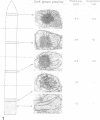
Abstract
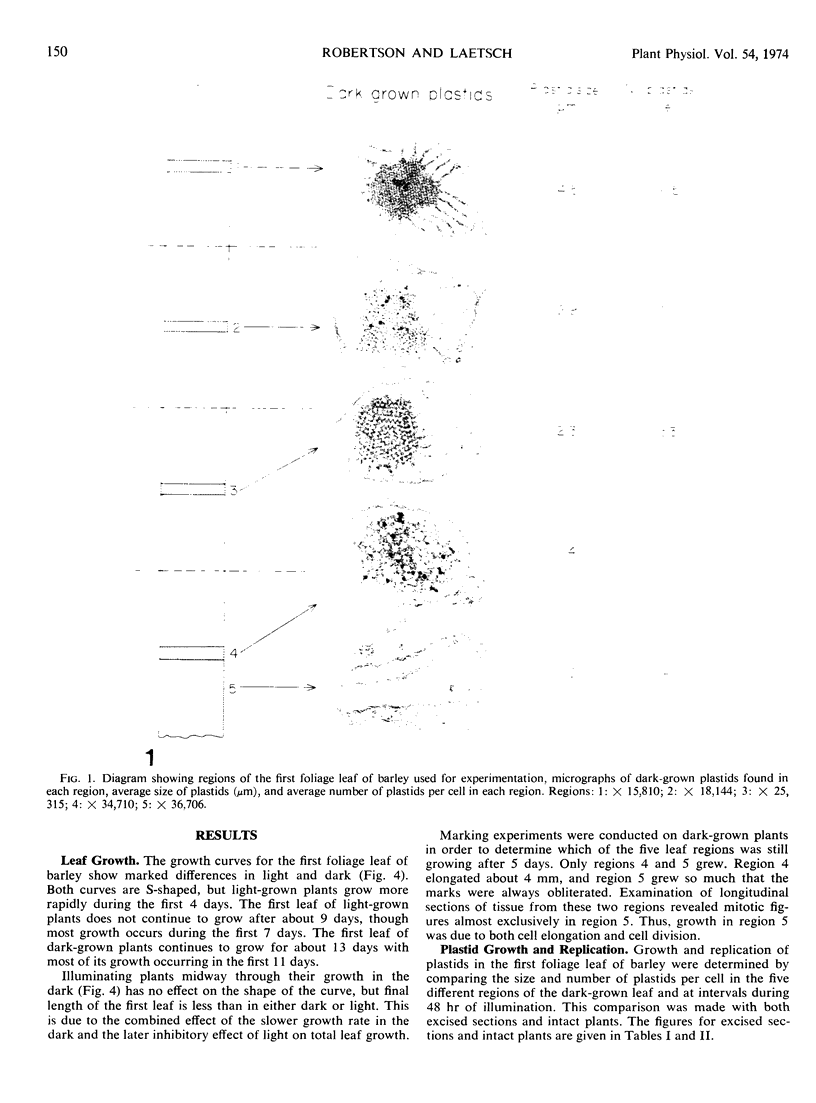
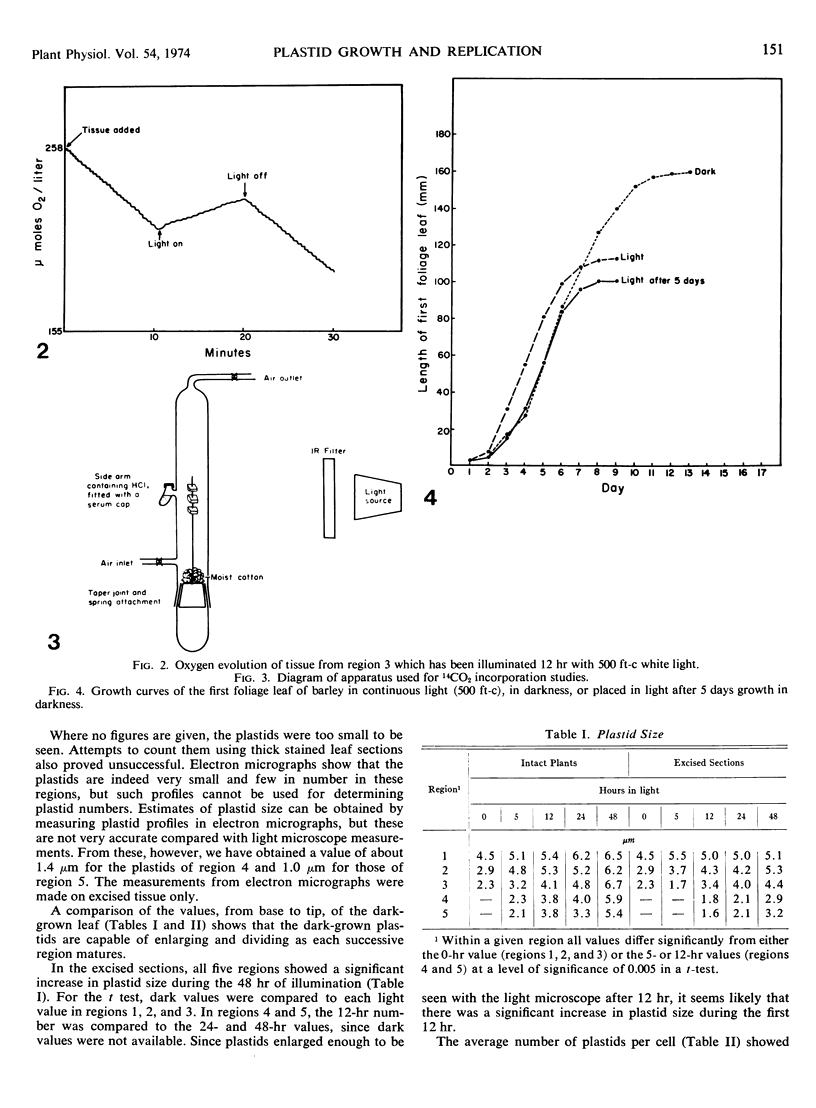
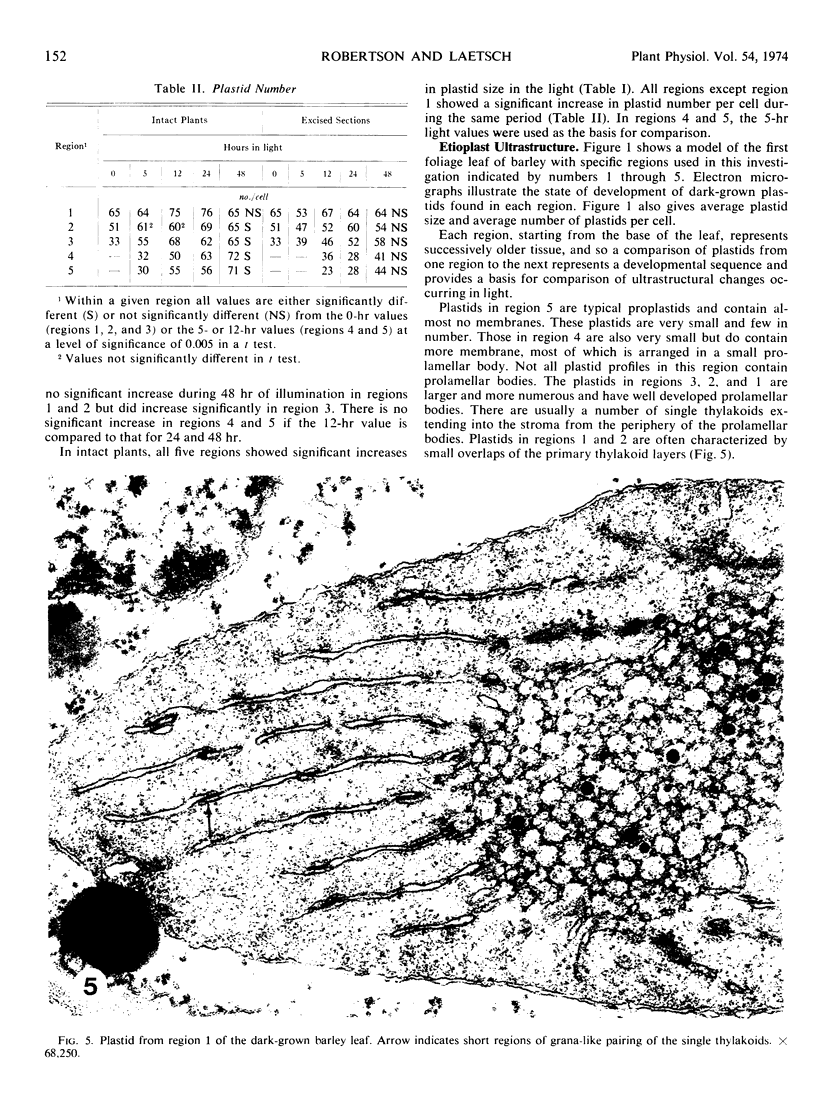
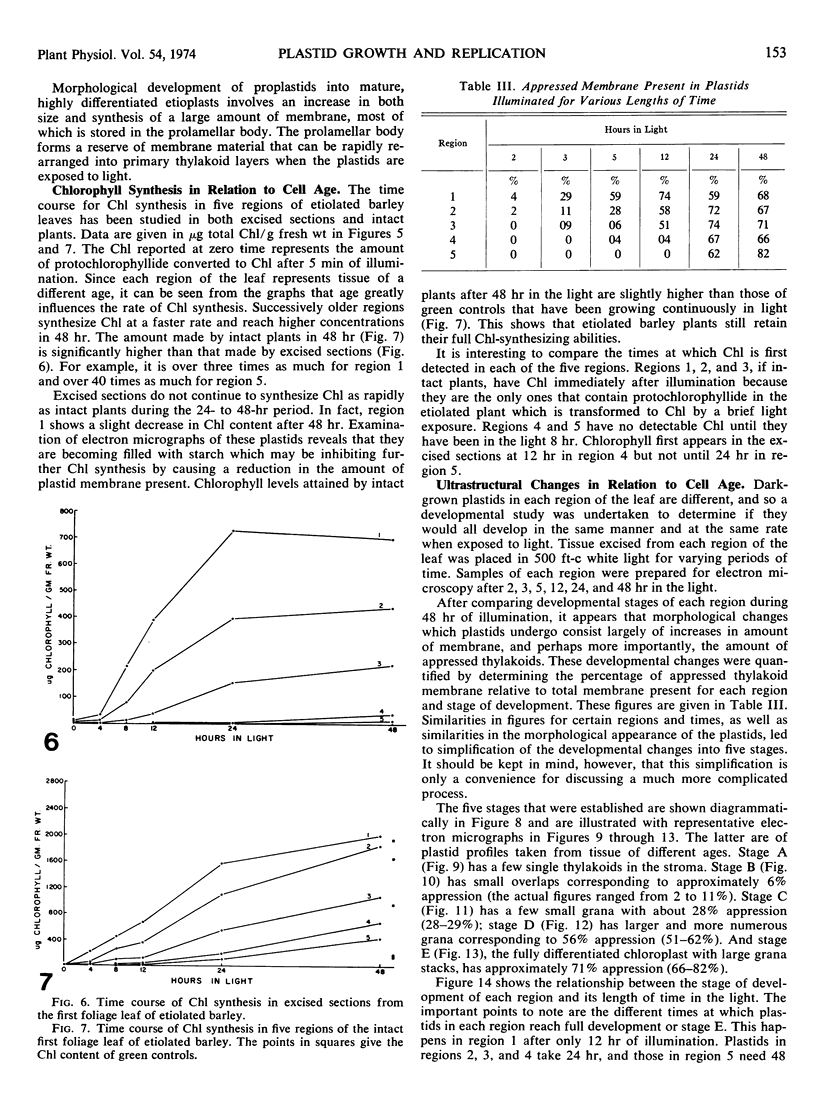
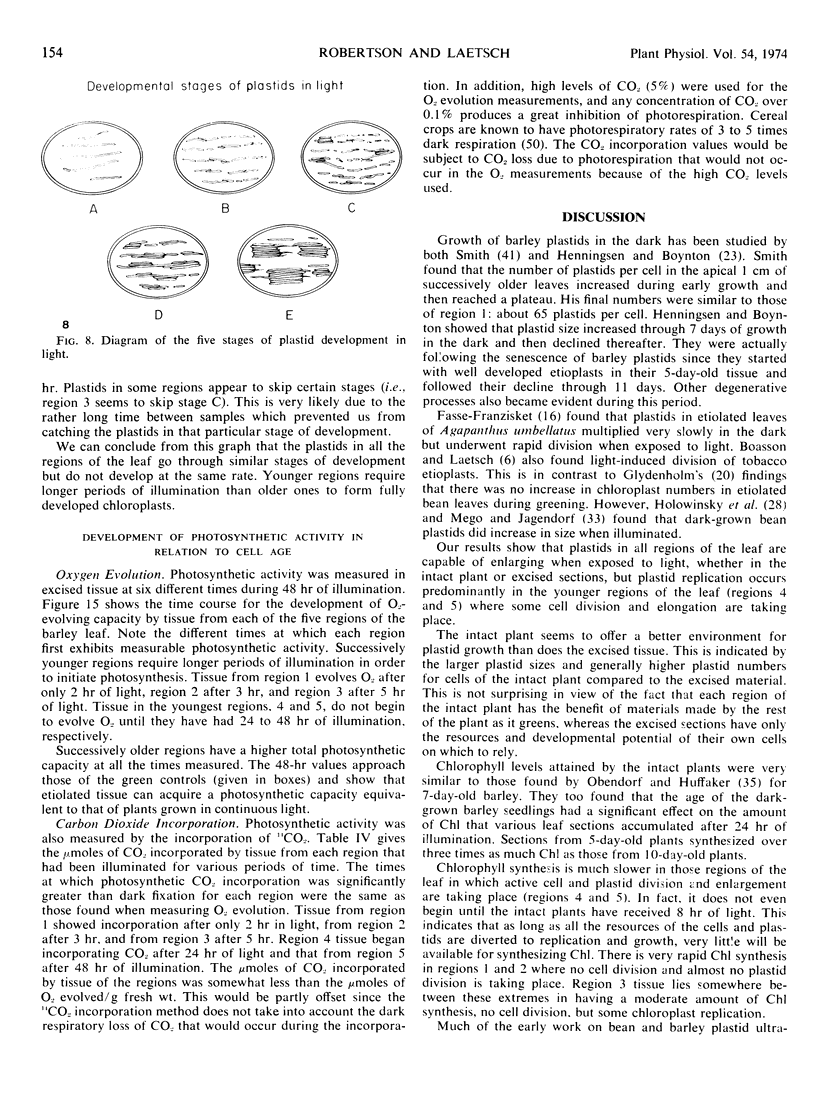
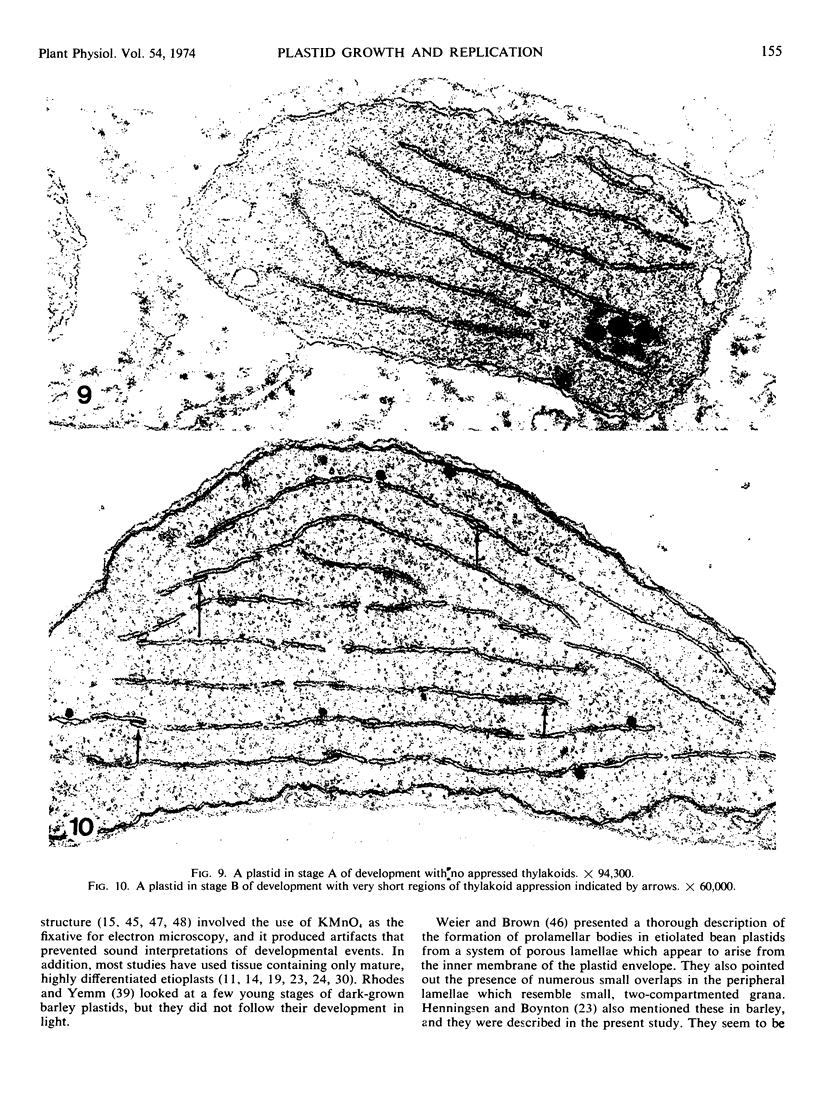
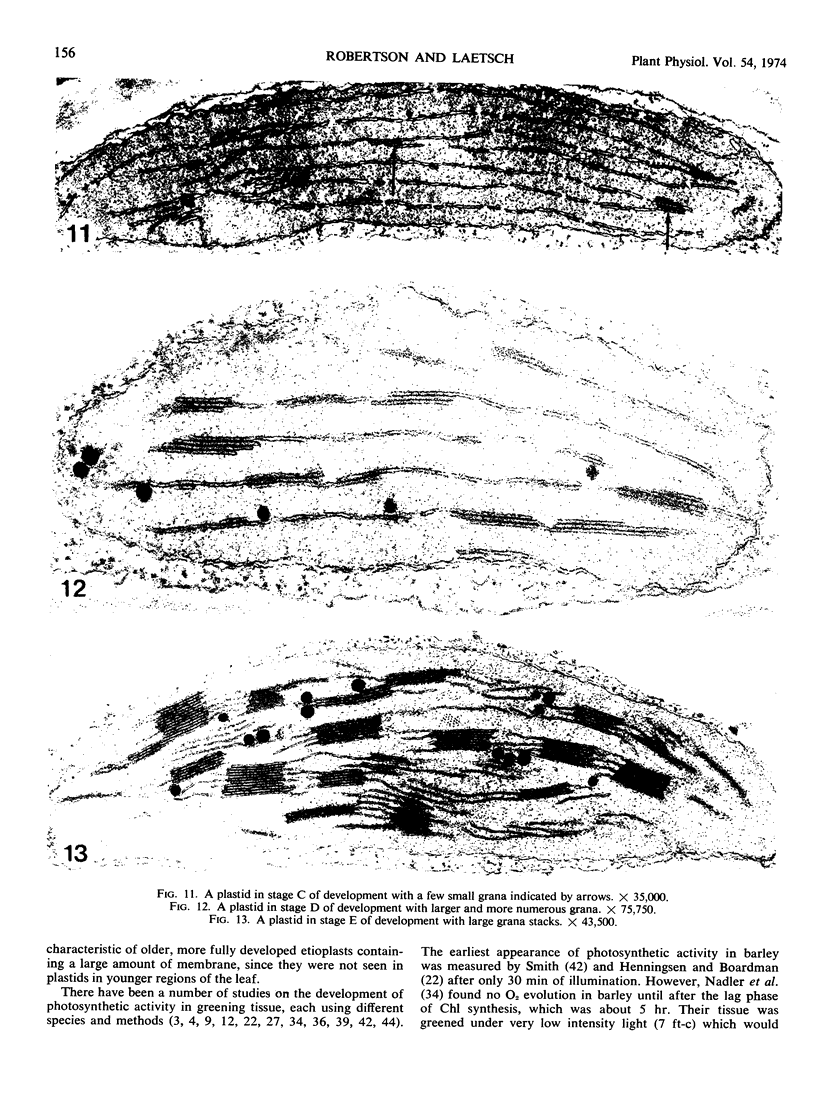
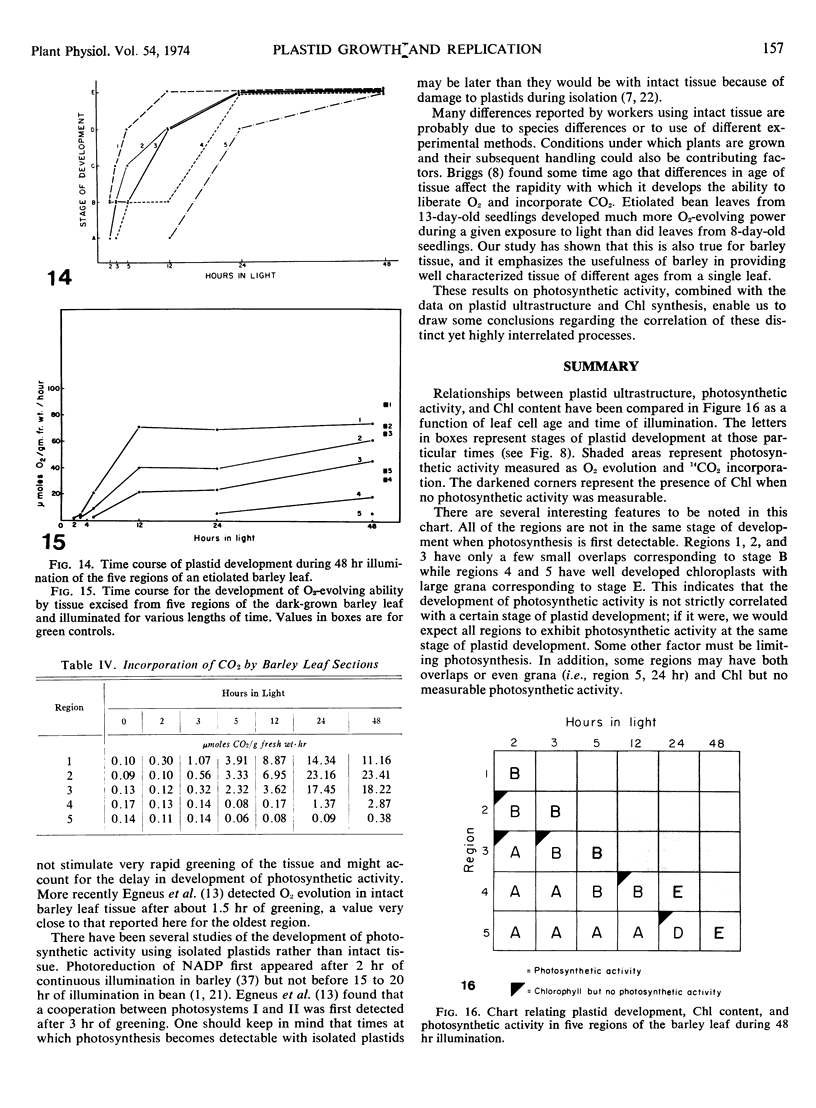
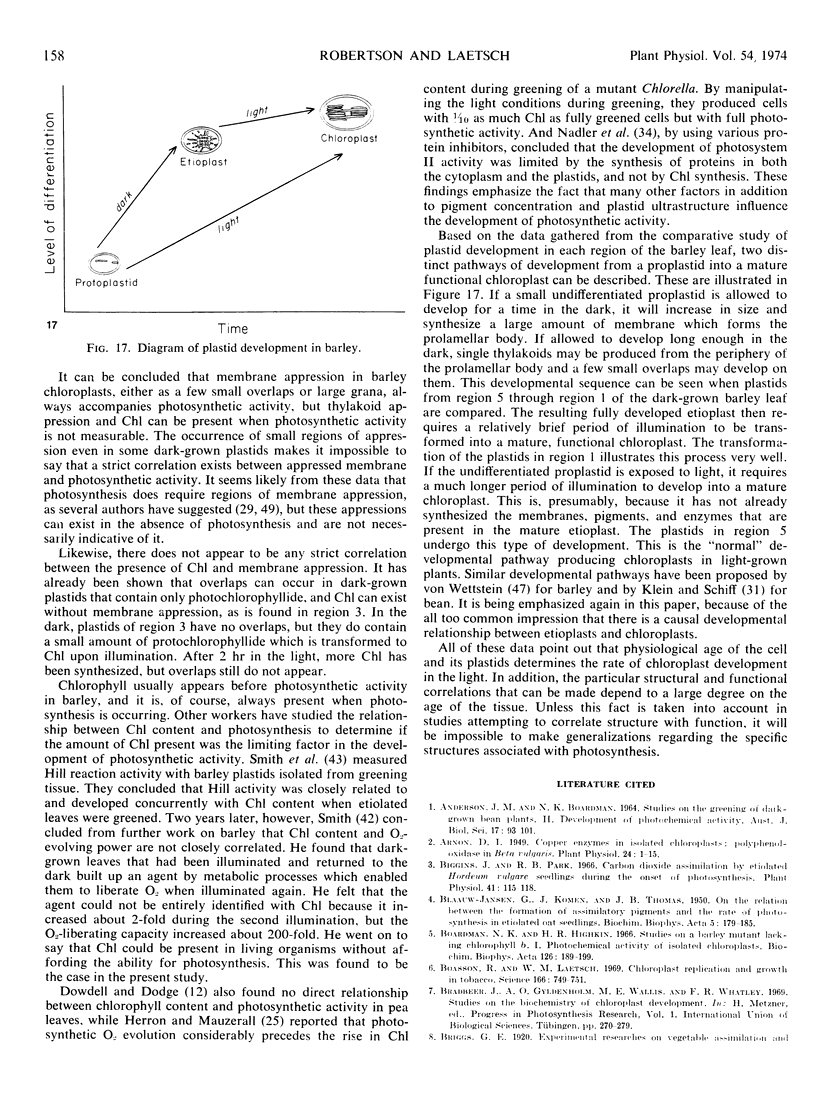
Five different regions of the first foliage leaf of etiolated barley seedlings were studied with respect to leaf growth, plastid growth and replication, differentiation of etioplasts, and conversion of etioplasts into chloroplasts upon illumination. Ultrastructural changes of the plastids were correlated with chlorophyll synthesis and development of photosynthetic activity as measured by 14CO2 incorporation and O2 evolution. The first foliage leaf has greater linear growth over a longer period of time in the dark than in the light. Only the bottom two regions (4 and 5) are still growing in the 5-day etiolated leaf. Region 4 grows by cell elongation, and region 5 grows by both cell division and elongation. Plastids in all five regions of the leaf are capable of enlarging when exposed to light. This is true both for the intact plant and for excised sections. Plastid replication occurs predominantly in the younger regions of the leaf (regions 3, 4, and 5). The amount of chlorophyll synthesized by different regions in the intact plant is significantly higher (3-40 times) than that made by excised sections. Ultrastructural changes occurring in each region when excised sections are illuminated were classified into five stages involving increased membrane synthesis and appression into grana, and these changes were correlated with the first appearance of photosynthetic activity. The earliest detectable photosynthetic activity occurs in region 1 after 2 hours of illumination when chloroplasts show only a few overlaps in the thylakoids. Plastids in younger regions of the leaf require up to 24 hours of light to form grana and develop photosynthetic activity. Plastids in each region of the leaf are in different stages of development when photosynthesis is initiated, indicating that development of photosynthetic activity is not strictly correlated with a certain stage of plastid development. Membrane appression is not indicative of photosynthetic activity since overlaps are formed in the dark, but it was always present when photosynthetic activity was detectable. Likewise, there does not appear to be any strict correlation between the presence of chlorophyll and membrane appression. These results show that the particular structural and functional correlations that can be made depend to a large degree on age of the tissue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Greef J., Butler W. L., Roth T. F. Greening of etiolated bean leaves in far red light. Plant Physiol. 1971 Apr;47(4):457–464. doi: 10.1104/pp.47.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild D. J., Highkin H. R., Boardman N. K. The fine structure of chloroplasts in a barley mutant lacking chlorophyll B. Exp Cell Res. 1966 Oct;43(3):684–688. doi: 10.1016/0014-4827(66)90045-0. [DOI] [PubMed] [Google Scholar]
- Goodenough U. W., Armstrong J. J., Levine R. P. Photosynthetic Properties of ac-31, a Mutant Strain of Chlamydomonas reinhardi Devoid of Chloroplast Membrane Stacking. Plant Physiol. 1969 Jul;44(7):1001–1012. doi: 10.1104/pp.44.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyldenholm A. O. Macromolecular physiology of plastids V. On the nucleic acid metabolism during chloroplast development. Hereditas. 1968;59(1):142–168. doi: 10.1111/j.1601-5223.1968.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Henningsen K. W., Boynton J. E. Macromolecular physiology of plastids. 8. Pigment and membrane formation in plastids of barley greening under low light intensity. J Cell Biol. 1970 Feb;44(2):290–304. doi: 10.1083/jcb.44.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsen K. W., Boynton J. E. Macromolecular physiology of plastids. VII. The effect of a brief illumination on plastids of dark-grown barley leaves. J Cell Sci. 1969 Nov;5(3):757–793. doi: 10.1242/jcs.5.3.757. [DOI] [PubMed] [Google Scholar]
- Herron H. A., Mauzerall D. The development of photosynthesis in a greening mutant of chlorella and an analysis of the light saturation curve. Plant Physiol. 1972 Jul;50(1):141–148. doi: 10.1104/pp.50.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highkin H. R., Frenkel A. W. Studies of growth & metabolism of a barley mutant lacking chlorophyll b. Plant Physiol. 1962 Nov;37(6):814–820. doi: 10.1104/pp.37.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller R. G., Boardman N. K. Light driven redox changes of cytochrome f and the development of photosystems I and II during greening of bean leaves. Biochim Biophys Acta. 1971 Dec 7;253(2):449–458. doi: 10.1016/0005-2728(71)90048-x. [DOI] [PubMed] [Google Scholar]
- Homann P. H., Schmid G. H. Photosynthetic reactions of chloroplasts with unusual structures. Plant Physiol. 1967 Nov;42(11):1619–1632. doi: 10.1104/pp.42.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Schiff J. A. The Correlated Appearance of Prolamellar Bodies, Protochlorophyll(ide) Species, and the Shibata Shift during Development of Bean Etioplasts in the Dark. Plant Physiol. 1972 Apr;49(4):619–626. doi: 10.1104/pp.49.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEGO J. L., JAGENDORF A. T. Effect of light on growth of Black Valentine bean plastids. Biochim Biophys Acta. 1961 Oct 28;53:237–254. doi: 10.1016/0006-3002(61)90437-1. [DOI] [PubMed] [Google Scholar]
- Nadler K. D., Herron H. A., Granick S. Development of chlorophyll and hill activity. Plant Physiol. 1972 Mar;49(3):388–392. doi: 10.1104/pp.49.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obendorf R. L., Huffaker R. C. Influence of age and illumination on distribution of several calvin cycle enzymes in greening barley leaves. Plant Physiol. 1970 May;45(5):579–582. doi: 10.1104/pp.45.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze-Karow H., Butler W. L. The development of photophosphorylation and photosynthesis in greening bean leaves. Plant Physiol. 1971 Nov;48(5):621–625. doi: 10.1104/pp.48.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sane P. V., Goodchild D. J., Park R. B. Characterization of chloroplast photosystems 1 and 2 separated by a non-detergent method. Biochim Biophys Acta. 1970 Aug 4;216(1):162–178. doi: 10.1016/0005-2728(70)90168-4. [DOI] [PubMed] [Google Scholar]
- Smith J. H., French C. S., Koski V. M. The Hill Reaction: Development of Chloroplast Activity During Greening of Etiolated Barley Leaves. Plant Physiol. 1952 Jan;27(1):212–213. doi: 10.1104/pp.27.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. H. The Development of Chlorophyll and Oxygen-evolving Power in Etiolated Barley Leaves When Illuminated. Plant Physiol. 1954 Mar;29(2):143–148. doi: 10.1104/pp.29.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Gailey F. B. Carbon Dioxide Fixation by Etiolated Plants after Exposure to White Light. Plant Physiol. 1955 Nov;30(6):491–499. doi: 10.1104/pp.30.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]