Abstract
Migraine headache affects about 12% of Western populations and is the third most common disease worldwide (sixth in terms of disability). In 1993, triptans were introduced in the United States as a new treatment for managing migraine attacks, but their use is limited by lack of response and safety concerns in some patients. Treatment options for patients with migraine who fail or cannot tolerate triptans include switching to another medication or adding an adjunctive medication. Desirable characteristics reported by patients for acute treatment of migraine attacks include complete pain relief, fast onset of action, and no pain recurrence. Diclofenac is a nonsteroidal anti-inflammatory medication that has been established as effective for acute treatment of migraine by the American Headache Society based on available evidence. Diclofenac potassium for oral solution is rapidly absorbed, achieving maximal plasma concentrations in 15 min, which coincides with a rapid onset of effect. In a comparison of diclofenac potassium for oral solution with diclofenac potassium tablets, the solution achieved a significant reduction in headache intensity beginning at 15 min compared with 60 min for the tablet. Across randomized clinical trials, approximately 25% of patients were pain free 2 h after administration of diclofenac oral solution and the effects were maintained over a 24-h period. Diclofenac potassium for oral solution is well tolerated; the most common adverse events are dizziness and gastrointestinal complaints, with incidences similar to placebo. No serious adverse events have been reported in clinical trials of diclofenac potassium for oral solution in the acute treatment of migraine. Diclofenac oral solution may offer rapid and sustained pain relief for patients who do not achieve pain resolution with other medications. In addition, patients who experience central sensitization with allodynia may benefit from the cyclooxygenase-blocking activity of diclofenac, which is needed in this advanced phase of migraine.
Keywords: diclofenac potassium for oral solution, efficacy, migraine, migraine treatment, pharmacokinetics, pharmacology, safety
Introduction
Migraine is a common, disabling disorder that affects approximately 12% of individuals in Western countries.1,2 The incidence of migraine is approximately three times higher in women than in men.3 Migraine is associated with moderate-to-severe headache pain intensity and substantial disability, ranking sixth in global years lived with disability, an increase of 46% from 1990 to 2013.1,4 Migraine without aura is a recurrent headache with specific features that lasts 4–72 h (Table 1).4 Migraine with aura is characterized by visual, sensory, speech, or other central nervous system symptoms that typically develop gradually, last 5–60 min but average 20–30 min, and are fully reversible (Table 2).4
Table 1.
Diagnostic criteria for migraine without aura.4
| Recurrent headache | • ⩾5 attacks |
| • Lasting 4–72 h (untreated or successfully treated) | |
| Headache has ⩾2 characteristics | • Unilateral location |
| • Pulsating quality | |
| • Moderate or severe pain intensity | |
| • Aggravated by or causing avoidance of routine physical activity | |
| Headache associated with ⩾1 other symptom | • Nausea and/or vomiting |
| • Photophobia and phonophobia | |
| Headache cannot be better accounted for by another diagnosis | |
Table 2.
Diagnostic criteria for migraine with aura.4
| At least two attacks meeting the following criteria: | |
|---|---|
| Headache has ⩾1 fully reversible aura symptom | • Visual |
| • Sensory | |
| • Speech or language | |
| • Motor | |
| • Brainstem | |
| • Retinal | |
| Headache has ⩾2 characteristics related to aura symptoms | • ⩾1 aura symptom that slowly spreads over a period of ⩾5 min, and/or ⩾2 symptoms that occur one after another |
| • Each symptom lasts 5–60 min | |
| • ⩾1 symptom is unilateral | |
| • Aura is concurrent with or followed by a headache within 60 min | |
| Headache cannot be readily attributed to another diagnosis | |
| Transient ischemic attack has been excluded | |
The acute treatment of migraine attacks evolved in the 1990s with the availability of selective serotonin 5-HT1 receptor agonists, commonly known as triptans.5,6 Sumatriptan first became available in 1993 in the United States and was formulated for self-administration as a subcutaneous injection.6 Despite their wide uptake and recommendation as standard care for migraine attacks, triptans do not work for all patients and their use for migraine is relatively low in the United States.5,7 In the longitudinal American Migraine Prevalence and Prevention (AMPP) study, 18.3% of respondents used triptans; only 21.7% of respondents who were taking triptans were using them as monotherapy, while the remainder combined triptans with nonsteroidal anti-inflammatory drugs (NSAIDs; 43.3%), over-the-counter (OTC) analgesics (34.1%), or other OTC medications (34.8%).7 This study found that triptan use was associated with higher levels of disability related to headache and higher headache frequency.7 Among the 5591 respondents of the AMPP study who had migraine for fewer than 15 days per month (i.e. episodic migraine), 40.7% had at least one unmet treatment need (Table 3).8 More than one-third of patients (37.4%) with an unmet treatment need were dissatisfied with their therapy for acute migraine.8
Table 3.
Unmet treatment needs among AMPP survey respondents.8
| Unmet treatment needs, n (%) | Respondents (n = 5591) |
|---|---|
| 1 unmet treatment need | 1467 (26.2) |
| 2 or more unmet treatment needs | 807 (14.4) |
| Total with 1 or more unmet treatment needs | 2274 (40.7) |
| Criteria for unmet needsa | (n = 2274) |
| Moderate or severe disability related to headache | 1069 (47.0) |
| Dissatisfied with acute treatment regimen | 851 (37.4) |
| Excessive opioid or barbiturate use and/or probable dependence | 728 (32.0) |
| History of cardiovascular events | 595 (26.2) |
| 2 or more visits to the emergency department or an urgent care clinic for headache | 129 (5.7) |
Respondents could be counted for more than 1 criterion.
AMPP, American Migraine Prevalence and Prevention.
When a patient fails to respond optimally to migraine treatment, several options are available for improving outcomes.9 The dose, timing, or route of administration of the current therapy can be changed in an effort to improve response.9 Other options include switching to a different medication or adding another treatment to the current regimen.9 Patients may require several trials of different medications to achieve a “one-and-done” treatment for migraine. One must administer the highest tolerable effective dose via the best route of administration within 30 min of the start of migraine to achieve lasting pain relief.2,6,9 In patient satisfaction studies, the most desirable effects reported for an acute migraine treatment include complete pain relief, rapid onset of action, and no recurrence of pain.5 The route of administration not only determines the onset of effect but may also be an important consideration for patients who are experiencing nausea and vomiting or who have poor absorption due to gastroparesis or slow gastrointestinal transit times.2,6 Nasal sprays, subcutaneous injections, and other routes of administration may be more effective and convenient for these patients or any patient who is not optimally responsive to tablets.
In the AMPP study, among patients who did not respond to triptans, switching to an opioid or barbiturate or switching between triptans was not associated with improvement in disability related to headache.9,10 The addition of NSAIDs reduced disability in patients taking triptans for episodic migraine of moderate frequency.9 Moreover, as pain severity increases over the course of a migraine attack, a treatment initiated later is less effective in terms of pain-free outcomes and risk of recurrence compared with an earlier treatment.5 The effectiveness of triptans may also be decreased in the presence of central sensitization and cutaneous allodynia (a clinical indicator of central sensitization) as the migraine attack progresses.11 In this advanced phase of migraine, levels of cyclooxygenases (COXs) in the spinal cord are increased; NSAIDs, such as diclofenac, inhibit the activity of these COXs and may offer benefit to migraineurs with allodynia and central sensitization.11
Here, we will review patient treatment goals, as well as the pharmacologic, pharmacokinetic, efficacy, and safety data for diclofenac potassium in patients with migraine, with a focus on the soluble formulation of diclofenac potassium (CAMBIA®, Depomed, Inc., Newark, CA, USA). In addition, this review will provide a clinical case analysis to demonstrate the potential role of diclofenac potassium for oral solution in the treatment paradigm for migraine patients.
Treatment goals and considerations
The goals of acute treatment of a migraine attack are to effectively and rapidly reduce pain and restore function while minimizing adverse events, recurrent headache, and the need for backup and rescue medication.3,12 Factors that are important to consider in the selection of acute migraine treatment include peak pain intensity, time to peak pain intensity, disability, comorbidities, concomitant medications, and the degree or presence of nausea for patients taking oral medications.6 The American Academy of Neurology recommends the initiation of oral triptans or dihydroergotamine for patients with moderate or severe migraine or those with mild-to-moderate headaches that respond poorly to NSAIDs or combination medications.3 We agree with this recommendation to use migraine-specific agents over analgesics as first-line treatment to achieve the best efficacy at the start of migraine pain.2 Patients will often try OTC analgesics and combination medications that include caffeine prior to consulting with a health care practitioner because of their availability and low cost.13 These products have the potential to cause medication-overuse headache and/or lead to incomplete headache relief and eventually to chronic migraine and caffeine dependency.2,13
Pathophysiology of migraine
Migraine is believed to develop as a result of cortical spreading depression and hypersensitivity of the cerebral cortex followed by peripheral sensitization involving the trigeminal nerve.14,15 Proinflammatory cytokines (interleukins 1, 6, and 8 and tumor necrosis factor-α), bradykinin, histamine, serotonin, and prostaglandin E2 promote the excitation and sensitization of nociceptors.14 Activation of sensory fibers triggers the release of vasodilatory neuropeptides peripherally in the meninges, such as calcitonin gene-related peptide (CGRP), which induce the release of the inflammatory mediators from immune cells, primarily mast cells in the meninges.14,16 In studies of plasma samples from patients with episodic and chronic migraine (with and without aura), levels of CGRP in peripheral blood were significantly (p ⩽ 0.005) increased compared with the controls.17,18 Moreover, intravenous injection of CGRP has been shown to cause delayed, migraine-like headaches in patients with a history of migraine with aura and early, nonmigraine headaches in the controls.15,19 Based on these findings, CGRP receptor antagonists have been developed for the treatment of migraine. A number of small-molecule oral agents have been shown to be effective with acute headache pain-relief response rates similar to the triptans, but concerns about liver toxicity related to drug metabolism have prevented their further development until recently.15 Several of these agents are now being developed for both acute and preventive migraine treatment. In addition, monoclonal antibodies to CGRP or its receptor are in development for the prevention of episodic migraine, chronic migraine, and cluster headache.20
Analysis of evidence
The American Headache Society updated their assessment of migraine therapy in 2015 and determined that triptans, ergots, NSAIDs, opioids, and combination medications are effective for acute therapy.21 Despite this assessment, we (along with many other headache specialists) rarely use opioids, as they do not work on the migraine process, they make triptan treatment less effective, and they do not alleviate migraine pain well.2 Although combination oral analgesics can occasionally be effective for patients with mild migraine, they are not appropriate for more severe cases and their use should be limited due to the potential for inadequate treatment, caffeine addiction, adverse events, and medication-overuse headache. We recommend that patients limit their use of combination analgesics to fewer than 4 days or 10 tablets per month.2 The International Classification of Headache Disorders-III beta defines medication-overuse headache as headache occurring with 10 days per month of combination analgesic use for >3 months in someone with 15 or more days of headache per month.4
In clinical studies of acute treatment of a migraine attack, typical endpoints that are used to determine efficacy include the proportion of patients (1) who are pain free at 2 h, (2) who have headache relief at 2 h [defined as a change from moderate or severe to none or mild on a visual analog scale (VAS) or via patient reporting], and (3) who maintain freedom from headache (sustained pain-free response for 48 h).5,21 The International Headache Society (IHS) recommends the primary outcome measure to be the proportion of patients who are pain free at 2 h.5 In a review of clinical drug trials in patients with migraine, the use of “pain free at 2 h” as the primary efficacy outcome occurred in 31% of acute, randomized, double-blind trials; it was used as a secondary outcome in 49% of these trials.5 Headache relief was used in 39% of trials, and sustained pain freedom for 48 h (IHS recommendation) was used in 3% of trials, but 47% of the trials modified this efficacy measure to evaluate the pain-free state at 24 hours.5 Commonly used secondary endpoints include measures of disability, absence of nausea or vomiting, or absence of photophobia or phonophobia.21 Recently, the US Food and Drug Administration began to require acute migraine treatment trials to use the combined endpoint of 2-h pain freedom and freedom from the most bothersome associated symptom (nausea, photophobia, or phonophobia).22 Using these primary and secondary outcome measures, the updated American Headache Society assessment of the efficacy of medications for acute treatment of migraine determined that the strength of evidence for diclofenac was Level A (established as effective).21
Pharmacology of diclofenac
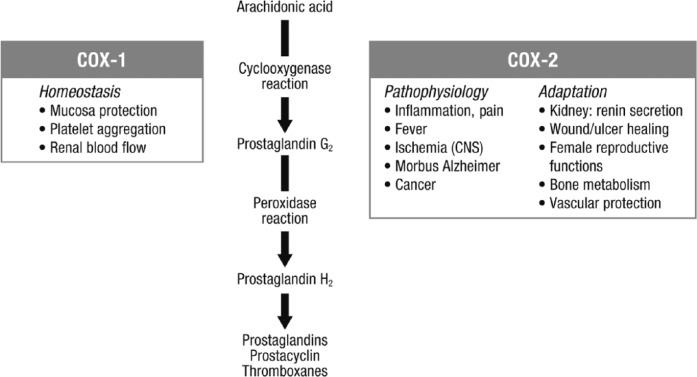
The pharmacologic action of NSAIDs is primarily based on their inhibition of the COX enzyme (Figure 1).23 COX exists in 2 isoforms: COX-1 is expressed in almost all tissues and mediates physiologic responses, and COX-2 is expressed by inflammatory cells and accounts for the effects of NSAIDs.23 Diclofenac is a phenylacetic acid derivative that has anti-inflammatory, antipyretic, and analgesic properties through inhibition of COX-1 and COX-2 activity and prostaglandin synthesis.24 Diclofenac is a potent COX inhibitor, with activity 3–1000 times greater than other NSAIDs.24 Diclofenac has a 4-fold higher selectivity for COX-2 compared with COX-1. However, at relevant therapeutic levels, 70% of COX-1 is inhibited; thus, it has low to moderate selectivity for COX-2 at therapeutic levels.25
Figure 1.
Arachidonic acid cascade and effects of COX enzymes. NSAIDs act by inhibiting the COX enzymes.23
CNS, central nervous system; COX, cyclooxygenase.
Pharmacokinetics of diclofenac
Oral formulations of diclofenac are salts of sodium or potassium.24 The sodium salt is a slow-release formulation that is indicated for chronic pain conditions, whereas the potassium salt was formulated to provide rapid uptake for acute pain.24 Diclofenac potassium for oral solution is packaged as a buffered powder that is dissolved in 30–60 ml of water prior to oral administration.26 The buffering agent, potassium bicarbonate, raises the pH of the aqueous solution, allowing diclofenac to remain soluble for rapid absorption in the upper small intestine (the jejunum).26 These characteristics provide rapid and consistent absorption, with a time to maximum plasma concentration (Tmax) of 0.25 h (range 0.17–0.67 h).27 When compared with the diclofenac potassium tablet, the oral solution reached maximum plasma concentrations approximately 80% faster; exposure was similar between the 2 formulations.26,28
Clinical studies of diclofenac for the acute treatment of a migraine attack
The efficacy and safety of diclofenac potassium in the acute treatment of a migraine attack has been evaluated in 4 placebo- or active-controlled studies, including 2 studies that used different formulations29,30 and 2 registration studies of diclofenac potassium for oral solution.31,32 Diclofenac potassium sugar-coated tablets were evaluated in 144 patients with migraine with or without aura.29 Headache pain at 2 h was significantly decreased with diclofenac potassium compared with placebo (difference in mm on VAS: 50 mg, −17.0; 100 mg, −18.6; p < 0.001 for both).29 The efficacy of diclofenac was maintained through the 8-h observation period.29 In a second study, diclofenac epolamine (DHEP), a more water-soluble and stable compound than the parent compound, was evaluated for efficacy and tolerability in 481 mild-to-moderate migraine attacks in 133 patients.30 Efficacy, measured as the proportion of attacks that were pain free within 2 h after drug administration, was reported in 45.8% of the migraine attacks treated with DHEP compared with 25.1% of attacks treated with placebo (p < 0.0001).30 Significant improvements in time to resolution of the migraine attack (4.5 h versus 6.8 h, respectively; p < 0.0001) and light and noise hypersensitivity (p ⩽ 0.01) were also observed with DHEP versus placebo.30 Working ability was improved for 54.4% of those receiving DHEP compared with 39.8% of those receiving placebo (p = 0.0001).30
Registration trials
There have been two registration studies conducted using diclofenac potassium in a sachet dissolved in water for oral administration. The first study was conducted in Europe and compared the oral solution with diclofenac potassium tablets and placebo.31 The other study was conducted in the United States and compared the oral solution with placebo.32 In the double-blind, crossover, European study, 328 patients with migraine of moderate-to-severe intensity with or without aura were randomized to receive three different treatments for three headaches, and 317 patients received at least one treatment.31 The percentage of patients who were pain free at 2 h was 24.7% for the oral solution, 18.5% for tablets, and 11.7% for placebo. The oral solution was significantly more effective than the tablets (p = 0.0035) or placebo (p < 0.0001).31 The oral solution provided significantly faster relief, as evidenced by an improvement in headache intensity (measured on VAS starting at 15 min and continuing to separate over the first 2 h post-dose), compared with placebo and the tablets (Figure 2).31 Significant improvements were also observed with diclofenac oral solution compared with tablets and placebo in the reduction in headache intensity (VAS) during the first 4 h and in sustained headache response and sustained pain-free response over 24 h (all p < 0.05).31
Figure 2.
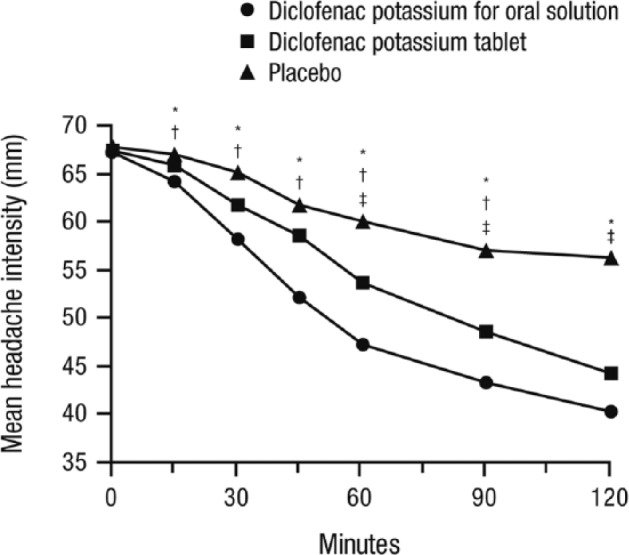
Mean VAS headache intensity over the first 2 h after drug administration. Significant improvements with diclofenac potassium for oral solution versus placebo were observed starting at 15 minutes. VAS from 0–100, with 0 = no headache to 100 = excruciating headache requiring bedrest. *p ⩽ 0.05 for diclofenac potassium for oral solution versus placebo; †p ⩽ 0.05 for diclofenac potassium for oral solution versus diclofenac potassium tablet; ‡p ⩽ 0.05 for diclofenac potassium tablet versus placebo. Figure reproduced with permission from Diener and colleagues.31
VAS, visual analog scale.
In the second trial, a large, double-blind, placebo-controlled, US study, 690 patients with migraine with or without aura were randomized and received treatment with diclofenac potassium for oral solution or placebo for the acute treatment of a single moderate or severe migraine attack.32 Significantly more patients were pain free at 2 h with diclofenac potassium for oral solution compared with placebo (25.1% versus 10.1%; p < 0.001).32 For the four co-primary endpoints for this trial, a significantly higher percentage of patients who received diclofenac potassium for oral solution experienced no nausea, photophobia, or phonophobia at 2 h than did those who received placebo (all p < 0.002).32 Significant improvements in pain intensity were observed 30 min after treatment with diclofenac potassium for oral solution (p = 0.013), and the improvements were maintained for the duration of the study period (24 h; p < 0.001).32
A recent Cochrane systematic review of clinical trials of diclofenac potassium for acute migraine provided summary outcomes versus placebo (Table 4).25 For all primary outcomes, the relative benefit versus placebo was similar or slightly higher for the soluble formulations of diclofenac potassium compared with diclofenac potassium overall.25
Table 4.
Primary outcomes for acute migraine patients receiving diclofenac potassium or placebo: systematic review of clinical trials.25
| Outcome (range), % | Diclofenac potassium | Placebo | Relative benefit |
|---|---|---|---|
| Pain free at 2 h | 22 (17–25) | 11 (10–13) | 2.0 (1.6–2.6)a |
| Headache relief at 2 hb | 55 (47–65) | 39 (36–41) | 1.5 (1.3–1.7)c |
| Sustained pain-free response (24 h) | 19 (15–22) | 8.2 (7.2–9.4) | 2.3 (1.7–3.0)d |
Soluble formulations only, 2.3 (1.6–3.1).
Reduction in headache pain from moderate or severe to none or mild without the use of rescue medication.
Soluble formulations only, 1.5 (1.3–1.7).
Soluble formulations only, 2.5 (1.8–3.6).
Safety of diclofenac in migraine attacks
Diclofenac was well tolerated in clinical studies of patients with migraine.25 In the two registration studies, the most common adverse events were nausea, vomiting, upper abdominal pain, diarrhea, dizziness, and dyspepsia.31,32 In the Cochrane review, the overall incidence of adverse events was similar with diclofenac potassium (18%) and placebo (16%).25 No serious adverse events were reported in any of the included trials in the Cochrane review, including the two registration studies.25,31,32
Clinical case analyses
Both patients described in the following cases provided written informed consent for their information to be reported in this article. Case 1: Karen is a 26-year-old female attorney and mother of two children. Her typical headaches are in the left frontal region and sometimes the right frontal and orbital region and can radiate to the occipital and neck area. The pain is described as pulsating, “like a heartbeat in my head,” and can last anywhere from 5–24 h. She prefers to avoid bright light, and loud noises can be irritating. She experiences mild nausea, and sometimes certain odors can trigger an attack. She prefers to lay down in a dark room during her attacks. Her current frequency is about 2–3 headache days per week. Quite often, she awakens with a headache in the morning.
She denies any nighttime clenching or grinding. She takes rizatriptan 10 mg tablets at the onset of her typical migraine attack and, depending on its severity, she gets partial relief only 30–40% of the time. Typically, the morning headaches are not responsive to medications in tablet form. These morning headaches are usually associated with what she calls “hair sensitivity,” and she prefers to let her hair down in these situations. She is sensitive to triptans and could not tolerate the side effects of oral or subcutaneous sumatriptan. She occasionally takes ibuprofen 200 mg. She reports taking ibuprofen and rizatriptan more often during menstruation. Her other medications include a hormonal contraceptive medication containing low estrogen with a placebo pill during the 7 days of menses. Frequent migraine triggers include certain foods, such as bacon and hot dogs, chocolate (which she actually craves just before her typical migraine), skipping lunch, and not sleeping well. There is no family history of any neurologic disorder, including migraine. Her neurologic exam is unremarkable. Screening blood work was normal. She has not had a computerized tomography or magnetic resonance imaging (MRI) scan of the brain. She does have some jaw popping on exam but no tenderness or pain. She has never kept headache diaries. She denies smoking or use of any illicit drugs. She sometimes has to travel and is more likely to get a migraine attack then.
Treatment plan
Karen appears to be sensitive to sumatriptan and has only a partial response to rizatriptan. Her morning headaches suggest an allodynic state or poor absorption of medication. She also appears to have a menstrual association to her migraine. She was started on diclofenac potassium for oral solution (CAMBIA®) at the start of all migraine attacks, especially the morning migraines. A low dose of nortriptyline (10 mg) was added at bedtime and was increased to 20 mg after 1 week. We discussed using an alternative non–estrogen-based contraceptive. After 6–8 weeks, her overall headache frequency was reduced to 2–3 migraine days monthly around menstruation. Her headaches were responsive to diclofenac potassium for oral solution within 30 min, without any side effects. She tolerated nortriptyline and noted that she was better able to deal with everyday stress and was sleeping better. She elected not to try an alternative contraceptive medication.
Case 2: Jane is a 48-year-old female secretary. She is currently in perimenopause and her headache frequency in the last 6 months has increased from 2–3 headache days per month to 16 headache days per month. Headache diaries reveal that about 8–9 headache days per month meet the criteria for migraine and that some of the other headaches have some features of migraine. Her last menstrual period was about 6 months ago. She frequently has hot flashes and night sweats. She denies any other triggering mechanism in the past 6 months, including head trauma, viral illness, or stressful life events. Her typical headaches are described as unilateral or bilateral, throbbing or steady, and are associated with nausea, rarely vomiting, and some noise and light sensitivity. About 5–12 h prior to her typical migraine, she has neck stiffness, irritability, and an increase in urination. Her triggers include foods that contain monosodium glutamate, skipping meals, and red wine. She has been taking a nonprescription combination of acetaminophen, aspirin, and caffeine (Excedrin® Migraine, Bristol-Myers Squibb Co., USA), four tablets per day, at least 4 days per week; ibuprofen 200 mg every 6 h, at least 3 days per week; and acetaminophen 500 mg, 2 days per week. She also tries to take naratriptan 2.5 mg at the onset of her headache. Typical relief is only mild to moderate and sometimes takes 4 h. Tablets of sumatriptan 50 mg or rizatriptan 10 mg caused palpitations and a flushed sensation in the face, with some jaw tightness. There is a strong family history of migraine in her mother and two siblings.
Her current medical conditions include obesity on a diet regimen and anxiety, for which she takes sertraline 25 mg daily. Her neurologic exam is unremarkable. An MRI scan of the brain with contrast 3 months ago was unremarkable. Laboratory evaluations for complete blood count, erythrocyte sedimentation rate, C-reactive protein, Lyme titer, and thyroid-stimulating hormone were also unremarkable.
Treatment plan
Jane has chronic migraine and medication-overuse headache. She also has a few risk factors for the migraine chronification, including obesity and anxiety as a comorbidity.33 She appears to be sensitive to fast-acting triptans. Diclofenac potassium for oral solution (CAMBIA®) was tried alone without other medicines, and she responded favorably. Migraine attacks can increase in frequency during perimenopause.34 Propranolol 60 mg increased to 120 mg and magnesium glycinate 400 mg once per day were added for migraine prevention. She preferred not to have a trial of topiramate due to potential side effects. She was also advised to slowly reduce her daily intake of all OTC medications and exercise for about 20 min, 5 days per week.
After 8 weeks, her overall frequency improved to 1–2 headache days per week, half of which met the criteria for migraine. Individual headaches were overall less severe, and there was less recurrence later in the day or the next day. The magnesium and propranolol have helped with some of her vasomotor symptoms of perimenopause.
Conclusion
The treatment of migraine requires a multimodal approach based on a thorough history, including the headache attack characteristics and the impact of headaches on a patient’s daily life. Headache diaries are essential, especially in complex patients with comorbidities and overuse of medications. Electronic diaries provide more accurate data than typical paper diaries. They enable patients and health care practitioners to recognize headache and acute care medication frequency, menstrual association, and migraine triggers as well as to track response to migraine treatments. Pharmacodynamic and pharmacokinetic characteristics of acute care medications, including route of administration, onset of effect, and recurrence rate, should be considered in the treatment of migraine attacks. Diclofenac potassium for oral solution is a nontriptan alternative for the acute treatment of migraine attacks, with a fast onset of action, sustained efficacy, and a favorable safety profile, with no triptan-type side effects.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Editorial support was provided by Megan Knagge, PhD, of MedErgy, and was funded by Depomed, Inc, Newark, CA, USA.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Joshi is on the advisory board for Eli Lilly and Company, Indianapolis, IN, USA, and is on the speakers bureau for Avanir Pharmaceuticals, Inc., Aliso Viejo, CA, USA; Depomed; Pernix Therapeutics, Inc., Morristown, NJ, USA; Allergan Inc., Irvine, CA, USA; Supernus Pharmaceuticals Inc, Rockville, MD, USA; and Teva Pharmaceuticals, Frazer, PA, USA. Dr Rapoport is on the speakers bureau for Avanir and Depomed and is a consultant to Autonomic Technologies, Inc., Redwood City, CA, USA; Avanir; Depomed; Dr Reddy’s Laboratories Ltd., Princeton, NJ, USA; electroCore, LLC, Basking Ridge, NJ, USA; Impax Laboratories, Inc., Bridgewater, NJ, USA; Pernix; Teva; and Zosano Pharma, Fremont, CA, USA.
Contributor Information
Shivang Joshi, Community Neuroscience Services, Associate Professor of Pharmacy Practice, MCPHS University, 33 Lyman Street, Suite 400, Westborough, MA 01581, USA.
Alan M. Rapoport, Department of Neurology, The David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
References
- 1. Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang M, Rapoport AM. Acute treatment of migraine headache. Tech Reg Anesth Pain Manag 2009; 13: 9–15. [Google Scholar]
- 3. American Academy of Neurology. Migraine headache, https://www.aan.com/guidelines/home/getguidelinecontent/120 (2016, accessed 15 August 2016).
- 4. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 5. Hougaard A, Tfelt-Hansen P. Are the current IHS guidelines for migraine drug trials being followed? J Headache Pain 2010; 11: 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rapoport AM. What happens to the old headache medicines? Headache 2012; 52: 701–706. [DOI] [PubMed] [Google Scholar]
- 7. Chu MK, Buse DC, Bigal ME, et al. Factors associated with triptan use in episodic migraine: results from the American Migraine Prevalence and Prevention study. Headache 2012; 52: 213–223. [DOI] [PubMed] [Google Scholar]
- 8. Lipton RB, Buse DC, Serrano D, et al. Examination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention (AMPP) study. Headache 2013; 53: 1300–1311. [DOI] [PubMed] [Google Scholar]
- 9. Buse DC, Serrano D, Reed ML, et al. Adding additional acute medications to a triptan regimen for migraine and observed changes in headache-related disability: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache 2015; 55: 825–839. [DOI] [PubMed] [Google Scholar]
- 10. Serrano D, Buse DC, Kori SH, et al. Effects of switching acute treatment on disability in migraine patients using triptans. Headache 2013; 53: 1415–1429. [DOI] [PubMed] [Google Scholar]
- 11. Jakubowski M, Levy D, Goor-Aryeh I, et al. Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache 2005; 45: 850–861. [DOI] [PubMed] [Google Scholar]
- 12. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55: 754–762. [DOI] [PubMed] [Google Scholar]
- 13. Cupini LM, Calabresi P. Medication-overuse headache: pathophysiological insights. J Headache Pain 2005; 6: 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burstein R, Jakubowski M, Rauch SD. The science of migraine. J Vestib Res 2011; 21: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol 2015; 55: 533–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med 2011; 13: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ashina M, Bendtsen L, Jensen R, et al. Evidence for increased plasma levels of calcitonin gene-related peptide in migraine outside of attacks. Pain 2000; 86: 133–138. [DOI] [PubMed] [Google Scholar]
- 18. Cernuda-Morollon E, Larrosa D, Ramon C, et al. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 2013; 81: 1191–1196. [DOI] [PubMed] [Google Scholar]
- 19. Hansen JM, Hauge AW, Olesen J, et al. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 2010; 30: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 20. Mitsikostas DD, Rapoport AM. New players in the preventive treatment of migraine. BMC Med 2015; 13: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55: 3–20. [DOI] [PubMed] [Google Scholar]
- 22. US Department of Health and Human Services, US Food and Drug Administration Center for Drug Evaluation and Research. Guidance for industry. Migraine: developing drugs for acute treatment, http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm419465.pdf. (2014, accessed 15 August 2016). [Google Scholar]
- 23. Hinz B, Brune K. Cyclooxygenase-2—10 years later. J Pharmacol Exp Ther 2002; 300: 367–375. [DOI] [PubMed] [Google Scholar]
- 24. Gan TJ. Diclofenac: an update on its mechanism of action and safety profile. Curr Med Res Opin 2010; 26: 1715–1731. [DOI] [PubMed] [Google Scholar]
- 25. Derry S, Rabbie R, Moore RA. Diclofenac with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev 2013; 4: CD008783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen C, Bujanover S, Kareht S, et al. Differential pharmacokinetics of diclofenac potassium for oral solution vs immediate-release tablets from a randomized trial: effect of fed and fasting conditions. Headache 2015; 55: 265–275. [DOI] [PubMed] [Google Scholar]
- 27. CAMBIA®. Diclofenac potassium for oral solution (package insert). Newark, CA: Depomed, Inc., 2014. [Google Scholar]
- 28. Marzo A, Dal BL, Verga F, et al. Pharmacokinetics of diclofenac after oral administration of its potassium salt in sachet and tablet formulations. Arzneimittelforschung 2000; 50: 43–47. [DOI] [PubMed] [Google Scholar]
- 29. The Diclofenac-K/Sumatriptan Migraine Study Group. Acute treatment of migraine attacks: efficacy and safety of a nonsteroidal anti-inflammatory drug, diclofenac potassium, in comparison to oral sumatriptan and placebo. Cephalalgia 1999; 19: 232–240. [DOI] [PubMed] [Google Scholar]
- 30. Vecsei L, Gallacchi G, Sagi I, et al. Diclofenac epolamine is effective in the treatment of acute migraine attacks. A randomized, crossover, double-blind, placebo-controlled, clinical study. Cephalalgia 2007; 27: 29–34. [DOI] [PubMed] [Google Scholar]
- 31. Diener HC, Montagna P, Gacs G, et al. Efficacy and tolerability of diclofenac potassium sachets in migraine: a randomized, double-blind, cross-over study in comparison with diclofenac potassium tablets and placebo. Cephalalgia 2006; 26: 537–547. [DOI] [PubMed] [Google Scholar]
- 32. Lipton RB, Grosberg B, Singer RP, et al. Efficacy and tolerability of a new powdered formulation of diclofenac potassium for oral solution for the acute treatment of migraine: results from the International Migraine Pain Assessment Clinical Trial (IMPACT). Cephalalgia 2010; 30: 1336–1345. [DOI] [PubMed] [Google Scholar]
- 33. Buse DC, Rupnow MF, Lipton RB. Assessing and managing all aspects of migraine: migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clin Proc 2009; 84: 422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin VT, Pavlovic J, Fanning KM, et al. Perimenopause and menopause are associated with high frequency headache in women with migraine: results of the American Migraine Prevalence and Prevention study. Headache 2016; 56: 292–305. [DOI] [PubMed] [Google Scholar]