Abstract
In hormone-dependent tissues such as breast and ovary, tumorigenesis is associated with an altered expression ratio between the two estrogen receptor (ER) subtypes. In this study, we investigated the effects of ERβ ectopic expression on 17β-estradiol (E2)-induced transactivation and cell proliferation in ERα-positive BG1 ovarian cancer cells. As expected, ERβ expression strongly decreased the mitogenic effect of E2, significantly reduced E2-dependent transcriptional responses (both on a stably integrated estrogen response element [ERE] reporter gene and on E2-induced mRNAs), and strongly enhanced the formation of ER heterodimers as evidenced by chromatin immunoprecipitation analysis. Inhibition by the ERα-selective ligand propyl pyrazole triol was less marked than with the pan-agonist (E2) or the ERβ-selective (8β-vinyl-estradiol) ligands, indicating that ERβ activation reinforced the inhibitory effects of ERβ. Interestingly, in E2-stimulated BG1 cells, ERβ was more efficient than ERα to regulate the expression of receptor-interacting protein 140 (RIP140), a major ERα transcriptional corepressor. In addition, we found that the RIP140 protein interacted better with ERβ than with ERα (both in vitro and in intact cells by fluorescence cross-correlation spectroscopy). Moreover, RIP140 recruitment on the stably integrated reporter ERE was increased upon ERβ overexpression, and ERβ activity was more sensitive to repression by RIP140. Finally, small interfering RNA-mediated knockdown of RIP140 expression abolished the repressive effect exerted by activated ERβ on the regulation of ERE-controlled transcription by estrogens. Altogether, these data demonstrate the inhibitory effects of ERβ on estrogen signaling in ovarian cancer cells and the key role that RIP140 plays in this phenomenon.
Steroid hormones, such as estrogens, are required for normal developmental and reproductive processes in vertebrates (1). Most of these events are modulated by 2 nuclear estrogen receptors (ERα and ERβ) (2). These two ERs are encoded by distinct genes and differ in their relative and absolute tissue distribution (3). Binding of estrogen or estrogen-like compounds induces a conformational change in the receptor, an event that promotes ER homo- or heterodimerization (4). Once ER protein complexes are bound to DNA, they regulate the expression of estrogen-responsive genes that only partially overlap in response to ER homo- or heterodimer activation (5–7).
Estrogens stimulate cell proliferation in normal developing breast tissues and in a large proportion of ERα-positive breast cancers (8, 9). It has been shown that the ERα/ERβ ratio is higher in breast tumors than in normal tissues due to lower expression of ERβ and that ERα and ERβ are antagonistic to each other. For example, ERβ appears to reduce the cell proliferation induced by ERα activation, as shown in transient or stable cell transfection studies performed in MCF-7 breast cancer cells, which have a high ERα/ERβ ratio (10) or in T47D cells, with ERβ tetracycline-dependent expression (11–13). It has been proposed that the effect of estrogen-like compounds on cell proliferation is dependent on the actual ERα/ERβ expression levels in the cells or tissues and on the potential of the estrogen agonists to activate ERα and/or ERβ. Since the discovery of the ERβ potential to reduce ERα transactivation and proliferation, it appears essential to better understand mechanisms of action and the biological role of ERβ as well as its therapeutic utility.
Ovarian cancer is, after breast cancer, the second most common gynecologic cancer in terms of incidence but the first one in terms of morbidity in Western countries (14). A loss of ERβ expression (or an increase in the ERα/ERβ ratio) has been consistently reported by several groups in ovarian cancer as compared with normal tissues (15–18). As for breast cancer, this loss of ERβ could thus constitute a crucial step in ovarian carcinogenesis and hormone unresponsiveness. Indeed, the loss of ERβ expression is associated with a shorter overall survival of ovarian cancer patients (19), and cytoplasmic expression of ERβ has been correlated to a poor outcome for patients with advanced serous ovarian cancer (20). Altogether, these findings strongly indicate that ERβ is a critical factor in ovarian tumor progression.
The overall objective of the present study was therefore to analyze the effects of ERβ on 17β-estradiol (E2) signaling in ovarian cancer cells. To this aim, we studied the regulation of cell proliferation, ERE-dependent transactivation, and gene expression by E2 and selective ER ligands in BG1 human epithelial ovarian cancer cells stably expressing various amounts of ERβ. Our data demonstrated that the intensity of E2-induced responses in ovarian cancer cells depends on the relative expression and activation of the 2 ER subtypes. Moreover, this work also suggested that the transcriptional corepressor RIP140 (receptor-interacting protein 140) is a key regulator of the negative effects of ERβ on E2 signaling in ovarian cancer cells.
Materials and Methods
Chemicals and materials
Culture media and fetal calf serum (FCS) were obtained from Life Technologies, Inc (Cergy-Pontoise). Geneticin and luciferin were purchased from Promega (Charbonnières). [3H]E2 (41.3 Ci/mmol specific activity) was purchased from NEN Life Science Products. 17β-estradiol (E2), puromycin, and methylthiazolyldiphenyl tetrazolium bromide were obtained from Sigma-Aldrich, Inc. 4,4′,4′-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT) was purchased from Tocris Bioscience (Ellisville), whereas 3,17-dihydroxy-19-nor-17α-pregna-1,3,5(10)-triene-21,16α-lactone (16α-LE2) and 8-vinylestra-1,3,5(10)-triene-3,17β-diol (8β-VE2) were from Research Laboratories of Schering AG. Stock solutions (10 mM) were prepared in dimethyl sulfoxide, and successive dilutions were performed in culture medium. Stock solutions were kept at −20°C, and dilution series were extemporaneously prepared before each experiment.
Reporter cell lines and culture conditions
The BELZ (BG1 ERE Luciferase Zeomycin) cell line was obtained by cotransfection of the estrogen-responsive reporter gene ERE3-TATA-Luc (gift of Ingemar Pongratz), and the zeocin resistance-conferring plasmid pCDNA3-zeo (Invitrogen) BELZ ERβ clones 1 and 2 were obtained by stable transfection of BELZ cells by the ERβ1 plasmid construct, which encodes the wild-type ERβ protein of 530 amino acids. BELZ cells were cultured at 37°C in DMEM, supplemented with 5% FCS, 1% penicillin-streptomycin (PS), and 0.25 mg/mL zeocin. BELZ ERβ cells were cultured in DMEM-F12, supplemented with 5% FCS, 1% antibiotics (PS), 0.25 mg/mL zeocin, 0.125 μg/mL puromycin, and 1 nM PPT. Because of phenol red and FCS estrogenic activity, experiments were performed in test culture medium: DMEM-F12 without phenol red, supplemented with 5% dextran-coated charcoal-FCS and 1% antibiotic in a 5% CO2 humidified atmosphere.
RT-quantitative PCR (qPCR)
Total RNA was extracted using Quick RNA Miniprep kit (Zymo Research Corp). For RT-PCR, 1 μg of total RNA was subjected to a reverse transcription step using the superscript III Reverse Transcriptase kit (Invitrogen). Real-time PCR quantification was then performed using a Light Cycler SYBR Green approach (Applied Biosystem). For each sample, specific mRNA levels were corrected for 28S mRNA levels (reference gene). Primer sequences are listed in Table 1.
Table 1.
Primer Sequences
| hERα-F | 5′-CCACCAACCAGTGCACCATT-3′ |
| hERα-R | 5′-GGTCTTTTCGTATCCCACCTTTC-3′ |
| hERβ-F | 5′-AAGAATATCTCTGTGTCAAGGCCATG-3′ |
| hERβ-R | 5′-GGCAATCACCCAAACCAAAG-3′ |
| hPR-F | 5′-CGCGCTCTACCCTGCACTC-3′ |
| hPR-R | 5′-TGAATCCGGCCTCAGGTAGTT-3′ |
| hFibulin-1-F | 5′-CCGGATGGCCACTCATCAGA-3′ |
| hFibulin-1-R | 5′-CCCATTCAGGTCCTGGCACA-3′ |
| hRARα-F | 5′-CCCGGCTTCACCACCCTCACCAT-3′ |
| hRARα-R | 5′-AGCCCCGTCTCCGCATCATCCATC-3′ |
| hCyclin D1-F | 5′-GTGCTGCGGGCCATGCTGAAGG-3′ |
| hCyclin D1-R | 5′-TCGGGCCGGATGGAGTTGTCG-3′ |
| hRIP140-F | 5′-GCTGGGCATAATGAAGAGGA-3′ |
| hRIP140-R | 5′-CAAAGAGGCCAGTAATGTGCTATC-3′ |
| h28S-F | 5′-CGATCCATCATCCGCAATG-3′ |
| h28S-R | 5′-AGCCAAGCTCAGCGCAAC-3′ |
F, forward; h, human; R, reverse; RAR, retinoic acid receptor.
Western blot
After hormonal treatments, BELZ, BELZ ERβ-1, and BELZ ERβ-2 cells were lysed in lysis buffer (25 mM Tris-HCl, pH 7.8; 2 mM EDTA; 2 mM dithiothreitol (DTT); 10% glycerol; 1% Triton, supplemented with protease inhibitors). The proteins were then diluted in Laemmli sample buffer, resolved by SDS-PAGE, transferred onto nitrocellulose membranes, and detected by Western blotting using primary antibodies against ERα (sc-543 from Santa-Cruz Biotechnology, Inc; dilution 1:4000) or the Flag epitope (F3165 from Sigma, dilution 1:2000) for Flag-ERβ. Normalization was performed using the actin antibody (A2066 from Sigma), and detection was achieved using horseradish peroxidase-linked secondary antibodies and enhanced chemiluminescence Western-blotting detection reagent.
Whole-cell ER competitive ligand-binding assay
BELZ cells were seeded at a density of 2.105 cells per well in 24-well tissue culture plates and grown in test culture medium. After 24 hours, cells were labeled with 1 nM [3H]E2 (41.3Ci/mmol specific activity) at 37°C for 3 hours in the absence or presence of unlabeled E2 (1 μM), 16α-LE2 (10 nM), and 8β-VE2 (10 nM). The final incubation volume was 500 μL, and each well was tested in duplicate. After incubation, unbound material was aspirated and cells were washed 3 times with 500 μL of cold PBS. Then, 250 μL lysis buffer (400 mM NaCl; 25 mM Tris phosphate, pH 7.8; 2 mM DTT; 2 mM EDTA; 10% glycerol; 1% Triton X-100) was added, and plates were shaken for 5 minutes. Total cell lysate (200 μL) was mixed with 4 mL of LSC-cocktail (Emulsifier-Safe, Packard BioScience), and [3H]-bound radioactivity was liquid scintillation counted (LS-6000-SC, Beckman-Coulter). Nonspecific binding was determined in the presence of 100 nM unlabeled E2. Specific binding was calculated by subtracting nonspecific binding from total binding. Bound radioactivity values were expressed in disintegrations per minute (dpm). Protein quantifications were carried out using the Bradford assay (Bio-Rad Laboratories).
Cell proliferation assay
Briefly, BELZ cells were seeded at a density of 2.103 cells per well in 96-well tissue culture plates and grown in test culture medium. Test compounds were added 24 hours after seeding. Cell lines were incubated for 7 days at 37°C in the absence or presence of tested ligands. Each concentration was performed in quadriplicate. After the incubation period, the medium was replaced by 200 μL of culture medium containing 0.4 mg/mL methylthiazolyldiphenyl tetrazolium bromide. After incubation (4 hours), the optical density of the solubilized dark blue insoluble formazan reaction product was measured at 560 nm. Results were expressed as induction factor with respect to the ligand-free control.
Luciferase assay in stable cell lines
Agonistic activities in stable reporter cells were measured in the presence of increasing ligand concentrations (1 pM to 1 μM) using a MicroBeta Trilux luminometer (EGG Wallac) as previously described (21). Tests were performed in quadruplicate for each concentration. In BELZ cell lines, ERE-induced transactivation by ligands was expressed relative to vehicle control (dimethyl sulfoxide) set at 1. The estrogenic potency corresponding to the concentration yielding half-maximal luciferase activity (EC50 value) was calculated.
Transient transfection assay
For transient luciferase assay, HeLa or BG1 cells were plated in 96-well plates (2.104 cells per well) 24 hours prior to DNA transfection with Jet-PEI (0.25 μg of total DNA). Luciferase (firefly and Renilla) values from transient transfection were measured as previously described (22), and all data were expressed as mean ± SD. Small interfering RNA (siRNA) transfections were performed in BELZ-ERβ2 cells with either nontargeting siRNA (siControl) or RIP140-specific siRNA (siRIP140) from Dharmacon using INTERFERin (Polyplus) transfection agent. Each transfection was performed in triplicate, and interference efficiencies were tested by qPCR. Luciferase activity was measured as described above for transient transfections.
Chromatin immunoprecipitation (ChIP) analysis
BELZ and BELZ ERβ-2 cells (60% confluent) were treated for 1 hour, with ethanol or E2 (final concentration 10−8 M), and cross-linked with 3.7% formaldehyde during 10 minutes at 37°C. The ChampionChIP One-Day Kit (SABiosciences) was used according to the manufacturer's recommendations with antibodies directed against ERα (HC20; sc-543 SantaCruz), the Flag epitope (M2-TAG A2220 Sigma) for Flag-ERβ or with no antibody as a control. qPCR was then performed using the Power SYBR Green PCR master mix, on an Applied Biosystems 7300 thermal cycler with 2 μL of material per point. Primers were 5′-GCATTCTAGTTGTGGTTTGTCCAA-3′ (forward) and 5′-AGCTTCGAGATCCAGACATGATAAG-3′ (reverse) for ERE amplification on reporter gene and 5′-CTCAGGTCTCCTTCCTAGTT-3′ (forward) and 5′-TGTGTCCTGCTTTAATGTTG-3′ (reverse) for ERE amplification on RIP140 gene. The input DNA fraction corresponded to 1% of the immunoprecipitation. For ChIP/reChIP experiments, after the first immunoprecipitation, the extracts were incubated with 25 μL of 10 mM DTT during 30 minutes at 37°C. We then added 500 μL of Buffer A (SABiosciences kit) with 4 μg of second antibody for the second immunoprecipitation.
In vitro interaction assays and anisotropy measurements
In vitro translation and glutathione-S-transferase (GST) pull-down assays were performed as previously described (23). Anisotropy experiments were performed essentially as described elsewhere (24) using a fluorescein-labeled ERE (F-ERE). Baculovirus-expressed human ERα and ERβ were purchased in 95% purified form from Panvera Corp. Anisotropy titrations were carried out using a Beacon 2000 Variable Temperature Fluorescence Polarization System (Panvera Corp) set at 21°C. For titrations onto the preformed ER/F-ERE complex, GST-RIP (27–439) was prepared as described elsewhere (24). Aliquots were successively added to 200 μL of a solution containing 1 nM F-ERE, 50 nM ER, and 1 μM E2 in 50 mM Tris, 200 mM NaCl, 0.1 mM DTT, pH 7.6.
Fluorescence cross-correlation spectroscopy (FCCS)
COS-7 cells were maintained in DMEM:F12 medium supplemented with 10% FCS serum (Invitrogen) and antibiotics (Invitrogen). The 2 ERs and RIP140 were transfected as N-terminal fusions with the blue cerulean fluorescent protein (cer-ER) and mCherry (mCherry-RIP140), respectively. The localization of the tagged version of ERs was previously reported (25), and the image obtained for separate expression of mCherry-RIP140 also revealed a uniquely nuclear localization, as expected (Supplemental Figure 1A).
For imaging, cells were deprived of steroids for 5 days in phenol-red free medium supplemented with 3% dextran-coated charcoal stripped serum (DCC-DMEM:F12), plated in Lab-Tek Chambered Coverglass 2-well dishes (Nunc Thermo Fisher Scientific) at 3.105 cells per well and transfected after 1 day using JetPEI or FuGene6 (Invitrogen). After 24 hours (and at least 2 hours before imaging), medium was replaced 2 times by PBS and then by DCC-DMEM:F12 supplemented with 10−8 M E2, or with the same volume of pure ethanol (1.5 μL) as control condition.
FCCS measurements were conducted as previously described using a Zeiss Axiovert 200 microscope (Zeiss). Data were analyzed as previously described using 3 curves generated by time-correlation of the fluorescence intensity fluctuations detected, 2 autocorrelation functions arising from the blue and red fluorophores, and 1 cross-correlation function, which accounts for the molecules in which the 2 fluorophores diffuse together (see Supplemental Figure 1B).
Statistical analysis
Student's t test was used for statistical analysis: *, P < .05; **, P < .01; and ***, P < .001.
Results
Characterization of ERβ expressing ovarian reporter cell lines
In order to decipher the role of ERβ on estrogen signaling in ovarian cancer cells, we developed stably transfected BG1 ovarian cancer cells (26). These cells endogenously express the ERα and were first stably transfected by an estrogen-responsive luciferase gene (BELZ cell line). In a second step, an ERβ expression plasmid was stably introduced and 2 clones (BELZ ERβ-1 and -2) were selected. In these 2 cellular clones, the expression of the 2 ERs was first quantified at the mRNA level. As shown in Figure 1A, RT-qPCR analysis indicated that ERβ expression was strongly increased in the 2 stably transfected cellular clones, the mRNA levels being slightly higher in BELZ ERβ-2 cells. By contrast, ERα expression in the 2 clones was comparable to the level observed in the parental BELZ cells.
Figure 1.
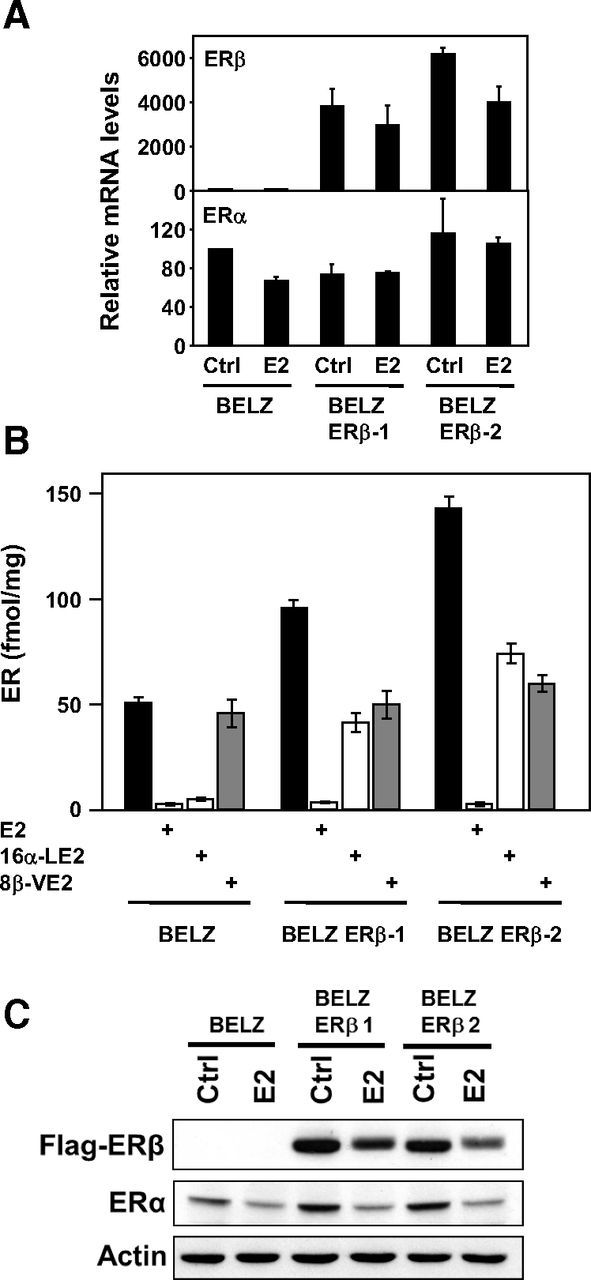
Expression of ERs in BELZ cell lines. A, mRNA quantification. RT-qPCR was performed as described in Materials and Methods in the 3 cell lines, treated (E2) or not (Ctrl) with 10 nM E2. Data are expressed as percent of the value obtained in control BELZ cells. B, Ligand binding assay. Binding of [3H]E2 to ERα and ERβ in the presence or absence of an excess of unlabeled E2 (1 μM), 16α-LE2 (10 nM) or 8β-VE2 (10 nM). Concentrations of ER are expressed in fentamoles/mg of protein. Values are means ± SD of 3 independent experiments. C, Western blot analysis. The levels of ERα and ERβ were quantified by Western blot analysis in BELZ, BELZ ERβ-1, and BELZ ERβ-2 cells, after 1 day of treatment with ethanol (Control) or estrogen (E2 10 nM). Equal loading was validated by measuring actin levels.
These observations were confirmed at the protein level by saturation whole-cell ligand-binding assay (LBA) in intact cells (Figure 1B). The LBA was performed using 1 nM [3H]E2, in the presence or absence of unlabeled specific ligands (16α-LE2 or 8β-VE2) used at 10 nM. This concentration was the most appropriated to specifically quantify the expression of ERα and ERβ because it raised the most significant differences in terms of E2 competition on the 2 receptors (21). These “whole-cell” LBAs confirmed the predominant expression of ERα in BELZ cells and the overexpression of ERβ in BELZ ERβ-1 and BELZ ERβ-2 clones. In BELZ ERβ-1, ERα and ERβ appeared to be expressed in a similar manner (∼50 fmol/mg of protein) whereas in BELZ ERβ-2, ERβ (∼75 fmol/mg of protein) is slightly more expressed than ERα. As shown in Figure 1C, these results were supported by Western blot analysis of ERα and ERβ expression. Altogether, these data indicated that these cell lines were fully adapted to analyze the role of ERβ in an ERα-positive context.
Estrogen regulation of cell proliferation in BELZ and BELZ ERβ cells
We first tested the effects of E2 and ER selective ligands on the proliferation of BELZ and BELZ ERβ cell lines. As shown in Figure 2A, E2 strongly increased BELZ cell proliferation (8-fold stimulation after 7 days of treatment). The ERα-selective ligand PPT was also able to fully induce proliferation with an induction identical to that observed with E2. In these cells, the ERβ-selective ligand 8β-VE2 was not able to stimulate proliferation at concentrations at which it only activated ERβ (ie, below 3 nM) but was fully mitogenic at 100 nM, a concentration at which it bound and activated ERα. These results thus confirmed that ERα is the main mediator of E2-induced proliferation in BG1 ovarian cancer cells.
Figure 2.
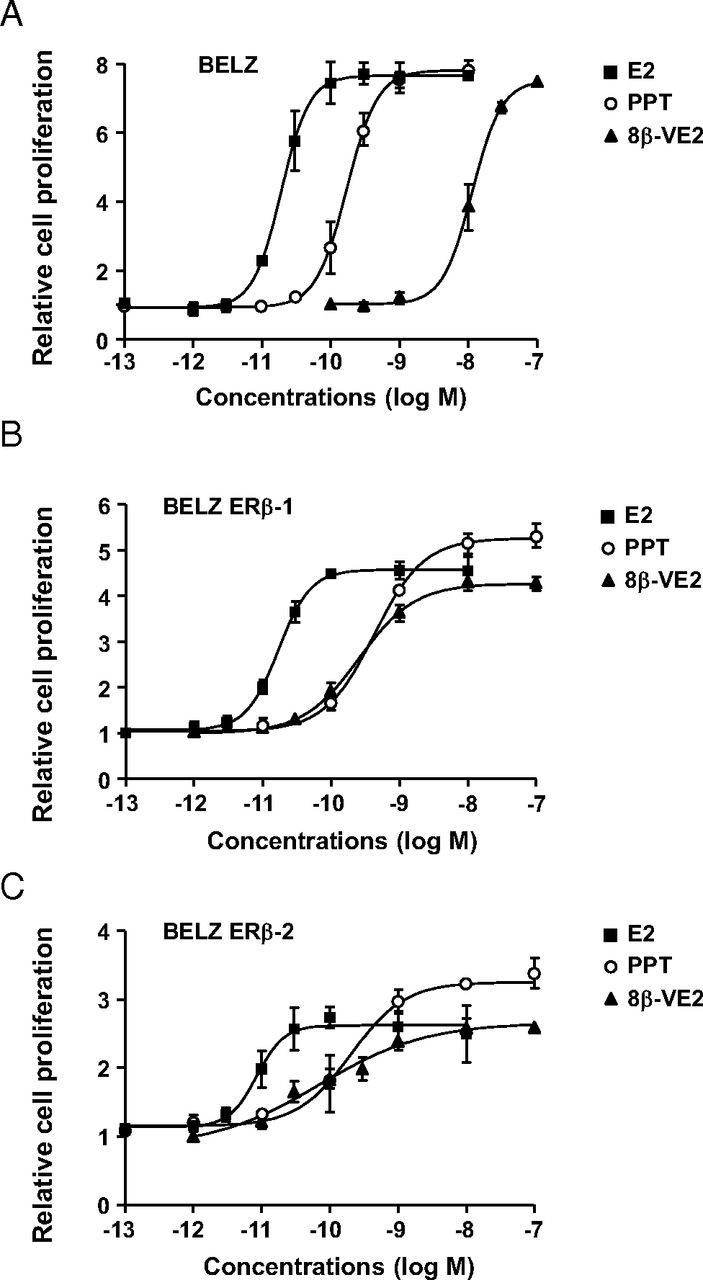
Effect of ER ligands on cell proliferation in BELZ cell lines. Cell proliferation was analyzed in BELZ (A), BELZ ERβ-1 (B), and BELZ ERβ-2 (C) cells following exposure during 7 days to increasing concentrations of E2 (■), PPT (○), and 8β-VE2 (▴). Data were normalized with respect to the proliferation of control cells set at 1. Each normalized data point represents the mean of 3 independent experiments ± SD.
Interestingly, as shown in Figure 2, B and C, ERβ expression strongly decreased the mitogenic effect of E2. This inhibition was observed in the BELZ ERβ-1 clone (4-fold stimulation of cell proliferation by E2) and was even stronger in the BELZ ERβ-2 clone expressing the highest levels of ERβ (2.5-fold stimulation by E2). The same effect of ERβ expression was observed when only ERα was activated (ie, in the presence of PPT) suggesting that the unliganded (apo) form of ERβ exerted an antiproliferative activity. However, activation of ERβ led to a stronger inhibition of ERα-stimulated cell proliferation. Indeed, treatment of BELZ ERβ cells with the pan-agonist ligand E2 or with the selective ERβ ligand 8β-VE2 at concentrations at which it also activated ERα led to a significant decreased stimulation of cell proliferation as compared with treatment with PPT (Figure 2, B and C). However, it should be noted that specific activation of ERβ stimulated cell proliferation because a mitogenic effect was observed upon treatment of BELZ ERβ cells with low concentrations (1 nM) of 8β-VE2 that selectively activated ERβ. These data thus demonstrated that ERβ exerted a mixed agonist/antagonist activity on E2-induced proliferation of BG1 ovarian cancer cells. Moreover, our results highlight a dual negative effect of ERβ with an antiproliferative effect of apo-ERβ and a further inhibition upon ligand activation.
Ligand-induced transactivation in BELZ and BELZ ERβ-cells
We then investigated the effects of the different ligands on ERE-mediated transcription in BELZ and BELZ ERβ-cell lines (Figure 3). In BELZ cells, we observed a strong dose-dependent transactivation by E2 and by ER-selective compounds with EC50 corresponding to the concentrations at which they bind ERα (Figure 3A). This confirmed again that in BG1 native cells, ERα is the main mediator of estrogenic responses. It should be noted that the 3 ligands were identically effective in transactivation (12-fold induction as compared with control without any ligand).
Figure 3.
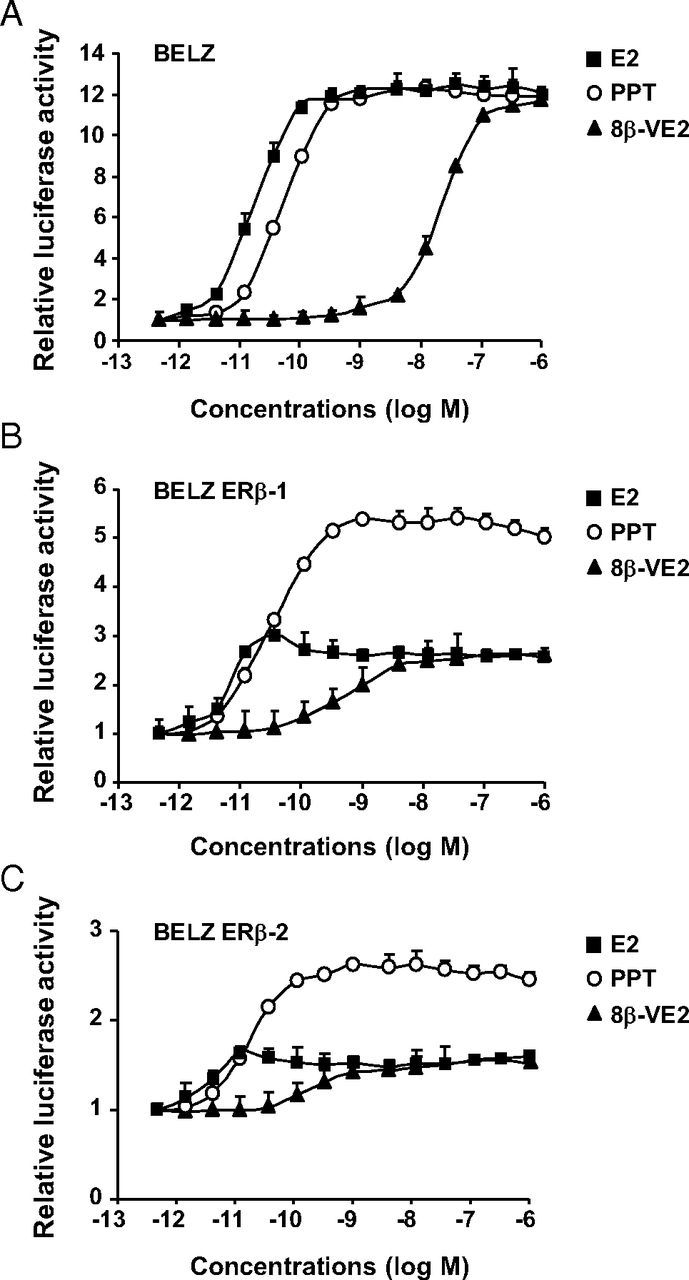
Transcriptional response of BELZ cell to ER ligands. The regulation of ERE-mediated luciferase activity was assessed in BELZ (A), BELZ ERβ-1 (B), and BELZ ERβ-2 (C) cells upon exposure to increasing concentrations of E2 (■), PPT (○), or 8β-VE2 (▴). The 3 cell lines were treated with the different ligands at the indicated concentrations for 24 hours. Luciferase activity was quantified as described in Materials and Methods. Results were expressed relative to control response set at 1. Data points represent the mean of 3 independent experiments ± SD.
By contrast, in the 2 BELZ ERβ clones (Figure 3, B and C), the ERα-selective compound was more active than E2 and the ERβ-selective ligand 8β-VE2. Indeed, the induction of luciferase activity in the BELZ ERβ clones was 2- to 3-fold higher with PPT than with E2 or 8β-VE2. Moreover, the induction factors obtained with the 3 different ligands were decreased as compared with BELZ cells, this effect being stronger in the BELZ ERβ-2 clone expressing the higher level of ERβ. As observed for cell proliferation, ERβ expression strongly diminished E2-dependent transactivation in BG1 cells. Interestingly, in the BELZ ERβ clones, the ERβ-selective compound (8β-VE2) induced luciferase expression at concentrations at which it only binds ERβ. These results suggested that, similarly to proliferation, both ERβ expression and activation strongly reduced the E2-dependent regulation of ERE-controlled luciferase expression by ERα.
Effects of combined treatments with ERα- and ERβ-selective ligands
These observations were supported by data shown in Figure 4, which analyzed the effects of combined stimulations with PPT (ERα-selective ligand) and increasing concentrations of 8β-VE2 (ERβ-selective ligand). As shown in Figure 4A, increasing doses of 8β-VE2 inhibited the effect of PPT on BELZ ERβ-2 cell proliferation, whereas no decrease was seen in BELZ cells. These results were confirmed on transactivation, the effects being even more intense than on cell proliferation. Indeed, as shown in Figure 4B, increasing doses of 8β-VE2 did not modify the transactivation obtained in response to PPT in BELZ cells whereas they significantly diminished the transcriptional response in the BELZ ERβ-2 clone.
Figure 4.
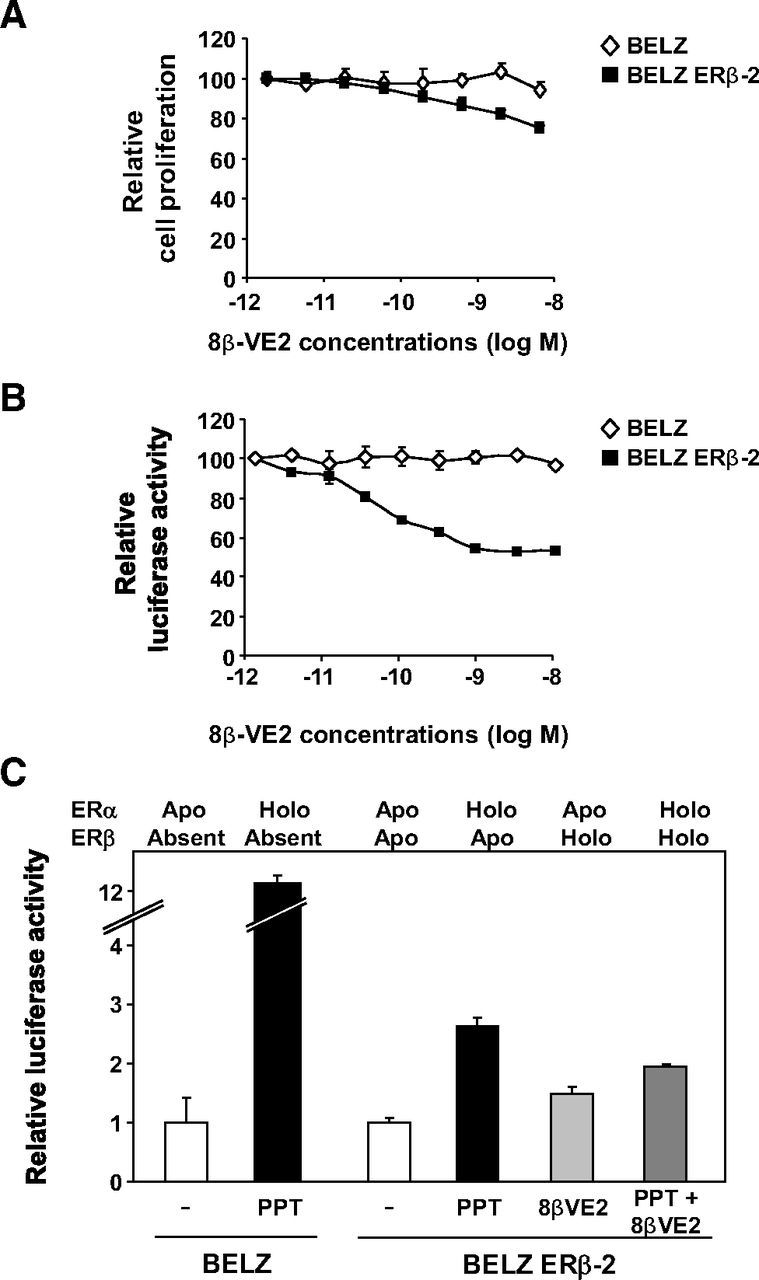
Transcriptional response of BELZ cells upon sequential activation of both ERs. A, Cell proliferation was quantified in BELZ (♢) and BELZ ERβ-2 (■) cells upon exposure during 7 days to PPT (10 nM) and increasing concentrations of 8β-VE2. Data are presented normalized with respect to the proliferation of control cells set at 100%. B, BELZ (♢) and BELZ ERβ-2 (■) cells were treated with PPT (10 nM) together with increasing concentrations of 8β-VE2. Luciferase activity was quantified as described in Materials and Methods and expressed as percent of control. C, Level of ERE-mediated luciferase activity in the BELZ and BELZ ERβ-2 cell lines treated with the indicated ligands. The status of ER activation (apo or holo status) is indicated. All experiments were performed and data expressed relative to control luciferase activity set at 1.
The effect of specific activation of the 2 ERs was also observed upon stimulation of BELZ ERβ-2 cells with ethinyl-estradiol which binds and activates ERα only at concentrations below 0.5 nM and both ERs at higher concentrations (21). Data shown in Supplemental Figure 2 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org clearly highlighted a significant breakdown in the transcriptional activation of the ERE-dependent luciferase activity at concentrations at which ethinyl-estradiol started to activate ERβ. Altogether, these data demonstrated the impact of ERβ expression and activation on ERα signaling in ovarian cancer cells. These results are summarized in Figure 4C, which shows the levels of ERE-mediated luciferase activity in BELZ cells expressing various combinations (see above the figure) of the apo or holo forms of ERα and ERβ.
Recruitment of ERα and ERβ on DNA
In order to decipher the underlying mechanisms, we first analyzed the recruitment of ERα and ERβ on DNA by performing ChIP experiments in the BELZ and BELZ ERβ-2 cell lines. As expected, ChIP analysis in BELZ ERβ-2 cells demonstrated a strong ligand-dependent interaction of ERβ (immunoprecipitated with the anti-FLAG antibody) with the ERE driving the expression of the luciferase gene, whereas no signal was obtained in BELZ cells (Figure 5A). Interestingly, the E2-dependent recruitment of ERα on this ERE that we observed in BELZ cells was altered in BELZ ERβ-2 cells when ERβ was expressed. As shown in Figure 5A, ChIP analysis revealed that ERβ expression increased the basal (ie, in the absence of ligand) interaction of ERα with DNA and slightly decreased its E2-dependent recruitment.
Figure 5.
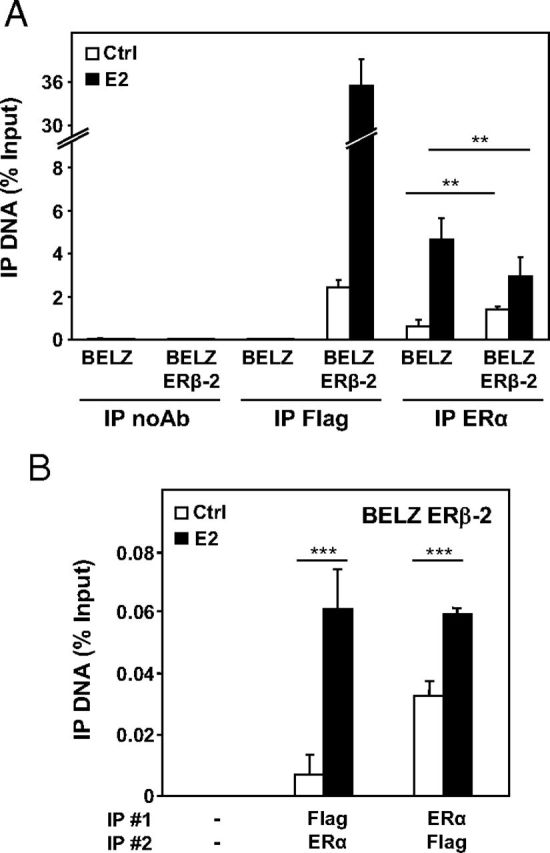
Interaction of ERs with DNA in BELZ cell lines. A, ChIP experiments using immunoprecipitation (IP) of Flag (ERβ) and ERα on the ERE sites of the estrogen-responsive luciferase gene, after treatment of BELZ cell lines with ethanol (Ctrl) or E2 (10 nM) during 1 hour. Control immunoprecipitation was performed with beads alone (IP noAb). IP values represent percentage of inputs. Data represent the mean of 2 independent experiments ± SD. B, ChIP analysis after 2 immunoprecipitations (IP 1 and 2) using Flag and ERα antibodies in BELZ ERβ-2 cell lines after 1 hour of estrogen treatment (E2 10 nM) or not (Ctrl). IP values represent percentage of inputs.
This observation may be due to the formation of ERα-ERβ heterodimers on DNA. To confirm this hypothesis, we performed ChIP/reChIP analysis for ERα and ERβ in BELZ ERβ-2 cells. As shown in Figure 5B, ERα/ERβ heterodimers were detected in control conditions when the ChIP/reChIP analysis was performed in the 2 ways (ie, immunoprecipitation of ERα, then ERβ or vice versa) and the recruitment of the heterodimer was significantly increased in the presence of E2 (between 2- and 6-fold). These results indicated that the effect of ERβ on ERα transactivation in our experimental conditions is, at least partly, the consequence of the formation of heterodimers between the 2 receptors that could impact the expression of target genes.
Effect of ERβ expression on E2 target genes
In order to support these results, we then analyzed the impact of ERβ on E2-regulated genes by quantifying the expression of several estrogen-regulated mRNAs. We first analyzed the effect of ERβ on the basal expression of progesterone receptor (PGR), fibulin-1, retinoic acid receptor-α, cyclin D1, and RIP140 genes. Data showed a significant inhibition of expression in the BELZ ERβ-2 clone, except for PGR and RIP140 genes (Figure 6A). As shown in Figure 6B, E2 treatment (10 nM) of BELZ cells significantly increased the levels of PGR, fibulin-1, retinoic acid receptor-α, and cyclin D1 mRNAs, and this regulation by E2 was significantly reduced in BELZ ERβ-2 cells. By contrast, RIP140 expression was not E2 regulated in BELZ cells but appeared significantly increased in BELZ ERβ-2 cells. These data therefore confirmed the impact of apo- and holo-ERβ on E2 signaling in ovarian cancer cells and revealed the atypical E2 regulation of the transcription coregulator RIP140 in these cells.
Figure 6.
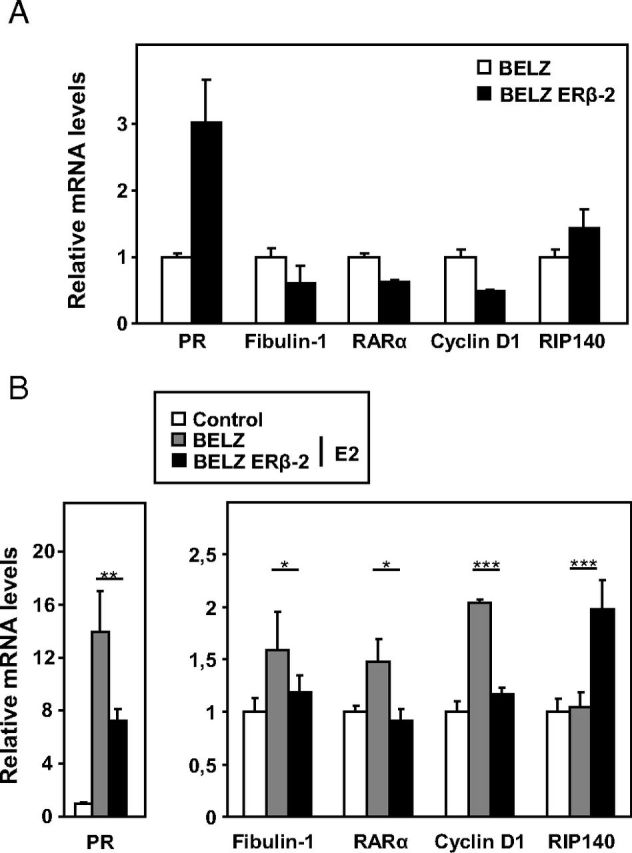
Regulation of endogenous ER-target genes in BELZ cell lines. The levels of PR, fibulin-1, retinoic acid receptor-α (RARα), cyclin D1, and RIP140 mRNA levels were measured by RT-qPCR in the BELZ and BELZ ERβ-2 cell lines, after 1 day of treatment with ethanol (Control) or estrogen (E2 10 nM). Results are expressed as fold vs level in BELZ cells in the absence of ligand (A) or as fold E2 induction (B).
Regulation of the RIP140 gene by the 2 ERs in BG1 cells
In order to further analyze the regulation of the RIP140 gene by E2 in BG1 ovarian cancer cells, we performed transient transfection experiments using the RIP140 gene reporter construct (27). As shown in Figure 7A, we found a higher level of luciferase activity in the presence of E2 in BELZ ERβ-2 cells as compared with BELZ cells, thus confirming the data obtained on the endogenous RIP140 mRNAs (Figure 6B).
Figure 7.
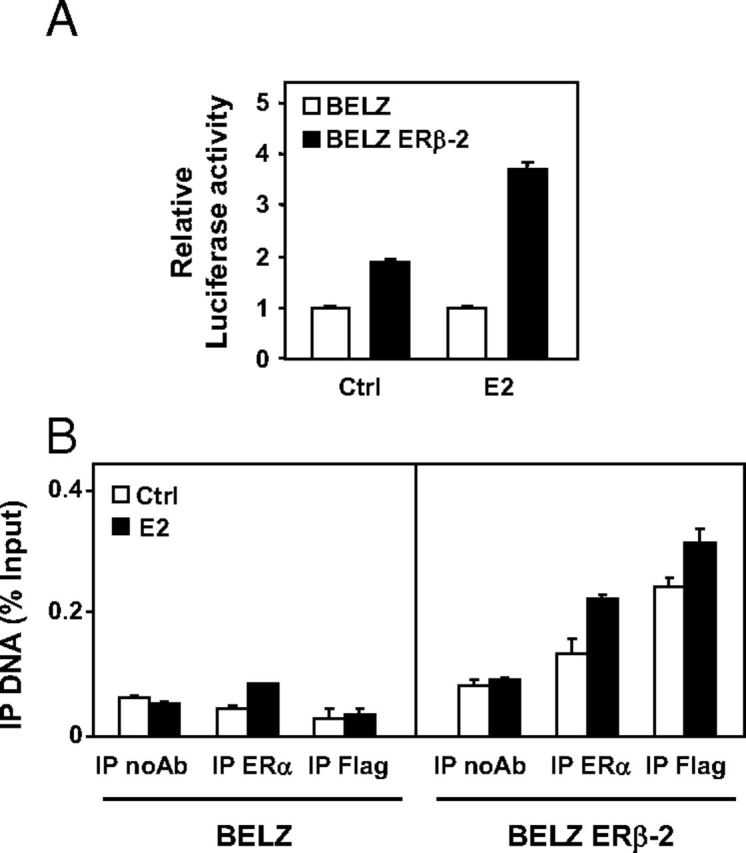
Effect of ERα and ERβ on RIP140 expression. A, BELZ and BELZ ERβ-2 cell lines were transiently transfected with the human RIP140 promoter reporter plasmids (25 ng). After 24 hours, cells were treated with E2. The luciferase activity in BELZ cell lines (Ctrl and E2) was normalized at 1. B, ChIP analysis with Flag or ERα immunoprecipitation on the ERE site of RIP140 promoter after treatment with ethanol (Ctrl) or E2 (10 nM) during 1 hour of BELZ and BELZ ERβ-2 cell lines. Immunoprecipitation with no antibody was used for negative control (IP noAb). IP values represent percentage of inputs.
We then performed ChIP analysis to investigate the recruitment of both ERs on the endogenous RIP140 gene (Figure 7B). Data demonstrated a very low E2-dependent binding of ERα in BELZ cells, which correlated with the lack of induction of the endogenous RIP140 mRNA in BELZ cells. Interestingly, results indicated that both ERα and ERβ recruitment was significantly increased in BELZ ERβ-2 cells. These data demonstrated that RIP140 preferentially cross talks with ERβ and suggested that this transcription coregulator might play a particular role in the ERα/ERβ antagonism in ovarian cancer cells.
Interaction of RIP140 with ERα and ERβ
RIP140 is an atypical coregulator that mainly acts an agonist-recruited transcriptional repressor (for a review see Ref. 28), and it is assumed that a higher expression and a stronger recruitment of RIP140 decreases the global transactivation of nuclear receptors as we previously showed for the androgen receptor (29). Although transcriptional inhibition by RIP140 was initially described on ERα (30), a direct effect of RIP140 on ERβ has not yet been evidenced, and the comparison of the effect of RIP140 on the 2 ERs has not been achieved.
To test whether RIP140 interacted differently with ERβ than with ERα, we analyzed the in vitro association of RIP140 with both receptors. We first used a GST-pull down assay to determine whether different regions of the cofactor were required for the binding to ERα or ERβ. Nine LxxLL motifs (leucine charged domain [LCD]) have been identified in the RIP140 protein and are involved in its hormone-dependent interaction with nuclear receptors (31). As shown in Figure 8A, several RIP140 domains efficiently interacted with in vitro expressed ERα and ERβ. Interestingly, in the absence of hormone, ERβ gave a stronger interaction than ERα with all GST constructs, the difference being greater with GST-RIP (429–582) and GST-RIP (683–916), which correspond to LCD6 and LCD7/8, respectively. We also carried out fluorescence anisotropy measurements to determine the relative affinity of RIP140 for DNA-bound ERα and ERβ using baculovirus-expressed purified human receptors and a fluorescein-labeled ERE. The anisotropy of the fluorescein-labeled ERE was measured with increasing concentrations of a purified GST-RIP140 protein. Data shown in Supplemental Figure 3 confirmed that ERβ interaction presented a higher affinity for GST-RIP140 than ERα.
Figure 8.
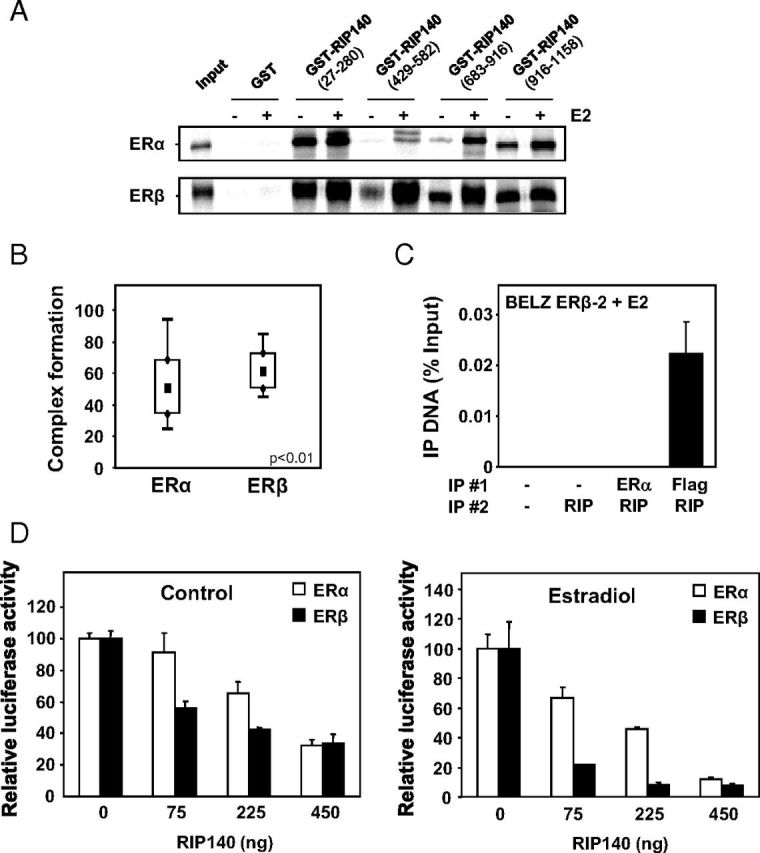
Interaction of RIP140 with ERα and ERβ. A, In vitro interaction of RIP140 with ERα and ERβ was assessed by GST pulldown assay between bacterially expressed GST-RIP proteins vs GST alone and 35S-labeled ERα or ERβ in the presence or not of E2 used at the concentration of 1 μM. B, Complex formation of RIP140 with ERα and ERβ was measured by FCCS in E2-treated COS-7 cells transfected with expression vectors for tagged-version of RIP140 and either ERα or ERβ. FCCS measurements and data analysis were conducted as described in Materials and Methods. Results are presented as percent complex formation between RIP140 and each of the ERs. C, RIP140 recruitment by the 2 ERs was performed using ChIP analysis. Two sequential immunoprecipitations (IP 1 and 2) were performed using RIP140 and Flag or ERα antibodies in BELZ ERβ-2 cell lines after 1 hour of E2 treatment (10 nM). The PCR amplification of the ERE site in front of the luciferase gene was performed as described in Materials and Methods. Values represent percentage of input. D, The effect of RIP140 on ERα and ERβ transactivation was assayed in transient transfection experiments. HeLa cells were transiently transfected with the ERE-βGlob-Luc reporter construct (500 ng) together with 500 ng of expression vector for ERα (white symbols) or ERβ (black symbols) and increasing concentrations of RIP140 expression plasmid (adjusted with empty vector). Cells were then treated with E2 for 24 hours, and luciferase activity was quantified as indicated in Materials and Methods. Results are expressed as relative luciferase activity (% of control) and are the mean (±SD) of 8 values from 3 independent experiments.
We previously reported the use of 2 photons, 2 colors, FCCS in transiently transfected cells to measure the interactions of ERα or ERβ with the transcriptional coactivator, transcriptional intermediary factor 2 (25). We used the same approach to study the interaction of RIP140 with the two ERs in living COS-7 cells (which lack endogenous ERs). The 2 ERs were expressed as N-terminal fusions with the cerulean blue fluorescent protein (cer-ER) cells whereas RIP140 was expressed as a fusion protein with mCherry (mCherry-RIP140) (Supplemental Figure 1A). FCCS acquisitions were performed on transfected cells as previously described (25), and correlation functions were obtained under estradiol treatment (Supplemental Figure 1B). As shown in Figure 8B, cross-correlation amplitudes in the presence of E2 were, on average, higher with ERβ than with ERα. These quantitative values for complex concentrations in a cellular context thus strengthen the in vitro data showing a stronger interaction of RIP140 with ERβ. Finally, to demonstrate a stronger recruitment of RIP140 by ERβ, we performed ChIP/reChIP experiments between ERs and RIP140 on ERE sequences in BELZ ERβ-2 cells. As shown in Figure 8C, a strong ligand-dependent interaction was observed when RIP140 immunoprecipitation was performed after the anti-FLAG immunoprecipitation, thus confirming that RIP140 preferentially interacted with ERβ.
Effect of RIP140 on ERα and ERβ activity
In order to compare the effect of RIP140 on the transcriptional activity of the 2 ERs, we performed transient transfection experiments. As shown in Figure 8D, the activity of both ERα and ERβ was negatively regulated when RIP140 expression vector was cotransfected. However, dose-response experiments performed with increasing amounts of RIP140 expression vector revealed a significant difference between the 2 receptors. Indeed, we observed that ERβ transcriptional activity was more sensitive than ERα to the repressive effect of RIP140 when low concentrations of RIP140 expression vector were transfected either in control conditions (left panel) or in the presence of 10 nM E2 (right panel). This result thus demonstrated that ERβ is more efficient to recruit RIP140 than ERα, and altogether our data supported an important role for this transcriptional corepressor in the negative effect of ERβ on ERα transactivation.
To experimentally demonstrate this point, we set up RIP140 knockdown experiments in BELZ ERβ-2 cells. As shown in Figure 9A, transient transfection of RIP140 siRNA significantly reduced the level of RIP140 mRNA by about 70% as compared with cells transfected with a control siRNA. When we assessed the ERE-mediated regulation of the luciferase reporter gene in these conditions, we found that the difference in luciferase activity obtained after treatment with the ERα-selective compound (PPT) and the pan-agonist ligand (E2) was totally abolished when RIP140 was knocked down (Figure 9B). These results thus demonstrated that RIP140 expression is required for the repressive effect exerted by activated ERβ on the regulation of ERE-controlled transcription by estrogens.
Figure 9.
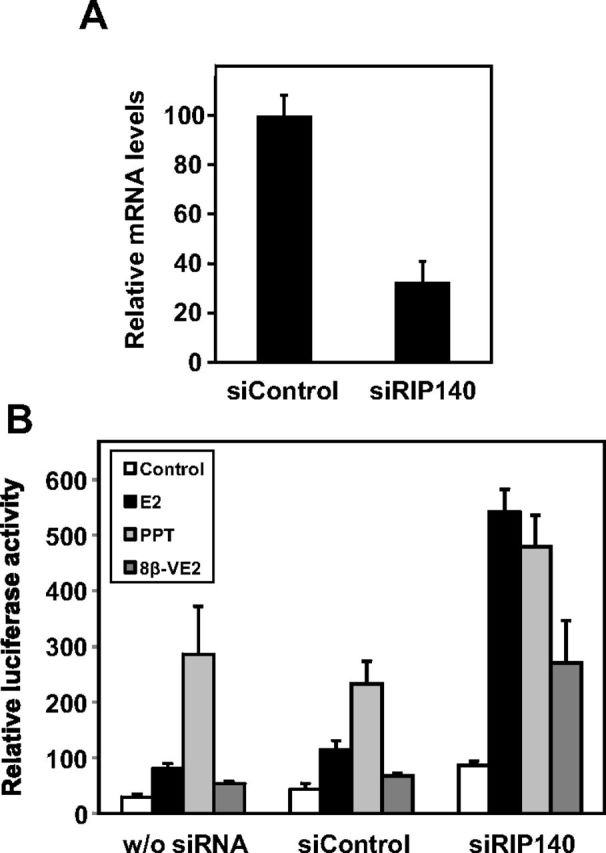
Knockdown of RIP140 expression in BELZ ERβ-2 cells. A, BELZ ERβ-2 cells were transfected with either small interfering control (siControl) or small interfering RIP140 (siRIP140) as indicated in Materials and Methods, and RIP140 mRNA expression was assessed by real time PCR. B, BELZ ERβ-2 cells were transfected with either siControl or siRIP140 or untransfected (w/o siRNA). After 24 hours, cells were treated with vehicle alone (EtOH), with 10−8 M E2 or PPT or with 10−9 M 8β-VE2. Luciferase activity was quantified as described in Materials and Methods. Results were expressed relative to control response set at 1.
Discussion
In hormone-dependent (breast and ovarian) cancers, ER expression is markedly altered between normal and tumoral epithelial cells (32, 33). Both in vitro and in vivo studies suggest that ERβ acts as a negative regulator of ERα action on gene expression and cell proliferation (34). However, the opposed effect of ERβ on ERα has been mainly studied in breast cancer cells (10–13, 35), and very few studies have been performed in other E2-responsive epithelial cells such as ovarian cell lines (36). The main objective of the present study was to determine the effect ERβ on the ability of ERα to regulate gene transactivation and cell proliferation in ovarian cancer cells. To this aim, we used ERα-positive BG1 ovarian cancer cells transfected with an ERE-driven luciferase gene (BELZ cell line). In these cells, we then combined overexpression of ERβ (leading to different ERα/ERβ ratios) with the use of selective ligands (able to activate specifically ERα or ERβ).
Our data first indicated that estrogen responses obtained in the parental BELZ cells are only mediated by ERα. Indeed, BELZ cells mostly expressed the ERα subtype and, in these cells, ERE-mediated transactivation and cell proliferation were fully activated by the ERα-selective agonist PPT. On the contrary, 8β-VE2 was not able to induce transactivation and cell proliferation at concentrations at which it bound and activated only ERβ. Interestingly, our results clearly demonstrated that, upon overexpression, ERβ exerted a mixed agonist/antagonist activity on E2-induced responses. In ERβ overexpressing cells (BELZ ERβ clones), we first noticed that the ERβ-selective compound 8β-VE2 was able to partially induce luciferase expression and cell proliferation at concentrations at which it only binds ERβ. Such partial agonist effects of ERβ-selective ligands were not observed in other studies performed in breast cancer cell lines (10–13) either due to a cell-specific effect or more probably, to a different expression ratio between ERα and ERβ.
Concerning the inhibitory activity of ERβ on E2 signaling, our data highlight a dual effect with a first action of the unliganded form of the receptor (apo-ERβ) and a further inhibition upon its ligand activation (see summary in Figure 4C). Transcriptomic analysis performed in U2OS cells has demonstrated that expression of ERβ in the absence of ligand regulated, either positively or negatively, an important set of genes (37). Moreover, a growth-inhibitory effect of the unliganded form of ERβ has also been reported in MCF-7 cells (38). Our results clearly demonstrated that in ovarian cancer cells, ERβ inhibited estrogen-induced transactivation and proliferation in a dose-dependent manner. These results are consistent with data obtained in breast (10–13) cancer cell lines, in which overexpression of ERβ resulted in reduced cell growth in the presence of E2. More recently, the tumor-suppressor properties of ERβ have been evidenced in ovarian cancer cells using in vitro assays and an orthotopic model of ovarian carcinogenesis (39). In this study, the authors suggested that ERβ exerted part of its effects by regulating ERα levels. Our results did not support such a mechanism, and the basis of this discrepancy remains to be determined.
Most of the above-mentioned studies have examined the different effects of ERα and ERβ action on cell proliferation, but few have investigated their roles on gene transactivation. Experiments performed with transient transfection in both ERα- and ERβ-negative cell lines have indicated that ERβ is generally less active than ERα (40, 41). On the contrary, stably reporter cell lines expressing ERα and ERβ established in our laboratory or by other groups have shown that, at least in HeLa and human embryonic kidney cells 293, ERβ was as transcriptionally active as ERα (21, 42).
Our results clearly show that the ERα/ERβ ratio is one of the major determinants of estrogen-induced responses such as regulation of transactivation and cell proliferation. The present study indicates that ERα and ERβ heterodimerize in BG1 cells and suggests that such heterodimer formation impinges estrogen signaling. Interaction of ERβ with ERα has been previously reported in MCF-7 breast cancer cells (43). In cells expressing both ERs, the extent of formation of ERα/β heterodimers depends on the relative amount of the 2 receptors (44). ERα/ERβ heterodimers and ERα homodimers have been shown to be preferentially formed in intact cells and to bind DNA estrogen-responsive elements (EREs) with higher affinity than ERβ homodimers (45). Using coexpression of the 2 ER subtypes in COS-7 cells followed by FCCS analysis, we previously reported substantial receptor heterodimer formation with estimated homo- and heterodimerization constants found to be in the low nanomolar range (25). More recently, ERα/β-heterodimer-selective compounds suggest that the ERα/β-heterodimer is growth inhibitory in HC11 and PC3 cells that coexpress ERα and ERβ (46).
A hypothesis that explains the inhibitory effect of ERβ on transactivation and cell proliferation involves a differential recruitment of transcriptional coregulators by the 2 ER subtypes. RIP140 is a nuclear protein identified as a transcription cofactor of nuclear receptors which, despite its recruitment in the presence of agonist ligands, exhibits a strong transcriptional repressive activity of various nuclear receptors (for a review see Ref. 28). Interestingly, using various approaches (GST pull down, anisotropy measurements, FCCS, and ChIP assays), we have clearly demonstrated that RIP140 was preferentially interacting with ERβ as compared with ERα. Moreover, our results obtained after siRNA-mediated knockdown of RIP140 expression demonstrated the key role of RIP140 in the repressive effect exerted by activated ERβ on the regulation of ERE-controlled transcription by estrogens. In addition, during the revision of this work, a paper reporting ChIP-SEQ analysis in MCF-7 cells nicely supported our conclusions (47). Indeed, the authors showed that the number of RIP140-binding sites was significantly increased upon ERβ expression, suggesting a preferential action of RIP140 with ERβ. Other coregulatory proteins have been shown to interact specifically with one receptor or the other. For instance, GIOT-4 and thyroid hormone receptor-associated protein 220 have been identified as ERβ-specific coactivators (48, 49), whereas BRG1- and hBRM-Associated Factor 57 or calmodulin selectively regulates ERα-mediated transcription (50, 51). More recently, a comparative computational analysis of the nuclear interactomes of the 2 ER subtypes revealed only 70 common proteins (on a total of 498 partners), thus likely explaining the distinct biological roles of the 2 ERs (52).
Our results indicate that RIP140 is engaged in a regulatory loop with ERβ because we found a preferential regulation of RIP140 expression by this receptor. Very interestingly, the recent study by Madak-Erdogan et al. (47), confirmed our data with a significantly stronger E2 induction of RIP140 expression in MCF-7 cells by ERβ than ERα. These data are in line with previous observations that revealed the existence of negative feedback loops involving RIP140 and various transcription factors such as other nuclear receptors (29) or E2Fs (22, 53) the activity of which is also repressed by RIP140. In addition, this demonstrates a dual mechanism for the repression of ERα activity by ERβ, which involves 1) the induction of RIP140 that can directly impinge ERα homodimer activity and 2) a stronger recruitment of the repressor on E2-target genes through ERα/ERβ heterodimer formation.
In conclusion, our findings demonstrate that the ER subtype ratio determines both quantitatively and qualitatively the functional ER response to selective ligands in ovarian cancer cells. These results also suggest that ERβ and RIP140 expression is functionally involved in ovarian tumorigenesis, at least in ERα-positive ovarian cancers. In this line, a signature associated with ERβ- and RIP140-binding sites was recently found to predict better prognosis for patients with breast cancer in several large clinical data sets (47). Altogether, these data point out the importance of ERα/ERβ ratio and RIP140 expression for the ultimate effects of hormones (or environmental ligands) on ovarian cancer cell proliferation.
Acknowledgments
We thank Dr I. Pongratz (Huddinge, Sweden) for plasmids and Dr Catherine Teyssier (IRCM, INSERM U896) for critical reading of the manuscript.
This work was supported by Institut National de la Santé et de la Recherche Médicale, University of Montpellier1, CRLC Val d'Aurelle.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BELZ
- BG1 ERE Luciferase Zeomycin
- DTT
- dithiothreitol
- E2
- 17-β-estradiol
- ER
- estrogen receptor
- ERE
- estrogen response element
- FCCS
- fluorescence cross-correlation spectroscopy
- FCS
- fetal calf serum
- GST
- glutathione-S-transferase
- LBA
- ligand-binding assay
- 16α-LE2
- 3,17-dihydroxy-19-nor-17α-pregna-1,3,5(10)-triene-21,16α-lactone
- PGR
- progesterone receptor
- PPT
- 4,4′,4′-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol
- qPCR
- quantitative PCR
- RIP140
- receptor-interacting protein 140
- siRNA
- small interfering RNA
- 8β-VE2
- 8-vinylestra-1,3,5(10)-triene-3,17β-diol.
References
- 1. Harris HA. Estrogen receptor-β: recent lessons from in vivo studies. Mol Endocrinol. 2007;21:1–13. [DOI] [PubMed] [Google Scholar]
- 2. Pearce ST , Jordan VC. The biological role of estrogen receptors α and β in cancer. Crit Rev Oncol Hematol. 2004;50:3–22. [DOI] [PubMed] [Google Scholar]
- 3. Nilsson S , Mäkelä S , Treuter E , Tujague M , Thomsen J , Andersson G, et al. . Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. [DOI] [PubMed] [Google Scholar]
- 4. Matthews J , Gustafsson JA. Estrogen signaling: a subtle balance between ER α and ER β. Mol Interv. 2003;3:281–292. [DOI] [PubMed] [Google Scholar]
- 5. Monroe DG , Getz BJ , Johnsen SA , Riggs BL , Khosla S , Spelsberg TC. Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERα or ERβ. J Cell Biochem. 2003;90:315–326. [DOI] [PubMed] [Google Scholar]
- 6. Monroe DG , Secreto FJ , Subramaniam M , Getz BJ , Khosla S , Spelsberg TC. Estrogen receptor α and β heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol Endocrinol. 2005;19:1555–1568. [DOI] [PubMed] [Google Scholar]
- 7. Stossi F , Barnett DH , Frasor J , Komm B , Lyttle CR , Katzenellenbogen BS. Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER)α or ERβ in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology. 2004;145:3473–3486. [DOI] [PubMed] [Google Scholar]
- 8. Heldring N , Pike A , Andersson S , Matthews J , Cheng G , Hartman J, et al. . Estrogen receptors: how do they signal and what are their targets. Physiol Rev 2007;87:905–931. [DOI] [PubMed] [Google Scholar]
- 9. Bai J , Uehara Y , Montell DJ. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000;103:1047–1058. [DOI] [PubMed] [Google Scholar]
- 10. Murphy LC , Peng B , Lewis A , Davie JR , Leygue E , Kemp A, et al. . Inducible upregulation of oestrogen receptor-β1 affects oestrogen and tamoxifen responsiveness in MCF7 human breast cancer cells. J Mol Endocrinol. 2005;34:553–566. [DOI] [PubMed] [Google Scholar]
- 11. Ström A , Hartman J , Foster JS , Kietz S , Wimalasena J , Gustafsson JA. Estrogen receptor β inhibits 17β-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA. 2004;101:1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sotoca AM , Ratman D , van der Saag P , Ström A , Gustafsson JA , Vervoort J, et al. . Phytoestrogen-mediated inhibition of proliferation of the human T47D breast cancer cells depends on the ERα/ERβ ratio. J Steroid Biochem Mol Biol. 2008;112:171–178. [DOI] [PubMed] [Google Scholar]
- 13. Sotoca AM , van den Berg H , Vervoort J , van der Saag P , Ström A , Gustafsson JA, et al. . Influence of cellular ERα/ERβ ratio on the ERα-agonist induced proliferation of human T47D breast cancer cells. Toxicol Sci. 2008;105:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenlee RT , Murray T , Bolden S , Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. [DOI] [PubMed] [Google Scholar]
- 15. Brandenberger AW , Tee MK , Jaffe RB. Estrogen receptor α (ER-α) and β (ER-β) mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: down-regulation of ER-β in neoplastic tissues. J Clin Endocrinol Metab. 1998;83:1025–1028. [DOI] [PubMed] [Google Scholar]
- 16. Pujol P , Rey JM , Nirde P , Roger P , Gastaldi M , Laffargue F, et al. . Differential expression of estrogen receptor-α and -β messenger RNAs as a potential marker of ovarian carcinogenesis. Cancer Res. 1998;58:5367–5373. [PubMed] [Google Scholar]
- 17. Rutherford T , Brown WD , Sapi E , Aschkenazi S , Muñoz A , Mor G. Absence of estrogen receptor-β expression in metastatic ovarian cancer. Obstet Gynecol. 2000;96:417–421. [DOI] [PubMed] [Google Scholar]
- 18. Chan KK , Wei N , Liu SS , Xiao-Yun L , Cheung AN , Ngan HY. Estrogen receptor subtypes in ovarian cancer: a clinical correlation. Obstet Gynecol. 2008;111:144–151. [DOI] [PubMed] [Google Scholar]
- 19. Halon A , Nowak-Markwitz E , Maciejczyk A , Pudelko M , Gansukh T , Györffy B, et al. . Loss of estrogen receptor β expression correlates with shorter overall survival and lack of clinical response to chemotherapy in ovarian cancer patients. Anticancer Res. 2011;31:711–718. [PubMed] [Google Scholar]
- 20. Drummond AE , Fuller PJ. The importance of ERβ signalling in the ovary. J Endocrinol. 2010;205:15–23. [DOI] [PubMed] [Google Scholar]
- 21. Escande A , Pillon A , Servant N , Cravedi JP , Larrea F , Muhn P, et al. . Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor α or β. Biochem Pharmacol. 2006;71:1459–1469. [DOI] [PubMed] [Google Scholar]
- 22. Docquier A , Augereau P , Lapierre M , Harmand P-O , Badia E , Annicotte J-S, et al. . The RIP140 gene is a transcriptional target of E2F1. PLoS ONE. 2012;7:e35839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castet A , Boulahtouf A , Versini G , Bonnet S , Augereau P , Vignon F, et al. . Multiple domains of the receptor-interacting protein 140 contribute to transcription inhibition. Nucleic Acids Res. 2004;32:1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thénot S , Bonnet S , Boulahtouf A , Margeat E , Royer CA , Borgna JL, et al. . Effect of ligand and DNA binding on the interaction between human transcription intermediary factor 1α and estrogen receptors. Mol Endocrinol. 1999;13:2137–2150. [DOI] [PubMed] [Google Scholar]
- 25. Savatier J , Jalaguier S , Ferguson ML , Cavaillès V , Royer CA. Estrogen receptor interactions and dynamics monitored in live cells by fluorescence cross-correlation spectroscopy. Biochemistry. 2010;49:772–781. [DOI] [PubMed] [Google Scholar]
- 26. Geisinger KR , Kute TE , Pettenati MJ , Welander CE , Dennard Y , Collins LA, et al. . Characterization of a human ovarian carcinoma cell line with estrogen and progesterone receptors. Cancer. 1989;63:280–288. [DOI] [PubMed] [Google Scholar]
- 27. Augereau P , Badia E , Fuentes M , Rabenoelina F , Corniou M , Derocq D, et al. . Transcriptional regulation of the human NRIP1/RIP140 gene by estrogen is modulated by dioxin signalling. Mol Pharmacol. 2006;69:1338–1346. [DOI] [PubMed] [Google Scholar]
- 28. Augereau P , Badia E , Carascossa S , Castet A , Fritsch S , Harmand PO, et al. . The nuclear receptor transcriptional coregulator RIP140. Nucl Recept Signal. 2006;4:e024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carascossa S , Gobinet J , Georget V , Lucas A , Badia E , Castet A, et al. . Receptor-interacting protein 140 is a repressor of the androgen receptor activity. Mol Endocrinol. 2006;20:1506–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cavaillès V , Dauvois S , L'Horset F , Lopez G , Hoare S , Kushner PJ, et al. . Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J. 1995;14:3741–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heery DM , Kalkhoven E , Hoare S , Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. [DOI] [PubMed] [Google Scholar]
- 32. Bardin A , Boulle N , Lazennec G , Vignon F , Pujol P. Loss of ERβ expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004;11:537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lazennec G. Estrogen receptor β, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Lett. 2006;231:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leitman DC , Paruthiyil S , Vivar OI , Saunier EF , Herber CB , Cohen I, et al. . Regulation of specific target genes and biological responses by estrogen receptor subtype agonists. Curr Opin Pharmacol. 2010;10:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas CG , Strom A , Lindberg K , Gustafsson JA. Estrogen receptor β decreases survival of p53-defective cancer cells after DNA damage by impairing G2/M checkpoint signaling. Breast Cancer Res Treat. 2011;127:417–427. [DOI] [PubMed] [Google Scholar]
- 36. Treeck O , Pfeiler G , Mitter D , Lattrich C , Piendl G , Ortmann O. Estrogen receptor β1 exerts antitumoral effects on SK-OV-3 ovarian cancer cells. J Endocrinol. 2007;193:421–433. [DOI] [PubMed] [Google Scholar]
- 37. Vivar OI , Zhao X , Saunier EF , Griffin C , Mayba OS , Tagliaferri M, et al. . Estrogen receptor β binds to and regulates three distinct classes of target genes. J Biol Chem. 2010;285:22059–22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paruthiyil S , Parmar H , Kerekatte V , Cunha GR , Firestone GL , Leitman DC. Estrogen receptor β inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423–428. [DOI] [PubMed] [Google Scholar]
- 39. Bossard C , Busson M , Vindrieux D , Gaudin F , Machelon V , Brigitte M, et al. . Potential role of estrogen receptor β as a tumor suppressor of epithelial ovarian cancer. PLoS ONE. 2012;7:e44787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kuiper GG , Carlsson B , Grandien K , Enmark E , Häggblad J , Nilsson S, et al. . Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. [DOI] [PubMed] [Google Scholar]
- 41. Delaunay F , Pettersson K , Tujague M , Gustafsson JA. Functional differences between the amino-terminal domains of estrogen receptors α and β. Mol Pharmacol. 2000;58:584–590. [DOI] [PubMed] [Google Scholar]
- 42. Barkhem T , Carlsson B , Nilsson Y , Enmark E , Gustafsson J , Nilsson S. Differential response of estrogen receptor α and estrogen receptor β to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54:105–112. [DOI] [PubMed] [Google Scholar]
- 43. Papoutsi Z , Zhao C , Putnik M , Gustafsson JA , Dahlman-Wright K. Binding of estrogen receptor α/β heterodimers to chromatin in MCF-7 cells. J Mol Endocrinol. 2009;43:65–72. [DOI] [PubMed] [Google Scholar]
- 44. Li X , Huang J , Yi P , Bambara RA , Hilf R , Muyan M. Single-chain estrogen receptors (ERs) reveal that the ERα/β heterodimer emulates functions of the ERα dimer in genomic estrogen signaling pathways. Mol Cell Biol. 2004;24:7681–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cowley SM , Hoare S , Mosselman S , Parker MG. Estrogen receptors α and β form heterodimers on DNA. J Biol Chem. 1997;272:19858–19862. [DOI] [PubMed] [Google Scholar]
- 46. Powell E , Shanle E , Brinkman A , Li J , Keles S , Wisinski KB, et al. . Identification of estrogen receptor dimer selective ligands reveals growth-inhibitory effects on cells that co-express ERα and ERβ. PLoS ONE. 2012;7:e30993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Madak-Erdogan Z , Charn TH , Jiang Y , Liu ET , Katzenellenbogen JA , Katzenellenbogen BS. Integrative genomics of gene and metabolic regulation by estrogen receptors α and β, and their coregulators. Mol Syst Biol. 2013;9:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kouzu-Fujita M , Mezaki Y , Sawatsubashi S , Matsumoto T , Yamaoka I , Yano T, et al. . Coactivation of estrogen receptor β by gonadotropin-induced cofactor GIOT-4. Mol Cell Biol. 2009;29:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49. Wärnmark A , Almlöf T , Leers J , Gustafsson JA , Treuter E. Differential recruitment of the mammalian mediator subunit TRAP220 by estrogen receptors ERα and ERβ. J Biol Chem. 2001;276:23397–23404. [DOI] [PubMed] [Google Scholar]
- 50. García Pedrero JM , Del Rio B , Martínez-Campa C , Muramatsu M , Lazo PS , Ramos S. Calmodulin is a selective modulator of estrogen receptors. Mol Endocrinol. 2002;16:947–960. [DOI] [PubMed] [Google Scholar]
- 51. García-Pedrero JM , Kiskinis E , Parker MG , Belandia B. The SWI/SNF chromatin remodeling subunit BAF57 is a critical regulator of estrogen receptor function in breast cancer cells. J Biol Chem. 2006;281:22656–22664. [DOI] [PubMed] [Google Scholar]
- 52. Nassa G , Tarallo R , Guzzi PH , Ferraro L , Cirillo F , Ravo M, et al. . Comparative analysis of nuclear estrogen receptor α and β interactomes in breast cancer cells. Mol Biosyst. 2011;7:667–676. [DOI] [PubMed] [Google Scholar]
- 53. Docquier A , Harmand PO , Fritsch S , Chanrion M , Darbon JM , Cavaillès V. The transcriptional coregulator RIP140 represses E2F1 activity and discriminates breast cancer subtypes. Clin Cancer Res. 2010;16:2959–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]


