Abstract
The TGF-β signaling pathway is involved with multiple processes in the mammalian ovary, including primordial follicle formation, granulosa cell (GC) proliferation, follicle atresia, ovulation, and feedback regulation between the pituitary and ovary. The transcriptional factor SMAD4 (Sma- and Mad-related protein 4) is the central component of the canonical TGF-β signaling pathway. Smad4 knockout (KO) using Amhr2-Cre, which is expressed in GCs of immature developing follicles, causes premature luteinization. In this study, we specifically depleted Smad4 in GCs of preovulatory follicles using Cyp19-Cre mice. As different from results with Smad4fl/fl;Amhr2-Cre mice, Smad4 depletion in preovulatory follicles did not cause premature luteinization or suppress GC proliferation; rather, it increased follicle atresia. In addition, Nppc and Npr2 expressions were reduced by Smad4 depletion; thus, their effect of maintaining oocyte meiotic arrest was weakened in Smad4 conditional KO mice. Smad4fl/fl;Cyp19-Cre female mice were subfertile and had irregular estrous cycles and ovulation defects. Smad4 KO also blocked LH-induced cumulus expansion and follicle rupture, but not oocyte meiotic resumption. Our results also indicated that SMAD4 was required for LH-stimulated activation of ERK1/2 and the expressions of ovulation-related genes. The defects arising from SMAD4 depletion could not be rescued by intraovarian mediators of LH actions, such as epidermal growth factor-like factors and prostaglandin E2. Furthermore, corpus lutea did not form in Smad4fl/fl;Cyp19-Cre female mice, indicating that SMAD4 was crucial for GCs terminal differentiation. Thus, by characterizing the ovarian phenotypes of preovulatory follicle-specific Smad4 KO mice, we identified the developmental stage-specific functions of the canonical TGF-β signaling pathway in ovulation and luteinization.
In mammals, ovulation is a multistep physiologic process that includes preovulatory follicle growth, oocyte meiotic maturation, cumulus-oocyte complex (COC) expansion, follicle rupture, and luteinization. The pituitary-secreted gonadotropins FSH and LH are major regulators of these events. During each estrous cycle, FSH facilitates the rapid growth of preantral and early antral follicles to the preovulatory stage. Then, a midestrous LH surge triggers the initiation of ovulation and the terminal differentiation of granulosa cells (GCs) into luteal cells (1). In addition to gonadotropins, ovarian local signaling factors also play crucial roles during specific steps of ovulation. For example, epidermal growth factor (EGF)-like factors are intrafollicular mediators of LH effects, including triggering oocyte germinal vesicle breakdown (GVBD) and COC expansion (2, 3). Granulosa and cumulus cell-produced prostaglandin E2 (PGE2) is important for organizing the COC matrix and in positive feedback regulation of EGF-like factors (3, 4).
It is now well accepted that the oocyte is not only a passenger carried by the follicle but is also an active regulator of follicle growth and ovulation. Oocyte-derived factors, including growth and differentiation factor 9 (GDF9) and bone morphogenic protein 15 (BMP15), promote COC expansion and the expressions of several key target genes involved in LH regulation of ovulation (Has2, Ptgs2, Tnfaip6, and Ptx3) (5). GDF9 and BMP15 are both signaling molecules of the TGF-β superfamily. They bind to their membrane receptors and trigger the serine/threonine protein kinase activity of these receptors (6). As a result, several members of the SMAD (Sma- and Mad-related protein) family of transcription factors become phosphorylated, SMAD1/5/8 in response to BMPs and SMAD2/3 in response to other TGF-β family ligands. Subsequently, phosphorylated SMAD1/5/8 and SMAD2/3 form heterodimers with SMAD4 in the cytoplasm, enter the nucleus, and regulate the expression of their target genes. This is the canonical TGF-β signaling pathway (7).
The TGF-β signaling pathway has multiple functions in the mammalian ovary. For example, in the ovaries of new born Gdf9 knockout (KO) mice, primordial follicles were formed, but they failed to develop beyond primary follicle stage and could not be maintained later in life, which indicates that this paracrine factor is essential for follicle formation (8). KO of inhibin and activin, TGF-β family ligands that are secreted by GCs, cause multiple ovarian defects by disrupting the feedback regulation between the pituitary and ovary (9). In both murine and human ovaries, the TGF-β family ligand anti-Mullerian hormone (AMH) is an intraovarian growth factor that regulates primordial follicle recruitment and the FSH sensitivity of growing follicles in an inhibitory manner (10).
The complexity of the TGF-β signaling pathway in ovarian functions was further demonstrated by mouse models with KOs for TGF-β receptors and SMADs. Conditional KO (cKO) of BMP receptor 1A/1B (BMPR1A/B) (11) or SMAD1/5/8 (12) in the GCs of developing follicles resulted in the oncogenic transformation of these cells. Deletion of both SMAD2 and -3 dramatically reduced female fertility, which was associated with the disruption of multiple ovarian processes, including follicular development, ovulation, and COC expansion (13). Most importantly, GC-specific depletion of SMAD4, the central component of the canonical TGF-β signaling pathway, caused premature luteinization of GCs followed by ovulation failure.
However, in all of these studies, GC-specific depletion of target genes was achieved using Amhr2-Cre mice. In this mouse strain, the expression of cAMP response element (CRE) recombinase is under the control of anti-Müllerian hormone type II receptor (Amhr2) promoter, and CRE activity is found in the ovary as early as embryonic day 17.5. Throughout the postnatal ovary, CRE activity is found in GCs of all secondary and small antral follicles. However, low CRE activity is also found in some theca cells (14). In addition, as expected, the muscular layer of the uterus is CRE positive because Amhr2 is expressed in the mesenchyme of the Müllerian duct that gives rise to the uterine musculature (15). Moreover, Amhr2-Cre recombinase activity in GCs does not appear to be sufficient to demonstrate any pronounced effects of depleting certain genes, such as Ctnnb1 (16).
To disrupt Smad4 more precisely in the ovary during the later stages of follicular development and luteinization and, thereby, analyze the effects of the canonical TGF-β pathway more precisely, we used our Cyp19-Cre mice in which the expression of CRE recombinase is high and limited to the GCs of preovulatory follicles (17, 18). In contrast to results observed with Smad4fl/fl;Amhr2-Cre mice, depleting Smad4 in preovulatory follicles did not cause premature luteinization. Smad4fl/fl;Cyp19-Cre female mice were subfertile and had ovulation defects. Our results also indicated that SMAD4 was required for LH-stimulated activation of ERK1/2 and the expressions of ovulation-related genes. Furthermore, corpus lutea (CLs) did not form in Smad4fl/fl;Cyp19-Cre female mice, which indicated that in mature follicles, SMAD4 was crucial for GCs terminal differentiation. These findings with this novel mouse model revealed developmental stage-specific functions of the canonical TGF-β signaling pathway in ovulation and luteinization.
Materials and Methods
Mice
Wild-type (WT) C57/BL6 mice were obtained from the Center of Experimental Animals, Zhejiang University. Mice with GC-specific KO of Smad4 (Smad4gc−/−) were generated by crossing Cyp19-Cre mice with previously reported Smad4fl/fl mice (19). Animals were housed under a 14-hour light, 10-hour dark schedule, provided food and water ad libitum, and were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
To study ovarian responses to exogenous gonadotropins, postnatal day (PD)21 female mice were analyzed to avoid the complexity of ovarian functions associated with estrous cycles and endogenous surges of gonadotropins. Immature mice were injected ip with 5 IU PMSG (pregnant mare serum gonadotropin; Calbiochem, La Jolla, California) to stimulate preovulatory follicle development followed 48 hours later with 5 IU human chorionic gonadotropin (hCG; American Pharmaceutical Partners, Schaumburg, Illinois) to stimulate ovulation and luteinization.
GC culture
GCs were harvested from PMSG-primed (24 h), PD21 mice as described previously (20, 21). Briefly, undifferentiated GCs were released from antral follicles by puncturing with a 26.5 gauge needle. Cells were cultured at a density of 1 × 106 cells in DMEM/F12 medium (Invitrogen, Carlsbad, California) containing 5% fetal bovine serum (Invitrogen), 100 U/mL penicillin, and 100 μg/mL streptomycin in 24-well culture dishes. After overnight culture, cells were washed and cultured in serum-free medium before any further treatments.
RNA isolation and real-time RT-PCR
Total RNA was extracted using Trizol (Invitrogen) according to the manufacturer's instructions. Real-Time PCR analysis used Q Tag SYBR Green Master Mix (Becton Dickinson Medical Devices Co., Franklin Lakes, New Jersey) and an Applied Biosystems (Foster City, California) 7500 Real-Time PCR System. Relative mRNA levels were calculated by normalizing to the levels of endogenous β-actin mRNA (used as an internal control) using Microsoft EXCEL. For each indicated gene, the relative transcript level of the control sample (left-hand bar of each graph) was set as 1. The relative transcript levels of other samples were compared with the control, and the fold-changes are shown in the graph. For each experiment, quantitative PCRs were done in triplicate. Primer sequences are available upon request to the authors.
TUNEL assay
TUNEL (terminal deoxynucleotidetransferase-mediated dUTP end labeling) assays were performed on 10% formalin-fixed paraffin-embedded sections using the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Serologicals Corp, Norcross, Georgia) according to manufacturer's instructions.
Histologic analysis and immunohistochemistry
Ovaries were fixed overnight in 10% PBS-buffered formalin and then embedded in paraffin. Ovary samples were serially sectioned at 5 μm thicknesses and were stained with hematoxylin and eosin. For immunohistochemistry, sections were deparaffinized and rehydrated, and were incubated with primary antibodies for 1 hour at room temperature, followed by biotin-labeled secondary antibodies for 30 minutes. Staining procedure was performed using the Vectastain ABC kit and 3,3′-diaminobenzidine peroxidase substrate kit (Vector Laboratories, Burlingame, California).
In vitro COC expansion assay
Fully grown, nonexpanded COCs were collected from ovaries of PMSG-primed immature WT or Smad4gc−/− mice. COCs (∼30) were plated in 100 μL of defined COC medium (MEM w/ES, 25 mM HEPES, 0.25 mM sodium pyruvate, 3 mM l-glutamine, 1 mg/ml BSA, 100 U/mL penicillin, 100 μg/ml streptomycin) with 1% fetal bovine serum under the cover of mineral oil and treated with amphiregulin (AREG, 100 ng/mL) or PGE2 (500 nM). Expansion status was evaluated by microscopic examination after overnight culture.
Western blot analysis
Protein extracts were dissolved in SDS sample buffer. Protein lysates (30 μg total protein per lane) were separated by SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride membranes (Millipore Corp, Bedford, Massachusetts). After probing with primary antibodies, the membranes were washed in Tris-buffered saline-Tween 20 and incubated with an horseradish peroxidase-linked secondary antibody. Finally, bands on the membranes were detected using an Enhanced Chemiluminescence Detection Kit (Amersham, Piscataway, New Jersey).
Hormone level assays
Mice were anesthetized by ip injection with phenobarbital sodium, and blood was collected by cardiac puncture. Serum and blood cells were separated by centrifugation. Serum hormone levels were determined by Di'an Medical Diagnostics Limited Corporation (Hangzhou, China).
Statistical analysis
Results are given as means ± SDs; each experiment included at least 3 independent samples and was repeated at least 3 times. Group comparisons were made by unpaired Student's 2-tailed t tests. P values of < .05 were considered significant.
Results
Selective Smad4 depletion in GC cells of antral follicles causes female subfertility
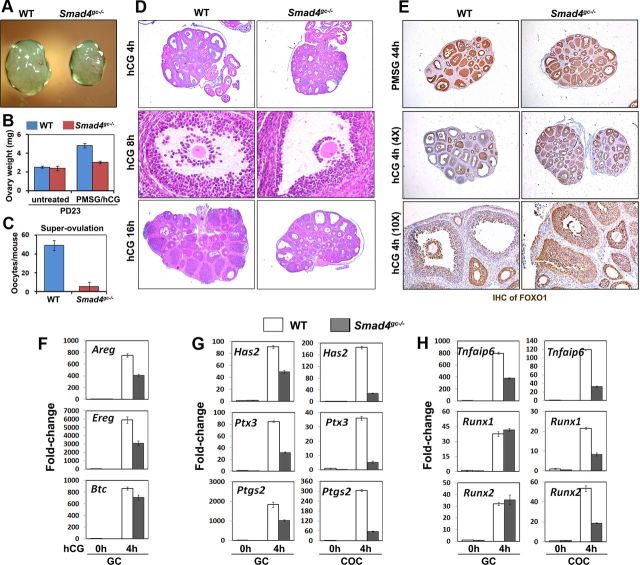
We first assessed SMAD4 expression patterns in mouse ovaries by immunohistochemistry. In pubertal mouse ovaries harvested at PD23, SMAD4 protein was highly expressed in the GCs of secondary, preantral, and early antral follicles as compared with ovarian stroma cells and theca cells (Figure 1A). When mice were treated with PMSG and hCG to induce rapid growth of antral follicles and ovulation, SMAD4 was continually expressed in the GCs of preovulatory follicles and in luteinizing GCs after ovulation (Figure 1A). The continuous high expression of SMAD4 in GCs (from secondary to ovulatory follicles) suggested that the canonical TGF-β signaling pathway played multiple roles during various stages of follicle development and ovulation.
Figure 1.
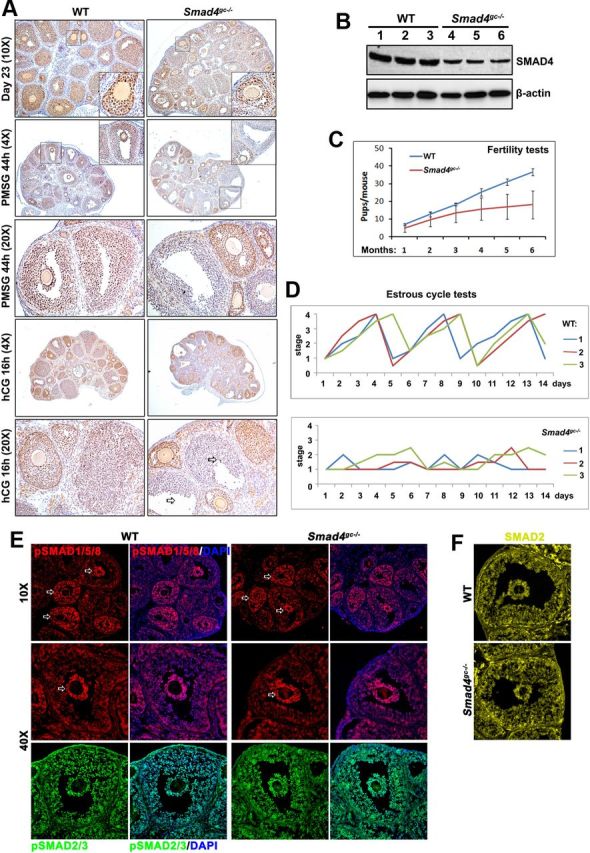
Selective Smad4 Depletion in GCs of Antral Follicles Causes Female Subfertility A, Immunohistochemistry results for SMAD4 expression in the ovaries of WT and Smad4gc−/− mice before and after ovulation. In nontreated pubertal ovaries, SMAD4 was highly expressed in GCs of secondary and early antral follicles. At 44 hours after WT PD21 mice were injected with PMSG, SMAD4 was also highly expressed in GCs of antral follicles. However, in Smad4gc−/− ovaries, SMAD4 expression was significantly decreased in antral follicles, although notable SMAD4 expression was still observed in smaller secondary follicles. In WT ovaries, luteinizing GCs continued to express SMAD4 in follicles after ovulation. However, in Smad4gc−/− ovaries, most antral follicle failed to ovulate and GCs were mostly negative for SMAD4 in these unovulated follicles. B, Western blotting results for SMAD4 expression in GCs isolated from WT and Smad4gc−/− ovaries. C, Continuous breeding assay demonstrating the subfertility of Smad4gc−/− females. D, Estrous cycle test results for WT and Smad4gc−/− females. The estrous status of mice at 2–3 months of age (n = 6 for each genotype) were determined by cytology examinations of vaginal cell smears. E, Immunofluorescence results for phosphorylated SMAD2/3 and SMAD1/5/8 in the ovaries of WT and Smad4gc−/− mice before ovulation. F, Immunofluorescence results for SMAD2 expression in the ovaries of WT and Smad4gc−/− mice before ovulation. DAPI, 4′,6-diamidino-2-phenylindole.
To study a specific role for SMAD4 in antral follicles, we crossed Smad4fl/fl mice with previously reported Cyp19-Cre transgenic mice. In Cyp19-Cre mice, the expression of Cre DNA recombinase is under the control of GC-specific Cyp19a1 promoter II (22, 23). CRE activity is primarily detected during the preantral/early antral stage of follicle development.
In the resulting Smad4 cKO mice (Smad4gc−/−), SMAD4 expression was largely intact in secondary and early antral follicles but was dramatically decreased in preovulatory follicles (Figure 1A). The reduction of SMAD4 protein level in GCs was further confirmed by Western blotting results (Figure 1B). However, SMAD4 was not totally depleted in GCs isolated from Smad4gc−/− mice. This might be due to the selective expression of Cyp19-Cre in a subset of antral follicles with active aromatase expression, but not all growing follicles (17, 18). The reduced expression of SMAD4 in preovulatory follicles indicated that we could use this mouse strain to study SMAD4 functions in follicle maturation and ovulation in vivo.
Furthermore, to rule out the possibility that SMAD4 deletion might differentially affect the activation of two groups of R-SMADs, we compared the levels and distribution patterns of phosphorylated SMAD2/3 and SMAD1/5/8, in WT and Smad4gc−/− ovaries. As shown in Figure 1E, relatively high levels of pSMAD1/5/8 were detected in GCs of preantral/early antral follicles and in cumulus cells of preovulatory follicles (indicated by arrows), with or without SMAD4 deletion. Phospho-SMAD2 was also detected in GCs and cumulus cells of antral follicles (Figure 1E), but its levels were not different between large and small follicles (data not shown). Neither the levels nor the nuclear localizations of pSMAD1/5/8 and pSMAD2/3 were affected by SMAD4 deletion. Total SMAD2 was also detected in WT and Smad4gc−/− ovaries (Figure 1F). SMAD2 signal was detected more in the GC cytoplasm than in the nuclei, with or without SMAD4 deletion. These results indicated that in Smad4 KO GCs, the TGF-β signaling was not affected at the level of R-SMAD phosphorylations and nuclear translocations.
Selective Smad4 depletion in GC cells impairs female fertility and increases follicle atresia
In a 6-month continuous breeding assay, Smad4gc−/− female mice (n = 8) had reduced fertility as compared with WT females (Figure 1C). Most Smad4gc−/− females became completely infertile at 6–7 months of age. In addition, whereas control females (3–4 mo old) had regular estrous cycles of 4–5 days, Smad4gc−/− females had irregular cycles (Figure 1D). This suggested that Smad4 depletion in antral follicles had caused endocrine disorders. Thus, we designed additional experiments to characterize the roles of Smad4 in preovulatory follicle growth, ovulation, and luteinization.
First, we examined the ovarian histology of Smad4gc−/− females at PD23. The ovaries of untreated animals did not appear to be different from their control littermates. This was primarily because Cyp19-Cre is not efficiently expressed in GCs at the pubertal stage (17, 18). Incomplete SMAD4 protein depletion in 23-day-old Smad4gc−/− ovaries was also shown in Figure 1A. Furthermore, we prepared serial sections for 23-day-old, non-PMSG/hCG-treated WT and Smad4gc−/− ovaries (n = 3 for each genotype) and then counted the numbers of all follicles that were beyond the secondary follicle stage. The results showed that the numbers of small antral follicles were similar in WT and Smad4gc−/− ovaries (∼50–60 follicles per ovary). PMSG stimulated the development of follicles to the antral stage in both WT and Smad4gc−/− ovaries. Importantly, premature luteinization has been reported in Smad4fl/fl;Amhr2-Cre mice. However, this phenotype was not observed in pubertal Smad4fl/fl;Cyp19-Cre mice. Furthermore, luteal cell marker genes, including Cyp11a1, Sfrp4, and Star, remained at low expression levels in pubertal Smad4gc−/− ovaries without hCG stimulation (Figure 5C). The serum levels of E2 and progesterone (P4) were comparable between Smad4gc−/− mice and their control littermates both before and after PMSG treatment (Figure 2G). Collectively, these results indicated that SMAD4 depletion in GCs during the antral stage did not cause precocious luteinization.
Figure 5.
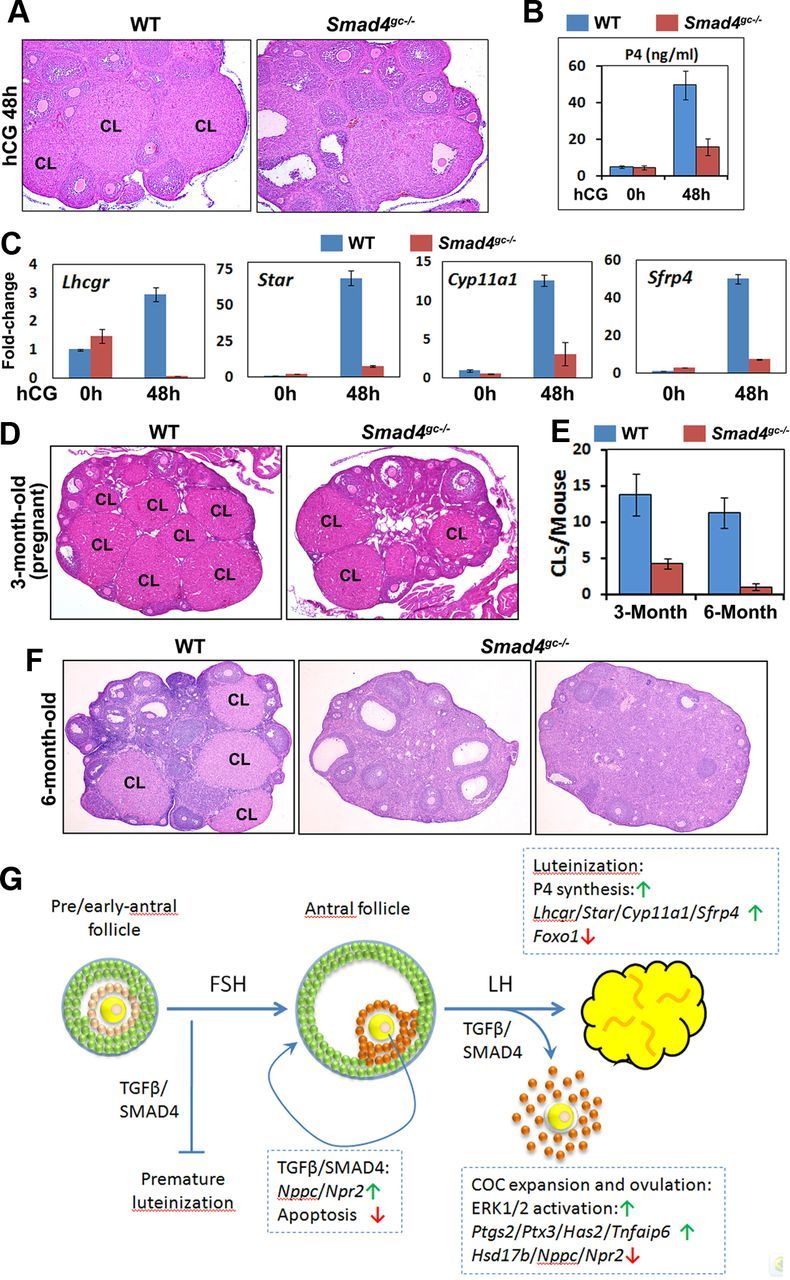
Effects of Smad4 cKO on Luteinization. A, Histology of WT and Smad4gc−/− ovaries at 48 hors after hCG injection. B, Serum P4 concentrations in WT and Smad4gc−/− mice before and after hCG injection. C, Quantitative RT-PCR results. The mRNA expression levels for luteal cell marker genes in WT and Smad4gc−/− ovaries before and after hCG treatments. D–F, Ovarian histology of adult WT and Smad4gc−/− mice (3 and 6 mo old, respectively). At 3 months of age, more CLs were observed in WT ovaries than in Smad4gc−/− ovaries. At 6 months, multiple CLs were observed in WT ovaries but not in Smad4gc−/− ovaries. G, Schematic diagram of SMAD4 functions in GCs during follicle development. The TGF-β family ligands GDF9 and BMP15 secreted by oocytes activate SMADs in nearby granulosa/cumulus cells. SMAD4 depletion in follicles before the antral stage causes premature luteinization of GCs. FSH stimulates follicles to grow beyond the early antral stage and establishes specific gene expression patterns, such as Nppc, Npr2, and 3β-HSD, in mural GCs and cumulus cells together with TGF-β/SMAD4 signaling. However, SMAD4 depletion in follicles beyond the antral stage no longer causes premature luteinization. Rather, SMAD4 is required for preventing follicle atresia at this stage. A TGF-β/SMAD4 signal is also required for LH-induced cumulus expansion, ovulation, and luteinization by inducing ERK1/2 phosphorylation and the expressions of key LH target genes.
Figure 2.
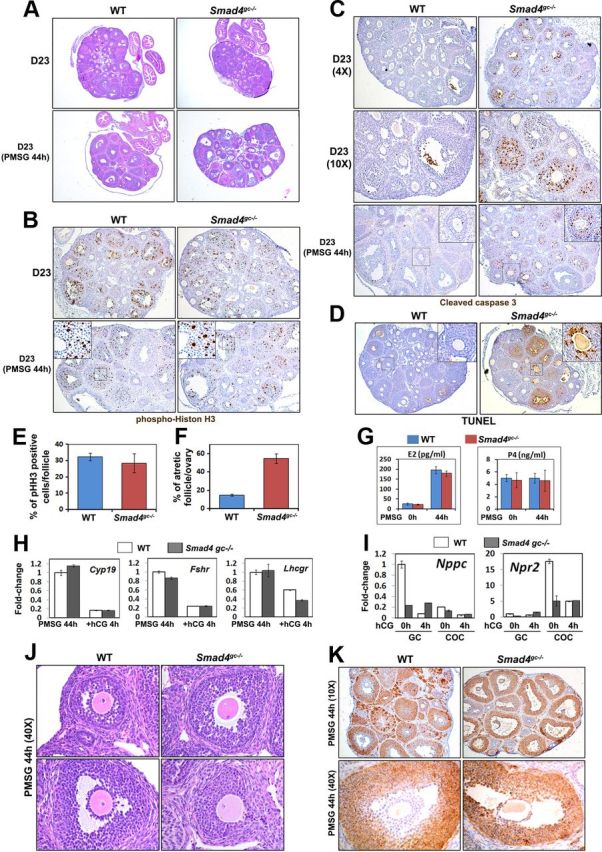
Effects of Smad4 cKO on Follicle Growth, Follicle Atresia, and FSH Target Genes Expression. A, Histology of WT and Smad4gc−/− ovaries at PD23, with or without PMSG treatment. B, Immunohistochemistry results for pHistone H3 (Ser10) indicating GC proliferation in WT and Smad4gc−/− ovaries, with or without PMSG treatment. C and D, Immunohistochemistry results for cleaved caspase 3 (C) and TUNEL assay (D) show an increase in GC apoptosis and follicle atresia in Smad4gc−/− ovaries. E and F, Quantification results of phospho-histone H3 (pHH3) positive cells (E) and TUNEL-positive atretic follicles (F) in WT and Smad4gc−/− ovaries at PD23 with PMSG treatment (44 h). G, Serum estrogen (E2) and P4 concentrations of WT and Smad4gc−/− mice before and after PMSG injections. H, Quantitative RT-PCR results using GCs isolated from WT and Smad4gc−/− ovaries (PMSG 44 h and PMSG 44 h+hCG 4 h) showing the expressions of the FSH target genes Cyp11a1, Fshr, and Lhcgr. I, Quantitative RT-PCR results for the effect of Smad4 depletion on Nppc/Npr2 expression. In WT ovaries before hCG treatment, Nppc was highly expressed in mural GCs and Npr2 was highly expressed in cumulus cells. After hCG treatment, Nppc and Npr2 mRNA levels were down-regulated in mural GCs and cumulus cells, respectively. However, this intricate regulation of Nppc/Npr2 expression was abolished in Smad4gc−/− ovaries. J, Hematoxylin and eosin staining results showing precocious oocyte meiotic resumption in Smad4gc−/− ovaries. K, Immunohistochemistry results for 3β-HSD expression patterns. This enzyme was predominantly expressed by mural GCs of antral follicles in WT ovaries, but was expressed in all GCs and cumulus cells in Smad4gc−/− ovaries.
GC proliferation was not affected by Smad4 depletion, as assessed by immunohistochemistry for the mitotic cell marker phosphohistone H3 (Figure 2, B and E). However, the numbers of apoptotic GCs and atretic follicles were significantly increased in Smad4gc−/− ovaries as assessed by immunohistochemical staining for cleaved caspase 3 and TUNEL assays (Figure 2, C, D, and F). Furthermore, the mRNA level of Cyp19a1, a well-established FSH target genes in GCs, was unaffected by Smad4 depletion (Figure 2H). The expression of genes encoding FSH and LH receptors, Fshr and Lhcgr, was only moderately decreased in Smad4gc−/− ovaries (Figure 2H).
TGF-β signaling is required for maintaining specific gene expression patterns in cumulus cells and for oocyte meiotic arrest
Recent studies showed that the C-type natriuretic peptide-natriuretic peptide receptor 2 (NPR2) signaling pathway was essential for maintaining high cGMP levels in COCs and for preventing precocious GVBD (24). Nppc is the gene that encodes for secreted factor C-type natriuretic peptide and is highly expressed in mural GCs. In contrast, its receptor gene, Npr2, is more prominently expressed in cumulus cells than in mural GCs (Figure 2I). The asymmetrical patterns of Nppc/Npr2 expression suggest that these genes might be regulated by oocyte-derived factors, such as GDF9 and BMP15, that are TGF-β family members.
In PMSG-primed Smad4gc−/− GCs and COCs, the mRNA levels of Nppc and Npr2 were dramatically decreased as compared with WT controls (Figure 2I). As a result, ≈ 10% of the fully grown oocytes in Smad4gc−/− ovaries underwent GVBD without PMSG/hCG stimulation (Figure 2J). We also found that Nppc and Npr2 expression was regulated by gonadotropins. At 4 hours post-hCG when most oocytes within preovulatory follicles underwent GVBD, the mRNA levels of Nppc and Npr2 were also dramatically down-regulated in mural GCs and in COCs, respectively. However, this hCG regulation of Nppc and Npr2 was absent in Smad4gc−/− ovaries (Figure 2I). In addition, 3β-hydroxysteroid dehydrogenase (HSD), the key enzyme for steroid hormone biosynthesis, was predominantly expressed in mural GCs of antral follicles in WT ovaries, but was ectopically expressed in cumulus cells of Smad4gc−/− ovaries (Figure 2K). Collectively, these results indicated that the distinct gene expression patterns in GCs and cumulus cells within preovulatory follicles were disrupted by Smad4 depletion.
SMAD4 is not required for LH-triggered oocyte meiotic resumption but is required for cumulus expansion and ovulation
In WT ovaries treated with hCG, the volumes of preovulatory follicles continued to increase until follicle rupture at ≈ 12 h after hCG injection. However, the sizes of Smad4gc−/− ovaries were consistently smaller than WT ovaries when measured at 4 hours after hCG injection (Figure 3, A and B). At 16 hours after hCG, Smad4gc−/− mice ovulated far fewer oocytes than controls (Figure 3C), which indicated that these mice had ovulation defects. Smad4gc−/− ovaries had fewer preovulatory follicles (5.7 ± 2.8 per section; n = 6) than control ovaries (15 ± 2.35 per section; n = 6; Figure 3D). Almost all oocytes within preovulatory follicles underwent GVBD at 8 hours after hCG in both control and Smad4gc−/− ovaries, which indicated that SMAD4 was not required by granulosa/cumulus cells for LH/hCG-triggered oocyte meiotic resumption (Figure 3D).
Figure 3.
Effects of Smad4 cKO on Ovulation. A and B, Size (A) and weight (B) comparisons between WT and Smad4gc−/− ovaries after hCG for 4 hours. C, Superovulation assay showing decreased ovulation in Smad4gc−/− mice. D, Hematoxylin and eosin staining showing histologic changes during hCG-induced ovulation in WT and Smad4gc−/− ovaries. E, Immunohistochemistry results for FOXO1 staining in WT and Smad4gc−/− ovaries after hormonal treatments. F, Quantitative RT-PCR results for the expression of 3 EGF-life factors (Areg, Ereg, and Btc) in GCs isolated from WT and Smad4gc−/− ovaries. G and H, Quantitative RT-PCR results for the expressions of LH target genes essential for cumulus expansion and ovulation in GCs and COCs isolated from WT and Smad4gc−/− ovaries (hCG 0 h and 4 h).
However, COC expansion was blocked in Smad4gc−/− ovaries, which confirmed the results in previous reports of essential roles for TGF-β signaling during this process (13, 25). When treated with hCG for 16 hours, most preovulatory follicles had ruptured in WT ovaries, whereas multiple, large preovulatory follicles could still be observed in Smad4gc−/− ovaries (Figure 3D). Previous studies showed that the transcription factor FOXO1 was highly expressed in growing follicles and was a negative regulator of steroidogenesis. LH/hCG induces degradation of FOXO1 before ovulation and stimulates steroidogenesis and GCs terminal differentiation (26, 27). We found that FOXO1 expression remained at high levels in Smad4gc−/− ovaries, even after hCG treatment (Figure 3E), which indicated that GCs differentiation was impaired after SMAD4 depletion.
Our previous studies showed that hCG triggered the expression of a large number of ovulation-related genes in both GCs and cumulus cells (28). Areg, Ereg, and Btc are early LH-induced genes that are intrafollicular mediators of LH activity for inducing oocyte maturation and COC expansion. As shown in Figure 3F, the expression levels of Areg and Ereg, but not that of Btc, decreased about 50% in Smad4-depleted GCs as compared with control cells at 4 hours after hCG.
We also examined the expressions of EGF-like factor-induced genes related to COC expansion and ovulation in both mural GCs and COCs that were harvested in vivo. As shown in Figure 3, G and H, Has2, Ptx3, Ptgs2, and Tnfaip6 mRNA levels were decreased in both mural GCs and COCs after Smad4 depletion; this effect was more pronounced in COCs. In contrast, Runx1 and 2 mRNA expressions were negatively affected by Smad4 KO only in COCs (Figure 3H). These results indicated that SMAD4 was required for LH-induced expression of ovulation-related genes in preovulatory follicles, particularly in cumulus cells.
Ovary defects resulting from Smad4 KO cannot be rescued by EGF-like factors or PGE2.
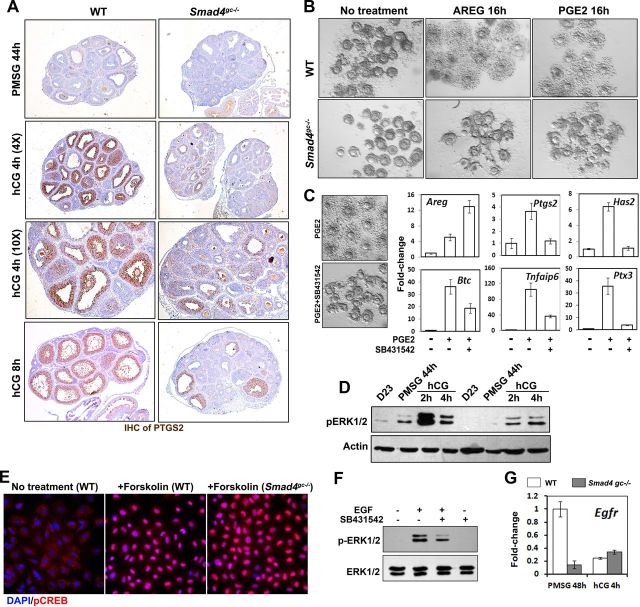
Prostaglandin synthase 2 (PTGS2) is the key enzyme for PGE2 biosynthesis and is essential for successful ovulation (29). Ptgs2 KO causes ovulation defects similar to the phenotypes we observed in Smad4gc−/− mice (30). Thus, we sought to confirm the defects of PTGS2 expression in Smad4gc−/− ovaries by immunohistochemistry. As shown in Figure 4A, PTGS2 expression was dramatically induced in WT preovulatory follicles after hCG injection, whereas its expression was greatly reduced in Smad4gc−/− ovaries. To test whether the COC expansion defect observed in Smad4gc−/− ovaries was caused by insufficient production of EGF-like factors or PGE2, we isolated COCs from WT and Smad4gc−/− mice and treated them with AREG (100 ng/mL) or PGE2 (500 nM). Cumulus expansion was observed in COCs from WT mice, but not in those from Smad4gc−/− mice (Figure 4B), which indicated that AREG or PGE2 was not sufficient to rescue the cumulus expansion defects caused by Smad4 depletion.
Figure 4.
Effects of Smad4 cKO on Signaling Pathways Downstream of EGF-Like Factors and PGE2 A, Immunohistochemistry results for PTGS2 expression, which was significantly induced by hCG in GCs of preovulatory follicles, but was blocked in Smad4gc−/− ovaries. B, In vitro cumulus expansion assay results. The COC expansion defect of Smad4gc−/− ovaries could not be rescued by exogenous AREG (100 ng/mL) or PGE2 (500 nM). Images were acquired 18–20 hours after culture. C, Quantitative RT-PCR results. SB431542 (10 μM) did not block the PGE2-induced expression of Areg but did prevent the induction of PGE2 downstream genes related to cumulus expansion (Ptgs2, Has2, Tnfaip6, and Ptx3). D, Western blot results. Gonadotropin-induced ERK1/2 phosphorylation was reduced in Smad4gc−/− ovaries, especially at 2 hours after hCG injection. E, Immunofluorescence results. Smad4 depletion did not affect PGE2-induced phosphorylation of cAMP responsive element binding protein (CREB) in cultured GCs. Primary GCs were isolated from the antral follicles of PMSG-primed WT and Smad4gc−/− mice and treated with PGE2 (500 nM) for 30 minutes. F, Western blot results. SB431542 blocked EGF-stimulated ERK1/2 phosphorylation in cultured GCs. Primary GCs were treated with EGF (20 ng/mL) or EGF plus SB431542 (10 μM) for 10 minutes. G, Quantitative RT-PCR results. EGF receptor (Egfr) mRNA was markedly down-regulated in GCs of PMSG-primed Smad4gc−/− ovaries. CREB, CRE-binding protein; DAPI, 4′,6-diamidino-2-phenylindole.
To test whether the COC expansion defect in Smad4gc−/− ovaries reflected a gradual accumulation of developmental deficiencies or if it was caused by direct inhibition of TGF-β signaling, we harvested intact COCs from PMSG-primed WT ovaries and induced COC expansion in vitro using PGE2 with or without SB431542, a selective inhibitor of the TGF-β receptor. As shown in Figure 4C, SB431542 blocked PGE2-induced COC expansion and also reduced PGE2-induced expressions of COC expansion-related genes (Ptgs2, Has2, Ptx3, and Tnfaip6). Previous studies showed that PGE2 also up-regulated the expressions of EGF-like factors in cumulus cells, which creates a positive feedback loop (3). Interestingly, PGE2-induced Areg expression (but not Btc) was not inhibited; rather, it was enhanced by SB431542 treatment. These results indicated that inhibiting TGF-β signaling, either by Smad4 KO or by TGF-β receptor inhibition, directly blocked COC expansion due to impaired expressions of key genes involved in this event.
Both the cAMP/protein kinase A pathway and the RAS/ERK1/2 cascade are activated by LH/hCG and are essential for ovulation (28, 31, 32). Therefore, we tested whether activation of these pathways was affected by Smad4 depletion. In cultured GCs isolated from Smad4gc−/− ovaries, PGE2-induced phosphorylation of cAMP responsive element-binding protein; a direct target of protein kinase A) at Ser133 was not affected as compared with WT cells (Figure 4E). In contrast, ERK1/2 phosphorylation was notably decreased in Smad4gc−/− ovaries, particularly at 2 hours after hCG treatment when pERK1/2 reached its highest level in WT ovaries (Figure 4D). Consistent with our in vivo results, EGF-induced ERK1/2 phosphorylation in cultured WT GCs was also decreased by SB431542 treatment (Figure 4F).
To determine a reason for why ERK1/2 activation was affected by Smad4 depletion, we examined EGF receptor gene (Egfr) mRNA levels. As shown in Figure 4G, Egfr mRNA expression was greatly reduced in Smad4-depleted GCs, which was consistent with previous findings for cumulus cells from Gdf9 KO mice (33).
SMAD4-depleted GCs fail to properly luteinize
Our results thus far showed that Smad4gc−/− mice had defects in ovulation and that unruptured preovulatory follicles persisted at 16 hours after hCG treatment, the time point at which WT mice had already ovulated. Therefore, we investigated the fates of these follicles that failed to ovulate. Hematoxylin and eosin staining showed that, in WT ovaries, the GCs of ovulated follicles had fully differentiated into luteal cells at 48 hours after hCG treatment. However, in Smad4gc−/− ovaries, the mural GCs in unruptured follicles showed signs of only partial luteinization, as characterized by lightly stained nuclei and increased cell sizes when compared with undifferentiated GCs, and the antral cavity and unovulated oocytes could still be seen (Figure 5A). In these mice, serum P4 levels were significantly lower than those of control mice (Figure 5B), which indicated that the endocrine function of CLs was defective.
To further confirm this, we examined the mRNA expression levels of 4 widely recognized luteal cell marker genes essential for CL functions: Lhcgr, Star, Cyp11a1, and Sfrp4, which respectively encode for the LH receptor, steroidogenic acute regulatory protein, cytochrome P450 side-chain cleavage enzyme, and secreted frizzled-related protein 4 (20, 21). The expressions of these genes were strongly induced by hCG during luteinization in WT ovaries but were induced at much lower levels in Smad4gc−/− ovaries (Figure 5C).
In addition to the defects observed in experimentally induced luteinization by exogenous gonadotropins, we also examined CL development in adult Smad4gc−/− mice. The 3-month-old pregnant Smad4gc−/− mice have fewer CLs in their ovaries, when compared with the age-matched pregnant WT mice (Figure 5, D and E), which is inconsistent with their reduced fertility. CLs at different developmental stages were seen in nonpregnant adult WT mice, whereas CL structures were absent in most adult Smad4gc−/− mice at 6–7 months of age, when they became almost infertile (n = 6). These CL-deficient Smad4gc−/− ovaries had fewer growing follicles than normal and sometimes contained large, unruptured follicles (more precisely, ovarian lesions; Figure 5F).
Discussion
The functions of Smad4 in GCs in vivo have been studied by cKO using Amhr2-Cre mice (25). Because CRE recombinase is expressed during the early stages of follicular development in Smad4fl/fl;Amhr2-Cre mice, analyzing the selective effects of SMAD4 in preovulatory follicles in this mouse strain is not ideal. Therefore, we generated a novel Smad4 cKO mouse model by mating Smad4fl/fl mice with our Cyp19-Cre mice. Cyp19-Cre mice have the advantage that CRE recombinase expression is GC specific and is enhanced in preovulatory follicles in a manner similar to that of the endogenous Cyp19a1 gene (18, 34). The Smad4fl/fl;Cyp19-Cre mice had phenotypes similar to Smad4fl/fl;Amhr2-Cre mice (25). However, these two Smad4 cKO mouse models also have important differences. In the ovaries of Smad4fl/fl;Amhr2-Cre mice, many of the large antral follicles underwent premature luteinization, and the GCs of these mice abnormally expressed a number of luteal markers after stimulation with PMSG. This suggested that one of the primary functions of the TGF-β superfamily in developing immature follicles was to regulate the timing of GCs terminal differentiation. However, our results showed that depleting Smad4 in the GCs of preovulatory follicles did not cause premature defects in follicular development. Rather, these GCs failed to express LH-target genes required for luteinization and terminal differentiation into luteal cells. As a result, CLs were absent in the ovaries of adult Smad4fl/fl;Cyp19-Cre female mice. By comparing these two mouse models, we propose that the functions of the TGF-β signaling pathway in ovarian GCs are stage specific.
The functions of the canonical TGF-β signaling pathway in ovarian GCs have been previously studied both in vitro and in vivo. For example, RNA interference depletion of SMAD4 impaired GC proliferation and estrogen biosynthesis, as well as TGF-β-induced expression of micro-RNAs (particularly miR-244) (35). In vitro studies also indicated that steroidogenic factor-1 is required for TGF-β3-mediated 17β-estradiol synthesis (36). However, there still remain some unanswered questions. The numbers of antral follicles decreased in the ovaries of both Smad2/3 and Smad4 cKO mice (13, 25). Yet, it has not been determined whether this phenotype is caused by retarded GC proliferation or by an increase in GC apoptosis. The Smad3 null mice have multiple defects in folliculogenesis, including decreased GC proliferations, FSH-target gene expressions, and ovulation (37). Our results indicated that GCs proliferation was not dramatically affected by Smad4 depletion. This might be because in the Smad4fl/fl;Cyp19-Cre mouse strain, Smad4 was deleted only in the late stage of folliculogenesis. Therefore, our results proved, for the first time, that the role of SMAD3/4 in ovulation was independent of their role in early follicular development. Rather, the numbers of atretic follicles increased, notably in Smad4gc−/− ovaries. It has been reported that SMAD7 mediates apoptosis induced by TGF-β in mouse GCs. TGF-β-induced SMAD7 expression in GCs in a TGF-β receptor 1-dependent but SMAD3-independent manner (38). Therefore, the role of SMAD4 in regulating Smad7 expression through other R-SMADs may also play a role in increased apoptosis seen in GCs. Thus, the TGF-β signaling pathway may support follicle development by preventing premature GCs apoptosis, as also suggested by a previous study of Nodal (39) (which belongs to TGF-β superfamily as well) in the ovary.
The TGF-beta family is divided into 2 prominent subfamilies TGF-β/Activin and the BMP families. Both are critically important to normal ovarian function at many stages of follicle development. At the level of the R-SMADs, mice deficient in both SMAD2 and SMAD3 in GCs have severe fertility defects and share some features with Smad4 cKO mice, such as impaired cumulus expansion, although luteinization defects are not as prominent and serum progesterone is not elevated (13). In contrast, the GC-specific abolishment of BMP signaling, including cKO of BMP receptors (BMPR1A and BMPR1B) (11) and SMAD1/5/8 (12), caused GC tumor formation as well as subfertility. SMAD1 and SMAD5 are also functionally redundant in the ovary. SMAD1/5 double-cKO females are subfertile and develop metastatic GC tumors, suggesting that SMAD1/5 signaling in GCs acts as a tumor suppressor pathway, potentially by antagonizing SMAD2/3 signaling. However, the previous reports did not describe the detailed ovulation defects in BMPR1A/B or SMAD1/5 cKO ovaries. Our Smad4gc−/− mice have ovulation defects but did not develop GC tumors even at very old ages, suggesting that the blockade of SMAD2/3 signaling contributed to these phenotypes more than the blockade of SMAD1/5/8 signaling.
Previous studies have shown that SMAD3/4 is critical for regulation of aromatase expression. Thus, Smad4 deletion might create a negative feedback loop in our system that reduces Cyp19a1 promoter activity (and therefore SMAD4 suppression). However, the beauty of our cKO system is that it is a hit-and-run system. As long as Cyp19-Cre is expressed before Smad4 deletion, CRE recombinase will target the flox sequence and knock out the Smad4 gene. After this recombination, continuous CRE expression is no longer needed because Smad4 KO is an irreversible phenomenon. Therefore, we did not have to worry about the possible negative feedback effect of Smad4 deletion on CRE expression.
The role of the TGF-β signaling pathway in cumulus cells during ovulation has long been a focus of study. However, its effects on oocyte meiotic arrest and resumption have not been adequately addressed. It was recently discovered that the GC ligand C-type natriuretic peptide and its receptor NPR2 in cumulus cells were essential for maintaining oocyte meiotic arrest before ovulation (24). It has also been suggested that the Npr2 mRNA levels in cumulus cells were regulated by oocyte-derived paracrine factors, including GDF9, BMP15, and fibroblast growth factor 8. Our current study showed that TGF-β signaling through SMAD4 was required for Nppc and Npr2 expression in both GCs and cumulus cells. The physiologic importance of TGF-β signaling for maintaining Nppc/Npr2 expression was demonstrated by the large percentage of oocytes that precociously resumed meiotic maturation in Smad4gc−/− ovaries.
Our previous study showed that LH-stimulated activation of the RAS-ERK1/2 signaling cascade was crucial for ovulation processes, including oocyte meiotic maturation, cumulus expansion, follicle rupture, and luteinization (18, 28). However, the possible interplay between the TGF-β signaling pathway and the ERK1/2 cascade was insufficiently discussed. Here, we report, for the first time, that LH-stimulated oocyte meiotic maturation was not affected in Smad4gc−/− ovaries, whereas the later events of ovulation, including cumulus expansion and luteinization, were severely impaired. These observations indicated that the TGF-β signaling pathway was involved in ovulation events later than ERK1/2. More importantly, hCG-stimulated ERK1/2 phosphorylation was significantly down-regulated by Smad4 depletion. We suggest that this is due to the reduced expression of EGF receptors by SMAD4-deficient GCs in preovulatory follicles. Furthermore, SMAD4 was required for ERK1/2 signaling propagation, as the expression of PTGS2 and, therefore, the production of intraovarian PGE2 was blocked in Smad4gc−/− ovaries. Not surprisingly, similar ovarian phenotypes were observed in our Smad4gc−/− mice and in previously reported Ptgs2 KO mice (30). Collectively, these results suggested that the RAS-ERK1/2 signaling cascade was the trigger of ovulation and that the TGF-β signaling pathway played an important permissive role in this process.
The possible permissive role of Smad4 in expression of LH-target genes within preovulatory follicles was further tested by real-time RT-PCR analyses. It appeared that expression of LH-target genes in cumulus cells was more dramatically down-regulated than in mural GCs after Smad4 depletion. An explanation for this observation could be that cumulus cells are closer to an oocyte and receive more stimulating signals by GDF9 and BMP15 and, therefore, depend on the transcription-inducing function of SMAD4 more so than mural GCs. In addition, Npr2 expression and 3β-HSD repression that occur in normal cumulus cells were also abolished by Smad4 depletion. As hypothesized by Diaz et al. (5), Sugiura et al (40), and Su et al (41), TGF-β signals from the center of a preovulatory follicle (ie, the oocyte) and FSH signals from the follicle periphery fine tune the gene expression patterns during ovulation. For the first time, our observations of Smad4gc−/− ovaries provide an in vivo model that proves the importance of this gradient of TGF-β signals in determining the gene expression patterns of cumulus cells.
Taken together, our findings demonstrate that disrupting signaling through SMAD4 in the GCs of preovulatory follicles does not cause premature luteinization. Rather, the reproductive defects observed for Smad4fl/fl;Cyp19-Cre female mice indicated that the canonical TGF-β signaling pathway played a permissive role in LH-induced cumulus expansion, ovulation, and luteinization. Therefore, these results demonstrate that the key in vivo roles of TGF-β superfamily signaling in GCs are developmentally stage specific (summarized in Figure 5G).
Acknowledgments
This work was supported by grants from National Basic Research Program of China (2011CB944504, 2012CB944403), National Natural Science Foundation of China (81172473), Natural Science Foundation of Zhejiang Province (R2100145), and Basic Scientific Research Funding of Zhejiang University (2011QN81001).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMH
- anti-Mullerian hormone
- AREG
- amphiregulin
- BMP
- bone morphogenic protein
- cKO
- conditional KO
- CLs
- corpus lutea
- COC
- cumulus-oocyte complex
- CRE
- cAMP response element
- EGF
- epidermal growth factor
- GC
- granulosa cell
- GDF9
- growth differentiation factor 9
- GVBD
- germinal vesicle breakdown
- KO
- knockout
- NPR2
- natriuretic peptide receptor 2
- P4
- progesterone
- PD
- postnatal day
- PGE2
- prostaglandin E2
- PMSG
- pregnant mare serum gonadotropin
- PTGS2
- prostaglandin synthase 2
- SMAD
- Sma- and Mad-related protein
- TUNEL
- terminal deoxynucleotidetransferase-mediated dUTP end labeling
- WT
- wild type.
References
- 1. Fan HY , Richards JS. Minireview: physiological and pathological actions of RAS in the ovary. Mol Endocrinol. 2010;24:286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Park JY , Su YQ , Ariga M , Law E , Jin SL , Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. [DOI] [PubMed] [Google Scholar]
- 3. Shimada M , Hernandez-Gonzalez I , Gonzalez-Robayna I , Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–1365. [DOI] [PubMed] [Google Scholar]
- 4. Ochsner SA , Day AJ , Rugg MS , Breyer RM , Gomer RH , Richards JS. Disrupted function of tumor necrosis factor-α-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology. 2003;144:4376–4384. [DOI] [PubMed] [Google Scholar]
- 5. Diaz FJ , Wigglesworth K , Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007;120:1330–1340. [DOI] [PubMed] [Google Scholar]
- 6. Pangas SA , Matzuk MM. The art and artifact of GDF9 activity: cumulus expansion and the cumulus expansion-enabling factor. Biol Reprod. 2005;73:582–585. [DOI] [PubMed] [Google Scholar]
- 7. Pangas SA. Regulation of the ovarian reserve by members of the transforming growth factor β family. Mol Reprod Dev. 2012;79:666–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dong J , Albertini DF , Nishimori K , Kumar TR , Lu N , Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. [DOI] [PubMed] [Google Scholar]
- 9. Pangas SA , Jorgez CJ , Tran M , et al. Intraovarian activins are required for female fertility. Mol Endocrinol. 2007;21:2458–2471. [DOI] [PubMed] [Google Scholar]
- 10. van Houten EL , Themmen AP , Visser JA. Anti-Mullerian hormone (AMH): regulator and marker of ovarian function. Ann Endocrinol (Paris). 2010;71:191–197. [DOI] [PubMed] [Google Scholar]
- 11. Edson MA , Nalam RL , Clementi C , et al. Granulosa cell-expressed BMPR1A and BMPR1B have unique functions in regulating fertility but act redundantly to suppress ovarian tumor development. Mol Endocrinol. 2010;24:1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pangas SA , Li X , Umans L , et al. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol. 2008;28:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Q , Pangas SA , Jorgez CJ , Graff JM , Weinstein M , Matzuk MM. Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo. Mol Cell Biol. 2008;28:7001–7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jorgez CJ , Klysik M , Jamin SP , Behringer RR , Matzuk MM. Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol Endocrinol. 2004;18:953–967. [DOI] [PubMed] [Google Scholar]
- 15. Jamin SP , Arango NA , Mishina Y , Hanks MC , Behringer RR. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. [DOI] [PubMed] [Google Scholar]
- 16. Hernandez Gifford JA , Hunzicker-Dunn ME , Nilson JH. Conditional deletion of β-catenin mediated by Amhr2cre in mice causes female infertility. Biol Reprod. 2009;80:1282–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan HY , Liu Z , Cahill N , Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol. 2008;22:2128–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fan HY , Shimada M , Liu Z , et al. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development. 2008;135:2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chu GC , Dunn NR , Anderson DC , Oxburgh L , Robertson EJ. Differential requirements for Smad4 in TGFβ-dependent patterning of the early mouse embryo. Development. 2004;131:3501–3512. [DOI] [PubMed] [Google Scholar]
- 20. Fan HY , Liu Z , Johnson PF , Richards JS. CCAAT/enhancer-binding proteins (C/EBP)-α and -β are essential for ovulation, luteinization, and the expression of key target genes. Mol Endocrinol. 2011;25:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fan HY , O'Connor A , Shitanaka M , Shimada M , Liu Z , Richards JS. β-Catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol Endocrinol. 2010;24:1529–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hinshelwood MM , Mendelson CR. Tissue-specific expression of the human CYP19 (aromatase) gene in ovary and adipose tissue of transgenic mice. J Steroid Biochem Mol Biol. 2001;79:193–201. [DOI] [PubMed] [Google Scholar]
- 23. Hinshelwood MM , Smith ME , Murry BA , Mendelson CR. A 278 bp region just upstream of the human CYP19 (aromatase) gene mediates ovary-specific expression in transgenic mice. Endocrinology. 2000;141:2050–2053. [DOI] [PubMed] [Google Scholar]
- 24. Zhang M , Su YQ , Sugiura K , Xia G , Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pangas SA , Li X , Robertson EJ , Matzuk MM. Premature luteinization and cumulus cell defects in ovarian-specific Smad4 knockout mice. Mol Endocrinol. 2006;20:1406–1422. [DOI] [PubMed] [Google Scholar]
- 26. Richards JS , Sharma SC , Falender AE , Lo YH. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol. 2002;16:580–599. [DOI] [PubMed] [Google Scholar]
- 27. Liu Z , Rudd MD , Hernandez-Gonzalez I , et al. FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthetic pathways in granulosa cells. Mol Endocrinol. 2009;23:649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fan HY , Liu Z , Shimada M , et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ochsner SA , Russell DL , Day AJ , Breyer RM , Richards JS. Decreased expression of tumor necrosis factor-α-stimulated gene 6 in cumulus cells of the cyclooxygenase-2 and EP2 null mice. Endocrinology. 2003;144:1008–1019. [DOI] [PubMed] [Google Scholar]
- 30. Lim H , Paria BC , Das SK , et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. [DOI] [PubMed] [Google Scholar]
- 31. Richards JS. New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol. 2001;15:209–218. [DOI] [PubMed] [Google Scholar]
- 32. Wayne CM , Fan HY , Cheng X , Richards JS. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol. 2007;21:1940–1957. [DOI] [PubMed] [Google Scholar]
- 33. Su YQ , Sugiura K , Li Q , Wigglesworth K , Matzuk MM , Eppig JJ. Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol Endocrinol. 2010;24:1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fan HY , Liu Z , Paquet M , et al. 2009 Cell type-specific targeted mutations of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult to ovarian surface epithelial cells. Cancer Res. 2009;69:6463–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yao G , Yin M , Lian J , et al. MicroRNA-224 is involved in transforming growth factor-β-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Mol Endocrinol. 2010;24:540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liang N , Xu Y , Yin Y , et al. Steroidogenic factor-1 is required for TGF-β3-mediated 17β-estradiol synthesis in mouse ovarian granulosa cells. Endocrinology. 2011;152:3213–3225. [DOI] [PubMed] [Google Scholar]
- 37. Gong X , McGee EA. Smad3 is required for normal follicular follicle-stimulating hormone responsiveness in the mouse. Biol Reprod. 2009;81:730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quezada M , Wang J , Hoang V , McGee EA. Smad7 is a transforming growth factor-β-inducible mediator of apoptosis in granulosa cells. Fertil Steril. 2012;97:1452–1459.e1–6. [DOI] [PubMed] [Google Scholar]
- 39. Wang H , Jiang JY , Zhu C , Peng C , Tsang BK. Role and regulation of nodal/activin receptor-like kinase 7 signaling pathway in the control of ovarian follicular atresia. Mol Endocrinol. 2006;20:2469–2482. [DOI] [PubMed] [Google Scholar]
- 40. Sugiura K , Su YQ , Diaz FJ , et al. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134:2593–2603. [DOI] [PubMed] [Google Scholar]
- 41. Su YQ , Sugiura K , Wigglesworth K , et al. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135:111–121. [DOI] [PubMed] [Google Scholar]