Financially motivated adulteration of spices is a long-standing and important public health problem worldwide.1 For example, in 1994, ground paprika adulterated with lead oxide resulted in the poisoning and hospitalization of >50 people in Hungary.2 Today, adulteration of turmeric with lead chromate, which is vibrant yellow, is a concern in India and Bangladesh. In this commentary, we summarize a growing body of evidence indicating that turmeric containing excessive concentrations of lead is available for purchase in US grocery stores and that childhood lead-poisoning cases attributable to consumption of contaminated turmeric have occurred in the United States. We hypothesize that turmeric is being intentionally adulterated with lead to enhance its weight, color, or both. Additionally, we review current regulations on spice safety and provide recommendations for consumers, public health professionals, and government agencies charged with ensuring the safety of the US food supply.
Evidence of Turmeric Contamination With Lead in the United States
Case Reports
In 2010, a report in Pediatrics detailed the case of a 12-month-old boy who was referred to the Pediatric Environmental Health Center at Boston Children’s Hospital with a blood lead level of 28 μg/dL,3 which exceeded the Centers for Disease Control and Prevention’s reference level of 5 μg/dL.4 After conducting a detailed investigation of the child’s home, the Massachusetts Department of Public Health determined that daily consumption of several lead-contaminated spices, including turmeric, was the primary pathway of exposure.3 Between 2010 and 2014, five other cases of childhood lead poisoning attributable to culinary spice consumption were reported in the United States. The cases were geographically diverse, and all were documented by departments of public health (Arizona,5 California,6 Colorado,7 Connecticut,8 and New York9).
Product Recalls
In the past several years, 13 brands of lead-contaminated turmeric have been recalled, all voluntarily. In 2011, companies based in Missouri and California initiated recalls of Archer Farms10 and Spice Hunter11 ground turmeric sold at stores nationwide because of excessive lead levels. Later that year, an online distributor recalled a powder-based dietary turmeric supplement (Dr Clark brand), which had been sold throughout the United States, Canada, Japan, Korea, and the United Kingdom.12 These recalls were followed by the voluntary recall of Pran ground turmeric in 2013 by 4 companies based in New York,13,14 Texas,15 and Michigan.16 Samples collected from these states had lead concentrations of 28-42 ppm, 53 ppm, and 48 ppm, respectively.13–16 After these recalls, the US Food and Drug Administration (FDA) issued an import alert, which allows ports to detain future shipments from specific importers, targeting turmeric from Pran (Bangladesh), Visakarega Trading (India), and IndoVedic Nutrients (India).17 In August 2016, seven brands of turmeric distributed by Gel Spice Inc were recalled because of elevated lead levels.18–20 The recalled turmeric had been distributed throughout the United States, including at a farmers market in Georgia. Coincidentally, 5 brands of curry powder—of which turmeric is a key ingredient—amounting to 337 000 pounds were recalled by the Florida-based Oriental Packing Company because of lead contamination.21 Most recently, 38 000 pounds of turmeric that were distributed to Florida and New York by Spices USA Inc were recalled because of elevated lead levels.22
Surveillance in India, Bangladesh, and the United States
A 2014 study published by researchers at Harvard University reported lead concentrations of up to 483 ppm in turmeric samples collected from 18 households in rural Bangladesh,23 where the allowable level of lead in turmeric is 2.5 ppm.24 Furthermore, several international media outlets have cited evidence that adulteration of turmeric with lead chromate is an ongoing problem. For example, the Times of India reported that during a raid by the Indian Food and Drug Authority in 2010, inspectors discovered >100 bags of raw turmeric contaminated with lead chromate at a spice-manufacturing plant.25 Similarly, a major Bangladeshi newspaper purchased turmeric from local markets in 2014 and found lead concentrations of up to 55 ppm in packaged powder samples and 182 ppm in dry turmeric roots that had been boiled and polished but not ground.26 When the newspaper interviewed a local turmeric grower, he reported that “traders use the artificial color [lead chromate] to hide the marks of pest attacks and other spots on raw turmeric. It is used during boiling and polishing to make the spice look brighter to attract big buyers, including spice processing firms.”26 Most turmeric sold in the United States is imported from India and Bangladesh.27
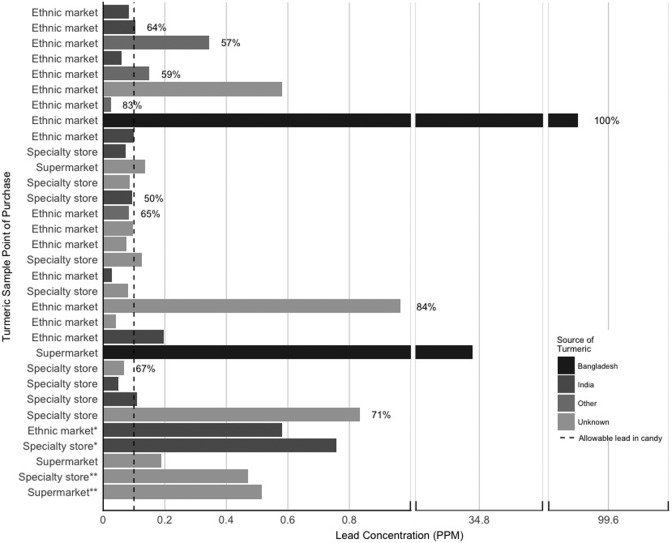
In 2011 and 2012, we purchased samples of turmeric from mainstream grocery stores, specialty stores, and ethnic markets throughout greater Boston. We analyzed 32 samples using inductively coupled plasma-mass spectrometry and found detectable levels of lead in all of the samples, with a median concentration of 0.11 ppm (range, 0.03-99.50 ppm; Figure). The FDA has published several recommended maximum levels of lead; however, the FDA has not established guidelines for lead levels in spices.28 Without such a guideline, we evaluated our results against the FDA’s maximum allowable level of lead in candy (0.1 ppm), which we concluded was the best available comparison food, despite differences in candy composition, consumption habits, and packaging. We found that concentrations in 16 of the 32 samples exceeded the FDA’s allowable level of lead in candy, with 2 samples (34.78 and 99.50 ppm) exceeding the level by 2 orders of magnitude. These were the only samples imported from Bangladesh. Lead is generally insoluble in soil and poorly absorbed by plants.29 Therefore, although detection of low lead levels in most of our samples may reflect uptake from soil during growth, it is unlikely that agricultural fields were the source of the excessive concentrations that we detected.
Figure.
Lead concentration (ppm = 1000 × ng [lead] / g [turmeric]) in 32 turmeric samples purchased in the Boston metropolitan area, 2011 and 2012. The percentages indicate the estimated bioaccessibility (ie, fraction that can be absorbed by the human gastrointestinal tract) of 10 samples; not all samples were tested for bioaccessibility. Bioaccessibility was calculated following the formula outlined in the US Environmental Protection Agency Simple Bioaccessibility Extraction Test (SBET) protocol.30 Asterisks indicate that 2 samples were the same brand.
Lead exists in several chemical forms, which along with the source of exposure (eg, ground spice, paint, soil) may influence the degree to which it is absorbed into the body. A measure of potential absorption, or gastrointestinal solubility, is bioaccessibility. To determine whether lead in turmeric powder can be readily absorbed by the human gastrointestinal tract, we measured bioaccessibility using the US Environmental Protection Agency’s simple bioaccessibility extraction test.30 We found that bioaccessibility ranged from 50% to 100% (mean, 70%). The sample with the highest concentration of lead was also the most bioaccessible; however, we did not find a correlation between lead concentration and bioaccessibility. We found greater lead bioaccessibility than that found by a 2010 study in Boston (mean, 49%)3 that examined a mixture of spices or by a 2014 study in Bangladesh (mean, 43%)23 that examined only turmeric. Interestingly, the Boston-based study determined that lead oxide was the primary lead form present, whereas the Bangladesh-based study detected levels of chromium in 3 samples (maximum, 235 ppm).
Turmeric Consumption in the United States
Concern for turmeric as a source of lead is heightened by its increased use in the United States. In 2014, approximately 12 million pounds of turmeric were imported, and per capita import rates increased 89% during the past 50 years, from 9 g in 1966 to 17 g in 2014.31 This increase likely reflects the growing diversity of the US population and the promotion of spices as healthy, flavor-enhancing alternatives to salt.32 Turmeric is used as a natural food-coloring agent for many foods, including cheeses, cereals, mustard, ice cream, and margarine33; as the demand for natural food additives rises,34 we expect its use in these products to increase. For example, in 2015 Kraft replaced synthetic colorants in its macaroni and cheese with turmeric and other spices.35 This upward trend in turmeric use suggests that exposure might be increasing for the US population, especially among Asian families, whose food culture often involves the use of large quantities of spice to prepare traditional meals. In addition to its culinary use, turmeric is being explored for medicinal use by research institutions and pharmaceutical companies because of its antioxidant, anti-inflammatory, and anticancer properties.36 Likewise, turmeric-based dietary supplements and beverages are widely sold in nutrition and grocery store chains; between 2013 and 2014, the most recent years for which data are available, turmeric was the best-selling herbal ingredient at independent and chain natural product retail stores across the United States.37
Spice Safety Regulations
The Bangladesh Standards and Testing Institution, the Bureau of Indian Standards, and the Indian Agricultural Produce Grading and Marking Act state that turmeric must be free from lead chromate and other artificial coloring matter. The allowable level of lead in turmeric powder is 2.5 ppm,24 10 ppm,38 and 2.5 ppm,33 according to these 3 agencies, respectively. Unlike agencies in Bangladesh and India, the FDA has not established a recommended maximum level for heavy metals (eg, lead, chromium) in spices in the United States.39,40 However, several existing regulatory tools have been conferred by the Federal Food, Drug and Cosmetics Act and the Food Safety Modernization Act for preventing contaminated spices from reaching consumers in the United States.40,41 Specifically, the FDA has the authority to (1) inspect domestic and foreign manufacturing, packing, and storage facilities; (2) test products at spice facilities and ports where spices are imported; and (3) detain shipments or deny entry of products from international facilities that refuse access to FDA or third-party inspectors. Additionally, the FDA can issue import alerts.
Recommendations
In December 2016, the FDA issued an import alert (#28-13) after inspectors from the New York State Department of Agriculture and Markets detected high concentrations of lead in ground turmeric during routine sampling.17 We support this alert and encourage the use of portable, fast, inexpensive, and reliable heavy metal screening tools, such as x-ray fluorescence instruments, at major ports.42 In addition to general surveillance, the FDA has the authority to issue targeted field assignments to better understand particular food safety problems. We recommend that the FDA conduct a targeted field assignment focused on lead contamination of turmeric. We further support recent initiatives by the FDA to improve spice safety, including its development of the International Food Protection Training Institute, which is focused on teaching international colleagues about vulnerabilities in food production pathways.41 We recommend that strategies for preventing and detecting lead contamination be incorporated into the programs developed by this institute.
By law, food production and manufacturing facilities must “identify hazards reasonably likely to occur” and “establish preventive controls for such hazards.”41 We recommend that spice facilities that repackage, store, and distribute turmeric in the United States incorporate lead-specific screening approaches as a component of their hazard analysis plans. Finally, although we support the FDA’s risk profile on pathogens and spices, we note that this risk analysis pertains only to microbial pathogens (ie, salmonella) and filth (ie, rodent hair).39 Given the potential for lead poisoning attributable to turmeric consumption, we recommend that this risk analysis be extended to include heavy metals and that a maximum allowable level of lead in spices be established.
At the local level, clinicians and public health officials should be aware of the potential for exposure to excessive levels of lead from consumption of processed turmeric. Public health agencies should consider adding turmeric and possibly other spices to guidance documents and protocols used during investigation and clinical management of lead-poisoning cases. Our recommendations for consumers are limited. If lead chromate is being used as an adulterant to polish turmeric roots before they are ground, it is plausible that the use of whole, unpeeled turmeric roots might avoid exposure to lead. Unfortunately, fresh turmeric is not available for purchase in many geographic regions.
Public Health Implications
The case reports, product recalls, and surveillance data reviewed in this commentary provide insight on a nontraditional source of lead exposure in the United States and potentially globally. Removal of lead from paint and gasoline and the reductions in blood lead levels that followed was one of the most important environmental health achievements of the past quarter-century. However, as we have observed, evidence exists that turmeric contamination with lead is a problem in India and Bangladesh, which are major global exporters of the spice. Future research investigating both lead and chromium would contribute to a better understanding of the pathways by which turmeric is contaminated with lead. Finally, government agencies tasked with maintaining the safety of the food supply should prioritize the development of policies aimed at preventing the distribution of contaminated turmeric throughout US commerce.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Moore JC, Spink J, Lipp M. Development and application of a database of food ingredient fraud and economically motivated adulteration from 1980 to 2010. J Food Sci. 2012;77(4):R118–R126. [DOI] [PubMed] [Google Scholar]
- 2. Kákosy T, Hudák A, Náray M. Lead intoxication epidemic caused by ingestion of contaminated ground paprika. J Toxicol Clin Toxicol. 1996;34(5):507–511. [DOI] [PubMed] [Google Scholar]
- 3. Lin CG, Schaider LA, Brabander DJ, Woolf AD. Pediatric lead exposure from imported Indian spices and cultural powders. Pediatrics. 2010;125(4):e828–e835. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Childhood lead poisoning prevention program. https://www.cdc.gov/nceh/lead/acclpp/blood_lead_levels.htm. Accessed February 13, 2017.
- 5. Arizona Department of Health Services, Office of Environmental Health. Targeted lead screening plan for the prevention of childhood lead poisoning. http://www.azdhs.gov/documents/preparedness/epidemiology-disease-control/childhood-lead/targeted-lead-screening-plan.pdf. Published 2014. Accessed March 5, 2015.
- 6. Seith A. Caution advised about lead in Indian spices and powders. http://newamericamedia.org/2010/04/caution-advised-about-lead-in-indian-spices-and-powders.php#. Published 2010. Accessed June 4, 2011.
- 7. Williams N. Lead-contaminated spices associated with elevated blood lead levels in Tri-County area. http://www.tchd.org/archivecenter/viewfile/item/113 . Published 2011. Accessed March 5, 2015.
- 8. Provenzano F. Lead in spices. EHS circular letter 2012-28. http://www.ct.gov/dph/lib/dph/environmental_health/lead/pdf/2012-28_lead_in_spices.pdf. Published 2012. Accessed March 5, 2015.
- 9. Erie County New York Department of Health. Elevated levels of lead in local child’s blood leads to nationwide recall of spice [news release]. http://www2.erie.gov/health/index.php?q=elevated-levels-lead-local-child’s-blood-leads-nationwide-recall-spice. Published October 21, 2013. Accessed March 5, 2015.
- 10. US Food and Drug Administration. B&M, Inc conducts voluntary nationwide recall of 6 lot numbers of Archer Farms ground turmeric due to excessive lead levels [press release]. http://www.fda.gov/safety/recalls/ucm251639.htm. Published April 15, 2011. Accessed June 4, 2011.
- 11. Food Safety News. Turmeric recalled due to lead levels. http://www.foodsafetynews.com/2011/04/turmeric-recalled-due-to-excessive-lead-levels/#.v91rj5mrjty. Published 2011. Accessed June 4, 2011.
- 12. US Food & Drug Administration. Enforcement report for October 5, 2011: recalls and field corrections: foods—class I. Recall F-1827-2011: Dr Clark store turmeric powder http://www.fda.gov/safety/recalls/enforcementreports/ucm274632.htm. Accessed December 12, 2011.
- 13. US Food & Drug Administration. OnTime Distribution Inc recalls PRAN brand spice powder turmeric due to excessive levels of lead [press release]. http://www.fda.gov/safety/recalls/ucm370702.htm. Published October 3, 2013. Accessed April 4, 2014.
- 14. US Food & Drug Administration. Asia Cash & Carry Inc recalls PRAN brand spice powder turmeric due to excessive levels of lead [press release]. http://www.fda.gov/Safety/Recalls/ucm370854.htm. Published October 8, 2013. Accessed April 4, 2014.
- 15. US Food & Drug Administration. Fahman Enterprises Inc recalls PRAN brand spice powder turmeric due to elevated levels of lead [press release]. http://www.fda.gov/Safety/Recalls/ucm371206.htm. Published October 17, 2013. Accessed April 4, 2014.
- 16. US Food & Drug Administration. Best Value Inc recalls PRAN brand turmeric powder due to elevated levels of lead [press release]. http://www.fda.gov/safety/recalls/ucm371042.htm. Published October 15, 2013. Accessed April 4, 2014.
- 17. US Food & Drug Administration. Detention without physical examination of turmeric due to lead contamination. Import alert 28-13. http://www.accessdata.fda.gov/cms_ia/importalert_1143.html. Published 2016. Accessed August 20, 2016.
- 18. US Food & Drug Administration. Gel Spice, Inc issues alert on elevated lead levels in one lot of Fresh Finds ground turmeric powder [news release]. http://www.fda.gov/safety/recalls/ucm513844.htm. Published July 28, 2016. Accessed August 20, 2016.
- 19. US Food & Drug Administration. Update: JM Exotic Foods, Inc recalls ground turmeric due to elevated levels of lead [news release]. http://www.fda.gov/Safety/Recalls/ucm515105.htm. Published August 5, 2016. Accessed August 20, 2016.
- 20. US Food & Drug Administration. Update: Gel Spice, Inc issues expanded recall of ground turmeric powder due to elevated lead levels [news release]. http://www.fda.gov/safety/recalls/ucm515328.htm. Published August 5, 2016. Accessed August 20, 2016.
- 21. US Food & Drug Administration. Oriental Packing Co., Inc issues alert on lead in curry powder [news release]. http://www.fda.gov/Safety/Recalls/ucm516541.htm. Published August 12, 2016. Accessed September 24, 2016.
- 22. US Food & Drug Administration. Spices USA Inc issues alert on elevated levels of lead in ground turmeric [news release]. http://www.fda.gov/Safety/Recalls/ucm523561.htm. Published September 26, 2016. Accessed December 29, 2016.
- 23. Gleason K, Shine JP, Shobnam N, et al. Contaminated turmeric is a potential source of lead exposure for children in rural Bangladesh. J Environ Public Health. 2014;24(730636). https://www.hindawi.com/journals/jeph/2014/730636. Accessed August 12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bangladesh Standards and Testing Institution. List of 155 products brought under mandatory certification marks scheme. Turmeric powder BDS 991: 2001 http://www.bsti.gov.bd/cert_mark_productList.html. Published November 2012. Accessed March 5, 2015.
- 25. Mishral A. This food could be injurious to health. http://timesofindia.indiatimes.com/city/kanpur/this-food-could-be-injurious-to-health/articleshow/5914081.cms. Published May 10, 2010. Accessed March 8, 2014.
- 26. Parvez S, Ahmed B. Brighter riskier: Star’s random sample test finds only wet turmeric safe. http://103.16.74.132/brighter-riskier-14586. Published March 8, 2014. Accessed March 5, 2015.
- 27. Buzzanell PJ, Dull R, Gray F. The spice market in the United States: recent developments and prospects. AIB-709 https://www.ers.usda.gov/publications/pub-details/?pubid=42049. Published July 1995. Accessed June 4, 2011.
- 28. US Food & Drug Administration, Center for Applied Safety and Applied Nutrition. Guidance for industry: lead in candy likely to be consumed frequently by small children: recommended maximum level and enforcement policy. http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ChemicalContaminantsMetalsNaturalToxinsPesticides/ucm077904.htm. Published November 2006. Accessed June 4, 2011.
- 29. Brown SL, Chaney RL, Hettiarachchi GM. Lead in urban soils: a real or perceived concern for urban agriculture? J Environ Qual. 2016;45(1):26–36. [DOI] [PubMed] [Google Scholar]
- 30. US Environmental Protection Agency. Standard Operating Procedure for an In Vitro Bioaccessibility Assay for Lead in Soil. Washington, DC: EPA; 2012. EPA 92002–86. [Google Scholar]
- 31. US Department of Agriculture, Economic Research Service. Food availability (per capita) data system: coffee, tea, cocoa, and spices. https://www.ers.usda.gov/data-products/food-availability-per-capita-data-system. Published 2016. Accessed February 24, 2017.
- 32. Hiza H. Availability of spices on the rise in the US food supply. Nutrition insight 39 http://www.cnpp.usda.gov/sites/default/files/nutrition_insights_uploads/insight39.pdf. Published 2008. Accessed June 4, 2011.
- 33. Plotto A. Turmeric: post-harvest operations. http://www.fao.org/3/a-ax446e.pdf. Published 2004. Accessed June 4, 2011.
- 34. Martins N, Roriz CL, Morales P, Barros L, Ferreira I. Food colorants: challenges, opportunities and current desires of agroindustries to ensure consumer expectations and regulatory practices. Trends Food Sci Technol. 2016;52:1–15. [Google Scholar]
- 35. Kell J. Kraft Mac & Cheese changed its recipe and nobody got mad. http://fortune.com/2016/03/07/kraft-mac-cheese-changed. Published 2016. Accessed February 24, 2017.
- 36. Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39(3):283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith T, Lynch ME, Johnson J, Kawa K, Bauman H, Blumenthal M. Herbal dietary supplement sales in US increase 6.8% in 2014. HerbalGram. 2015;107:52–59. [Google Scholar]
- 38. Bureau of Indian Standards. Spices and condiments—turmeric, whole and ground—specification (third revision). https://law.resource.org/pub/in/bis/S06/is.3576.2010.pdf. Published 2010. Accessed March 5, 2015.
- 39. US Food & Drug Administration, Center for Food Safety and Applied Nutrition. Draft risk profile: pathogens and filth in spices. http://www.fda.gov/downloads/Food/FoodScienceResearch/RiskSafetyAssessment/UCM367337.pdf. Published 2013. Accessed August 20, 2016.
- 40. Pub Law No 75-717, USC Title 21.
- 41. Pub Law No 111-353 (2011).
- 42. Lynch RA, Boatright DT, Moss SK. Lead-contaminated imported tamarind candy and children’s blood lead levels. Public Health Rep. 2000;115(6):537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]