 Therefore, the data does suggest that while cEBRT may be reasonably efficacious in achieving partial pain relief, its efficacy may be inferior to that of spine SBRT when it comes to complete pain relief. It should be noted, however, that there is currently no completed randomized study comparing treatment outcomes from spine SBRT to those from conventional fractionation. An ongoing phase II/III RTOG study is randomizing patients with up to 3 separate sites of spinal metastases to 8 Gy in 1 fraction of cEBRT versus SBRT to a dose of 16 Gy or 18 Gy in a single fraction.53 The feasibility of successfully delivering image-guided SBRT in this trial setting has been reported.54 The primary objective of the phase III component of the study is to evaluate pain response rates as measured by the 11-point Numerical Rating Pain Scale (NRPS) at 3 months after study entry. More recently, a multi-center NCIC phase II randomized study has been launched which examines 20 Gy in 5 fractions of cEBRT versus SBRT to a dose of 24 Gy in 2 fractions.55 It is anticipated that the results of these studies will provide higher quality outcome data with respect to spine SBRT in de novo metastases.
Table 3.
Selected Spine SBRT Series for Spinal Metastases With No Prior History of Radiation.
| Study Authors (Year) | Study Design | No. of Tumors/No. of Patients | No. of Postoperative Tumors | Histology | Total Dose (Range)/No. of Fractions (Range) | Follow-up in Months (Range) | Local Control | Overall Survival | Pain Response |
|---|---|---|---|---|---|---|---|---|---|
| Gerszten et al52 (2007) | Prospective | 156/naa | 9 | Mixed | Mean: 20 Gy (12.5-25 Gy)/1 | Median: 21 (3-53) | 90% (crude) | na | 86% reported long-term improvement |
| Yamada et al127 (2008) | Retrospective | 103/93 | 0 | Mixed | Median: 24 Gy (18-24 Gy)/1 | Median: 15 (2-45) | 90% (15 months) | Median: 15 months | na |
| Sahgal et al59 (2009) | Retrospective | 23/14 | 0 | Mixed | Median: 24 Gy (7-40 Gy)/3 (1-5) | Median: 9 (1-26) | 85% (1 year)/69% (2 years) | 45% (2 years) | na |
| Nguyen et al49 (2010) | Prospective | na/22a | 0b | Renal cell carcinoma | Median: 27 Gy (24-30 Gy)/3 (1-5) | Median: 13.1 (3.3-54.5) | 82% (1 year)c | 72% (1 year)c | BPI: no pain 23% (baseline) to 52% (12 months) |
| Wang et al76 (2012) | Prospective | 166/149 | 0d | Mixed | 27-30 Gy/3 | Median: 15.9 (1.0-91.6) | 80.5% (1 year)/72.4% (2 years) | 68.5% (1 year)/46.4% (2 years) | BPI: no pain 26% (baseline) to 54% (6 months) |
| Ahmed et al75 (2012) | Retrospective | 63/46a | 0 | Mixed | Median: 24 Gy (10-40 Gy)/3 (1-5) | Mean: 8.2 | 91.2% (1 year) | 59% (1 year) | na |
| Thibault et al128 (2014) | Retrospective | 60/37a | 10 | Renal cell carcinoma | Median: 24 Gy (18-30 Gy)/2 (1-5) | Median: 12.3 (1.2-55.4) | 83.4% (1 year)/66.2% (2 years) | 64.1% (1 year)/45.6% (2 years) | na |
| Guckenberger et al74 (2014) | Retrospective | 387/301 | 0 | Mixed | Median: 24 Gy (10-60 Gy)/3 (1-20) | Median: 11.8 (0-105) | 89.9% (1 year)/83.9% (2 years) | 64.9% (1 year)/43.7% (2 years) | na |
| Sohn et al129 (2014) | Retrospective | 13/13 | 0 | Renal cell carcinoma | Mean: 38.0 Gy/median: 4 | na | 85.7% (1 year) | Median: 15 months | 23.1% complete; 53.8% partial |
| Folkert et al73 (2014) | Retrospective | 108/88a | 33 | Sarcoma | Median: 24 Gy (18-24 Gy)/1 or median: 28.5 Gy (24-36 Gy)/3 (3-6) | Median: 12.3 (1-80.7) | 87.9% (1 year) | 60.6% (1 year) | na |
| Park et al130 (2014) | Retrospective | 45/28a | 1 | Mixed | Median: 27 Gy (18-35 Gy)/3 (1-5) | Median: 7.4 (1.1-42.5) | 93.2% (1 year)/93.2% (2 years) | 47.4% (1 year)/27.9% (2 years) | VAS: median 4 (pre-SBRT) to 1 (1-3 months post-SBRT) |
| Anand et al50 (2015) | Retrospective | 76/52e | 8 | Mixed | Median: 24 Gy (24-27 Gy)/3 (1-3) | Median: 8.5 (3.0-40.0) | 94% (1 year)/82.6% (2 years) | 68% (1 year)/45.4% (2 years) | 92.3% complete; 5.8% partial |
| Sellin et al131 (2015) | Retrospective | 40/37 | 0 | Renal cell carcinoma | Median: 24 Gy (24-30 Gy)/1 (1-5) | Median: 49.0 (38.2-75.8) | 57% | Median: 16.3 months | VAS: 41.4% improved pain |
| Bishop et al132 (2015) | Retrospective | 332/285f | 0 | Mixed | Median (tumor dose): 43 Gy (biologically equivalent dose, alpha/beta = 10) | Median: 19 (0-111) | 88% (1 year)/82% (3 years) | 64% (1 year)/33% (3 years) | na |
| Bate et al71 (2015) | Retrospective | 48/36a | 0 | Mixed | 16-23 Gy/1 or 20-30 Gy/2-5 | Median: 9.8 | 95.8% (1 year) | 44% (crude) | na |
| Azad et al72 (2015) | Retrospective | 25/25 | 0 | Mixed | Median: 20 Gy (15-25.5)/2 (1-5) | Median: 18 (1-81) | 84.2% (crude) | Median: 28 months | na |
Abbreviations: SBRT, stereotactic body radiotherapy; BPI, Brief Pain Inventory; VAS, 10-point Visual Analogue Scale; na, not available.
aWhere available, re-irradiated lesions were removed from the total number of tumor count in each series.
bTwenty-eight of 48 patients from entire cohort (de novo and re-irradiated) underwent prior spine surgery.
cData based on combined de novo and re-irradiation cases.
dForty patients received prior radiotherapy alone, 22 patients received prior surgery alone, and 39 patients received prior radiotherapy and surgery.
eFour patients received prior radiotherapy.
fIncludes both de novo and re-irradiated lesions but breakdown not reported.
Re-irradiation Spine SBRT
Repeat radiation treatment in the scenario of symptomatic bone metastases within the spine is a well-established practice for which the overall pain response rate with cEBRT is approximately 58% based on a meta-analysis of 7 studies.56 A recent randomized study comparing 2 conventional fractionation schedules in the re-irradiation setting showed improved quality of life with less functional interference associated with pain in responders; however, a relatively low 11% to 14% complete pain response rate was observed in those patients who received their assigned treatment.57,58 The challenge with re-irradiation has been in respecting the total biologically effective dose to the spinal cord. As a result, the dose given in the second course of radiation, with conventional techniques, is equal to or less than the initial course of cEBRT to limit spinal cord dose exposure. This practice is nonideal when the intent is to maximize local control. Spine SBRT is uniquely suited to this task with respect to re-irradiation as it allows dose escalation to the tumor while attaining rapid dose falloff to minimize spinal cord doses. Similar to the de novo situation, we expect greater rates of local control and complete pain response than conventional re-irradiation external beam radiotherapy.
Local control rates in re-irradiation spine SBRT reported in the literature range from 66% to 92% at 1 year; selected series are summarized in Table 4. These numbers appear to be comparable to those achieved in de novo cases;59 however, it is acknowledged given differences in patient, tumor, and treatment factors published in various de novo and retreatment cohorts, retrospective comparisons are only hypothesis-generating. A prospective cohort of 59 patients re-irradiated at the MD Anderson Cancer Center reported an actuarial 1-year local control and overall survival of 76% for both outcomes.60 Freedom from neurologic deterioration from any cause was 92% at 1 year. High-quality pain response data unfortunately is limited within the literature in this population of patients; however, published series suggest overall response rates of 65% to 79%.61–64
Table 4.
Selected Re-Irradiation Spine SBRT Series for Spinal Metastases.
| Study Authors (Year) | Study Design | No. of Tumors/No. of Patients | Histology | Total Dose (Range)/No. of Fractions (Range) | Prior RT Dose (Range)/No. of Fractions (Range) | Follow-up in Months (Range) | Local Control | Overall Survival | Pain Response |
|---|---|---|---|---|---|---|---|---|---|
| Sahgal et al59 (2009) | Retrospective | 37/25 | Mixed | Median: 24 Gy (7-40 Gy)/3 (1-5) | Median: 36 Gy/14 | Median: 7 (1-48) | 92% (1 year) | 45% (2 years)a | na |
| Mahadevan et al64 (2010) | Retrospective | 81/60 | Mixed | Median: 24 Gy (24-30 Gy)/3 (3-5) | Median: 30 Gy (8-46 Gy)/10 (1-25) | Median: 12 (4-36) | Median: 9 months | Median: 11 months | 64.7% reported pain response; 18% complete response |
| Choi et al61 (2010) | Retrospective | 51/42 | Mixed | Median: 20 Gy (10-30 Gy)/2 (1-5) | Median: 40 Gy (30-40 Gy)/20 (10-20) | Median: 7 (2-47) | 73% (1 year) | 68% (1 year) | 65% reported pain response |
| Garg et al60 (2011) | Prospective | 63/59 | Mixed | Median: 27 Gy (20-30 Gy)/3 (3-5) | Median: 30 Gy/na | Median: 13 (0.9-67.5) | 76% (1 year) | 76% (1 year) | na |
| Damast et al62 (2011) | Retrospective | 97/95 | Mixed | Median: 30 Gy (16-30 Gy)/5 (4-6) | Median: 30 Gy (8-66 Gy)/na | Median: 12.1 (0.2-63.6) | 66% (1 year) | 52-59% (1 year); median: 13.6 months | 77% reported pain response |
| Thibault et ala,128 (2014) | Retrospective | 11/37 | Renal cell carcinoma | Median: 24 Gy (18-30 Gy)/2 (1-5) | Median: 30 Gy (8-30 Gy)/10 (1-10) | Median: 12.3 (1.2-55.4) | 83.4% (1 year)/66.2% (2 years) | 64.1% (1 year)/45.6% (2 years) | na |
| Thibault et al65 (2015) | Retrospective | 56/40 | Mixed | Median: 30 Gy (20-35 Gy)/4 (2-5) | Median (SBRT): 24 Gy (20-35 Gy)/2 (1-5); median (cEBRT, n = 24): 22.5 Gy (20-30 Gy)/5 (5-40) | Median: 6.8 (0.9-39) | 80.6% (1 year)/71.5% (2 years) | 48% (1 year) | na |
| Kawashiro et al63 (2015) | Retrospective | 23/23 | Mixed | Median: 24.5 Gy (14.7-50 Gy)/5 (3-25) | Median: 30 Gy (30-40 Gy)/10 (10-20) | Median: 10 (1-54) | 88% (1 year)/75% (2 years) | 50% (1 year)/20% (2 years) | 78.9% reported pain relief |
Abbreviations: SBRT, stereotactic body radiotherapy; na, not available.
aData based on combined de novo and re-irradiation cases.
Specific to the setting of salvage SBRT, defined as imaging-confirmed local tumor progression at a previously radiation-treated site for which re-irradiation SBRT is offered, Sahgal et al reported an excellent local control rate of 96% at 1 year.59 The management of failures after an initial course of SBRT and subsequent retreatment outcomes were largely unknown prior to the recent report by Thibault et al. The authors analyzed the outcomes specific to the management of SBRT failures with a salvage second course of spine SBRT,65 in which 56 spinal segments in 40 patients were evaluated from a prospective database. The median salvage second SBRT dose fractionation was 30 Gy in 4 fractions. Overall survival and local control at 1 year were found to be 48% and 81%, respectively.
Postoperative Spine SBRT
The role of decompressive surgery in patients with symptomatic single-level MESCC was established by Patchell et al.66 This landmark article in the spine literature demonstrated the superiority of surgery and postoperative radiation over radiation as a standalone in the treatment of MESCC. Improvements in ambulatory status, neurologic status, and survival were shown in this practice defining randomized controlled trial. Moreover, in a recent multicenter, prospective study, Fehlings et al demonstrated that surgery, when combined adjunctively with radiation, improved quality of life outcomes as well as pain control and neurological status.67
Traditionally, cEBRT has been the standard postoperative therapy as SBRT has only been recently developed. Local failure rates as high as 69.3% at 1 year have been reported and this prompted early adopters of SBRT to apply it to the postoperative indication.68 Three larger series to date have reported on long-term outcomes of postoperative spine SBRT. Al-Omair et al reviewed 80 patients from a prospective database who were treated with surgery followed by postoperative SBRT to a median dose of 24 Gy in 2 fractions (44% treated with 18-26 Gy in 1 to 2 fractions, 56% treated with 18-40 Gy in 3 to 5 fractions) at the University of Toronto (UofT).69 One-year local control and overall survival were 84% and 64%, respectively. Multivariate proportional hazards analysis showed the ability of patients to receive post-SBRT systemic therapy to be an important predictor of overall survival. Higher dose per fraction and the degree of epidural disease involvement as characterized by the Bilksy (ESCC) grade (1 or less) were significant predictors of local control. Furthermore, high-grade (ESCC grade 2 or 3) disease that was downgraded to 0 or 1 postoperatively had superior local control rates compared to those of postoperative grade 2 or higher. Laufer et al reported on 186 patients with MESCC treated at Memorial-Sloan Kettering Cancer Center with surgical decompression, instrumentation, and postoperative spine SBRT using a number of different dose fractionation schemes.70 Durable local control was achieved with a rate similar to that of the UofT series at 83.6% at 1 year. High-dose hypofractionated SBRT, defined as 24 to 30 Gy in 3 fractions, was found to improve local control compared to low-dose hypofractionated SBRT, defined as 18 to 36 Gy in 5 or 6 fractions. This finding echoes the results of the UofT series where local control increases with total radiation dose. Bate et al reviewed 57 patients with 69 lesions treated with spine SBRT for spinal metastases, of which 48 lesions (70%) were treated with radiotherapy alone, and 21 (30%) were treated with surgery prior to radiation.71 The rates of local control were excellent at 94.2% for all patients and 90.5% for those treated with surgery and postoperative SBRT at 1 year. The authors did not identify any statistically significant variables in predicting recurrence on univariate regression analysis. In all 3 series, adverse events were uncommon and the treatment procedures were well tolerated. Table 5 provides a summary of selected published postoperative spine SBRT series. Given the complexity of the patient population and rapid advances in the technology of SBRT delivery, prospective studies with higher quality health-related quality of life (HRQoL) data is currently unavailable. Notwithstanding, these important series have established the safety and efficacy of combining a surgical approach with postoperative SBRT.
Table 5.
Selected Postoperative Spine SBRT Series for Spinal Metastases.
| Study Authors (Year) | Study Design | No. of Tumors/No. of Patients | Histology | Total Dose (Range)/No. of Fractions (Range) | Follow-up in Months (Range) | Local Control | Overall Survival | Pain Response |
|---|---|---|---|---|---|---|---|---|
| Gerszten et al123 (2005) | Prospective | 26/26 | Mixed | Mean: 18 Gy to 80% isodose line (16-20 Gy)/1 | Median: 16 (11-24) | na | na | VAS: 92% long-term improvement |
| Rock et al133 (2006) | Retrospective | 18/18 | Mixed | Mean: 11.4 Gy (6-16)/1 | Median: 7 (4-36) | na | na | na |
| Gerszten et al95 (2009) | Prospective | 11/11 | Mixed | Mean: 19 Gy (16-22.5 Gy)/1 | Median: 11 (7-44) | na | na | VAS: 100% long-term improvement |
| Moulding et al134 (2010) | Retrospective | 21/21 | Mixed | Median: 24 Gy (18-24 Gy)/1 | Median: 10.2 (1.2-54.0) | 90.5% (1 year) | Median: 10.2 months | na |
| Massicotte et al96 (2012) | Retrospective | 10/10 | Mixed | Median: 24 Gy (18-35 Gy)/3 (1-5) | Median: 13 (3-18) | 70% (crude) | na | na |
| Al-Omair et al69 (2013) | Retrospective | 80/80 | Mixed | Median: 24 Gy (18-40 Gy)/2 (1-5) | Median: 8.3 (0.13-39.1) | 84% (1 year) | 64% (1 year) | na |
| Laufer et al92 (2013) | Retrospective | 186/186 | Mixed | 24 Gy/1 (21.5%) or 24-30 Gy/3 (19.9%), or 18-36 Gy/5-6 (58.6%) | Median: 7.6 (1.0-66.4) | 83.6% (1 year) | 29.0% (crude); median among patients who died: 6.1 months | na |
| Bate et al71 (2015) | Retrospective | 21/21 | Mixed | 16-22 Gy/1 or 20-30 Gy/2-5 | Median: 13.7 | 90.5% (1 year) | 44%a (crude) | na |
Abbreviations: SBRT, stereotactic body radiotherapy; na, not available.
aData based on combined SBRT alone and surgery + SBRT cases.
Toxicity of Spine SBRT
In general, acute toxicity is mild and very limited in spine SBRT with 5% or less reported rates of severe and undesirable (grade 3 or higher) adverse events.69–76 Concerns regarding potential hardware failure as a result of postoperative spine SBRT have not been substantiated by the major series.69–71 Laufer et al and Al-Omair et al reported 4 of 186 total treated (2.2%) cases and 1 of 51 instrumented (2.0%) cases of hardware failure, respectively. In fact, a small series has suggested a trend toward higher fusion rates and lower incidence of instrumentation failure with spine SBRT as compared to cEBRT;77 however, larger prospective studies are lacking. It is important to note that the risk of a transient increase in pain during or shortly after SBRT, known as a pain flare, is nontrivial and in the order of 23% to 68%,78,79 which may be ameliorated with the short-term use of dexamethasone.80 A prospective observational study showed a pain flare incidence of 19% when patients were treated prophylactically with oral 4 mg or 8 mg of dexamethasone, implying a reduction in the incidence of pain flare from 68% in a previously reported steroid-naïve cohort.80 Ultimately, a randomized controlled study would be required for definitive recommendations with respect to prophylactic steroid therapy.
Radiation-induced myelopathy, the most feared late complication, can be a serious and debilitating adverse event. Fortunately, in the modern era of spine SBRT, the occurrence of this complication is extremely rare. Sahgal et al analyzed the maximum point dose and to small volumes of the thecal sac and reported clear guidelines for spinal cord tolerance in the setting of both de novo irradiation and reirradiation.11,12 Practitioners of spine SBRT are strongly recommended to consult these published guidelines in the planning and evaluation of radiation treatment.
A more common late effect of spine SBRT is radiation-induced VCF (an illustrative example is shown in Figure 3). Early single institutional reports demonstrated crude risks of this complication from 11% to 39%.81–83 In order to clarify the risk of VCF, a multi-institutional spine SBRT analysis based on 410 spinal segments was reported in 2013, in which the risk of the development of VCF was determined to be 14%.39 VCF was defined as either a new (de novo) fracture or progression of an existing fracture. The median time to fracture was 2.5 months. A dose-complication relationship was observed such that patients treated with 24 Gy in 1 fraction had nearly a 40% risk of VCF. In general, doses ≥20 Gy per fraction must be used with great caution as they are associated with significantly higher risks of VCF. Preexisting risk factors should be taken into account in the overall management decision, as 3 of 6 SINS criteria including baseline VCF, lytic tumor, and spinal deformity have been identified in the multi-institutional study to be significant predictors of VCF. A recent post hoc analysis of 2 prospective cohorts confirms the utility of SINS as a predictive tool in this regard, demonstrating high SINS (7-12) as a statistically significant risk factor for VCFs and symptomatic fractures (hazard ratio 5.6 and hazard ratio 5.3, respectively).40 Nonetheless, it is important to note that the data suggests only 50% of VCFs are symptomatic requiring any intervention, the majority of which will involve cement augmentation alone.
Figure 3.
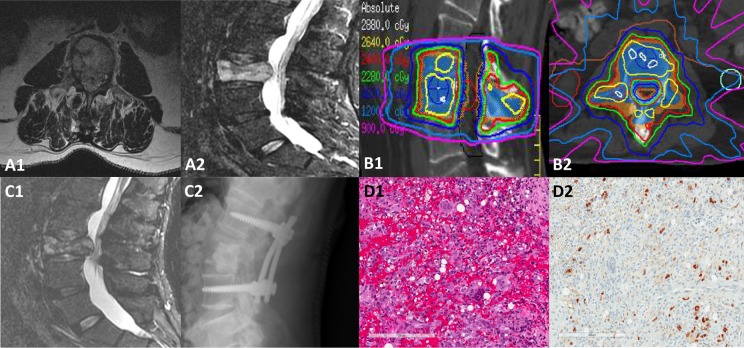
Illustrative case of 61-year-old male patient with metastatic melanoma to T8 and L3 who was undergoing curative external beam radiation therapy for head and neck squamous cell carcinoma when he presented with mechanical back pain and right leg paresthesia along the L3 nerve root distribution. The diagnostic MRI shown in (A1-A2) demonstrated diffuse involvement of L3, a VCF with retropulsion, and extra osseous soft tissue extending into the anterior epidural space. The patient was treated with spine SBRT with minimal interruption to the head and neck cancer treatment with the target volume encompassing the entire vertebral body and bilateral posterior elements to a prescription dose of 24 Gy in 2 fractions, limiting the thecal sac to 18.7 Gy point max. Panel (B1-B2) illustrates the radiation plan in 2 planes, highlighting the tight isodose lines around the spinal canal with a rapid dose falloff gradient to limit exposure to normal tissues. Two and a half weeks after SBRT, the patient demonstrated fracture progression and underwent a posterior spinal decompression of circumferential disease, L3 vertebroplasty, and pedicle screw instrumentation L2 through L4, as shown in panel (C1-C2). Panel (D1-C2) indicates the hematoxylin and eosin stain and the SOX10 IHC, respectively, showing the tissue to be mostly reactive in nature with foamy histiocytes, hemorrhage, and multinucleated giant cells with only a few atypical nuclei in the background representing residual melanoma.
Posttherapy Follow-up
Patterns of Failure
Early published reports suggested that SBRT failures occur via 2 primary mechanisms: (1) marginal in the bone adjacent to the site of prior treatment and (2) in the epidural space adjacent to the spinal cord.84,85 Al-Omair et al confirmed this finding in a detailed postoperative spine SBRT series where of the 21 failures, the most common site of progression was within the epidural space (71%).69 A recent study evaluating the outcomes of salvage spine SBRT following in-field failure of initial SBRT also found 85% of progressors involved the epidural space, lending further support to the risk of failure in this area.65 Furthermore, Al-Omair and colleagues showed that if baseline epidural disease is surgically downgraded then local control is improved.
This observation has now been shown by several investigators and could be a result of relative underdosing in the epidural space in order to respect the spinal cord dose tolerance, geographic miss, or tumor biology. Therefore, improving local control rates and reducing epidural progression rates may be achieved via one or more of the following strategies: (1) maximizing epidural disease resection to achieve optimal surgical downgrading, (2) increasing the allowable dose to the spinal cord considering that preliminary guidelines may be too conservative since our technique and imaging standards have improved dramatically from the early days of spine SBRT, and (3) combining SBRT with systemic therapy. These are all active areas of investigation.
Response Assessment
Assessment of tumor and treatment response after SBRT is challenging given that no consensus guidelines exist as to the optimal method of determining radiologic and pain response. Considering imaging-based outcomes are increasing in importance with local control as a primary endpoint, we are only in the early phase of understanding MRI signal changes in the spine following high-dose SBRT, where evaluation may be further complicated by VCF or pseudoprogression, a phenomenon well-documented in brain glioma literature, which may be defined as subacute radiological appearance of tumor progression that subsequently subsides without further treatment and does not represent true progressive disease.86 CT alone is inadequate in the assessment of spinal metastases when compared to MRI with significantly lower sensitivity (66.2% vs 98.5%). This is possibly due to the lack of malignant bone marrow replacement prior to the occurrence of osteoblastic or osteolytic changes in a proportion of cases.87 The role of the CT, however, requires careful consideration given that MRI alone is unable to characterize blastic versus lytic lesions. Pain assessment may be complex in the spine, as an understanding of mechanical pain is required in addition to biologic tumor pain. Furthermore, patients often have multiple sites of spinal disease, which may be contributing to the symptomatic area. Therefore, determining the causal vertebral segment with respect to pain and documenting response is a major challenge.
A recent publication by the SPIne response assessment in Neuro-Oncology (SPINO) group has put forth suggested preliminary recommendations for imaging follow-up, follow-up frequency, and pain response evaluation based on the survey results from a panel of international experts in spine SBRT.88 The preferred imaging modality for follow-up and tumor response assessment is MRI, T1- and T2-weighted axial non–contrast-enhanced sequences, ideally with a slice thickness of 1 to 2 mm. Gadolinium may be considered in select cases with extra-osseous extension into the paraspinal and/or epidural region. At the present time, T1- or T2-weighted signal changes posttherapy are to be interpreted with caution, and advanced MRI sequences such as diffusion-weighted imaging or DCE-MRI show promise but remain investigational. Validated criteria specifically designed for the assessment of radiographic tumor response in the spine have not yet been developed and the applicability of Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 in this setting is unfortunately limited. Table 6 summarizes the SPINO recommendations in image interpretation of tumor response and follow-up frequency. Spinal MRIs are recommended for follow-up every 2 to 3 months after SBRT for the first 12 to 18 months, and every 3 to 6 months thereafter. The first MRI should be done at 2 to 3 months after SBRT for response and toxicity assessment, especially considering the risk of VCF within this time frame.39
Table 6.
Consensus SPINO Recommendations for Spine SBRT Response Assessment88.
| Imaging-based local tumor response |
|
| Pain response |
|
| Imaging follow-up frequency |
|
Abbreviations: SPINO, SPIne response assessment in Neuro-Oncology group; SBRT, stereotactic body radiotherapy; MRI, magnetic resonance imaging; RECIST, Response Evaluation Criteria in Solid Tumors; BPI, Brief Pain Inventory.
Evaluation of pain response requires a validated pain assessment instrument such as the Brief Pain Inventory, and an appropriately chosen time point as the endpoint of interest, most commonly at 3 months after SBRT administration. The International Bone Metastases Consensus Working Party has outlined formal criteria with respect to endpoints in palliative radiotherapy specific to bone metastases.89,90
Surgical Approach to Spinal Metastases
Surgical indications in a patient with spinal metastases include preservation or restoration of neurologic function, reduction in intractable tumor-related pain, spinal stabilization, and tumor control in select cases.91,92 In general, experts agree that appropriate candidates should have a life expectancy of at least 3 months. Complex patients warrant thorough consideration in a multidisciplinary setting involving the surgeon, radiation oncologist, and medical oncologist.93
Prior to the use of radiotherapy for MESCC, simple laminectomy, which entailed removal of the posterior elements of the spinal column, was the only available treatment, but this failed to demonstrate any benefit over that of conventional radiation alone.94 Moreover, simple laminectomy used without instrumentation carries the risk of creating spinal instability. The subsequent development of direct decompressive surgery, often combined with reconstruction to provide stabilization culminated in the landmark Patchell randomized trial demonstrating the superiority of surgery plus cEBRT over that of cEBRT alone with respect to patients’ ability to walk post treatment (84% vs 57%), the duration of time over which ambulation was retained (median 122 days vs 13 days), and the percentage of patients regaining the ability to walk who were nonambulatory prior to therapy (62% vs 19%).66 The need for opioid analgesics and corticosteroids was also reduced in the surgery plus radiation group compared to that of the radiation alone group. More recently, Fehlings et al reported on both clinical-assessed criteria and patient-reported functional and health-related quality of life outcomes following surgical intervention in these patients in a major prospective trial led by the AOSpine North American Clinical Research Network.67 This multicenter observational cohort study evaluated 142 patients with a single symptomatic MESCC lesion who were treated surgically for intractable pain (38.7%), neurological deficits (40.2%), and/or imminent or overt spinal instability (21.1%). The surgeries involved a median of 5 vertebral levels with spinal construction using spinal devices performed in 134 patients (94.4%). Postoperative radiotherapy was considered for all patients and deemed beneficial and administered in 121 patients (85.2%). The results showed a median survival of 7.7 months, and 30-day and 12-month morality rates of 9% and 62%, respectively. The incidence of wound complications was 10%. Statistically significant improvements were seen in ambulatory status at 6 months, in Oswestry Disability Index, EQ-5D, and pain interference at 6 weeks and at 3, 6, and 12 months. The American Spinal Injury Association impairment scale grade also improved at 3 months after surgery. SF-36 Short Form Health Survey improved postoperatively in 6 of 8 scales. This was the largest study to date to provide high-quality prospective HRQoL outcomes in patients with MESCC, and offers favorable evidence for surgical intervention including spinal reconstruction, as an adjunct to radiation and chemotherapy to achieve prompt and sustained pain relief, and improvements in neurologic, functional, and HRQoL outcomes in this population of patients with at least a 3-month life expectancy and a single focal lesion causing MESCC. As new surgical instrumentation and techniques have emerged, a wider spectrum of interventions have become available to patients.95–98
Surgical Techniques
Traditional open surgery may require extensive soft tissue exposure, and an anterior, posterior, lateral, or combined approach may be undertaken. The choice of approach depends on multiple factors such as tumor location, reconstruction options, and the optimal route of access. Tumors most often arise anteriorly within the vertebral body and subsequently extend posteriorly. Therefore, anterior approach commonly provides the most direct access. On the other hand, the posterior approach is more familiar to most surgeons and avoids the morbidity of an anterior approach. A simultaneous anterior-posterior approach may be utilized in cases where the tumor involves both the anterior and posterior columns as it facilitates direct visualization of adjacent neurovascular structures, the ability to perform a complete resection, and the placement of ventral and dorsal stabilization hardware in one session.99 However, a combined approach is more extensive and rarely performed for metastatic disease. Irrespective of the approach, circumferential decompression of the spinal cord is the key element. As those surgeries can be destabilizing, reconstruction is critical. Multiple studies have shown the benefit of decompressive surgery combined with stabilization in terms of improved pain, neurologic function, and quality of life.66,100–105 A number of reconstruction instrumentation systems have been used in practice. In this population where life expectancy is limited, reconstruction of the anterior column is often performed with polymethylmethacrylate (PMMA). This option is inexpensive and offers immediate stability. Cages and auto/allograft are usually reserved when long-term survival is anticipated. Posteriorly, stabilization usually involves multilevel point of fixation using a screw-rod system.106
Some tumors are hypervascular and as the tumor capsule is violated to accomplish surgical decompression, significant blood loss can be expected. To minimize blood loss, preoperative embolization may be considered for vascular malignancies such as renal cell carcinoma, thyroid carcinoma, and hepatocellular carcinoma. Early case series supported the practice of preoperative embolization and its benefit of reducing the risk of intraoperative hemorrhage in hypervascular tumors, particularly of renal cell origin.107,108 A randomized controlled trial of 45 patients reported in 2015 showed no overall statistically significant difference in intraoperative blood loss and allogeneic red blood cell transfusion between the arms assigned to preoperative embolization and preoperative angiography only (control).109 The validity of their results may be limited by the fact that no corpectomy/anterior decompression was performed, which is the part of the surgery where most blood loss is encountered. Even with this limitation, a subgroup analysis did demonstrate a significant decrease in blood loss in the embolization arm in moderate and pronounced hypervascular tumors as graded based on angiography. Furthermore, surgery time was significantly shorter in the embolization group.
As a cautionary note, surgery for spinal metastases can be associated with significant morbidity, underscoring the importance of proper patient selection. Lau et al reported an overall complication rate of 21.7% in a cohort of 106 patients who underwent surgery for spinal metastasis.110 Predictors for higher rates of complication included age >40 and metastatic lesion involving 3 or more contiguous levels. Jansson et al reported a similar rate of complication of 20% in a series of 282 patients.111 A systematic review of the literature from 1970 to 2007 showed an overall complication rate of 29% and a 30-day mortality rate of 5%. In a prospective study of the general spine population, Street et al observed a mean of 3.1 and 4.7 adverse events (AEs) per patient for the elective and emergency oncologic population, respectively.112 More recently, using the same prospective database but only focusing on the emergent oncologic population, Dea et al reported that 76.2% of the patients experienced at least one AE and intraoperative AE occurred in 32% of the patients.113 Mortality within the same admission was 10.9%. This data emphasized that patient counseling and selection is primordial.
Minimally Invasive Spine Surgery (MISS) and Percutaneous Augmentation Procedures
The guiding principle behind MISS is minimization of injury to the normal anatomy without negatively affecting the ability to address the patient’s specific pathology. MISS, using tubular retraction techniques, were initially applied to spinal stenosis, disc herniation, and pedicle screw fixation;114 however, applications are now being increasingly seen in the setting of spinal malignancies. Molina et al performed a systematic review of MISS in the management of metastatic spine disease, which included 5 studies addressing the use of video-assisted thoracotomy (VAST) or endoscopy-assisted posterior decompression, and 6 studies addressing the use of minimal access spine surgery (MASS).115 VAST and MASS techniques both yielded similar results in achieving pain and neurologic dysfunction relief, but the data suggested that MASS may be associated with lower operative times, shorter hospital stay, and decreased blood loss; however, it was uncertain if these differences were statistically significant. Miscusi et al reviewed 2 series of patients treated at the same institution with one group having undergone standard open surgery and the other MISS for acute myelopathy secondary to spine metastases and showed no significant differences between the 2 groups with regard to neurological outcomes and complications.116 Furthermore, the MISS cohort demonstrated reduced blood loss, shorter operative time and bed rest length, which was associated with more rapid functional recovery and discharge from hospital. One of the earliest series combining MASS and postoperative spine SBRT was described by Massicotte et al, where the surgical technique was based on a tubular retraction system and stabilization achieved using methylmethacrylate applied under direct visualization.96 Decompression of the thecal sac was attained in 8 of 10 cases and intraoperative blood loss was minimal. Local control based on imaging with a median follow-up of 13 months was 70%. Improvements were observed in pain control, disability, and quality of life.
“Separation surgery” refers to a limited approach where only the epidural component of the tumor is decompressed and stabilization is achieved to facilitate postoperative radiation. It allows for high-dose radiation to be delivered with minimal risk to the spinal cord. Fixation is typically percutaneous with screws 2 levels above and 2 levels below the affected level, and decompression performed through a limited approach. Amankulor et al reviewed 318 patients who underwent posterolateral decompressive surgery and posterior segmental instrumentation without anterior column reconstruction to assess the incidence of symptomatic hardware failure.117 The authors found 9 (2.8%) cases of symptomatic hardware failure and identified prior chest wall resection and construct lengths spanning 6 or more contiguous vertebral segments as risk factors for failure. The data suggests that “separation surgery” offers an effective treatment. A novel approach to managing tumors within the epidural space that has recently been reported is laser interstitial thermotherapy (LITT).118–120 The procedure entails CT-image guided placement of laser catheters via a lateromedial transpedicular trajectory at a distance of 5 to 6 mm from the dural edge. Thermal ablation of tumor is performed under real-time monitoring using thermal MRI up to a threshold of 50°C at the interface of the tumor and dura to avoid damage to the neural elements. Subtraction of the nonenhancing tissue from the pre- and postcontrast images allows direct estimation of the area of coagulative necrosis immediately after treatment. In combination with postprocedure spine SBRT, this procedure has been shown to be feasible and in a preliminary study of 11 patients, in whom pain reduction and downgrading of ESCC scale were demonstrated.120 The median hospital stay was 2 days and 1 case of transient limb paresthesia was observed, which resolved after 4 weeks. These early reports suggests that LITT combined with postprocedure spine SBRT may represent a potential alternative to “separation surgery” in patients without neurological deficits prior to SBRT, particularly in cases where conventional surgery may be morbid and lead to prolonged delays in the delivery of subsequent oncological treatments. However, higher quality prospective data is required to establish the safety and efficacy of this approach.
Vertebroplasty and kyphoplasty are conservative techniques wherein cement, most commonly PMMA, is injected into the vertebral body via a percutaneous needle under fluoroscopy guidance. Kyphoplasty differs from vertebroplasty in that an inflatable balloon is first employed to create a cavity into which cement may be administered with the goal of restoring vertebral body height. PMMA provides structural support to maintain anterior column stability, and in patients who have symptomatic VCFs, kyphoplasty has been shown to improve pain and back-specific functional status in a randomized study.98 Fourney et al reported in a series of 56 patients marked or complete pain relief in 84% of those who underwent vertebroplasty or kyphoplasty.121 Asymptomatic cement extravasation was observed in 9.2% of the vertebroplasty cohort and none in the kyphoplasty cohort. In general, cement leakage beyond the vertebral body is uncommon and typically does not require further intervention. Based on available evidence, the Spine Oncology Study Group endorsed a strong recommendation for cement augmentation in patients with painful compression fractures due to metastatic spine disease.122 The efficacy and safety of a combined approach with kyphoplasty followed by spine SBRT has also been reported.123 However, in select cases with epidural disease the possibility of tumor extravasation must be taken into account as it has been recently illustrated by Cruz et al.124 Of note, in the setting of mechanical instability secondary to tumor involvement, especially in the presence of posterior element fractures (pedicles and/or joints), vertebroplasty or kyphoplasty alone may be insufficient to provide vertebral column support. In such cases, percutaneous pedicle screw fixation alone or a combinatorial approach with screw fixation and cement augmentation may be appropriate.125,126 In the series reported by Moussazadeh et al, 44 patients underwent cement-augmented percutaneous spinal fixation with a median SINS of 10 (range 8-15), and complete pain resolution was observed in 29 (66%) patients.126 Complications were limited with one adjacent-level fracture responsive to kyphoplasty and one case of asymptomatic screw pullout.
Conclusion
The optimal management of patients with spinal metastases is complex and requires multidisciplinary assessment from an oncologic team that is familiar with the shifting paradigm as a consequence of evolving techniques in surgery and stereotactic radiation, as well as new developments in systemic agents. SINS and the ESCC (Bilsky) grading systems are useful tools that facilitate communication among oncologic team members and can direct management by providing a baseline assessment of risks prior to therapy. Spine SBRT is a highly effective treatment that is capable of delivering ablative doses to the target while sparing the critical organs-at-risk, chiefly the critical neural tissues, within a short and manageable schedule. At the same time, surgery occupies an important role in select patients, particularly with the expanding availability and expertise in minimally invasive techniques. The combined multimodality approach with “separation surgery” followed by postoperative spine SBRT achieves thecal sac decompression, improves tumor control, and avoids complications that may be associated with more extensive surgery. With rapid adoption of spine SBRT in centers outside of the academic setting, it is imperative for the practicing oncologist to understand the relevance and application of these evolving concepts.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Arjun Sahgal has received honorarium for previous educational seminars from Medtronic Kyphoplasty Division and Elekta AB, research grants from Elekta AB. Dr Hany Soliman has received travel support from Elekta AB for research conferences. Dr Simon Lo has received research support through International Oligometastasis Consortium from Elekta AB, and travel expenses and honorarium for speaking in a users’ meeting from Accuray. There are no other conflicts of interest to disclose.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Cole JS, Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol. 2008;7:459–466. doi:10.1016/S1474-4422(08)70089-9. [DOI] [PubMed] [Google Scholar]
- 2. Galasko CS. Skeletal metastases. Clin Orthop Relat Res. 1986;(210):18–30. [PubMed] [Google Scholar]
- 3. Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2014;385:977–1010. doi:10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE–5-a population-based study. Lancet Oncol. 2014;15:23–34. doi:10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 5. Marshall DC, Webb TE, Hall RA, Salciccioli JD, Ali R, Maruthappu M. Trends in UK regional cancer mortality 1991-2007. Br J Cancer. 2016;114:340–347. doi:10.1038/bjc.2015.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 7. Keegan THM, Ries LAG, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016; 122:1009–1016. doi:10.1002/cncr.29869. [DOI] [PubMed] [Google Scholar]
- 8. Sahgal A, Roberge D, Schellenberg D, et al. The Canadian Association of Radiation Oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin Oncol (R Coll Radiol). 2012;24:629–639. doi:10.1016/j.clon.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 9. Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14:e28–e37. doi:10.1016/S1470-2045(12)70510-7. [DOI] [PubMed] [Google Scholar]
- 10. Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010;40:107–111. doi:10.1093/jjco/hyp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sahgal A, Ma L, Gibbs I, et al. Spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:548–553. doi:10.1016/j.ijrobp.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 12. Sahgal A, Ma L, Weinberg V, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:107–116. doi:10.1016/j.ijrobp.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 13. Sahgal A, Weinberg V, Ma L, et al. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys. 2013;85:341–347. doi:10.1016/j.ijrobp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 14. Medin PM, Boike TP. Spinal cord tolerance in the age of spinal radiosurgery: lessons from preclinical studies. Int J Radiat Oncol Biol Phys. 2011;79:1302–1309. doi:10.1016/j.ijrobp.2010.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phys. 2008;71:652–665. doi:10.1016/j.ijrobp.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 16. Sahgal A, Bilsky M, Chang EL, et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine. 2011;14:151–166. doi:10.3171/2010.9.SPINE091005. [DOI] [PubMed] [Google Scholar]
- 17. Husain ZA, Thibault I, Letourneau D, et al. Stereotactic body radiotherapy: a new paradigm in the management of spinal metastases. CNS Oncol. 2013;2:259–270. doi:10.2217/cns.13.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lo SSM, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16:9–19. doi:10.1089/jpm.2012.0376. [DOI] [PubMed] [Google Scholar]
- 19. Hyde D, Lochray F, Korol R, et al. Spine stereotactic body radiotherapy utilizing cone-beam CT image-guidance with a robotic couch: intrafraction motion analysis accounting for all six degrees of freedom. Int J Radiat Oncol Biol Phys. 2012;82:e555–e562. doi:10.1016/j.ijrobp.2011.06.1980. [DOI] [PubMed] [Google Scholar]
- 20. Tseng CL, Sussman MS, Atenafu EG, et al. Magnetic resonance imaging assessment of spinal cord and cauda equina motion in supine patients with spinal metastases planned for spine stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2015;91:995–1002. doi:10.1016/j.ijrobp.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 21. Chan MW, Thibault I, Atenafu EG, et al. Patterns of epidural progression following postoperative spine stereotactic body radiotherapy: implications for clinical target volume delineation. J Neurosurg Spine. 2016;24:652–659. doi:10.3171/2015.6.SPINE15294. [DOI] [PubMed] [Google Scholar]
- 22. Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88:254–262. doi:10.1016/j.ijrobp.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi:10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi:10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 25. Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18:240–243. doi:10.1016/j.semradonc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 26. Kocher M, Treuer H, Voges J, Hoevels M, Sturm V, Müller RP. Computer simulation of cytotoxic and vascular effects of radiosurgery in solid and necrotic brain metastases. Radiother Oncol. 2000;54:149–156. doi:10.1016/S0167-8140(99)00168-1. [DOI] [PubMed] [Google Scholar]
- 27. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi:10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 28. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi:10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi:10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 30. Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res. 2012;177:311–327. doi:10.1667/RR2773.1. [DOI] [PubMed] [Google Scholar]
- 31. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 2005;30:2186–2191. [DOI] [PubMed] [Google Scholar]
- 32. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976). 2001;26:298–306. [DOI] [PubMed] [Google Scholar]
- 33. Lee CH, Chung CK, Jahng TA, et al. Which one is a valuable surrogate for predicting survival between Tomita and Tokuhashi scores in patients with spinal metastases? A meta-analysis for diagnostic test accuracy and individual participant data analysis. J Neurooncol. 2015;123:267–275. doi:10.1007/s11060-015-1794-1. [DOI] [PubMed] [Google Scholar]
- 34. Park S, Lee C, Chung S, Lee K. How accurately can Tokuhashi score system predict survival in the current practice for spinal metastases? J Spinal Disord Tech. 2015;28: E219–E224. doi:10.1097/BSD.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 35. Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18:744–751. doi:10.1634/theoncologist.2012-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976). 2010;35: E1221–E1229. doi:10.1016/j.yneu.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 37. Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011;29:3072–3077. doi:10.1200/JCO.2010.34.3897. [DOI] [PubMed] [Google Scholar]
- 38. Fisher CG, Schouten R, Versteeg AL, et al. Reliability of the Spinal Instability Neoplastic Score (SINS) among radiation oncologists: an assessment of instability secondary to spinal metastases. Radiat Oncol. 2014;9:69 doi:10.1186/1748-717X-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sahgal A, Atenafu EG, Chao S, et al. Vertebral compression fracture after spine stereotactic body radiotherapy: a multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J Clin Oncol. 2013;31:3426–3431. doi:10.1200/JCO.2013.50.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee SH, Tatsui CE, Ghia AJ, et al. Can the spinal instability neoplastic score prior to spinal radiosurgery predict compression fractures following stereotactic spinal radiosurgery for metastatic spinal tumor? A post hoc analysis of prospective phase II single-institution trials. J Neurooncol. 2016;126:509–517. doi:10.1007/s11060-015-1990-z. [DOI] [PubMed] [Google Scholar]
- 41. Lam TC, Uno H, Krishnan M, et al. Adverse outcomes after palliative radiation therapy for uncomplicated spine metastases: role of spinal instability and single-fraction radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93:373–381. doi:10.1016/j.ijrobp.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 42. Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13:324–328. doi:10.3171/2010.3.SPINE09459. [DOI] [PubMed] [Google Scholar]
- 43. Rades D, Stalpers LJA, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23:3366–3375. doi:10.1200/JCO.2005.04.754. [DOI] [PubMed] [Google Scholar]
- 44. Rades D, Lange M, Veninga T, et al. Preliminary results of spinal cord compression recurrence evaluation (score-1) study comparing short-course versus long-course radiotherapy for local control of malignant epidural spinal cord compression. Int J Radiat Oncol Biol Phys. 2009;73:228–234. doi:10.1016/j.ijrobp.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 45. Mizumoto M, Harada H, Asakura H, et al. Radiotherapy for patients with metastases to the spinal column: a review of 603 patients at Shizuoka Cancer Center Hospital. Int J Radiat Oncol Biol Phys. 2011;79:208–213. doi:10.1016/j.ijrobp.2009.10.056. [DOI] [PubMed] [Google Scholar]
- 46. Campos S, Presutti R, Zhang L, et al. Elderly patients with painful bone metastases should be offered palliative radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1500–1506. doi:10.1016/j.ijrobp.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 47. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25:1423–1436. doi:10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 48. Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol. 2012;24:112–124. doi:10.1016/j.clon.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 49. Nguyen QN, Shiu AS, Rhines LD, et al. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1185–1192. doi:10.1016/j.ijrobp.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 50. Anand AK, Venkadamanickam G, Punnakal AU, et al. Hypofractionated stereotactic body radiotherapy in spinal metastasis: with or without epidural extension. Clin Oncol. 2015;27:345–352. doi:10.1016/j.clon.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 51. Ryu S, Jin R, Jin JY, et al. Pain control by image-guided radiosurgery for solitary spinal metastasis. J Pain Symptom Manage. 2008;35:292–298. doi:10.1016/j.jpainsymman.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 52. Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976). 2007;32:193–199. doi:10.1016/S0276-1092(08)79346-5. [DOI] [PubMed] [Google Scholar]
- 53. Radiation Therapy Oncology Group. Image-guided radiosurgery or stereotactic body radiation therapy in treating patients with localized spine metastasis (ClinicalTrials.gov NLM Identifier: NCT00922974). Bethesda, MD: National Library of Medicine; 2000. https://clinicaltrials.gov/ct2/show/NCT00922974 . Accessed February 1, 2017. [Google Scholar]
- 54. Ryu S, Pugh SL, Gerszten PC, et al. RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1-3) spine metastases: phase 2 results. Pract Radiat Oncol. 2014;4:76–81. doi:10.1016/j.prro.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. NCIC Clinical Trials Group. Feasibility study comparing stereotactic body radiotherapy vs conventional palliative RT in spinal metastases (ClinicalTrials.gov NLM Identifier: NCT02512965). Bethesda, MD: National Library of Medicine; 2000. https://clinicaltrials.gov/ct2/show/NCT02512965 Accessed February 1, 2017. [Google Scholar]
- 56. Huisman M, van den Bosch MAAJ, Wijlemans JW, van Vulpen M, van der Linden YM, Verkooijen HM. Effectiveness of reirradiation for painful bone metastases: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2012;84:8–14. doi:10.1016/j.ijrobp.2011.10.080. [DOI] [PubMed] [Google Scholar]
- 57. Chow E, van der Linden YM, Roos D, et al. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15:164–171. doi:10.1016/S1470-2045(13)70556-4. [DOI] [PubMed] [Google Scholar]
- 58. Chow E, Meyer RM, Chen BE, et al. Impact of reirradiation of painful osseous metastases on quality of life and function: a secondary analysis of the NCIC CTG SC.20 randomized trial. J Clin Oncol. 2014;32:3867–3873. doi:10.1200/JCO.2014.57.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sahgal A, Ames C, Chou D, et al. Stereotactic body radiotherapy is effective salvage therapy for patients with prior radiation of spinal metastases. Int J Radiat Oncol Biol Phys. 2009;74:723–731. doi:10.1016/j.ijrobp.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 60. Garg AK, Wang XS, Shiu AS, et al. Prospective evaluation of spinal reirradiation by using stereotactic body radiation therapy: The University of Texas MD Anderson Cancer Center experience. Cancer. 2011;117:3509–3516. doi:10.1002/cncr.25918. [DOI] [PubMed] [Google Scholar]
- 61. Choi CYH, Adler JR, Gibbs IC, et al. Stereotactic radiosurgery for treatment of spinal metastases recurring in close proximity to previously irradiated spinal cord. Int J Radiat Oncol Biol Phys. 2010;78:499–506. doi:10.1016/j.ijrobp.2009.07.1727. [DOI] [PubMed] [Google Scholar]
- 62. Damast S, Wright J, Bilsky M, et al. Impact of dose on local failure rates after image-guided reirradiation of recurrent paraspinal metastases. Int J Radiat Oncol Biol Phys. 2011;81:819–826. doi:10.1016/j.ijrobp.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 63. Kawashiro S, Harada H, Katagiri H, et al. Reirradiation of spinal metastases with intensity-modulated radiation therapy: an analysis of 23 patients. J Radiat Res. 2016;57:150–156. doi:10.1093/jrr/rrv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mahadevan A, Floyd S, Wong E, Jeyapalan S, Groff M, Kasper E. Stereotactic body radiotherapy reirradiation for recurrent epidural spinal metastases. Int J Radiat Oncol Biol Phys. 2011;81:1500–1505. doi:10.1016/j.ijrobp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 65. Thibault I, Campbell M, Tseng CL, et al. Salvage stereotactic body radiotherapy (SBRT) following in-field failure of initial SBRT for spinal metastases. Int J Radiat Oncol Biol Phys. 2015;93:353–360. doi:10.1016/j.ijrobp.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 66. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648. doi:10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 67. Fehlings MG, Nater A, Tetreault L, et al. Survival and clinical outcomes in surgically treated patients with metastatic epidural spinal cord compression: results of the prospective multicenter AOSpine study. J Clin Oncol. 2016;34:268–276. doi:10.1200/JCO.2015.61.9338. [DOI] [PubMed] [Google Scholar]
- 68. Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neurochir. 1998;140:957–967. [DOI] [PubMed] [Google Scholar]
- 69. Al-Omair A, Masucci L, Masson-Cote L, et al. Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro Oncol. 2013;15:1413–1419. doi:10.1093/neuonc/not101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Laufer I, Iorgulescu JB, Chapman T, et al. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine. 2013;18:207–214. doi:10.3171/2012.11.SPINE12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bate BG, Khan NR, Kimball BY, Gabrick K, Weaver J. Stereotactic radiosurgery for spinal metastases with or without separation surgery. J Neurosurg Spine. 2015;22:409–415. doi:10.3171/2014.10.SPINE14252. [DOI] [PubMed] [Google Scholar]
- 72. Azad TD, Esparza R, Chaudhary N, Chang SD. Stereotactic radiosurgery for metastasis to the craniovertebral junction preserves spine stability and offers symptomatic relief [published online October 30 2015]. J Neurosurg Spine. doi:10.3171/2015.6.SPINE15190. [DOI] [PubMed] [Google Scholar]
- 73. Folkert MR, Bilsky MH, Tom AK, et al. Outcomes and toxicity for hypofractionated and single-fraction image-guided stereotactic radiosurgery for sarcomas metastasizing to the spine. Int J Radiat Oncol Biol Phys. 2014;88:1085–1091. doi:10.1016/j.ijrobp.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 74. Guckenberger M, Mantel F, Gerszten PC, et al. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: a multi-institutional analysis. Radiat Oncol. 2014;9:226 doi:10.1186/s13014-014-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ahmed KA, Stauder MC, Miller RC, et al. Stereotactic body radiation therapy in spinal metastases. Int J Radiat Oncol Biol Phys. 2012;82:e803–e809. doi:10.1016/j.ijrobp.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 76. Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1-2 trial. Lancet Oncol. 2012;13:395–402. doi:10.1016/S1470-2045(11)70384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Harel R, Chao S, Krishnaney A, Emch T, Benzel EC, Angelov L. Spine instrumentation failure after spine tumor resection and radiation: comparing conventional radiotherapy with stereotactic radiosurgery outcomes. World Neurosurg. 2010;74:517–522. doi:10.1016/j.wneu.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 78. Pan HY, Allen PK, Wang XS, et al. Incidence and predictive factors of pain flare after spine stereotactic body radiation therapy: secondary analysis of phase 1/2 trials. Int J Radiat Oncol Biol Phys. 2014;90:870–876. doi:10.1016/j.ijrobp.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 79. Chiang A, Zeng L, Zhang L, et al. Pain flare is a common adverse event in steroid-naïve patients after spine stereotactic body radiation therapy: a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2013;86:638–642. doi:10.1016/j.ijrobp.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 80. Khan L, Chiang A, Zhang L, et al. Prophylactic dexamethasone effectively reduces the incidence of pain flare following spine stereotactic body radiotherapy (SBRT): a prospective observational study. Support Care Cancer. 2015:2937–2943. doi:10.1007/s00520-015-2659-z. [DOI] [PubMed] [Google Scholar]
- 81. Cunha MVR, Al-Omair A, Atenafu EG, et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int J Radiat Oncol Biol Phys. 2012;84: e343–e349. doi:10.1016/j.ijrobp.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 82. Boehling NS, Grosshans DR, Allen PK, et al. Vertebral compression fracture risk after stereotactic body radiotherapy for spinal metastases. J Neurosurg Spine. 2012;16:379–386. doi:10.3171/2011.11.SPINE116. [DOI] [PubMed] [Google Scholar]
- 83. Rose PS, Laufer I, Boland PJ, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol. 2009;27:5075–5079. doi:10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Koyfman SA, Djemil T, Burdick MJ, et al. Marginal recurrence requiring salvage radiotherapy after stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2012;83:297–302. doi:10.1016/j.ijrobp.2011.05.067. [DOI] [PubMed] [Google Scholar]
- 85. Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151–160. doi:10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 86. Taylor DR, Weaver JA. Tumor pseudoprogression of spinal metastasis after radiosurgery: a novel concept and case reports. J Neurosurg Spine. 2015;22:534–539. doi:10.3171/2014.10.SPINE14444. [DOI] [PubMed] [Google Scholar]
- 87. Buhmann Kirchhoff S, Becker C, Duerr HR, Reiser M, Baur-Melnyk A. Detection of osseous metastases of the spine: comparison of high resolution multi-detector-CT with MRI. Eur J Radiol. 2009;69:567–573. doi:10.1016/j.ejrad.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 88. Thibault I, Chang EL, Sheehan J, et al. Response assessment after stereotactic body radiotherapy for spinal metastasis: a report from the SPIne response assessment in Neuro-Oncology (SPINO) group. Lancet Oncol. 2015;16:e595–e603. doi:10.1016/S1470-2045(15)00166-7. [DOI] [PubMed] [Google Scholar]
- 89. Chow E, Wu JSY, Hoskin P, Coia LR, Bentzen SM, Blitzer PH. International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Radiother Oncol. 2002;64:275–280. doi:10.1016/j.ijrobp.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 90. Chow E, Hoskin P, Mitera G, et al. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys. 2012;82:1730–1737. doi:10.1016/j.ijrobp.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 91. Kaloostian PE, Yurter A, Zadnik PL, Sciubba DM, Gokaslan ZL. Current paradigms for metastatic spinal disease: an evidence-based review. Ann Surg Oncol. 2014;21:248–262. doi:10.1245/s10434-013-3324-8. [DOI] [PubMed] [Google Scholar]
- 92. Laufer I, Sciubba DM, Madera M, et al. Surgical management of metastatic spinal tumors. Cancer Control. 2012;19:122–128. [DOI] [PubMed] [Google Scholar]
- 93. Bilsky MH, Laufer I, Burch S. Shifting paradigms in the treatment of metastatic spine disease. Spine (Phila Pa 1976). 2009;34(22 suppl): S101–S107. doi:10.1097/BRS.0b013e3181bac4b2. [DOI] [PubMed] [Google Scholar]
- 94. Young RF, Post EM, King GA. Treatment of spinal epidural metastases. Randomized prospective comparison of laminectomy and radiotherapy. J Neurosurg. 1980;53:741–748. doi:10.3171/jns.1980.53.6.0741. [DOI] [PubMed] [Google Scholar]
- 95. Gerszten PC, Monaco EA. Complete percutaneous treatment of vertebral body tumors causing spinal canal compromise using a transpedicular cavitation, cement augmentation, and radiosurgical technique. Neurosurg Focus. 2009;27(6):E9 doi:10.3171/2009.9.FOCUS09184. [DOI] [PubMed] [Google Scholar]
- 96. Massicotte E, Foote M, Reddy R, Sahgal A. Minimal access spine surgery (MASS) for decompression and stabilization performed as an out-patient procedure for metastatic spinal tumours followed by spine stereotactic body radiotherapy (SBRT): first report of technique and preliminary outcomes. Technol Cancer Res Treat. 2012;11:15–25. [DOI] [PubMed] [Google Scholar]
- 97. Tancioni F, Navarria P, Pessina F, et al. Early surgical experience with minimally invasive percutaneous approach for patients with metastatic epidural spinal cord compression (MESCC) to poor prognoses. Ann Surg Oncol. 2012;19:294–300. doi:10.1245/s10434-011-1894-x. [DOI] [PubMed] [Google Scholar]
- 98. Berenson J, Pflugmacher R, Jarzem P, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12:225–235. doi:10.1016/S1470-2045(11)70008-0. [DOI] [PubMed] [Google Scholar]
- 99. Fourney DR, Abi-Said D, Rhines LD, et al. Simultaneous anterior-posterior approach to the thoracic and lumbar spine for the radical resection of tumors followed by reconstruction and stabilization. J Neurosurg. 2001;94(2 suppl):232–244. [DOI] [PubMed] [Google Scholar]
- 100. Pointillart V, Vital JM, Salmi R, Diallo A, Quan GM. Survival prognostic factors and clinical outcomes in patients with spinal metastases. J Cancer Res Clin Oncol. 2011;137:849–856. doi:10.1007/s00432-010-0946-0. [DOI] [PubMed] [Google Scholar]
- 101. Ibrahim A, Crockard A, Antonietti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine. 2008;8:271–278. doi:10.3171/SPI/2008/8/3/271. [DOI] [PubMed] [Google Scholar]
- 102. Quan GMY, Vital JM, Aurouer N, et al. Surgery improves pain, function and quality of life in patients with spinal metastases: a prospective study on 118 patients. Eur Spine J. 2011;20:1970–1978. doi:10.1007/s00586-011-1867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fujibayashi S, Neo M, Miyaki K, Nakayama T, Nakamura T. The value of palliative surgery for metastatic spinal disease: satisfaction of patients and their families. Spine J. 2010;10:42–49. doi:10.1016/j.spinee.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 104. Falicov A, Fisher CG, Sparkes J, Boyd MC, Wing PC, Dvorak MF. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine (Phila Pa 1976). 2006;31:2849–2856. doi:10.1097/01.brs.0000245838.37817.40. [DOI] [PubMed] [Google Scholar]
- 105. Wai EK, Finkelstein JA, Tangente RP, et al. Quality of life in surgical treatment of metastatic spine disease. Spine (Phila Pa 1976). 2003;28:508–512. doi:10.1097/01.BRS.0000048646.26222.FA. [DOI] [PubMed] [Google Scholar]
- 106. Witham TF, Khavkin YA, Gallia GL, Wolinsky JP, Gokaslan ZL. Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat Clin Pract Neurol. 2006;2(2):87–94. doi:10.1038/ncpneuro0116. [DOI] [PubMed] [Google Scholar]
- 107. Olerud C, Jónsson H, Löfberg AM, Lörelius LE, Sjöström L. Embolization of spinal metastases reduces peroperative blood loss. 21 patients operated on for renal cell carcinoma. Acta Orthop Scand. 1993;64:9–12. [DOI] [PubMed] [Google Scholar]
- 108. Manke C, Bretschneider T, Lenhart M, et al. Spinal metastases from renal cell carcinoma: effect of preoperative particle embolization on intraoperative blood loss. AJNR Am J Neuroradiol. 2001;22:997–1003. [PMC free article] [PubMed] [Google Scholar]
- 109. Clausen C, Dahl B, Frevert SC, Hansen LV, Nielsen MB, Lönn L. Preoperative embolization in surgical treatment of spinal metastases: single-blind, randomized controlled clinical trial of efficacy in decreasing intraoperative blood loss. J Vasc Interv Radiol. 2015;26:402–412.e1. doi:10.1016/j.jvir.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 110. Lau D, Leach MR, Than KD, Ziewacz J, La Marca F, Park P. Independent predictors of complication following surgery for spinal metastasis. Eur Spine J. 2013;22:1402–1407. doi:10.1007/s00586-013-2706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jansson KA, Bauer HCF. Survival, complications and outcome in 282 patients operated for neurological deficit due to thoracic or lumbar spinal metastases. Eur Spine J. 2006;15:196–202. doi:10.1007/s00586-004-0870-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Street JT, Lenehan BJ, DiPaola CP, et al. Morbidity and mortality of major adult spinal surgery. A prospective cohort analysis of 942 consecutive patients. Spine J. 2012;12:22–34. doi:10.1016/j.spinee.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 113. Dea N, Versteeg A, Fisher C, et al. Adverse events in emergency oncological spine surgery: a prospective analysis. J Neurosurg Spine. 2014;21:698–703. doi:10.3171/2014.7.SPINE131007. [DOI] [PubMed] [Google Scholar]
- 114. Smith ZA, Fessler RG. Paradigm changes in spine surgery: evolution of minimally invasive techniques. Nat Rev Neurol. 2012;8:443–450. doi:10.1038/nrneurol.2012.110. [DOI] [PubMed] [Google Scholar]
- 115. Molina CA, Gokaslan ZL, Sciubba DM. A systematic review of the current role of minimally invasive spine surgery in the management of metastatic spine disease. Int J Surg Oncol. 2011;2011:598148 doi:10.1155/2011/598148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Miscusi M, Polli FM, Forcato S, et al. Comparison of minimally invasive surgery with standard open surgery for vertebral thoracic metastases causing acute myelopathy in patients with short- or mid-term life expectancy: surgical technique and early clinical results. J Neurosurg Spine. 2015;22:518–525. doi:10.3171/2014.10.SPINE131201. [DOI] [PubMed] [Google Scholar]
- 117. Amankulor NM, Xu R, Iorgulescu JB, et al. The incidence and patterns of hardware failure after separation surgery in patients with spinal metastatic tumors. Spine J. 2014;14:1850–1859. doi:10.1016/j.spinee.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 118. Ghia AJ, Rebueno NC, Li J, Brown PD, Rhines LD, Tatsui CE. The use of image guided laser interstitial thermotherapy to supplement spine stereotactic radiosurgery to manage metastatic epidural spinal cord compression: proof of concept and dosimetric analysis. Pract Radiat Oncol. 2016;6(2):e35–e38. doi:10.1016/j.prro.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 119. Ahrar K, Stafford RJ. Magnetic resonance imaging-guided laser ablation of bone tumors. Tech Vasc Interv Radiol. 2011;14:177–182. doi:10.1053/j.tvir.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 120. Tatsui CE, Stafford RJ, Li J, et al. Utilization of laser interstitial thermotherapy guided by real-time thermal MRI as an alternative to separation surgery in the management of spinal metastasis. J Neurosurg Spine. 2015;23:400–411. doi:10.3171/2015.2.SPINE141185. [DOI] [PubMed] [Google Scholar]
- 121. Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(1 suppl):21–30. [DOI] [PubMed] [Google Scholar]
- 122. Mendel E, Bourekas E, Gerszten P, Golan JD. Percutaneous techniques in the treatment of spine tumors: what are the diagnostic and therapeutic indications and outcomes? Spine (Phila Pa 1976). 2009;34(22 suppl):S93–S100. doi:10.1097/BRS.0b013e3181b77895. [DOI] [PubMed] [Google Scholar]
- 123. Gerszten PC, Germanwala A, Burton SA, Welch WC, Ozhasoglu C, Vogel WJ. Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. J Neurosurg Spine. 2005;3:296–301. doi:10.3171/spi.2005.3.4.0296. [DOI] [PubMed] [Google Scholar]
- 124. Cruz JP, Sahgal A, Whyne C, Fehlings MG, Smith R. Tumor extravasation following a cement augmentation procedure for vertebral compression fracture in metastatic spinal disease. J Neurosurg Spine. 2014;21:372–377. doi:10.3171/2014.4.SPINE13695. [DOI] [PubMed] [Google Scholar]
- 125. Kim CH, Chung CK, Sohn S, Lee S, Park SB. Less invasive palliative surgery for spinal metastases. J Surg Oncol. 2013;108:499–503. doi:10.1002/jso.23418. [DOI] [PubMed] [Google Scholar]
- 126. Moussazadeh N, Rubin DG, McLaughlin L, Lis E, Bilsky MH, Laufer I. Short-segment percutaneous pedicle screw fixation with cement augmentation for tumor-induced spinal instability. Spine J. 2015;15:1609–1617. doi:10.1016/j.spinee.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 127. Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi:10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 128. Thibault I, Al-Omair A, Masucci GL, et al. Spine stereotactic body radiotherapy for renal cell cancer spinal metastases: analysis of outcomes and risk of vertebral compression fracture. J Neurosurg Spine. 2014;21:711–718. doi:10.3171/2014.7.SPINE13895. [DOI] [PubMed] [Google Scholar]
- 129. Sohn S, Chung CK, Sohn MJ, et al. Stereotactic radiosurgery compared with external radiation therapy as a primary treatment in spine metastasis from renal cell carcinoma: a multicenter, matched-pair study. J Neurooncol. 2014:121–128. doi:10.1007/s11060-014-1455-9. [DOI] [PubMed] [Google Scholar]
- 130. Park HJ, Kim HJ, Won JH, Lee SC, Chang AR. Stereotactic Body Radiotherapy (SBRT) for spinal metastases: who will benefit the most from SBRT? Technol Cancer Res Treat. 2015;14:159–166. doi:10.7785/tcrt.2012.500411. [DOI] [PubMed] [Google Scholar]
- 131. Sellin JN, Reichardt W, Bishop AJ, et al. Factors affecting survival in 37 consecutive patients undergoing de novo stereotactic radiosurgery for contiguous sites of vertebral body metastasis from renal cell carcinoma. J Neurosurg Spine. 2015;22:52–59. doi:10.3171/2014.9.SPINE1482. [DOI] [PubMed] [Google Scholar]
- 132. Bishop AJ, Tao R, Rebueno NC, et al. Outcomes for spine stereotactic body radiation therapy and an analysis of predictors of local recurrence. Int J Radiat Oncol. 2015;92:1016–1026. doi:10.1016/j.ijrobp.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 133. Rock JP, Ryu S, Shukairy MS, et al. Postoperative radiosurgery for malignant spinal tumors. Neurosurgery. 2006;58:891–897. doi:10.1227/01.NEU.0000209913.72761.4F. [DOI] [PubMed] [Google Scholar]
- 134. Moulding HD, Elder JB, Lis E, et al. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J Neurosurg Spine. 2010;13:87–93. doi:10.3171/2010.3.SPINE09639. [DOI] [PubMed] [Google Scholar]