Abstract
The testis is an immune privileged organ in which the tissue-specific cells have adopted effective innate immune functions against microbial pathogens. Toll-like receptors (TLRs) mediate innate immune response in the testis. The current study demonstrates that melanoma differentiation-associated protein 5 (MDA5) and retinoic acid-inducible gene I (RIG-I) initiate the testicular innate antiviral response. Both MDA5 and RIG-I are expressed in Leydig cells, and MDA5 is also expressed in spermatids. Polyinosinic-polycytidylic acid [poly(I:C)], a common agonist of MDA5 and RIG-I, significantly induces the expression of type I interferons (IFN-α/β) and antiviral proteins, including IFN-stimulated gene 15, 2′5′-oligoadenylate synthetase 1, and Mx GTPase 1, in primary TLR3-deficient (TLR3−/−) Leydig and germ cells. Moreover, major proinflammatory cytokines, including TNF-α and IL-6, are significantly up-regulated by poly(I:C) in these testicular cells. The poly(I:C)-induced innate antiviral response in the testicular cells is significantly reduced by knockdown of individual MDA5 and RIG-I using specific small interfering RNA. We also provide evidence that local injection of poly(I:C) induces antiviral response in the testis of TLR3−/− mice. These data provide novel insights into the mechanisms underlying testicular antiviral response.
The mammalian testis represents a remarkable immune privileged site, in which both allo- and autoantigens can be tolerated by systemic immunity. Although local testicular cells have adopted effective innate immune functions against microbial pathogens, infectious orchitis is one of the etiological factors of the male reproductive dysfunction (1, 2). Viral infection of the male germ cells impairs fertility and may also lead to the sexual dissemination of the pathogens (3, 4). Moreover, virus replication in the testis facilitates testicular cancer (5). Therefore, understanding the mechanisms underlying innate antiviral response in the testis is important to develop preventive and therapeutic approaches for testicular inflammation due to viral infections.
Type 1 interferon (IFN-α/β) production after viral infections is one of the primary innate responses against viruses (6). IFN-α/β-inducible antiviral proteins, including IFN-stimulating gene 15 (ISG15), 2′5′-oligoadenylate synthetase (OAS1), double-stranded RNA (dsRNA)-activated protein kinase (PKR), and Mx GTPase 1 (MX1), inhibit viral replication at multiple levels (7). IFN-α/β also enhance adaptive immune responses against viruses (8). Previous studies have shown that various rat testicular cells display antiviral responses by producing IFN-α/β (9–12). By contrast, human testicular cells exhibit relatively weak innate antiviral activities compared with their rat counterparts (13). The absence of natural viral infectious orchitis in rats and the presence of several viruses causing orchitis in men may be explained by the differential antiviral responses in the testis of the 2 species. Therefore, further understanding of the antiviral response mechanisms in the murine testis may provide novel strategies to develop therapeutic and preventive approaches for human viral orchitis.
Pattern recognition receptors (PRRs) belong to a superfamily of molecules that recognize conserved molecular structures of microbial pathogens, termed “pathogen-associated molecular patterns,” and trigger innate and adaptive immune responses against microbes (14, 15). Toll-like receptors (TLRs) are the best characterized PRRs, which belong to a subfamily of transmembrane receptors that recognize a broad range of pathogen-associated molecular patterns (16). To date, 13 TLR members have been identified in mammals. Four of them (TLR3, TLR7, TLR8, and TLR9) recognize diverse pathogen-derived nucleic acids (17). For example, TLR3 recognizes dsRNA that may be produced by different types of viruses during their replication, and synthetic dsRNA analog, poly(I:C) (polyinosinic-polycytidylic acid) (18). The important functions of TLRs in testicular innate immunity have been revealed (19, 20). Several TLR members are expressed in testicular cells (21). TLR functions in murine Sertoli cells have been extensively studied (22–25). Notably, TLR3-mediated antiviral responses in mouse Sertoli, Leydig, and germ cells have been recently reported (24, 26, 27).
The critical cytosolic viral sensors, retinoic acid-inducible gene I (RIG-I)-like receptors, including melanoma differentiation-associated protein 5 (MDA5) and RIG-I, induce innate antiviral response upon recognition of viral dsRNA (28). The activation of MDA5 and RIG-I triggers the IFN-β promoter stimulator-1-dependent signaling pathway, which results in the activation of IFN-regulatory factor 3 (IRF3) and nuclear factor-κB (NF-κB), thereby inducing expression of IFN-α/β and proinflammatory factors (29). Although TLR3 also recognizes viral dsRNA, it triggers Toll/IL-1 receptor domain containing adaptor-inducing IFN-β-dependent pathway. Both IFN-β promoter stimulator-1- and Toll/IL-1 receptor domain containing adaptor-inducing IFN-β-dependent signaling mediate antiviral response through 2 parallel pathways (30). The present study aims to evaluate MDA5/RIG-I-triggered innate antiviral response in the testis of TLR3−/− mice. We demonstrate that MDA5 and RIG-I are constitutively expressed in Leydig cells, and MDA5 is also expressed in round and elongating spermatids. MDA5/RIG-I signaling mediates innate antiviral response in these testicular cells. These results provide novel insights into the mechanisms underlying antiviral response in the testis.
Materials and Methods
Animals
TLR3−/− mice (B6/129S1-TLR3tm1Flv/J) with a genetic background of 50% C57BL/6 and 50% 129S1 were purchased from The Jackson Laboratory. Wild-type (WT) mice were obtained by mating C57BL/6 mice and 129S1. These mice were inbred under specific pathogen-free conditions and fed freely with food and water on a natural day/night light cycle. The mice were treated in accordance with the Guidelines for Care and Use of Laboratory Animal approved by the Chinese Council of Animal Care.
Antibodies and major reagents
Rabbit anti-MDA5 (antibody [ab]69983), anti-RIG-I (ab45428), anti-TLR3 (ab13915), and anti-OAS1 (ab86343) polyclonal antibodies were purchased from Abcam. Rabbit anti-phospho-IRF3 (no. 4947), anti-phospho-NF-κBp65 (no. 3031), and anti-ISG15 (no. 2743) polyclonal antibodies and anti-NF-κBp65 (no. 4764) monoclonal antibody were purchased from Cell Signaling Technology. Rabbit anti-IRF3 (sc-9082), anti-MX1 (sc-50509), and anti-PKR (sc-708) polyclonal antibodies were purchased from Santa Cruz Biotechnology. Poly(I:C) (tlr-pic), BAY11-7082 (tlrl-b82), and BX795 (tlr-bx7) were purchased from InvivoGen. Small interfering RNA (siRNA) targeting mouse RIG-I (sc-61481), MDA5 (sc-61011), and control siRNA (sc-37007) were purchased from Santa Cruz Biotechnology.
Isolation and culture of Leydig and germ cells
Leydig and germ cells were isolated from 5-week-old mice based on previously described procedures (26, 31). Briefly, mice were anesthetized with CO2 and humanely destroyed by cervical dislocation. For each isolation, the testes of 3 mice were decapsulated and incubated with 0.5 mg/mL collagenase type 1 (Sigma) at room temperature for 15 minutes with gentle oscillation. The suspensions were filtered through 80-μm copper meshes to separate the interstitial cells and the seminiferous tubules. The interstitial cells were collected in F12/DMEM (Life Technologies, Inc) supplemented with sodium bicarbonate (1.2 mg/mL), antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin), and 10% fetal calf serum (Life Technologies, Inc). The cells were incubated in a humidified atmosphere with 5% CO2 at 32°C for 30 minutes to allow the testicular macrophages to adhere on the culture dishes. Nonadherent and loosely adherent cells were collected from the supernatants by gently rinsing the culture dishes twice with medium. The cells were cultured for 48 hours for the proliferation of Leydig cells and then treated with 0.125 g/mL of trypsin for 5 minutes. Majority of the Leydig cells can be detached, whereas minor contaminated macrophages remain adherent during this treatment. The purity of the Leydig cells was more than 95% based on staining for 3β-hydroxysteroid dehydrogenase, a marker of Leydig cells (32).
The seminiferous tubules were resuspended in collagenase type 1 at room temperature for an additional 15 minutes to remove the peritubular myoid cells. The tubules were cut into small pieces (∼1 mm), and then incubated with 0.5 mg/mL hyaluronidase (Sigma) at room temperature for 10 minutes with gentle pipetting to separate Sertoli cells and germ cells. The cell suspensions were cultured in F12/DMEM at 32°C for 6 hours. The germ cells were recovered by collecting nonadherent cells. The purity of the germ cells was more than 97% based on the nuclear morphology analysis after staining with 4′,6-diamidino-2-phenylindole (DAPI). Sertoli cells were cultured for an additional 24 hours and then treated with a hypotonic solution (20 mM Tris, pH 7.4) for 1 minute to remove germ cells adhering to Sertoli cells. The purity of the Sertoli cells was more than 95% based on immunostaining for Wilms' tumor nuclear protein 1, a marker of Sertoli cells (33).
Three stages of germ cells, including spermatogonia, spermatocytes, and spermatids, were isolated from the testes of mice at 7, 21, and 35 days of age, respectively, by velocity sedimentation following a previously description (34). The purity of each cell type was more than 90% based on assessment of cell nuclear morphology after staining with DAPI.
Isolation of macrophages
The resident peritoneal macrophages were isolated based on a previously described procedure (35). Briefly, the peritoneal cavities of mice were lavaged with 5 mL ice-cold 1× PBS. The peritoneal cavity cells were cultured in RPMI 1640 medium (Life Technologies) supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, and 10% fetal calf serum in a humidified atmosphere containing 5% CO2 at 37°C. After 24 hours, nonadherent cells were removed by washing with PBS. More than 98% of the adherent cells were macrophages based on immunostaining for F4/80, a marker of macrophages (36).
Transfection
For poly(I:C) stimulation, Leydig cells were seeded in 6-well plates at a density of 5 × 105 cells/well and cultured for 24 hours. The cells were starved in serum-free F12/DMEM for 2 hours, and then transfected with 2 μg/mL poly(I:C) using 2 μL lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instruction. Fresh isolated germ cells were cultured in serum-free F12/DMEM at a density of 1 × 106 cells/well and transfected with poly(I:C).
In the experiments for gene silence with siRNA, Leydig cells were seeded in 6-well plates at a density of 2 × 105 cells/well. At 24 hours after culture, the cells were transfected with 50 nM siRNA using approaches described above. Fresh isolated germ cells were seeded at a density of 1 × 106 cells/well and transfected with siRNA. Twenty-four hours later, the cells were transfected with poly(I:C).
3-(4, 5)-Dimethylthiahiazolyl-2)-2,5-diphenyte-trazolium bromide (MTT) assay
Cell viability was assessed using MTT assay kit (American Type Culture Collection) according to the manufacturer's instructions. Briefly, cells were seeded in 96-well microplates at a density of 2 × 104 cells/well. After treatments with the same condition as those for the induction of antiviral response, the cells were incubated with 10 μL MTT solution for 2 hours. After removal of medium, 100 μL detergent reagent was added to each well. Absorbance at 570 nm was counted using a microplate reader (BioTek). The percentage of the absorbance value vs control represents cell viability.
Real-time quantitative RT-PCR (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. After treatment with RNase-free DNase I (Invitrogen) to remove genomic DNA contamination, the RNA (1 μg) was reverse transcribed into cDNA in 20 μL reaction mixture containing 2.5 μM random hexamers, 2 μM deoxynucleotide triphosphate, and 200 U Moloney murine leukemia virus reverse transcriptase (Promega Corp). PCR was performed in 20 μL reaction mixture containing 0.2 μL cDNA, 0.5 μM forward and reverse primers, and 10 μL 2× Power SYBR Green PCR Master Mix (Applied Biosystems) using an ABI PRISM 7300 real-time cycler (Applied Biosystems). The mRNA levels of the target genes were normalized to β-actin using the comparative threshold cycle method described in Applied Biosystems User Bulletin No. 2 (P/N 4303859). The primers for PCRs are listed in Table 1.
Table 1.
Primer Pairs (5′→3′) Used for Real-Time PCR
| Target Genes | Forward | Reverse |
|---|---|---|
| MDA5 | AGATCAACACCTGTGGTAACACC | CTCTAGGGCCTCCACGAACA |
| RIG-I | CCACCTACATCCTCAGCTATATGA | TGGGCCCTTGTTGTTCTTCT |
| TLR3 | TTGTCTTCTGCACGAACCTG | CGCAACGCAAGGATTTTATT |
| TLR7 | GGAAATTGCCCTCGATGTTA | CAAAAATTTGGCCTCCTCAA |
| TLR8 | GAAGCATTTCGAGCATCTCC | GAAGACGATTTCGCCAAGAG |
| TLR9 | ACTGAGCACCCCTGCTTCTA | AGATTAGTCAGCGGCAGGAA |
| IFN-α | GACCTCCACCAGCAGCTCAA | ACCCCCACCTGCTGCAT |
| IFN-β | GACGTGGGAGATGTCCTCAAC | GGTACCTTTGCACCCTCCAGTA |
| TNF-α | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| IL-6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA |
| ISG15 | CCAGTCTCTGACTGTGAGAGC | GCATCACTGTGCTGCTGGGAC |
| OAS1 | ATTACCTCCTTCCCGACACC | CAAACTCCACCTCCTGATGC |
| MX1 | GACCATAGGGGTCTTGACCAA | AGACTTGCTCTTTCTGAAAAGCC |
| PKR | GGAAAATCCCGAACAAGGAG | CCCAAAGCAAAGATGTCCAC |
| β-Actin | GAAATCGTGCGTGACATCAAAG | TGTAGTTTCATGGATGCCACAG |
Western blot analysis
Cells or tissues were lysed using a lysis buffer (Applygen Technologies Inc). The protein concentration of the lysates was determined using the bicinchonic acid protein assay kit (Pierce Biotechnology). Equal amount of proteins (20 μg) were separated on 10% SDS-PAGE gel and subsequently electrotransferred onto polyvinyl difluoride membranes (Millipore Corp). The membranes were then blocked for 1 hour in Tris-buffered saline (pH 7.4) containing 5% nonfat milk, and incubated with the primary antibodies overnight at 4°C. After washing twice with Tris-buffered saline containing 0.1% Tween-20, the membranes were incubated with the appropriate peroxidase-conjugated secondary antibodies (Zhongshan Biotechnology Co) at room temperature for 1 hour. Antigen-antibody complexes were visualized using an enhanced chemiluminescence detection kit (Zhongshan Biotechnology Co).
Immunofluorescence staining
Leydig cells cultured on Lab-Tek chamber slides (Nunc) were fixed with precold methanol at −20°C for 3 minutes and subsequently permeabilized with 0.2% Triton X-100 in 1× PBS for 15 minutes. After blocking with 10% normal goat serum in PBS for 30 minutes at room temperature, the cells were incubated with the primary antibodies at 37°C in a humid chamber for 1 hour. Afterward, the cells were washed 3 times with PBS and incubated with appropriate fluorescein isothiocyanate-conjugated secondary antibodies (Zhongshan Biotechnology Co) for 30 minutes. Negative controls were incubated with preimmune rabbit serum instead of the primary antibodies. The cells were mounted with a mounting solution (Vector Laboratories, Inc) for observation under a fluorescence microscope (IX-71; Olympus). The staining was repeated 3 times, and 300 cells were counted for each staining.
The testes of 10-week-old mice were fixed in 4% paraformaldehyde for 24 hours. After cryoprotection in 30% sucrose, frozen sections were cut to a thickness of 7 μm using Leica CM1950 (Leica Biosystems). The sections were then incubated with 1× PBS containing 3% H2O2 for 15 minutes to inhibit the endogenous peroxidase activity. Subsequently, the sections were blocked with 5% preimmune rabbit serum in PBS for 1 hour at room temperature, followed by incubation with primary antibodies overnight at 4°C. After rinsing with PBS, the sections were incubated with tetramethylrhodamine isothiocyanate-conjugated antirabbit IgG antibodies (Zhongshan Biotechnology Co) at room temperature for 30 minutes. Negative controls were incubated with the preimmune rabbit serum instead of the primary antibodies. The sections were costained with DAPI and then mounted with a mounting solution.
ELISA
The cells were cultured in 6-well plates at a density of 5 × 105 Leydig cells or 1 × 106 germ cells per well, and then transfected with poly(I:C) in a serum-free F12/DMEM medium. At 24 hours after the poly(I:C) transfection, cytokine levels in the culture medium were measured using ELISA kits (eBioscience) according to the manufacturer's instruction.
Injection of poly(I:C) into the testis
Mice were anesthetized with pentobarbital sodium (50 mg/kg), and the testes were surgically exposed. One testis was injected with a mixture containing 0.3 μg poly(I:C), 1 μL lipofectamine RNAiMAX, and 10 μL of 1× PBS, whereas the other testis was injected with an equal volume of PBS containing 1 μL lipofectamine RNAiMAX as control. The testes were recovered at 6 hours and 24 hours after poly(I:C) injection for mRNA and protein analyses, respectively.
Statistical analysis
Data are presented as mean ± SEM. Student's t test was used to determine the significance between cell treatments. One-way ANOVA test with Bonferroni's correction was used for multiple comparisons. The data were analyzed using statistic software SPSS version 11.0. Values of P < .05 were considered of statistical significance.
Results
Expression of MDA5 and RIG-I in the testis
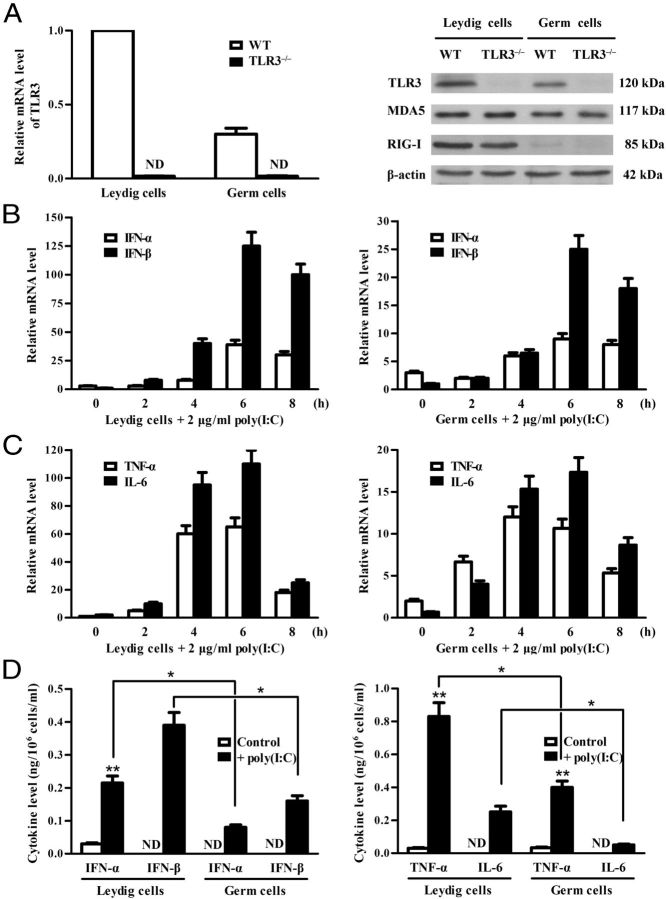
The expression of PRRs that recognize viral nucleic acids was examined in the testis and peritoneal macrophages from the same mice. Real-time qRT-PCR results showed that the testis of adult mice expresses relatively high mRNA levels of MDA5 and RIG-I compared with the macrophages (Figure 1A). TLR3 mRNA level was comparable in the testis and the macrophages. By contrast, TLR7, TLR8, and TLR9 mRNA levels were markedly low in the testis compared with the macrophages. We focused our study on MDA5 and RIG-I because the expression and function of TLR3 in the testis have already been investigated. MDA5 and RIG-I proteins in the testis were confirmed by Western blot (Figure 1B). Immunofluorescence staining showed that MDA5 (Figure 1C, upper panels) is predominantly located in the interstitial cells (asterisk), round spermatids (open arrow), and acrosomes of elongating spermatids (closed arrow), but is not detected in spermatogonia (open arrowhead) and spermatocytes (closed arrowhead), whereas RIG-I was exclusively located in the interstitial cells (asterisk) (Figure 1C, lower panels). The cell-specific expression of MDA5 and RIG-I was further confirmed in primary testicular cells at both mRNA (Figure 1D, left panel) and protein levels (Figure 1D, right panel). MDA5 and RIG-I were evident in Leydig cells. Significant MDA5 level was also detected in germ cells. By contrast, faint MDA5 and RIG-I levels were observed in Sertoli cells.
Figure 1.
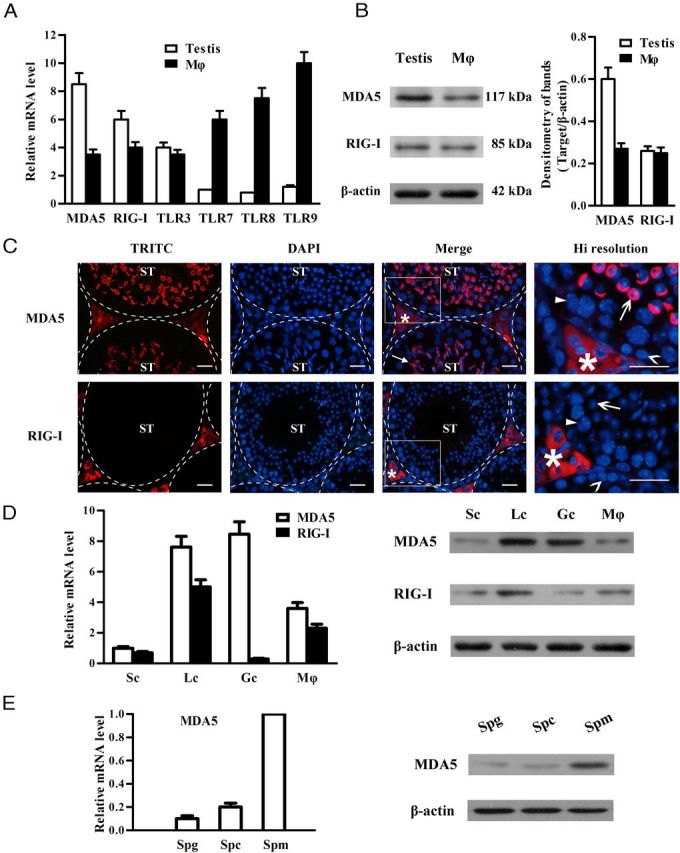
Expression of pattern recognition receptors that recognize viral nucleic acids in the testis. A, Total RNA was extracted from the testis and peritoneal macrophage (Mϕ) of 5-week-old WT mice. The expression of MDA5, RIG-I, and the indicated TLRs was examined at mRNA levels using real-time qRT-PCR. B, Protein levels of MDA5 and RIG-I. The lysates of the testis and Mϕ were analyzed via Western blot using specific antibodies. C, Localization of MDA5 and RIG-I. Immunofluorescence staining was performed in frozen testicular sections of 5-week-old mice using specific antibodies and tetramethylrhodamine isothiocyanate (TRITC)-conjugated secondary antibodies. The sections were costained with DAPI. The seminiferous tubules (ST) were delineated by dotted lines. The square areas were magnified for high (Hi) resolution (right panels). Asterisk, closed arrow, open arrow, closed arrowhead, and open arrowhead indicate the interstitial cells, acrosome, round spermatid, spermatocyte, and spermatogonium, respectively. D, Cell-specific expression of MDA5 and RIG-I. Sertoli cells (Sc), Leydig cells (Lc), germ cells (Gc), and Mϕ were isolated from 5-week-old WT mice. The mRNA levels of MDA5 and RIG-I in these cells were analyzed using real-time qRT-PCR (left panel), and their protein levels were determined using Western blot (right panel). E, Germ cell stage-specific expression of MDA5. Spermatogonia (Spg), spermatocytes (Spc), and spermatids (Spm) were isolated. The mRNA levels (left panel) and protein levels (right panel) of MDA5 in the 3 stages of germ cells were analyzed using real-time qRT-PCR and Western blot, respectively. β-Actin was used as loading control for Western blot. Images are representatives of at least 3 experiments. Data are the mean ± SEM of the 3 independent experiments. Bar, 20 μm.
Germ cell stage-specific expression of MDA5 was investigated in isolated primary spermatogonia, spermatocytes, and spermatids. The levels of MDA5 mRNA and protein were much higher in spermatids than spermatogonia and spermatocytes (Figure 1E). By contrast, RIG-I was absent in the 3 germ cell types (data not shown).
Poly(I:C) induces cytokine expression in Leydig and germ cells
Given that poly(I:C) induces the expression of IFN-α/β and proinflammatory cytokines through TLR3, MDA5, and RIG-I activation, and that TLR3 mediates the cytokine expression in Leydig and germ cells (26, 27), we examined the roles of MDA5 and RIG-I in mediating cytokine expression in Leydig and germ cells of TLR3−/− mice. Real-time qRT-PCR confirmed that TLR3 was null in TLR3−/− cells (Figure 2A, left panel), whereas, MDA5 and RIG-I were comparable at protein levels in TLR3−/− and WT cells (Figure 2A, right panel). Poly(I:C) transfection up-regulated mRNAs of IFN-α/β and IFN-β in the TLR3−/− Leydig and germ cells in a time-dependent manner (Figure 2B). Peak mRNA levels were observed 6 hours after transfection. Notably, poly(I:C) induced a relatively high level of the cytokines in Leydig cells (Figure 2B, left panel) compared with germ cells (Figure 2B, right panel). Moreover, poly(I:C) induced expression of TNF-α and IL-6 in the Leydig and germ cells (Figure 2C). ELISA results confirmed that Leydig and germ cells secrete significantly elevated IFN-α and IFN-β (Figure 2D, left panel), as well as TNF-α and IL-6 (Figure 2D, right panel) in the culture media at 24 hours after poly(I:C) transfection. The poly(I:C)-induced cytokine secretion by Leydig cells was significantly higher than germ cells.
Figure 2.
Poly(I:C)-induced expression of cytokines. A, Expression of TLR3, MDA5, and RIG-I. Leydig and germ cells were isolated from 5-week-old WT and TLR3-deficient (TLR3−/−) mice. The absence of TLR3 mRNA in TLR3−/− cells was confirmed by real-time qRT-PCR (left panel). The proteins of TLR3, MDA5, and RIG-I in WT and TLR3−/− cells were determined by Western blot (right panel). B and C, Poly(I:C)-induced up-regulation of cytokines. Total RNA was extracted from TLR3−/− Leydig and germ cells after transfection with poly(I:C) for the specified duration. The mRNA levels of the indicated cytokines were analyzed using real-time qRT-PCR. D, Cytokine secretion in culture media. The cells were seeded in 6-well plates at a density of 5 × 105 Leydig cells or 1 × 106 germ cells per well and transfected with poly(I:C). After 24 hours, the media were measured for cytokine concentration using ELISA. Data are the mean ± SEM of the 3 experiments. *, P < .05; **, P < .01. ND, not detectable.
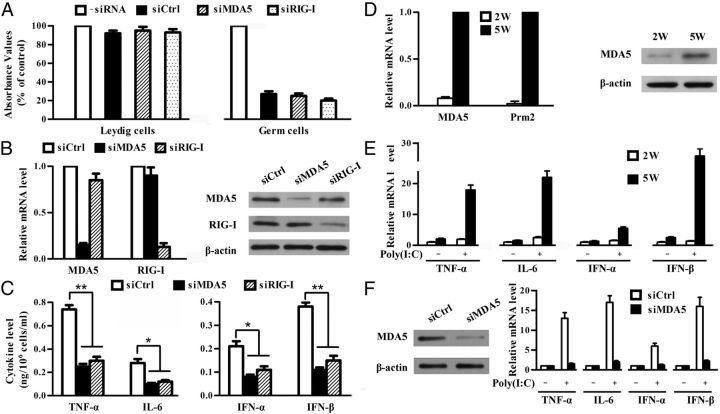
Role of MDA5 and RIG-I in poly(I:C)-induced cytokine expression
To prove involvement of MDA5 and RIG-I in poly(I:C)-induced cytokine expression, we tried to examine the poly(I:C)-induced cytokine expression in TLR3−/− cells after knockdown of individual MDA5 and RIG-I using specific siRNA. MTT assay results showed that Leydig cell viability was not apparently affected by siRNA transfection (Figure 3A, left panel). However, the germ cell viability was dramatically reduced 24 hours after siRNA transfection (Figure 3A, right panel). Each siRNA specifically reduced more than 70% target mRNA at 24 hours after transfection (Figure 3B, left panel). Dramatic decreases in the protein levels of MDA5 and RIG-I by siRNA were confirmed (Figure 3B, right panel). The cells were then transfected with poly(I:C). At 24 hours after poly(I:C) transfection, siRNA targeting either MDA5 or RIG-I significantly reduced the cytokine secretion by Leydig cells (Figure 3C). These results indicate that MDA5 and RIG-I cooperatively mediate the cytokine expression in TLR3−/− Leydig cells in response to poly(I:C).
Figure 3.
The contribution of MDA5 and RIG-I to poly(I:C)-induced cytokine expression. A, Cell viability. Leydig cells (left panel) and germ cells (right panel) were transfected with individual siRNAs targeting scrambled sequence (siCtrl), MDA5 (siMDA5), and RIG-I (siRIG-I). At 24 hours after transfection, cell viability was analyzed by MTT assay. B, Silence of target genes using specific siRNA. Leydig cells were transfected with individual siRNA. At 24 hours after siRNA transfection, MDA5 and RIG-I levels were assessed by real-time qRT-PCR (left and middle panels) and Western blot (right panel). C, Cytokine secretion. Leydig cells were transfected with siRNA. Twenty-four hours later, the cells were transfected with poly(I:C). At 24 hours after the poly(I:C) transfection, the cytokine levels in medium were measured using ELISA. D, Expression of MDA5. Germ cells were isolated from 2-week-old (2W) and 5-week-old (5W) TLR3−/− mice. MDA5 and protamine 2 (Prm2) mRNAs were analyzed by real-time qRT-PCR (left panel). MDA5 proteins in the 2 germ cell preparations were determined by Western blot (right panel). E, Poly(I:C)-induced cytokine expression. The germ cells from 2W and 5W TLR3−/− mice were transfected with 2 μg/mL poly(I:C). At 6 hours after transfection, total RNA was extracted, and relative mRNA levels of the indicated cytokines were analyzed by real-time qRT-PCR. F, Knockdown of MDA5 in germ cells. The germ cells from 5-week-old TLR3−/− mice were transfected with siCtrl or siMDA5. Twenty-four hours later, expression of MDA5 was determined by Western blot (left panel). Then the cells were transfected with poly(I:C), and the cytokine expression was examined using real-time qRT-PCR 6 hours after transfection. Images of Western blot are representatives of at least 3 independent experiments. Data represent the mean ± SEM of the 3 experiments. *, P < .05; **, P < .01.
To prove the role of MDA5 in poly(I:C)-induced antiviral response in the germ cells, we first compared the effects of poly(I:C) on the cytokine expression in the germ cells isolated from 2- and 5-week-old TLR3−/− mice. The germ cells of 5-week-old mice express protamine 2 (a marker for spermatids) and MDA5 (Figure 3D), whereas, the germ cells from 2-week-old mice do not express these 2 genes. Poly(I:C) dramatically induced cytokine expression in the germ cells from 5-week-old mice, but not in the cells from 2-week-old mice (Figure 3E). These results support speculation that poly(I:C) induces innate antiviral response in spermatids expressing MDA5. Further, MDA5 in the germ cells from 5-week-old mice was knocked down by siRNA (Figure 3F, left panel), which almost completely blocked the poly(I:C)-induced cytokine expression (Figure 3F, right panel). These results indicate that MDA5 in spermatids mediates the cytokine expression in response to poly(I:C) treatment.
Activation of IRF3 and NF-κB by poly(I:C)
Given that MDA5 and RIG-I up-regulate IFN-α/β, TNF-α, and IL-6 through the activation of IRF3 and NF-κB (37), we examined the effect of poly(I:C) on the phosphorylation of the 2 transcription factors in TLR3−/− Leydig and germ cells. We demonstrated that poly(I:C) induces the phosphorylation of both IRF3 and NF-κBp65 (p65) in Leydig cells (Figure 4A). The peak levels of phosphorylated IRF3 and p65 were detected in Leydig cells 2 hours after poly(I:C) transfection. Similarly, IRF3 and p65 were phosphorylated in the germ cells by poly(I:C) (Figure 4B). The phosphorylated IRF3 and p65 must translocate into nuclei to induce cytokine expression. Immunofluorescence staining showed that both IRF3 and p65 efficiently translocate into the nuclei of Leydig cells at 2 hours after poly(I:C) transfection (Figure 4C, left panel). The percentages of IRF3 or p65 positive nuclei peaked at 2 hours and sharply decreased at 3 hours after transfection (Figure 4C, right panel). The immunofluorescence staining was not performed on the germ cells because they could not be firmly attached to the slides for the staining process.
Figure 4.
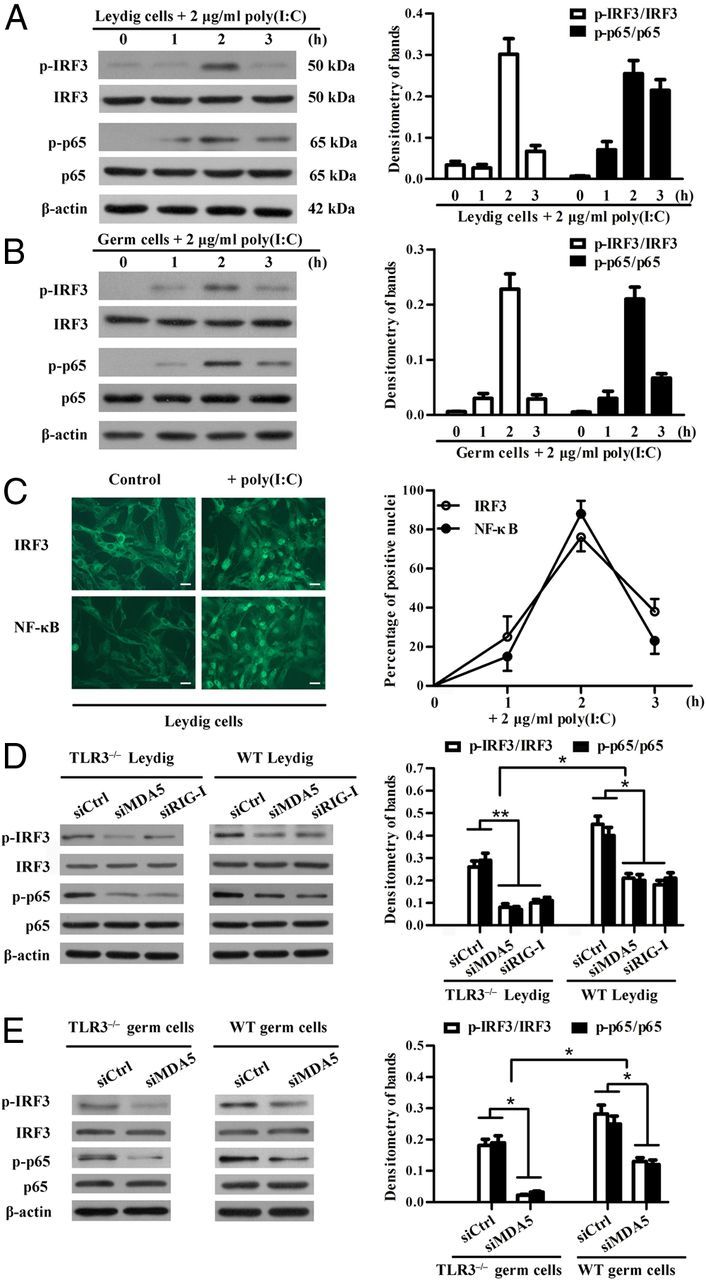
Activation of IRF3 and NF-κB. A and B, Phosphorylation of IRF3 and NF-κBp65 (p65) in Leydig and germ cells. TLR3−/− Leydig cells (A) and germ cells (B) were transfected with 2 μg/mL poly(I:C) for the specified duration. The cell lysates were analyzed by Western blot using specific antibodies against phospho-IRF3 (p-IRF3), IRF3, phospho-p65 (p-p65), and p65. β-Actin was used as loading control. C, Poly(I:C)-induced translocation of IRF3 and NF-κB from cytoplasm to the nuclei of Leydig cells. Leydig cells were transfected with poly(I:C) or transfection reagent alone (control) for 2 hours. Immunofluorescence staining was performed using antibodies against IRF3 and p65 (left panel). Scale bar, 20 μm. The time-dependent efficacy of nuclear translocation was quantitatively analyzed based on the immunofluorescence staining (right panel). D and E, Effects of silencing MDA5 and RIG-I on the poly(I:C)-induced phosphorylation of IRF3 and p65. TLR3−/−, and WT Leydig cells (D) and germ cells (E) were transfected with the indicated siRNAs. Twenty-four hours later, the cells were transfected with poly(I:C). The phosphorylation of IRF3 and p65 was determined by Western blot at 2 hours after poly(I:C) transfection. Images are representatives of 3 independent experiments, and data represent the mean ± SEM of the 3 experiments. *, P < .05; **, P < .01.
Moreover, we investigated the phosphorylation of IRF3 and p65 after the knockdown of MDA5 or RIG-I. The siRNAs targeting MDA5 or RIG-I significantly reduced the poly(I:C)-induced phosphorylation of IRF3 and p65 in both TLR3−/− and WT Leydig cells (Figure 4D). Notably, the phosphorylation levels of IRF3 and p65 were significantly higher in WT Leydig cells than in TLR3−/− cells. Similar results were observed in the germ cells after the knockdown of MDA5 (Figure 4E). These results suggest that TLR3, MDA5, and RIG-I mediate parallel pathways.
Expression of antiviral proteins
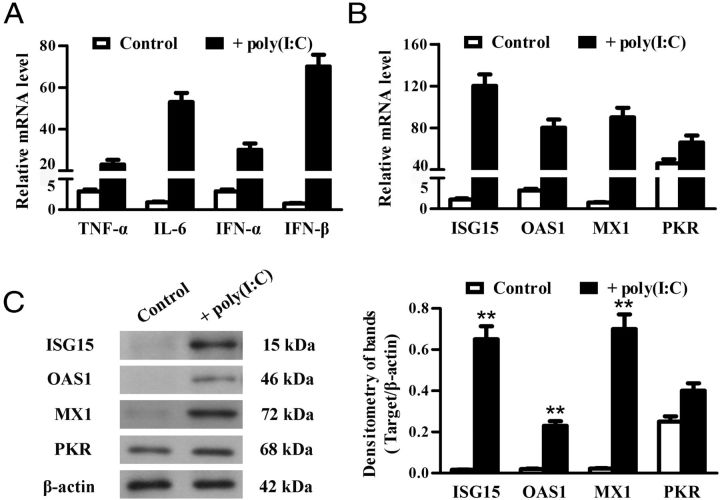
Given that IFN-α/β-inducible antiviral proteins are critical for intracellular response against viral infections (38), we examined the effect of poly(I:C) on the expression of the best-characterized antiviral proteins, including ISG15, OAS1, PKR, and MX1, in TLR3−/− Leydig and germ cells. Real-time qRT-PCR results showed that poly(I:C) dramatically up-regulates the mRNA of ISG15, OAS1, and MX1 in the Leydig cells in a time-dependent manner (Figure 5A). The peak levels of mRNA were observed in the Leydig cells at 8 hours after transfection with poly(I:C). Similar dynamics of antiviral protein expression were induced by poly(I:C) in the germ cells (Figure 5B). However, the poly(I:C)-induced mRNA levels of the antiviral proteins were relatively low in the germ cells compared with the Leydig cells. Notably, PKR was constitutively expressed and moderately up-regulated by poly(I:C) in the 2 cell types (Figure 5, A and B). The antiviral protein expression was confirmed by Western blot at protein levels 24 hours after poly(I:C) transfection. ISG15, OAS1, and MX1 proteins were dramatically induced by poly(I:C) in Leydig cells (Figure 5C) and germ cells (Figure 5D), whereas significant PKR was detected at basal conditions and remained consistent in the cells transfected with poly(I:C). Further, the poly(I:C)-induced antiviral proteins (except PKR) in the Leydig cells were significantly reduced by knockdown of individual MDA5 and RIG-I using siRNA (Figure 5E). Similarly, the knockdown of MDA5 significantly reduced the antiviral protein expression in the germ cells after poly(I:C) transfection (Figure 5F).
Figure 5.
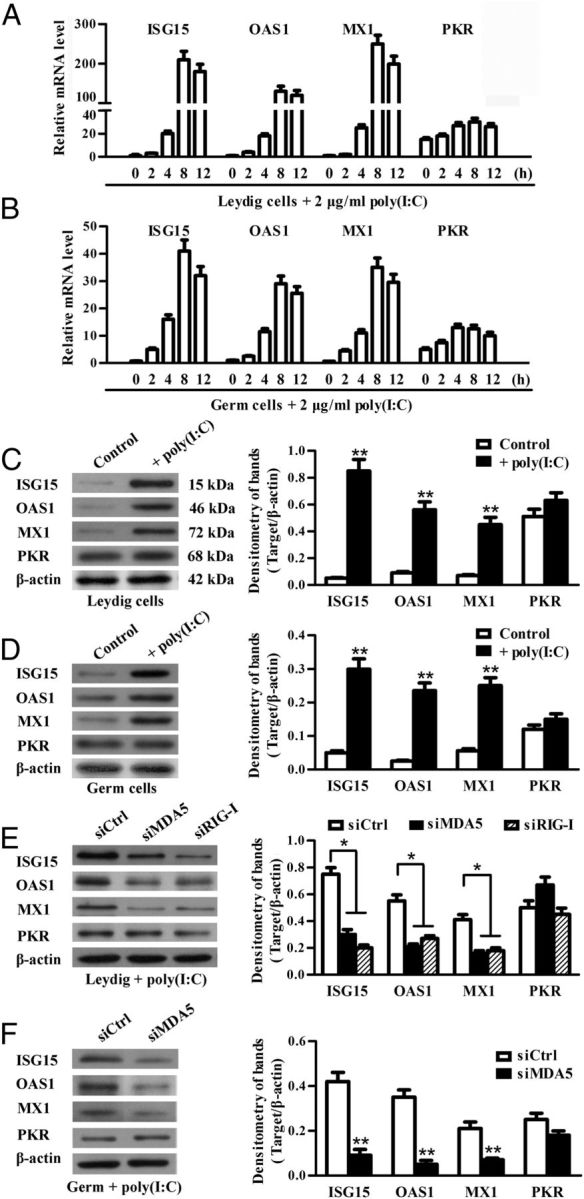
Poly(I:C)-induced expression of antiviral proteins. A and B, Relative mRNA levels of antiviral proteins. TLR3−/− Leydig cells (A) and germ cells (B) were transfected with poly(I:C). Total RNA was extracted at the indicated time points after transfection. Relative mRNA levels of ISG15, OAS1, MX1, and PKR were analyzed using real-time qRT-PCR. C and D, Protein levels of the antiviral proteins. Leydig cells (C) and germ cells (D) were transfected with poly(I:C) or with transfection reagent alone (control). At 24 hours after transfection, the cell lysates were subjected to Western blot using specific antibodies. E, Effect of siRNA on poly(I:C)-induced antiviral protein expression in Leydig cells. Leydig cells were transfected with 100 nM each siRNA. Twenty-four hours later, the cells were transfected with 2 μg/mL poly(I:C). At 24 hours after the poly(I:C) transfection, the antiviral protein levels were determined using Western blot. F, Effect of silencing MDA5 on the poly(I:C)-induced antiviral protein expression in germ cells. The germ cells were treated using siRNA targeting MDA5. The antiviral protein levels in the germ cells were determined using Western blot. β-Actin was used as loading control for Western blot. Images are representatives of the 3 experiments. Data represent the mean ± SEM of at least 3 experiments. *, P < .05; **, P < .01.
Involvement of IRF3 and NF-κB activation in poly(I:C)-induced antiviral response
To clarify the pathways that induce the antiviral response, we examined the effect of chemical inhibitors of IRF3 and NF-κB on the poly(I:C)-induced cytokine and antiviral protein expression. Poly(I:C) evidently induced the phosphorylation of IRF3 and p65 in TLR3−/− Leydig cells 2 hours after transfection, which was significantly inhibited by a 2-hour pretreatment of the cells with BX795 (an inhibitor of IRF3 activation) and BAY11-7082 (an inhibitor of NF-κB activation), respectively (Figure 6A). Similar results were obtained in the germ cells (data not shown). Notably, BX795 dramatically reduced the poly(I:C)-induced mRNA levels of IFN-α and IFN-β, but did not significantly influence TNF-α and IL-6 levels in Leydig cells (Figure 6B, left panel) and germ cells (Figure 6B, right panel). By contrast, BAY11-7082 inhibited the poly(I:C)-induced TNF-α and IL-6 and did not affect IFN-α and IFN-β in the 2 cell types. Accordingly, ISG15, OAS1, and MX1 were dramatically up-regulated by poly(I:C) in Leydig cells (Figure 6C, left panel) and germ cells (Figure 6C, right panel), which were significantly reduced by the presence of BX795. BAY11-7082 did not influence the expression of the antiviral proteins. The viability of Leydig and germ cells was not significantly affected by the treatment with poly(I:C) and the chemical inhibitors (Figure 6D).
Figure 6.
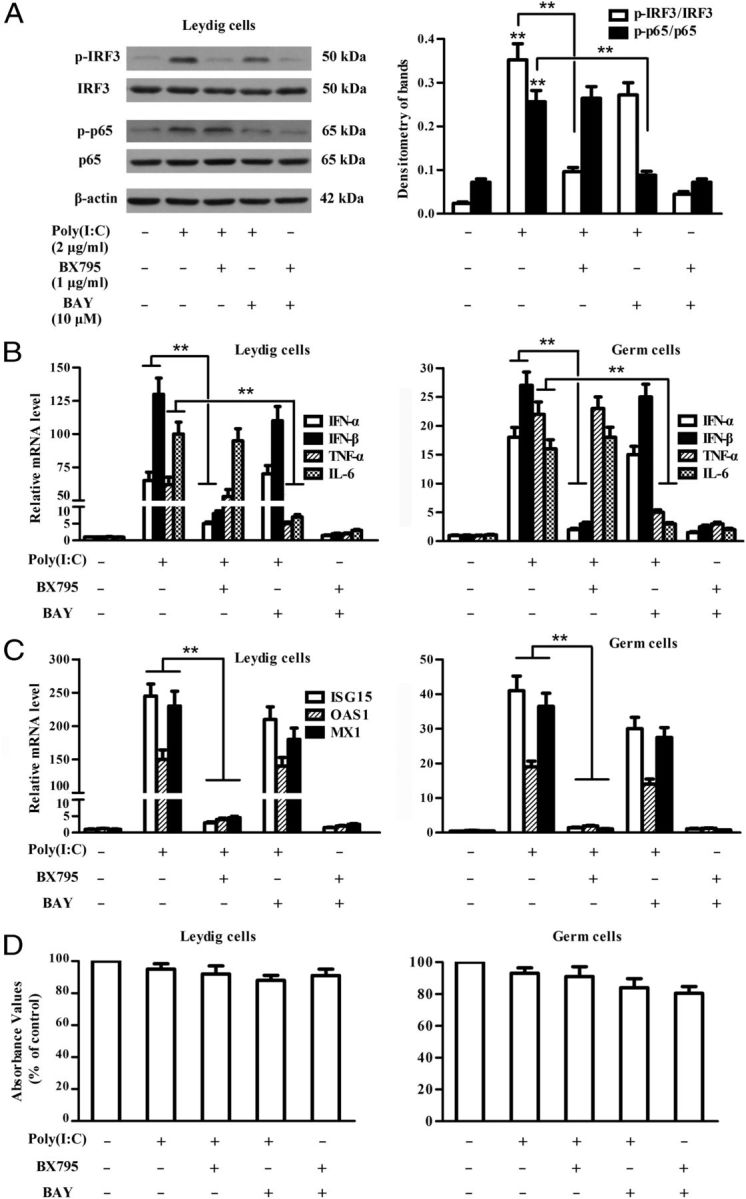
Involvement of IRF3 and NF-κB activation in gene expression. A, Inhibition of IRF3 and NF-κB activation by chemical inhibitors. TLR3−/− Leydig cells were transfected with poly(I:C), or with poly(I:C) after a 2-hour preincubation with inhibitors (1 μg/mL BX795 for IRF3, or 10 μM BAY11-7082 [BAY] for NF-κB). At 2 hours after transfection, the cell lysates were subject to Western blot to probe the indicated proteins using specific antibodies. B, Expression of inflammatory cytokines. Leydig cells (left panel) and germ cells (right panel) were transfected with poly(I:C) or with poly(I:C) after a 2-hour preincubation with the inhibitors. At 6 hours after transfection, relative mRNA levels of the indicated cytokines were analyzed using real-time qRT-PCR. C, Expression of antiviral proteins. Leydig cells and germ cells were treated as described in panel B. Relative mRNA levels of ISG15, OAS1, and MX1 in the cells 8 hours after poly(I:C) transfection were analyzed using real-time qRT-PCR. D, Effect of chemical inhibitors on cell viability. Leydig cells (left panel) and germ cells (right panel) were treated with the indicated inhibitors for 2 hours, and then transfected with poly(I:C) for 6 hours. The cell viability was examined using MTT assay. Images of Western blot are representatives of at least 3 separate experiments. Data represent the mean ± SEM of the 3 experiments. *, P < .05; **, P < .01.
Poly(I:C) induces testicular antiviral response in vivo
To determine whether poly(I:C) induces innate antiviral responses in the testis, we examined the expression of the cytokines and antiviral proteins after local injection of poly(I:C). The injection of a mixture of poly(I:C) and lipofectamine RNAiMAX into the testis significantly up-regulates TNF-α, IL-6, IFN-α, and IFN-β mRNAs 6 hours after injection (Figure 7A). Moreover, the mRNA levels of ISG15, OAS1, and MX1 were dramatically up-regulated in the testis after poly(I:C) injection (Figure 7B). By contrast, PKR was constitutively expressed in the testis and slightly up-regulated by poly(I:C). The protein levels of ISG15, OAS1, and MX1 were significantly up-regulated in the testis 24 hours after poly(I:C) injection (Figure 7C). PKR remained consistent at the protein level.
Figure 7.
Poly(I:C)-induced testicular innate antiviral response in vivo. A and B, Expression of inflammatory cytokines and antiviral proteins in vivo. A mixture of poly(I:C) and lipofectamine RNAiMAX was locally injected into one testis of 5-week-old TLR3−/− mice, whereas lipofectamine RNAiMAX alone was injected into the other testis (control) of the same mice. At 6 hours after injection, total RNA was extracted from the testes, and relative mRNA levels of the cytokines (A) and the antiviral proteins (B) were analyzed by real-time qRT-PCR. C, Induction of antiviral proteins at protein levels in vivo. The testes were injected as described above. At 24 hours after injection, the testicular lysates were subject to Western blot to probe the antiviral proteins using specific antibodies. Images are representatives of at least 3 experiments. Data represent the mean ± SEM of the 3 experiments. **, P < .01.
Discussion
A wide range of viruses is capable of infecting the testis (39–42). Viral infections in the testis may lead to chronic orchitis that impairs male fertility (1). The testis is an immune privileged site, where the local cell-initiated innate immunity is important to overcome immune privilege and mount appropriate responses against invading microbial pathogens. Understanding the mechanisms underlying testicular innate antiviral responses would aid in the development of preventive and therapeutic approaches against viral infections. In this study, we investigated the role of 2 cytosolic viral sensors, MDA5 and RIG-I, in initiating testicular innate antiviral response.
The mammalian testis is composed of interstitial spaces that contain mainly Leydig cells and macrophages, and seminiferous tubules that are composed of developing germ cells associated with Sertoli cells. The blood-testis barrier (BTB), comprised of various junctions between the neighboring Sertoli cells, divides the seminiferous tubules into basal and adluminal compartments. Both the interstitial spaces and seminiferous tubules may be infected by viruses (39). In addition to the interstitial macrophages, which are believed to construct the first line of defense against microbial pathogens from the blood circulation (43), other testicular cells can initiate innate immune responses to invading microbes. In particular, the immune cells rarely pass through the BTB and enter the adluminal compartment of the seminiferous tubules. Therefore, the innate immune responses initiated by testicular cells within the tubules are particularly important in the defense against microbes from ascending infections of the male genitourinary tracts (44). Sertoli cell-initiated innate immune responses through TLR activation have been recently revealed (19). Our recent studies demonstrated that TLR3 mediates innate antiviral response in mouse Leydig cells and early stages of germ cells, including spermatogonia and spermatocytes (26, 27). The present study provides evidence that MDA5 and RIG-I mediate antiviral response in Leydig cells and late stage of germ cells. The results extend our understanding of the mechanisms underlying the antiviral response in the testis.
The best characterized antiviral PRRs are TLR3, TLR7, TLR8, TLR9, MDA5, and RIG-I (45). TLR3 is expressed in the testis and initiates testicular innate antiviral response in different testicular cells, including Sertoli cells, Leydig cells, and germ cells (24, 26, 27, 46). In this study, we demonstrated that MDA5 and RIG-I cooperatively mediate testicular antiviral response. TLR3, MDA5, and RIG-I have common agonists, viral dsRNA and synthetic poly(I:C) (47). We used Leydig and germ cells of TLR3−/− mice to exclude the function of TLR3. PRR-induced IFN-α/β are crucial cytokines to direct the antiviral response. This study shows that IFN-α/β are dramatically up-regulated in TLR3−/− Leydig and germ cells after transfection with poly(I:C). Notably, the exogenously added poly(I:C) without transfection agent did not induce IFN-α/β expression in TLR3−/− cells (data not shown). Our recent study showed that the exogenously added poly(I:C) significantly induces antiviral response in Leydig and germ cells through TLR3 activation (26, 27). These observations suggest that TLR3 can be activated by exogenous poly(I:C), and internalized poly(I:C) is necessary to trigger MDA5/RIG-I signaling in Leydig and germ cells. We used total germ cells from 5-week-old mice in this study based on the following 2 reasons: 1) the testis of 5-week-old mice contains a great portion of round and elongating spermatids, in which MDA5 is expressed; and 2) although the purified different stages of germ cells can be ideal to distinguish the cell stage-specific response, we found that a large portion (> 40%) of spermatids isolated through a velocity sedimentation underwent apoptosis and necrosis (data not shown).
MDA5/RIG-I signaling induces inflammatory cytokine expression through IRF3 and NF-κB activation (48). Accordingly, we showed that poly(I:C) activates IRF3 and NF-κB in both Leydig and germ cells. The levels of poly(I:C)-induced phospho (p)-IRF3 and p-p65 were significantly reduced by knockdown of MDA5 or RIG-I in WT and TLR3−/− Leydig and germ cells (Figure 4, D and E). Moreover, the p-IRF3 and p-p65 levels were significantly higher in WT cells than TLR3−/− cells in response to poly(I:C) transfection with and without knockdown of MDA5 or RIG-I. Accordingly, the expression levels of the cytokines and antiviral proteins was higher in WT cells than TLR3−/− cells (data not shown). The results correspond to the previous observations that TLR3, MDA5, and RIG-I mediate antiviral response through parallel signaling pathways (30). Moreover, we demonstrated that IFN-α/β induction depends on IRF3 activation, whereas TNF-α and IL-6 up-regulation is attributable to NF-κB activation. The poly(I:C)-induced cytokine expression in Leydig cells is mediated by MDA5 and RIG-I because knockdown of individual MDA5 and RIG-I significantly reduced the cytokine expression. We showed that MDA5 is expressed in spermatids from WT mice. We confirmed that MDA5 was detected in the germ cells of 5-week-old TLR3−/− mice that contain spermatids but absent in the germ cells of 2-week-old mice that lack spermatids. Accordingly, the poly(I:C)-induced antiviral response was evident in the germ cells of 5-week-old mice. This response was absent in the germ cells of 2-week-old mice. Further, the knockdown of MDA5 in the germ cells of 5-week-old TLR3−/− mice almost completely abolish poly(I:C)-induced cytokine expression. Taken together, the results suggest that poly(I:C) induces antiviral response through MDA5 in spermatids.
IFN-α/β are pleiotropic cytokines that act against viruses by inducing the expression of antiviral proteins and facilitating adaptive immune responses (49). IFN-inducible various antiviral proteins, including ISG15, OAS1, PKR, and MX1, inhibit viral replication at multiple levels (7). We demonstrated that poly(I:C) transfection dramatically induces the expression of ISG15, OAS1, and MX1 in both Leydig and germ cells. Moreover, the local injection of poly(I:C) in the testis of TLR3−/− mice significantly induced the expression of IFN-α/β and antiviral proteins, suggesting that MDA5 and RIG-I initiate the testicular antiviral response in vivo. Notably, poly(I:C)-induced antiviral response in Leydig cells is significantly higher than that in germ cells. This difference can be caused by 2 facts: 1) Leydig cells express both MDA5 and RIG-I, which cooperatively mediate antiviral response. In contrast, only MDA5 is expressed in spermatids; and 2) germ cell viability is relatively low compared with Leydig cells during culture in vitro (data not shown), which could reduce germ cell response to poly(I:C).
Although resident macrophages in the interstitial spaces are believed to constitute the first line of testicular immune defense against pathogens from the circulation system (43), increasing evidence shows that the resident testicular macrophages display a reduced capacity of immune responses by producing less inflammatory cytokines compared with macrophages from other tissue (50, 51). By contrast, Leydig cell-initiated innate antiviral response may have an important role in the protection of the testis from invading viruses. Our recent and present studies have shown that different stages of germ cells display antiviral activities. These observations are interesting because the immune cells and cytokines in the interstitial spaces have limited access to the adluminal compartments of the seminiferous tubules due to BTB. Therefore, local germ cell-initiated innate responses should be important for the testis to counteract invading pathogens within the tubules. The innate antiviral response of the germ cells in the human testis remains elusive. Interestingly, several viruses may cause orchitis in humans. By contrast, natural viral orchitis is absent in mice. Whether the human testicular cells possess weaker antiviral capacities than the murine counterparts should be an interesting issue that is worthy of being clarified.
In conclusion, we demonstrated that mouse Leydig cells and spermatids display innate antiviral capacities through MDA5/RIG-I signaling in response to poly(I:C) stimulation, which could contribute to the testicular defense against viral infections in the interstitial spaces and the seminiferous tubules. The data provide novel insights into the mechanisms underlying innate antiviral response in the testis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants 31071270, 31171445, and 31261160491).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ab
- antibody
- BTB
- blood-testis barrier
- DAPI
- 4′, 6-diamidino-2-phenylindole
- dsRNA
- double-strand RNA
- IFN
- interferon
- IRF3
- IFN-regulatory factor 3
- ISG15
- IFN-stimulating gene 15
- MDA5
- melanoma differentiation-associated protein 5
- MTT
- 3-(4, 5)-dimethylthiahiazolyl-2)-2,5-diphenytetrazolium bromide
- MX1
- Mx GTPase 1
- NF-κB
- nuclear factor-κB
- OAS1
- 2′5′-oligoadenylate synthetase
- PKR
- dsRNA-activated protein kinase
- poly(I:C)
- polyinosinic-polycytidylic acid
- PRR
- pattern recognition receptor
- qRT-PCR
- quantitative RT-PCR
- RIG-I
- retinoic acid-inducible gene I
- TLR
- Toll-like receptor
- WT
- wild-type.
References
- 1. Schuppe HC , Meinhardt A , Allam JP , Bergmann M , Weidner W , Haidl G. Chronic orchitis: a neglected cause of male infertility? Andrologia. 2008;40:84–91. [DOI] [PubMed] [Google Scholar]
- 2. Ludwig M. Diagnosis and therapy of acute prostatitis, epididymitis and orchitis. Andrologia. 2008;40:76–80. [DOI] [PubMed] [Google Scholar]
- 3. Xu X. [The possible role of sperm in family HBV infection]. Zhonghua Liu Xing Bing Xue Za Zhi. 1992;13:337–339. [PubMed] [Google Scholar]
- 4. Baccetti B , Benedetto A , Burrini AG, et al. HIV-particles in spermatozoa of patients with AIDS and their transfer into the oocyte. J Cell Biol. 1994;127:903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dejucq N , Jégou B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev. 2001;65:208–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katze MG , He Y , Gale M. Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–687. [DOI] [PubMed] [Google Scholar]
- 7. Sadler AJ , Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. [DOI] [PubMed] [Google Scholar]
- 9. Dejucq N , Dugast I , Ruffault A , van der Meide PH , Jégou B. Interferon-α and -γ expression in the rat testis. Endocrinology. 1995;136:4925–4931. [DOI] [PubMed] [Google Scholar]
- 10. Dejucq N , Chousterman S , Jégou B. The testicular antiviral defense system: localization, expression, and regulation of 2′5′ oligoadenylate synthetase, double-stranded RNA-activated protein kinase, and Mx proteins in the rat seminiferous tubule. J Cell Biol. 1997;139:865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dejucq N , Lienard MO , Guillaume E , Dorval I , Jégou B. Expression of interferons-α and -γ in testicular interstitial tissue and spermatogonia of the rat. Endocrinology. 1998;139:3081–3087. [DOI] [PubMed] [Google Scholar]
- 12. Melaine N , Liénard MO , Guillaume E , Ruffault A , Dejucq-Rainsford N , Jégou B. Production of the antiviral proteins 2′5′oligoadenylate synthetase, PKR and Mx in interstitial cells and spermatogonia. J Reprod Immunol. 2003;59:53–60. [DOI] [PubMed] [Google Scholar]
- 13. Le Tortorec A , Denis H , Satie AP, et al. Antiviral responses of human Leydig cells to mumps virus infection or poly I:C stimulation. Hum Reprod. 2008;23:2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumar H , Kawai T , Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. [DOI] [PubMed] [Google Scholar]
- 15. Iwasaki A , Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takeda K , Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. [DOI] [PubMed] [Google Scholar]
- 17. Barbalat R , Ewald SE , Mouchess ML , Barton GM. Nucleic acid recognition by the innate immune system. Annu Rev Immunol. 2011;29:185–214. [DOI] [PubMed] [Google Scholar]
- 18. Matsumoto M , Oshiumi H , Seya T. Antiviral responses induced by the TLR3 pathway. Rev Med Virol. 2011;21:67–77. [DOI] [PubMed] [Google Scholar]
- 19. Hedger MP. Toll-like receptors and signalling in spermatogenesis and testicular responses to inflammation–a perspective. J Reprod Immunol. 2011;88:130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhushan S , Schuppe HC , Tchatalbachev S, et al. Testicular innate immune defense against bacteria. Mol Cell Endocrinol. 2009;306:37–44. [DOI] [PubMed] [Google Scholar]
- 21. Bhushan S , Tchatalbachev S , Klug J, et al. Uropathogenic Escherichia coli block MyD88-dependent and activate MyD88-independent signaling pathways in rat testicular cells. J Immunol. 2008;180:5537–5547. [DOI] [PubMed] [Google Scholar]
- 22. Riccioli A , Starace D , Galli R, et al. Sertoli cells initiate testicular innate immune responses through TLR activation. J Immunol. 2006;177:7122–7130. [DOI] [PubMed] [Google Scholar]
- 23. Wu H , Wang H , Xiong W , Chen S , Tang H , Han D. Expression patterns and functions of toll-like receptors in mouse Sertoli cells. Endocrinology. 2008;149:4402–4412. [DOI] [PubMed] [Google Scholar]
- 24. Starace D , Galli R , Paone A, et al. Toll-like receptor 3 activation induces antiviral immune responses in mouse Sertoli cells. Biol Reprod. 2008;79:766–775. [DOI] [PubMed] [Google Scholar]
- 25. Winnall WR , Muir JA , Hedger MP. Differential responses of epithelial Sertoli cells of the rat testis to Toll-like receptor 2 and 4 ligands: implications for studies of testicular inflammation using bacterial lipopolysaccharides. Innate Immun. 2011;17:123–136. [DOI] [PubMed] [Google Scholar]
- 26. Shang T , Zhang X , Wang T , Sun B , Deng T , Han D. Toll-like receptor-initiated testicular innate immune responses in mouse Leydig cells. Endocrinology. 2011;152:2827–2836. [DOI] [PubMed] [Google Scholar]
- 27. Wang T , Zhang X , Chen Q, et al. Toll-like receptor 3-initiated antiviral responses in mouse male germ cells in vitro. Biol Reprod. 2012;86:106. [DOI] [PubMed] [Google Scholar]
- 28. Loo YM , Gale M. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ireton RC , Gale M. RIG-I like receptors in antiviral immunity and therapeutic applications. Viruses. 2011;3:906–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meylan E , Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol Cell. 2006;22:561–569. [DOI] [PubMed] [Google Scholar]
- 31. Wang H , Wang H , Xiong W, et al. Evaluation on the phagocytosis of apoptotic spermatogenic cells by Sertoli cells in vitro through detecting lipid droplet formation by Oil Red O staining. Reproduction. 2006;132:485–492. [DOI] [PubMed] [Google Scholar]
- 32. Klinefelter GR , Hall PF , Ewing LL. Effect of luteinizing hormone deprivation in situ on steroidogenesis of rat Leydig cells purified by a multistep procedure. Biol Reprod. 1987;36:769–783. [DOI] [PubMed] [Google Scholar]
- 33. Sharpe RM , McKinnell C , Kivlin C , Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. [DOI] [PubMed] [Google Scholar]
- 34. Yu Z , Guo R , Ge Y, et al. Gene expression profiles in different stages of mouse spermatogenic cells during spermatogenesis. Biol Reprod. 2003;69:37–47. [DOI] [PubMed] [Google Scholar]
- 35. Chong MM , Metcalf D , Jamieson E , Alexander WS , Kay TW. Suppressor of cytokine signaling-1 in T cells and macrophages is critical for preventing lethal inflammation. Blood. 2005;106:1668–1675. [DOI] [PubMed] [Google Scholar]
- 36. Hume DA , Perry VH , Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localisation of antigen F4/80: macrophages associated with epithelia. Anat Rec. 1984;210:503–512. [DOI] [PubMed] [Google Scholar]
- 37. Yoneyama M , Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev. 2009;227:54–65. [DOI] [PubMed] [Google Scholar]
- 38. Staeheli P. Interferon-induced proteins and the antiviral state. Adv Virus Res. 1990;38:147–200. [DOI] [PubMed] [Google Scholar]
- 39. Csata S , Kulcsár G. Virus-host studies in human seminal and mouse testicular cells. Acta Chir Hung. 1991;32:83–90. [PubMed] [Google Scholar]
- 40. Lang ZW. [Distribution of hepatitis B virus in testicle tissue in patients with hepatitis B infection.] Zhonghua Yi Xue Za Zhi. 1993;73:329–331. [PubMed] [Google Scholar]
- 41. Liu FH , Tian GS , Fu XX. [Detection of plus and minus strand hepatitis C virus RNA in peripheral blood mononuclear cells and spermatid.] Zhonghua Yi Xue Za Zhi. 1994;74:284–286. [PubMed] [Google Scholar]
- 42. Nuovo GJ , Becker J , Simsir A , Margiotta M , Khalife G , Shevchuk M. HIV-1 nucleic acids localize to the spermatogonia and their progeny. A study by polymerase chain reaction in situ hybridization. Am J Pathol. 1994;144:1142–1148. [PMC free article] [PubMed] [Google Scholar]
- 43. Bryniarski K , Szczepanik M , Maresz K , Ptak M , Ptak W. Subpopulations of mouse testicular macrophages and their immunoregulatory function. Am J Reprod Immunol. 2004;52:27–35. [DOI] [PubMed] [Google Scholar]
- 44. Li N , Wang T , Han D. Structural, cellular and molecular aspects of immune privilege in the testis. Front Immunol. 2012;3:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thompson MR , Kaminski JJ , Kurt-Jones EA , Fitzgerald KA. Pattern recognition receptors and the innate immune response to viral infection. Viruses. 2011;3:920–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun B , Qi N , Shang T , Wu H , Deng T , Han D. Sertoli cell-initiated testicular innate immune response through toll-like receptor-3 activation is negatively regulated by Tyro3, Axl, and mer receptors. Endocrinology. 2010;151:2886–2897. [DOI] [PubMed] [Google Scholar]
- 47. Kawai T , Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann NY Acad Sci. 2008;1143:1–20. [DOI] [PubMed] [Google Scholar]
- 48. Bruns AM , Horvath CM. Activation of RIG-I-like receptor signal transduction. Crit Rev Biochem Mol Biol. 2012;47:194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Randall RE , Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. [DOI] [PubMed] [Google Scholar]
- 50. Hedger MP. Macrophages and the immune responsiveness of the testis. J Reprod Immunol. 2002;57:19–34. [DOI] [PubMed] [Google Scholar]
- 51. Bhushan S , Hossain H , Lu Y, et al. A Uropathogenic E. coli induce different immune response in testicular and peritoneal macrophages: implications for testicular immune privilege. PLoS One. 2011;6:e28452. [DOI] [PMC free article] [PubMed] [Google Scholar]