Abstract
Background
Ischaemic colitis (IC) is the most common form of intestinal ischaemia with a wide spectrum of severity, with possible risk of death.
Objective
The purpose of this study was to evaluate predictive factors of in-hospital and short-term mortality, in a cohort of patients with IC.
Methods
Retrospective analysis of IC cases diagnosed between 2008–2013 in a single tertiary centre, with assessment of factors at the time of diagnosis associated with in-hospital and 90-day mortality.
Results
Of the 203 patients included (132 women), 47 (23%) died during the follow-up (median: 16 months). There were 21 patients (45%) who died during hospitalization and at 90 days there were 30 deaths (64% of total). In multivariate analysis, need for vasopressor support (odds ratio (OR) 11.21; 95% confidence interval (CI): 2.31–54.24; p = 0.01), Intermediate or Intensive Care Unit (ICU): admission (OR 7.01; 95% CI: 1.48–33.16; p = 0.014) and atrial fibrillation (OR 4.99; 95% CI: 1.1–26.23; p = 0.048) were independently and significantly associated with in-hospital mortality. Using the coefficients of the estimated logistic model, we calculated a scoring model to predict the occurrence of in-hospital mortality. The presence of all three risk factors predicted a probability of death of 32% with an area under the receiver operating characteristic curve (AUROC) of 0.89 (95% CI 0.80–0.98. At 90 days, the presence of chronic kidney disease (OR 7.46; 95% CI: 1.87–29.73; p = 0.002), and male sex (OR 5.85; 95% CI: 1.57–21.83; p = 0.009) were also independently associated with mortality.
Conclusions
Most deaths in ischaemic colitis occur in the first 90 days after admission, sharing similar risk factors. Assessment of the presence of atrial fibrillation, need of vasopressor support or hospitalization in the intermediate/intensive care unit provides a useful tool to estimate in-hospital mortality and to establish the management for patients admitted for ischaemic colitis.
Keywords: Ischaemic colitis, prognosis, mortality, predictive model, management
Introduction
The term ischaemic colitis (IC) was primarily introduced by Marston et al. in their article published in 19661 and was preceded by the description of reversible colonic vascular occlusion by Boley and colleagues in 1963.2 IC encompasses a number of clinical entities, all with an end result of insufficient blood supply to a segment or the entire colon, resulting in ischaemic necrosis of varying severities that can range from superficial mucosal involvement to full-thickness transmural necrosis in up to 15% of cases. The incidence of IC in Western populations ranges from 4.5–44 cases per 100,000 person-years, and accounts for one in 1000 hospitalizations among the general hospital population.3 IC is often misdiagnosed and previous publications concerning presumed IC have been limited by absence of diagnostic confirmation, so a strict definition of IC will require biopsy confirmation of each diagnosis. Several conditions predispose to IC, most often affecting the elderly.3 However, the disorder usually develops in the absence of major vessel occlusion and the cause is not defined clearly in most cases.4 Most mortality cases occur during hospitalization. Less severe cases usually resolves without sequelae,5 such as stricture or chronic IC. The outcome of IC seems to depend on numerous factors, including its severity, location, rate of progression of the ischaemic insult, timing of treatment and patients’ comorbidities and chronic medications.6 To date, there are few published studies addressing the issue of prognosis of IC and most of them were heterogeneous in terms of patient characteristics, physical examination findings, medical comorbidities, serology, thresholds of various factors considered and use of nontraditional endpoints.7 In addition, some of these studies included patients whose diagnosis was not confirmed by histology, and only based on imaging studies. As far as we know, no specific predictive model of in-hospital mortality was developed to date, which could be a useful tool in approaching the patient admitted for IC, thus potentially reducing mortality at a high risk stage. As such, we conducted a study to identify factors associated with in-hospital and short-term (up to 90 days) mortality for IC and to develop a scoring model for assessing the probability of in-hospital mortality.
Materials and methods
A retrospective study was conducted on all patients with a diagnosis of IC in our hospital for a period of six years (January 2008–December 2013). Inclusion criteria were: diagnosis confirmed by colonoscopy or surgical specimen with compatible histopathology and in-hospital staying superior to 24 h. Histological findings consistent with ischaemic colitis included haemorrhage, crypt destruction, capillary thrombosis, granulation tissue with crypt abscesses. Exclusion criteria were diagnosis of inflammatory bowel disease, recent antibiotics use or positive stool sampling for enteric infections.
Clinical, laboratory, imaging, endoscopic and surgical data were collected from the medical records documented at the time of diagnosis of IC. Patients admitted with IC and those who developed IC during hospitalization were included.
Clinical variables included were age, sex, presenting symptoms, vital signs, medical history, previous abdominal surgeries or intra-vascular procedures (up to three months before) and chronic medication potentially implied in IC (antihypertensives, diuretics, anti-platelet agents, non-steroidal anti-inflammatories, hormonal therapy and digitalis).
The laboratory variables studied included blood analysis with a complete blood count, pH, lactate, lactic dehydrogenase, serum albumin, sodium (presence of hyponatraemia if Na+<135 mmol/l)), urea, creatinine and reactive C-protein.
Computerized tomography (CT) findings typical of IC were collected, including segmental or circumferential wall thickening and homogenous or heterogeneous wall enhancement.
Endoscopic variables included degree of clinical suspicion, timing of its completion (<12 h, 12–24 h, 24–48 h, 48 h), severity of the lesions (severe if evidence of necrosis or deep ulceration), second endoscopy and location (left colon, right colon, rectum, pancolitis, segment).
The surgical variables included indication for surgery (peritonitis/sepsis, gangrene, fulminant colitis, massive haemorrhage, clinical worsening) and type of surgery (Hartmann procedure, colectomy with ileostomy, colectomy segment or subtotal colectomy).
Treatment and follow-up variables included vasopressor support, fluid therapy, parenteral nutrition, antibiotic therapy, the treatment site (medical ward, surgical ward, intermediate/intensive care unit), complications, length of hospital stay (days), in-hospital and 90-day mortality.
Statistical analysis
Data were collated in a SPSS database (20.0, SPSS, Chicago, Illinois, USA). Hypotheses were tested about the distribution of continuous variables with non-normal distribution by using the non-parametric Mann-Whitney and Kruskal-Wallis tests, depending on the nature of the hypothesis.
Categorical data were expressed as absolute frequencies (n) and relative frequencies (%). Continuous data were described as mean, median and percentiles, according to its distribution.
Univariate analyses using chi-square or Fisher’s exact tests and Student t or Mann-Whitney’s U tests were performed to assess the association between patients’ characteristics and in-hospital mortality and short-term (90 days) mortality.
A logistic regression model was fitted to select the best subset of predictors of in-hospital mortality and short-term mortality. The factors showing a clinically and statistically significant association in univariate analysis were selected for the initial model. The final model was fitted using a step-wise forward method based on improvement in model likelihood ratios. Significance levels to enter and drop model variables were adopted as 5% and 10%, respectively. Accuracy of the resulting model to predict mortality was assessed by estimating and comparing the corresponding areas under the receiver operating characteristic curves (AUROCs). The significance level was set at p < 0.05.
Results
In total 223 patients were evaluated, and 20 patients were excluded. In five cases there was not enough clinical information in hospital records in order to be analysed, and in 15 cases a concomitant infectious disease could not be excluded. The remainder 203 patients were included, of whom the majority were female (65%, n = 132). Table 1 shows the baseline characteristics of the population.
Table 1.
Summary of patient demographics and comorbid conditions (n = 203)
| Age (years) | 76 ± 21 |
| Female | 132 (64.4%) |
| In-hospital onset | 20 (9.9%) |
| Clinical manifestations | |
| Rectal bleeding | 137 (66.8%) |
| Abdominal pain | 42 (20.5%) |
| Diarrhoea | 18 (8.8%) |
| Distention | 2 (1.0%) |
| Other | 4 (2.0%) |
| Haemoglobin (g/dl) | 12.69 ± 2.31 |
| White blood count (×109/ul) | 13.84 ± 9.99 |
| Lactate dehydrogenase (IU/l) | 278.87 ± 244.66 |
| Urea (mg/dl) | 68.02 ± 56.24 |
| Creatinine (mg/dl) | 1.62 ± 1.61 |
| Hyponatraemia (<130 mmol/l) | 46 (22.4%) |
| Endoscopic severitya | |
| Mild | 151 (80.7%) |
| Severe | 36 (19.3%) |
| Disease extension | |
| Left | 130 (63.7%) |
| Segmental | 57 (27.9%) |
| Right (isolated) | 10 (4.9%) |
| Pancolonic | 7 (3.4%) |
For categorical variables, the entries are number (%) of patients with the characteristic. aEndoscopic findings were considered severe if evidence of necrosis or deep ulceration. For continuous variables, the entries are means ( ± standard deviations).
The average age was 75 years (±20 years). Almost all patients were from the emergency department (88%, n = 179), and the remaining derived cases of patients hospitalized for other reasons. In 13 cases the patients were in intensive/intermediate care units, six patients were in surgical wards (general surgery, orthopaedics and vascular surgery), and five patients were in the internal medicine ward. The median time of hospital stay was eight days (interquartile range (IQR): 5.0–14.0), and the mean follow-up time since the diagnosis was 22 ± 24 months. The overall mortality rate was 23% (47 patients). Of those patients who died, 21 patients died in the hospital (45% of total deaths), while at the end of 90 days, 30 patients were dead (64% of total deaths). Table 2 shows the causes of in-hospital deaths.
Table 2.
Causes of in-hospital mortality
| Cause | n (%) |
|---|---|
| Cardiogenic shock | 5 (24) |
| Acute coronary syndrome | 2 (10) |
| Respiratory failure | 1 (5) |
| Surgery | 2 (10) |
| Sepsis | 5 (24) |
| Haemorrhagic shock | 6 (29) |
| Total | 21 (100) |
The symptoms most frequently reported by the patients at presentation were rectal bleeding/haematochezia (67%, n = 136), abdominal pain in lower quadrants (20%, n = 41) and non-bloody diarrhoea (9%, n = 18). Rarely, patients presented with an acute abdomen, bloating or other symptoms (up to 1% of cases). Upon admission, patients usually showed slight leukocytosis (mean 13850 ± 10020 leucocytes/µl) without associated anaemia (mean haemoglobin 12.68 ± 2.31 g/dl), and often accompanied by acute kidney injury (mean serum creatinine 1.62 ± 1.61 mg/dl), the latter representing the most common complication observed in the study population (see below).
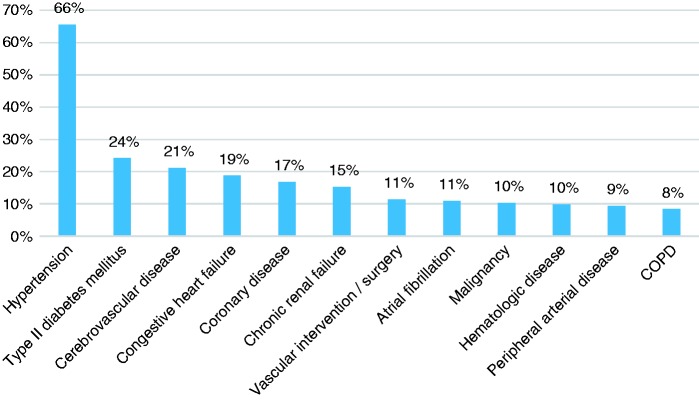
Cardiovascular diseases and subsequent complications were the most prevalent medical problems in this cohort. In particular, essential hypertension was present in more than two-thirds of the patients (n = 144, 71%), followed by type 2 diabetes mellitus, cerebrovascular disease, congestive heart failure, coronary heart disease and chronic renal failure. Figure 1 lists frequencies of all the comorbidities.
Figure 1.
Comorbidities. COPD: chronic obstructive pulmonary disease.
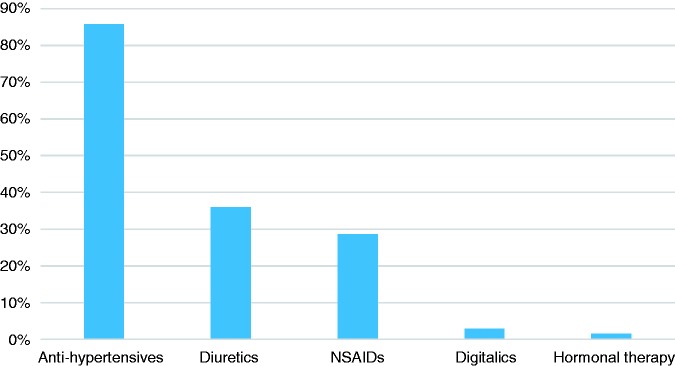
In relation to chronic medications previously described as potentially associated with IC, the vast majority of patients were treated with drugs such as antihypertensives (n = 174, 86%), followed by diuretics (n = 73, 36%) and anti-platelet agents or non-steroidal anti-inflammatories (n = 58, 29%), as shown in Figure 2.
Figure 2.
Chronic medications. NSAID: non-steroidal anti-inflammatory drug.
In 87% (n = 177) of the cases, the initial diagnosis method was the colonoscopy, while in only 9% (n = 18) of cases an image study (namely, angioCT) was previously performed, reflecting cases of atypical presentation, namely the absence of blood loss. Rarely, laparotomy was necessary to clarify the situation, including very severely ill patients with other associated conditions (n = 8, 4%). Approximately three-quarters (n = 131, 74%) of patients undergoing colonoscopy revealed mild endoscopic findings, with no evidence of necrosis or deep ulceration. Combining endoscopic, imaging and surgical findings, the left colon (distal to the splenic flexure) was involved in most cases (n = 130, 64%), followed by isolated involvement of the sigmoid colon (n = 57, 28%). In only 5% of cases (n = 10) the right colon (proximal to the splenic flexure) was involved, and also in a minority number of cases the whole colon was involved (pancolitis: n = 6, 3%). Notably, in five patients (3% of the cases) there was involvement of the rectum, i.e. proctocolitis.
Surgical treatment was necessary in 22 patients (11%). The evidence of peritonitis (n = 10) or intestinal gangrene (n = 5) were the main reasons for surgery. Less common causes were deterioration of clinical condition (n = 3), persistent bleeding (n = 2), and irreversible stenosis (n = 2).
After diagnosis of IC, the majority of cases (n = 40, 69%) were admitted in a surgical ward. In milder cases, patients remained on surveillance in the emergency department for a relatively short period and were discharged without the need for effective hospitalization. However, about one out of five patients showed severity criteria requiring hospitalization in intensive/intermediate care units (n = 39, 19%) and in many cases vasopressor support was initiated (69% of the hospitalizations in the ICU, 12% of the total). The same proportion of patients were submitted to, at least, one red blood cell transfusion. About 80% of all patients started empirical antibiotherapy.
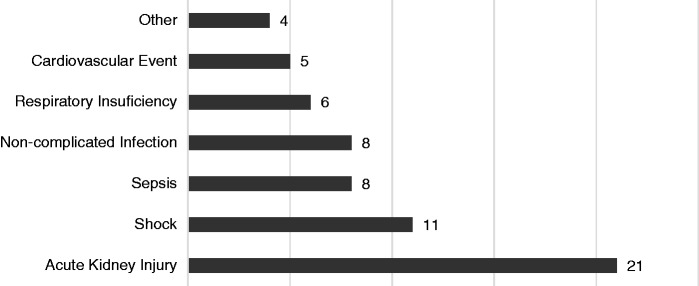
During hospitalization, the most common complications seen were the development of acute kidney injury and shock in 10% and 5% of cases, respectively (Figure 3).
Figure 3.
Complications during hospitalization.
After statistical analysis, there were several variables that correlated with in-hospital mortality. Univariate regression analyses showed a significant association (p < 0.05) with in-mortality for 14 variables, including: elevation of LDH, urea or creatinine, length of hospital stay, male gender, atrial fibrillation, bowel occlusion, recent vascular intervention, endoscopic severity, surgical intervention, vasopressor support, parenteral nutrition, empirical antibiotherapy and ICU admission (Table 3).
Table 3.
Variables that correlated with in-hospital mortality – univariate analysis
| lactate dehydrogenase (LDH) (mean value U/l) | Death/no death | 505.46 vs 253.37 (p = 0.004) |
| Blood urea nitrogen (BUN) (mean value mg/dl) | Death/no death | 86.10 vs 66.44 (p < 0.001) |
| Creatinine (mean value mg/dl) | Death/no death | 2.14 vs 1.57 (p = 0.001) |
| Hospitalization length (mean value in days) | Death/no death | 19.86 vs 14.03 (p = 0.006) |
| Male sex | Yes/no | 18.3% vs 6.1% (p = 0,006) |
| Atrial fibrillation | Yes/no | 22.7% vs 8.3% (p = 0.03) |
| Intestinal occlusion | Yes/no | 50% vs 8.2% (p < 0.001) |
| Recent intravascular intervention | Yes/no | 40% vs 8.3% (p = 0.001) |
| Endoscopic severity | Yes/no | 16.7% vs 5.4% (p = 0.02) |
| Surgical intervention | Yes/no | 45.5% vs 6.1% (p < 0.001) |
| Vasopressor support | Yes/no | 56% vs 3.9% (p < 0.001) |
| Parenteral nutrition | Yes/no | 50% vs 7.4% (p < 0.001) |
| Antibiotics | Yes/no | 13% vs 0% (p = 0.013) |
| Intermediate or Intensive care unit admission | Yes/no | 42.1% vs 2.5% (p < 0.001) |
BUN: blood urea nitrogen.
Predictors of mortality were then assessed by comparing patients who died during hospitalization and who survived. Of the clinical and laboratory parameters introduced in the final regression model (elevation of LDH, elevation of urea or creatinine, length of hospital stay, male sex, atrial fibrillation, bowel occlusion, recent vascular intervention, endoscopic severity, surgical intervention, vasopressor support, parenteral nutrition, empirical antibiotherapy and ICU admission), only the need for vasopressor support (odds ratio (OR) 11.21; 95% CI: 2.31–54.24; p = 0.01), ICU admission (OR 7.01; 95% CI: 1.48–33.16; p = 0.014) and atrial fibrillation (OR 4.99; 95% CI: 1.1–26.23; p = 0.048) were independently and significantly associated with in-hospital mortality. Using the coefficients of the estimated logistic model, we calculated a scoring model to predict the occurrence of in-hospital mortality (Y) based on the three significant clinical variables: vasopressor support (VS), ICU admission (ICU) and atrial fibrillation (AF). In the final model,
The resulting logistic regression model exhibited a high overall accuracy for the prediction of in-hospital mortality, with an AUROC of 0.89 (95% CI 0.80–0.98) (Figure 4). The best cut-off was −3.28 points, predicting in-hospital mortality with high sensitivity (89%) and specificity (78%).
Figure 4.
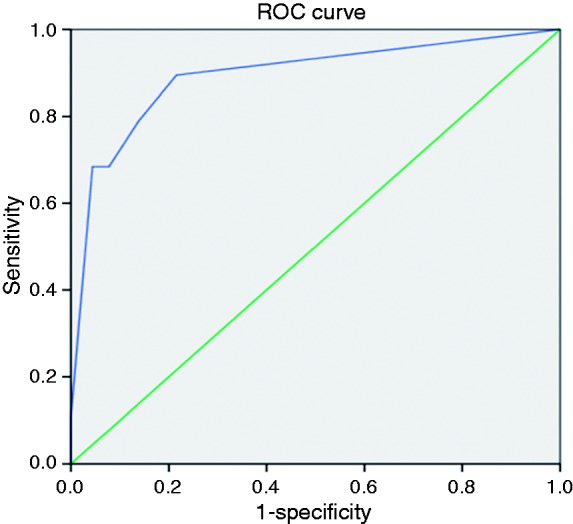
Receiver-operating characteristic (ROC) curve of the ischaemic colitis in-hospital mortality predictive model. The area under the curve was 0.89 (95% confidence interval (CI) 0.80–0.98). Mortality of 32% for values>–3.28).
Mortality at 30 days was 10.2% (n = 20), while the mortality at 90 days was 14.7% (n = 28). At 90 days, univariate analysis revealed associations similar to those described in the in-hospital mortality (Table 4). In multivariate analysis, need for vasopressor support (OR 22.68; 95% CI: 5.1–100.93; p < 0.001), atrial fibrillation (OR 16.07; 95% CI: 3.61–71.5; p < 0.001), chronic kidney disease (OR 7.46; 95% CI: 1.87–29.73; p = 0.002), and male sex (OR 5.85; 95% CI: 1.57–21.83; p = 0.009) and were independently and significantly associated with 90-day mortality.
Table 4.
Variables that correlated with 90-day mortality – univariate analysis
| Haemoglobin (mean value g/dl) | Death/no death | 11.78 vs 12.86 (p = 0.006) |
| lactate dehydrogenase (LDH) (mean value U/l) | Death/no death | 442.86 vs 253.27 (p = 0.004) |
| Blood urea nitrogen (BUN) (mean value mg/dl) | Death/no death | 92.90 vs 60.63 (p < 0.001) |
| Creatinine (mean value mg/dl) | Death/no death | 2.26 vs 1.48 (p < 0.001) |
| Hospitalization length (mean value in days) | Death/no death | 20.63 vs 13.53 (p = 0.002) |
| Male sex | Yes/no | 29% vs 8.3% (p < 0.001) |
| Atrial fibrillation | Yes/no | 36.4% vs 12.6% (p = 0.004) |
| Chronic kidney disease | Yes/no | 34.5% vs 11.9% (p = 0.002 |
| Intestinal occlusion | Yes/no | 71.4% vs 13.2% (p < 0.001) |
| Recent intravascular intervention | Yes/no | 44.4% vs 13.9% (p = 0.013) |
| Hyponatraemia | Yes/no | 28.6% vs 12.2% (p = 0.01) |
| Endoscopic severity | Severe/mild | 25% vs 9.3% (p = 0.014) |
| Surgical intervention | Yes/no | 59.1% vs 10.1% (p < 0.001) |
| Blood transfusion | Yes/no | 33.3% vs 13.3% (p = 0.012) |
| Vasopressor support | Yes/no | 68% vs 7.9% (p < 0.001) |
| Parenteral nutrition | Yes/no | 64.3% vs 11.9% (p < 0.001) |
| Antibiotics | Yes/no | 19.5% vs 0% (p = 0.004) |
| Intermediate or Intensive care unit admission | Yes/no | 50% vs 6.7% (p < 0.001) |
BUN: blood urea nitrogen.
Discussion
In our large cohort, similar to previous ones,8 the first symptoms of IC were haematochezia and abdominal pain that usually arise in patients with concomitant cardiovascular risk factors, namely arterial hypertension and type II diabetes, which were present in more than 60% of patients. In-hospital mortality (21/203, 10%) was similar to previous reports.5 The remaining characteristics of the population under study were not significantly different from those described in previous epidemiological studies, with a preponderance of diseases and medications commonly observed in older populations.4 As the population ages, there may be a significant increase in IC incidence and associated morbidity and mortality. So, it is fundamental to identify patients at increased risk of IC, as a high index of suspicion is needed to make this diagnosis.3
To date, there are few studies concerning poor prognostic factors in IC. Furthermore, these studies were heterogeneous in terms of patient characteristics, physical examination findings, medical comorbidities, serology, and thresholds of various factors considered. Surgical intervention and mortality are the most commonly used endpoints in studies that assess factors associated with poor outcome in IC and are used to define severe or complicated disease. These studies all conducted multivariate analyses and found various significant predictors of outcome including epidemiologic factors, clinical presentation of disease, vital signs, serologic values and disease distribution.
The epidemiologic factors that were associated with poor outcome included antimicrobial therapy, hepatitis C positivity, history of cancer, male gender and warfarin use at the time of diagnosis.9,10 We also found male gender and use of antibiotics to be associated with in-hospital mortality (p = 0.006 and p = 0.013, respectively). The association between death and the use of antibiotics is almost universal but does not appear to be justified from a pathophysiological point of view, most likely corresponding to an empirical reaction to the clinical severity of the situation and not a direct deleterious effect of these drugs. Also the presence of atrial fibrillation was associated with in-hospital mortality in univariate analysis (p = 0.03), a fact not reported in previous studies.
Regarding clinical factors at diagnosis, associated with worse outcomes, prior studies are relatively homogeneous, indicating that abdominal pain without rectal bleeding, non-bloody diarrhoea, peritoneal signs, heart rate of >100 beats per min and systolic blood pressure of <90 mm Hg at the time of diagnosis are associated with the need for surgical intervention and/or mortality.8,10,11 In our cohort, the clinical manifestations at presentation as well as the findings on physical examination were not significantly associated with in-hospital mortality. However, the need for vasopressor support (p < 0.001), ICU admission (p < 0.001) and surgery (p < 0.001) indicates, indirectly, that more seriously ill patients on admission had worse outcomes.
In addition to the clinical manifestations, studies indicate that many laboratory parameters at diagnosis are associated with worse outcomes, namely Hb < 12 g/dl, hyponatraemia (Na < 136 mEq/l), LDH > 450 U/l, and blood urea nitrogen (BUN) (>28 mg/dl).12,13 Our population showed results consistent with this findings. In univariate analysis, higher levels of LDH (p = 0.004), BUN (p < 0.001) and serum creatinine (p = 0.001) were also associated with in-hospital mortality.
Finally, the distribution of lesions in the colon and the severity of endoscopic findings have prognostic information, in particular the cases with involvement of the entire colon or isolated right colon involvement.10,11,12 Interestingly, the extent of disease revealed no association with mortality in our cohort, probably due to the rare cases of isolated right colon or pancolonic involvement (5% and 3%, respectively). The presence of deep ulceration or gangrene in the endoscopic evaluation was associated with higher mortality (p = 0.02), which is consistent with the results of Matsumoto et al.14 As mentioned above, to date only one study developed a predictive model for poor outcomes in patients diagnosed with IC.15 Chung et al. assessed 152 patients with clinically confirmed IC (only 74.5% with histologically confirmation) to analyse significant risk factors for poor outcome (improvement delayed by more than two weeks, 30-day colectomy, and 30-day mortality) and used these factors to design a prognostic scoring system. After multivariate analysis, ulceration on colonoscopy, shock within 24 h of admission and tachycardia at the time of diagnosis were independent predictors of those outcomes. However, given their inclusion of patients with nonpathologically confirmed IC and including nontraditional endpoints for severe disease it is somewhat difficult to generalise its results, despite the model's ease of use. Thus, a scoring model for systematic prediction of short-term mortality of IC is needed. Our study aimed to address this issue.
Initially, we analysed the relation of clinical, laboratory, imaging, endoscopic and surgical factors with in-hospital mortality. We found that the presence of elevation of LDH, urea or creatinine, length of hospital stay, male gender, atrial fibrillation, bowel occlusion, recent vascular intervention, endoscopic severity, surgical intervention, vasopressor support, parenteral nutrition, empirical antibiotherapy and ICU admission were associated with a higher in-hospital mortality rate in univariate analyses. However in multivariate regression analyses, only the need for vasopressor support, ICU admission and atrial fibrillation were identified as significant independent factors for the prediction of in-hospital mortality. Therefore, based on these data we calculated our scoring model, which exhibited a high overall accuracy for the prediction of in-hospital mortality. However, despite the high significance of the three variables in our model, we observed wide ranges for the CIs.
Notwithstanding our large sample, more studies are warranted with prospective designs and external validation in other institutions in order to assess the power of the presented predictive model.
Regarding mortality at 90 days, univariate analysis revealed associations similar to those observed in in-hospital mortality, adding that the male sex and history of chronic kidney disease was associated independently, probably reflecting a subgroup of patients with more comorbidities and consequently less functional reserve, as previously reported in nonpostoperative IC patients,16 suggesting that they deserve special attention and aggressive therapy. However after multivariate analysis, it was not possible to build a predictive model similar to that previously described by the lack of statistical power. More importantly, however, is that about two-thirds of the deaths have occurred up until this time period, revealing that during this phase, patients should be closely monitored for early diagnosis of clinical worsening and detection and treatment complications.
Our study has several limitations. Data was obtained retrospectively, which may have affected the results because of the drawbacks of such an investigational design. This bias was somewhat reduced by selecting only patients with histopathologically proven IC. Also the number of severely ill patients was still small. Despite the high significance of the several variables in multivariate analysis and in the predictive model, we observed wide ranges for CIs, which can also be attributed to the limited number of patients with severe ischaemic colitis. Future studies with prospective application of the predictive model are needed before clinicians can apply it in clinical practice.
Conclusion
Multiple clinical and analytical factors easily measured on admission are associated with in-hospital mortality from IC. Most deaths occur up to 90 days after diagnosis, which is the highest risk period in which patients should be monitored more regularly. Based on some variables that include AF, vasopressor support and admission in ICU we created a predictive model of in-hospital mortality with high sensitivity and specificity, which could be a useful tool in approaching the patient diagnosed with IC, thus potentially reducing mortality in a high risk stage. Mortality at 90 days had similar risk factors, with anaemia severity and chronic kidney failure being additional independent risk factors.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Marston A, Pheils MT, Thomas ML. Ischaemic colitis. Gut 1966; 7: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boley SJ, Schwartz S, Lash J, et al. Reversible vascular occlusion of the colon. Surg Gynecol Obstet 1963; 116: 53–60. [PubMed] [Google Scholar]
- 3.Higgins PD, Davis KJ, Laine L. Systematic review: The epidemiology of ischaemic colitis. Aliment Pharmacol Ther 2004; 19: 729–738. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi SK, Hanson MM, Vernava AM, et al. Ischemic colitis. Dis Colon Rectum 1996; 39: 88–100. [DOI] [PubMed] [Google Scholar]
- 5.Greenwald DA, Brandt LJ. Colonic ischemia. J Clin Gastroenterol 1998; 27: 122–128. [DOI] [PubMed] [Google Scholar]
- 6.Longo WE, Ward D, Vernava AM, et al. Outcome of patients with total colonic ischemia. Dis Colon Rectum 1997; 40: 1448–1454. [DOI] [PubMed] [Google Scholar]
- 7.Brandt LJ, Feuerstadt P, Longstreth GF, et al. ACG clinical guideline: Epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia (CI). Am J Gastroenterol 2015; 110: 18–44. [DOI] [PubMed] [Google Scholar]
- 8.Añón R, Bosca MM, Sanchiz V, et al. Factors predicting poor prognosis in ischemic colitis. World J Gastroenterol 2006; 12: 4875–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huguier M, Barrier A, Boelle PY, et al. Ischemic colitis. Am J Surg 2006; 192: 679–684. [DOI] [PubMed] [Google Scholar]
- 10.Mosele M, Cardin F, Inelmen EM, et al. Ischemic colitis in the elderly: Predictors of the disease and prognostic factors to negative outcome. Scand J Gastroenterol 2010; 45: 428–433. [DOI] [PubMed] [Google Scholar]
- 11.O’Neill S, Elder K, Harrison SJ, et al. Predictors of severity in ischaemic colitis. Int J Colorectal Dis 2012; 27: 187–191. [DOI] [PubMed] [Google Scholar]
- 12.Longstreth GF, Yao JF. Epidemiology, clinical features, high-risk factors, and outcome of acute large bowel ischemia. Clin Gastroenterol Hepatol 2009; 7: 1075–1080. [DOI] [PubMed] [Google Scholar]
- 13.Montoro MA, Brandt LJ, Santolaria S, et al. Clinical patterns and outcomes of ischaemic colitis: Results of the Working Group for the Study of Ischaemic Colitis in Spain (CIE study). Scand J Gastroenterol 2011; 46: 236–246. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto S, Tsuji K, Shirahama S. Clinical investigation of 41 patients with IC accompanied by ulcer. World J Gastroenterol 2007; 13: 1236–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung JW, Cheon JH, Park JJ, et al. Development and validation of a novel prognostic scoring model for ischemic colitis. Dis Colon Rectum 2010; 53: 1287–1294. [DOI] [PubMed] [Google Scholar]
- 16.Lee TC, Wang HP, Chiu HM, et al. Male gender and renal dysfunction are predictors of adverse outcome in nonpostoperative ischemic colitis patients. J Clin Gastroenterol 2010; 44: e96–e100. [DOI] [PubMed] [Google Scholar]