Abstract
Background
Despite differences between men and women in incidence of colorectal cancer (CRC) and its precursors, screening programs consistently use the same strategy for both genders.
Objective
The objective of this article is to illustrate the effects of gender-tailored screening, including the effects on miss rates of advanced neoplasia (AN).
Methods
Participants (age 50–75 years) in a colonoscopy screening program were asked to complete a fecal immunochemical test (FIT) before colonoscopy. Positivity rates, sensitivity and specificity for detection of AN at multiple cut-offs were determined. Absolute numbers of detected and missed AN per 1000 screenees were calculated.
Results
In total 1,256 individuals underwent FIT and colonoscopy, 51% male (median age 61 years; IQR 56–66) and 49% female (median age 60 years; IQR 55–65). At all cut-offs men had higher positivity rates than women, ranging from 3.8% to 10.8% versus 3.2% to 4.8%. Sensitivity for AN was higher in men than women; 40%–25% and 35%–22%, respectively. More AN were found and missed in absolute numbers in men at all cut-offs.
Conclusion
More AN were both detected and missed in men compared to women at all cut-offs. Gender-tailored cut-offs could either level sensitivity in men and women (i.e., lower cut-off in women) or level the amount of missed lesions (i.e., lower cut-off in men).
Keywords: Colorectal cancer, fecal immunochemical test, gender, screening, miss rates
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-related death in the Western world.1,2 Detection of occult blood in feces by guaiac fecal occult blood testing (gFOBT) has been proven to reduce CRC-related mortality.3 In recent years, fecal immunochemical testing (FIT) has become the preferred method of detecting fecal occult blood for CRC screening. FIT is more sensitive for the detection of CRC and its precursors.4,5 In addition, FIT is easier to handle than gFOBT.6 Consequently, screening participation rates increase.4,7,8 Also, FIT analysis can be automated and quantitated. Quantitative FITs enable adjustment of cut-off to vary test characteristics and match demand with available resources, in particular colonoscopy capacity.9
Men and women differ with respect to the prevalence of advanced colorectal neoplasia, with men having substantial higher prevalence of advanced adenomas and CRC than women.10,11 Repeated biennial gFOBT screening leads to a higher overall mortality reduction in men than in women.3 In fact, a recent gFOBT-based study showed that the prevalence of colorectal neoplasms was higher in men with a negative test than in women with a positive test.12 Moreover, male gender seems to be a stronger predictor of CRC than a positive gFOBT.13 Results from the national gFOBT screening program in Scotland showed a lower proportion of interval cancers for men compared to women.14 One study, using FIT, showed that men had higher positivity rates, as well as a higher detection rate.15 However, this study was limited as only FIT-positive (i.e., a fecal hemoglobin concentration >10 µg Hb/g feces) screenees underwent colonoscopy.
As more screening programs are being implemented worldwide, these gender differences become more apparent. Despite these differences, screening programs consistently use the same strategies for both genders with regards to cut-off and screening intervals,10,16 even though the use of different cut-offs in men and women would allow tailored screening strategies for each gender and improve CRC screening efficacy.
Most studies on gender differences in CRC screening used gFOBT or FIT with one cut-off for both genders. Results were often based on assessing equal sensitivity of the test for men and women, thereby not taking into account gender differences regarding detection rate and miss rate of lesions in absolute numbers. Therefore, we aimed to illustrate the effect of gender-tailored FIT screening including the detection and miss rates of advanced neoplasia.
Methods
The protocol of this population-based screening pilot (trialregister.nl; identifier NTR3549) has been described previously in detail.17,18 All authors had access to the study data and reviewed and approved the final manuscript.
Study population
Between June 2009 and July 2010, 6600 asymptomatic individuals aged 50–75 years, living in the Amsterdam and Rotterdam regions, were randomly selected from regional municipal administration registrations. They were invited for colonoscopy screening as primary screening modality or invited for computed tomography colonography. For the purpose of this manuscript only data of the population undergoing colonoscopy were used.
Individuals with a history of inflammatory bowel disease or CRC, as well as those who had undergone a full colonic examination in the past five years, those with an estimated life expectancy of <5 years, and people who were unable to give informed consent were excluded from the study. As there was no CRC screening program at the time of the trial in the Netherlands, the target population was screening-naive when first approached.
Fecal occult blood screening and colonoscopy
Eligible individuals who gave informed consent for colonoscopy screening were asked to complete one sample FIT (OC-sensor, Eiken, Japan) before colonoscopy. Participants were instructed to perform FIT at home, within 48 hours before the colonoscopy, but before starting the bowel preparation. No dietary restrictions were given. All patients underwent subsequent colonoscopy by experienced endoscopists. Research staff attended all colonoscopies and prospectively documented colonoscopy quality indicators and data on CRC and polyp detection.
Histology
Experienced pathologists classified all removed lesions as non-neoplastic, serrated polyp, adenoma (tubular, tubulovillous or villous) or carcinoma. Dysplasia was defined as low-grade or high-grade. Advanced adenomas were defined as an adenoma larger than 10 mm, an adenoma with villous histology (>25%) and/or an adenoma with high-grade dysplasia. Advanced neoplasia (AN) included both AA and CRC.
Statistical analysis
All screening participants who completed a FIT and subsequently underwent colonoscopy were included in the analysis. Baseline characteristics were described using descriptive statistics. The Chi-square test was used for comparing proportions of AN between men and women. The Mann-Whitney U test was used for non-parametric distributions. The sensitivity, specificity, positive and negative predictive value (PPV/NPV), and detection rate (DR) of AN were calculated for the most commonly used cut-offs: 10 (FIT10), 20 (FIT20), 30 (FIT30) and 40 (FIT40) µg Hb/g feces. These values correspond to 50, 100, 150 and 200 ng Hb/ml buffer. Following, sensitivity and specificity for fecal Hb concentrations for all cutoffs between 0 and 100 µg Hb/g feces were calculated. Absolute numbers of detected and missed AN per 1000 individuals screened were calculated for men and women.
Results
Baseline characteristics and colonoscopy outcome
In total 1,256 invitees underwent FIT and colonoscopy, 638 men and 618 women. Men (61 years, interquartile range (IQR) 56–66) were slightly older than women (60 years, IQR 55–65). Gender-specific findings at colonoscopy are described in Table 1. AN detection rate was slightly higher in men than in women, 10.6% (68/638) versus 8.3% (51/618) (p = 0.146). CRC was detected in five (0.8%) men and in three (0.5%) women. No differences between men and women were seen in location of AN or number of AN per participant. In individuals with CRC, the median fecal hemoglobin concentration was 61 µg Hb/g feces (range 0–251 µg Hb/g) in men and 77 µg Hb/g feces (range 13–448 µg Hb/g) in women (p = 0.76). In individuals with AN, men had a median fecal Hb concentration of 3.2 µg Hb/g feces (range 0–485 µg Hb/g) and women 2.6 µg Hb/g feces (IQR 0–670 µg Hb/g) (p = 0.94).
Table 1.
Findings at colonoscopy in men and women
| Men | Women | ||
|---|---|---|---|
| n = 638 | n = 618 | p value | |
| Most advanced finding at colonoscopya | |||
| No histology | 17 (2.7%) | 3 (0.5%) | |
| No abnormalities | 303 (47.5%) | 357 (57.8%) | |
| SSA < 10 mm | 19 (3.0%) | 17 (2.8%) | |
| HP | 82 (12.8%) | 77 (12.4%) | |
| TA < 10 mm | 149 (23.4%) | 113 (18.3%) | |
| TA ≥ 10 mm | 21 (3.3%) | 16 (2.6%) | |
| SSA ≥ 10 mm | 1 (0.2%) | 1 (0.2%) | |
| TVA | 32 (5.0%) | 25 (4.1%) | |
| VA | 2 (0.3%) | 0 | |
| HGD | 7 (1.1%) | 6 (0.9%) | |
| CRC | 5 (0.8%) | 3 (0.5%) | |
| Total advanced neoplasia | 68 (10.6%) | 51 (8.3%) | 0.15 |
| Location of most advanced neoplasiab | |||
| Distal/proximal (%) | 51 (75)/17 (25) | 38 (75)/13 (25) | 0.95 |
| Number of advanced neoplasia per participant n (%)b | 0.55 | ||
| 1 | 53 (77.9%) | 42 (82.4%) | |
| >1 | 15 (22.1%) | 9 (17.6) |
No histology: removed polyp not retrieved for histology; SSA: sessile-serrated adenoma; HP: hyperplastic polyp; TA: tubular adenoma; TVA: tubular villous adenoma; VA villous adenoma; HGD: high-grade dysplasia; CRC: colorectal cancer.
Only individuals with advanced neoplasia included (men n = 68 and women n = 51).
Test characteristics
Performance characteristics of FIT for AN are provided in Table 2. Differences in test characteristics were not significant. At each of the pre-specified cut-offs, men had slightly higher positivity rates than women. The positivity rates ranged from 3.2% to 10.8% for the highest and lowest cut-off. The sensitivity for AN ranged from 40% (95% confidence interval (CI) 29%–52%) at FIT10 to 25% (95% CI 16%–37%) at FIT40 in men, and from 35% (95% CI 24%–49%) at FIT10 to 22% (95% CI 12%–35%) at FIT40 in women. The specificity of FIT for AN tended to be lower in men when compared to women up to cut-offs of 20 µg Hb/g. The detection rate of AN was higher in men than women at all cut-offs. False-positivity rates ranged from 1.1% (95% CI 0.5%–2.3%) to 6.6% (95% CI 4.9%–8.8%) for men and from 1.5% (95% CI 0.8%–2.8%) to 5.5% (95% CI 4.0%–7.6%) for women. True positivity rates ranged from 2.7% (95% CI 1.7%–4.2%) to 4.2% (95% CI 2.9%–6.1%) for men and from 1.8% (95% CI 1.0%–3.2%) to 2.9% (95% CI 1.8%–4.6%) for women. Sensitivity and specificity were calculated for the study population at multiple cut-offs ranging from 0 to 100 µg Hb/g feces (Figure 1). At an increasing cut-off, in both genders there is a relatively more rapid decline in sensitivity than an increase in specificity. Overall, men had slightly higher sensitivities than women. For example, at a commonly used cut-off of 10 µg Hb/g feces women should have a lower cut-off to reach the same sensitivity and specificity as men.
Table 2.
Positivity rate, sensitivity, specificity, positive predictive value, negative predictive value, detection rate for men and women at different cutoffs
| Men |
Women |
|||
|---|---|---|---|---|
| % | (95% CI) | % | (95% CI) | |
| FIT 10 | ||||
| PR | 10.8 | (8.6–13.5) | 8.4 | (6.5–10.9) |
| Sensitivity | 39.7 | (28.8–51.7) | 35.3 | (23.5–49.2) |
| Specificity | 92.6 | (90.2–94.5) | 94.0 | (91.7–95.7) |
| PPV | 39.1 | (28.4–51.0) | 34.6 | (23.0–48.8) |
| NPV | 92.7 | (90.4–94.7) | 94.2 | (91.9–95.8) |
| DR | 4.2 | (2.9–6.1) | 2.9 | (1.8–4.6) |
| FIT 20 | ||||
| PR | 6.3 | (4.6–8.4) | 5.0 | (3.5–7.0) |
| Sensitivity | 29.4 | (19.8–41.2) | 33.3 | (21.8–47.2) |
| Specificity | 96.4 | (94.6–97.7) | 97.5 | (95.9–98.5) |
| PPV | 50.0 | (35.0–65.0) | 54.8 | (37.4–71.1) |
| NPV | 92.0 | (89.5–93.9) | 94.2 | (92.0–95.8) |
| DR | 3.1 | (2.0–4.8) | 2.8 | (1.7–4.4) |
| FIT 30 | ||||
| PR | 5.0 | (3.6–7.0) | 3.6 | (2.4–5.3) |
| Sensitivity | 29.4 | (19.8–41.2) | 25.5 | (15.4–39.1) |
| Specificity | 98.0 | (96.3–98.8) | 98.4 | (97.0–99.2) |
| PPV | 62.5 | (44.9–77.3) | 59.0 | (38.2–77.2) |
| NPV | 92.1 | (89.6–94.0) | 93.6 | (91.4–95.3) |
| DR | 3.1 | (2.9–4.8) | 2.1 | (1.2–3.6) |
| FIT 40 | ||||
| PR | 3.8 | (2.5–5.6) | 3.2 | (2.1–5.0) |
| Sensitivity | 25.0 | (16.1–36.6) | 21.6 | (12.4–34.9) |
| Specificity | 98.8 | (97.4–99.4) | 98.4 | (97.0–99.2) |
| PPV | 70.8 | (50.2–85.4) | 55.0 | (33.6–74.7) |
| NPV | 91.7 | (89.2–93.6) | 93.3 | (91.0–95.1) |
| DR | 2.7 | (1.7–4.2) | 1.8 | (1.0–3.2) |
FIT: fecal immunochemical test; FIT 10: cutoff level 10 µg/g feces; FIT 20: cutoff level 20 µg/g feces; FIT 30: cutoff level 30 µg/g feces; FIT 40: cutoff level 40 µg/g feces; PR: positivity rate; PPV: positive predictive value; NPV: negative predictive value; DR: detection rate; CI: confidence interval.
Figure 1.
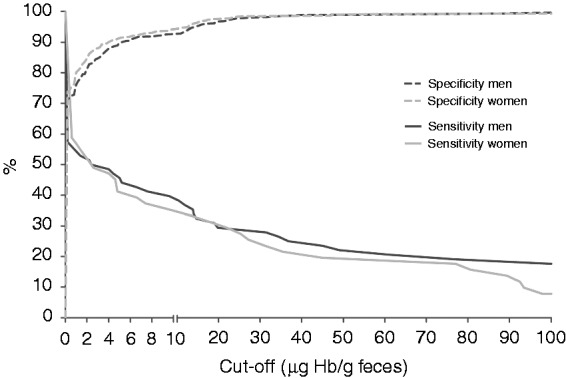
Sensitivity and specificity for men and women for all cut-offs ranging from 0 to 100 μg Hb/g feces.
Detected lesions in absolute numbers
At all cut-offs, more lesions were detected as well as missed in men than in women (Figure 2(a) and (b)). For all cut-offs the number needed to screen to identify one screenee with AN was higher in women than in men. It ranged from 38 to 56 participants in women and from 24 to 38 participants in men. Stepwise-lowering cut-offs for men from FIT40 to respectively FIT30, FIT20 and FIT10 successively resulted in the additional detection of 3, 0, and 7 AN. This required 8, 8, and 29 additional colonoscopies. Stepwise-lowering the cut-offs for women from FIT40 to FIT10 successively resulted in the additional detection of two, four and one AN. This required 2, 4 and 21 additional colonoscopies.
Figure 2.
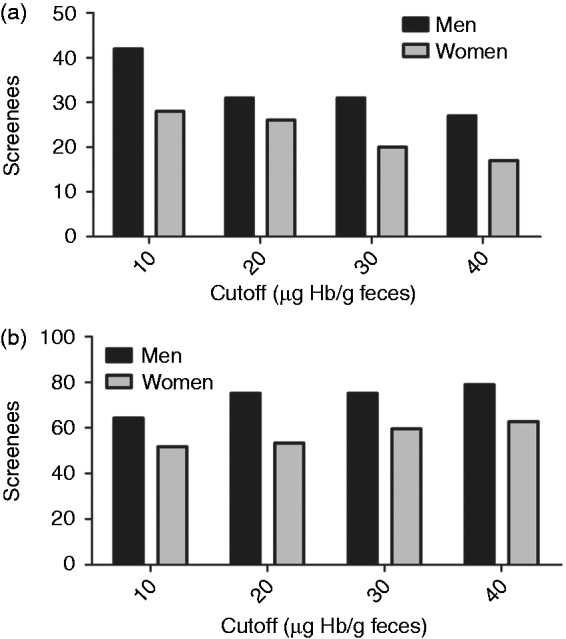
(a) Detected advanced neoplasia per 1000 screenees (for 100% participation in absolute numbers). (b) Missed advanced neoplasia per 1000 screenees (for 100% participation in absolute numbers).
Discussion
In this colonoscopy-based screening program, we evaluated gender differences with respect to the efficacy of FIT screening in average-risk individuals. Furthermore, we illustrated the effect of using different cut-offs on a broad spectrum of screening outcomes. Our study demonstrated that FIT had a higher sensitivity and lower specificity for AN in men than in women. By increasing the cut-off, a relatively more rapid decline in sensitivity was found than an increase in specificity for both genders. Furthermore, FIT had an overall higher PPV in men. When looking at diagnostic yield in absolute numbers, men had higher detection rates and miss rates of AN than women at all cut-offs. This last finding is of particular interest as in current literature little attention has been given to gender-specific miss rates of lesions.
A strength of this study is that this cohort was set in a population-based screening setting, making these results representative for average-risk screening populations. Also, as all participants underwent both colonoscopy and FIT, it is a very suitable population to demonstrate actual differences and to estimate the number of missed lesions. However, to appreciate our findings some limitations need to be discussed. Firstly, this cohort consists of relatively small numbers and was not powered to detect differences in men and women. Another limitation is that only individuals willing to undergo colonoscopy as a primary screening method participated, which could have led to a selection bias resulting in a population that is not representative of FIT participants. Only 22% of all invitees decided to participate in colonoscopy screening, while FIT screening generally has much higher participation rates around 60%.19,20 Nevertheless, the population includes only screening-naive average-risk participants, and therefore we think that the risk of selection bias is limited.
The introduction of fecal immunochemical testing was an important step forward in population-based CRC screening. FIT enables simple, low-burden primary screening at relatively low costs and has a high uptake. For this reason, an increasing number of countries have implemented FIT-based screening programs or are in the process of doing so.21 This is mostly associated with a marked increase in colonoscopy demand. This asks for a strong focus on optimal use of limited resources.
Differences between men and women in terms of number of advanced lesions, location of lesions and fecal hemoglobin concentrations are becoming more evident. Dissimilarities in prevalence of AN between men and women have been well described, with men having a substantially higher prevalence of AN than women.10,11 Consequently, research on tailored screening strategies become of significant importance. We are the first to describe the detection and miss rate of lesions in absolute numbers, showing that in men more lesions were both detected and missed for all cut-offs. This was especially the case at higher cut-offs. A previous Polish study showed that the number needed to screen in colonoscopy-based CRC screening to identify one screenee with AN was considerably higher in women than in men.10 Our data show that these numbers also apply to CRC screening programs based on FIT. At each FIT cut-off, 14 to 18 more women needed to be screened to find one case of AN compared to men.
Differences in FIT screening between men and women can be explained by a combination of factors. It has been suggested that because men have a higher hemoglobin concentration in general, blood from bleeding polyps will contain more globin.22 As FIT specifically detects globin in feces, blood from these polyps could be detected more frequently in men. This is supported by the fact that differences in fecal Hb concentration have been found in men and women.15,23 A second explanation could be that women have more right-sided lesions, as it is known that fecal occult blood testing may not be as sensitive for proximal lesions as it is for distal lesions.14,22,24 Yet, our data did not show differences in location of AN between men and women. Another reason for gender differences in FIT test characteristics could be the differences in colonic transit time between men and women, with women having slower transit times.25 A slower transit time could lead to more degradation of Hb and could decrease the likelihood of blood being detected by FIT.
An important question to be answered is how these results can be applied in CRC screening programs. Essentially, for gender-adjusted cut-offs in FIT-based CRC screening programs three scenarios are possible. These are the use of the same cut-off in both genders or using a higher cut-off in men than women or vice versa. An increase of the cut-off for men compared to women can lead to a similar proportional sensitivity for detection of AN in both groups. As a consequence the difference in PPV between men and women would increase, with men having a substantially higher PPV. Also, a higher cut-off in men would lead to a further increase in miss rates of AN in men in absolute numbers and thus to a further increase in difference of miss rates in terms of absolute numbers of advanced lesions compared to women. Furthermore, using a lower cut-off for women would result in a higher rate of false-positive tests in women. The opposite strategy, i.e., increasing the cut-off for women compared to men, can lead to a similar miss rate in terms of absolute numbers, and to a similar PPV in both genders. It would, however, result in decreased sensitivity and detection rates for women. In this scenario a larger proportion of the colonoscopy capacity would be used for men. However, such a strategy could make sense given that men are at higher risk of AN and subsequently the development of CRC.
Other gender-based CRC screening strategies besides adjusting the cut-off include the use of different age ranges for screening, changing screening modality, or the use of different screening intervals. A German study showed that women reached equivalent levels of CRC-related mortality as men at a four- to eight-year higher age.26 Gender differences in other screening modalities, such as colonoscopy, sigmoidoscopy, fecal biomarkers and fecal DNA, have not yet been extensively investigated. However, using different methods or combinations of tests for men and women could optimize screening efficacy and should be further investigated.
With regard to gender differences in patient-education, there is still much to gain. Information on miss rates of advanced lesions is an important issue in client information. At present, men and women are informed in the same manner about FIT-based CRC screening. These results help to accurately inform the client about the gender-dependent risk of miss rates and detection of advanced lesions in a FIT-based CRC screening program.
To conclude, CRC screening using FIT with the same cut-off for both genders results in a higher sensitivity and lower specificity for AN in men than in women. In absolute numbers more AN are detected and missed in men for all cut-offs. Therefore, tailored cut-off based on gender could either level sensitivity in men and women by using a lower cut-off in women, or level the amount of missed lesions when using a lower cut-off in men. Adjusting cut-offs based on gender can contribute to the efficacy of FIT-based CRC screening programs and optimize the use of available endoscopy resources. In addition, individuals invited to attend a FIT-based CRC screening should be informed accordingly about these gender differences.
Acknowledgments
E.J. Grobbee and M.C.W. Spaander conceived the idea for this study. E.J. Grobbee, M.C.W. Spaander and E.J. Kuipers designed and conceptualized the study. M.C.W. Spaander supervised execution of the study. E.M. Stoop and T.R. de Wijkerslooth were responsible for data entry. E.J. Grobbee, E. Wieten, M.C.W. Spaander, E.J. Kuipers and I. Lansdorp-Vogelaar performed analysis and interpretation of data. E.J. Grobbee drafted the manuscript. E.Wieten, T.R. de Wijkerslooth, I. Lansdorp-Vogelaar, E. Dekker, P.M. Bossuyt, M.C.W. Spaander and E.J. Kuipers provided critical revision of the manuscript for important intellectual content.
Declaration of conflicting interests
None declared.
Funding
This work was supported by The Netherlands Organization for Health Research and Development (ZonMW 120720012) and by the Center for Translational Molecular Medicine (CTMM DeCoDe-project).
References
- 1.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev 2009; 18: 1688–1694. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116: 544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013; 369: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 4.von Karsa L, Patnick J, et al. European Colorectal Cancer Screening Guidelines Working Group. European guidelines for quality assurance in colorectal cancer screening and diagnosis: Overview and introduction to the full supplement publication. Endoscopy 2013; 45: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: Random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. Br J Cancer 2009; 100: 1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tinmouth J, Lansdorp-Vogelaar I, Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: What clinicians and colorectal cancer screening programme organisers need to know. Gut 2015; 64: 1327–1337. [DOI] [PubMed] [Google Scholar]
- 7.Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer 2013; 49: 3049–3054. [DOI] [PubMed] [Google Scholar]
- 8.Halloran SP, Launoy G, Zappa M, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Faecal occult blood testing. Endoscopy 2012; 44(Suppl 3): SE65–SE87. [DOI] [PubMed] [Google Scholar]
- 9.Kuipers EJ, Rosch T, Bretthauer M. Colorectal cancer screening—optimizing current strategies and new directions. Nat Rev Clin Oncol 2013; 10: 130–142. [DOI] [PubMed] [Google Scholar]
- 10.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med 2006; 355: 1863–1872. [DOI] [PubMed] [Google Scholar]
- 11.Brenner H, Hoffmeister M, Stegmaier C, et al. Risk of progression of advanced adenomas to colorectal cancer by age and sex: Estimates based on 840,149 screening colonoscopies. Gut 2007; 56: 1585–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenner H, Hoffmeister M, Birkner B, et al. Men with negative results of guaiac-based fecal occult blood test have higher prevalences of colorectal neoplasms than women with positive results. Int J Cancer 2014; 134: 2927–2934. [DOI] [PubMed] [Google Scholar]
- 13.Ferlitsch M, Heinze G, Salzl P, et al. Sex is a stronger predictor of colorectal adenoma and advanced adenoma than fecal occult blood test. Med Oncol 2014; 31: 151–151. [DOI] [PubMed] [Google Scholar]
- 14.Steele RJ, McClements P, Watling C, et al. Interval cancers in a FOBT-based colorectal cancer population screening programme: Implications for stage, gender and tumour site. Gut 2012; 61: 576–581. [DOI] [PubMed] [Google Scholar]
- 15.Kapidzic A, van der Meulen MP, Hol L, et al. Gender differences in fecal immunochemical test performance for early detection of colorectal neoplasia. Clin Gastroenterol Hepatol 2015; 13: 1464–1471. e4. [DOI] [PubMed] [Google Scholar]
- 16.Ferlitsch M, Reinhart K, Pramhas S, et al. Sex-specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. JAMA 2011; 306: 1352–1358. [DOI] [PubMed] [Google Scholar]
- 17.de Wijkerslooth TR, de Haan MC, Stoop EM, et al. Study protocol: Population screening for colorectal cancer by colonoscopy or CT colonography: A randomized controlled trial. BMC Gastroenterol 2010; 10: 47–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. Am J Gastroenterol 2012; 107: 1570–1578. [DOI] [PubMed] [Google Scholar]
- 19.de Wijkerslooth TR, de Haan MC, Stoop EM, et al. Burden of colonoscopy compared to non-cathartic CT-colonography in a colorectal cancer screening programme: Randomised controlled trial. Gut 2012; 61: 1552–1559. [DOI] [PubMed] [Google Scholar]
- 20.Kapidzic A, Grobbee EJ, Hol L, et al. Attendance and yield over three rounds of population-based fecal immunochemical test screening. Am J Gastroenterol 2014; 109: 1257–1264. [DOI] [PubMed] [Google Scholar]
- 21.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: A global overview of existing programmes. Gut 2015; 64: 1637–1649. [DOI] [PubMed] [Google Scholar]
- 22.Brenner H, Haug U, Hundt S. Sex differences in performance of fecal occult blood testing. Am J Gastroenterol 2010; 105: 2457–2464. [DOI] [PubMed] [Google Scholar]
- 23.Auge JM, Pellise M, Escudero JM, et al. Risk stratification for advanced colorectal neoplasia according to fecal hemoglobin concentration in a colorectal cancer screening program. Gastroenterology 2014; 147: 628–636. e1. [DOI] [PubMed] [Google Scholar]
- 24.Chacko L, Macaron C, Burke CA. Colorectal cancer screening and prevention in women. Dig Dis Sci 2015; 60: 698–710. [DOI] [PubMed] [Google Scholar]
- 25.Meier R, Beglinger C, Dederding JP, et al. Influence of age, gender, hormonal status and smoking habits on colonic transit time. Neurogastroenterol Motil 1995; 7: 235–238. [DOI] [PubMed] [Google Scholar]
- 26.Brenner H, Hoffmeister M, Arndt V, et al. Gender differences in colorectal cancer: Implications for age at initiation of screening. Br J Cancer 2007; 96: 828–831. [DOI] [PMC free article] [PubMed] [Google Scholar]


