Abstract
Case summary
A 16-year-old, castrated male, feline immunodeficiency virus (FIV)-positive, domestic shorthair cat developed multiple skin lesions. Most of these were Bowenoid carcinoma in situ and contained DNA sequences consistent with Felis catus papillomavirus type 2. Two additional lesions that developed in the skin and subcutaneous tissues between the digital and carpal pads on the left forelimb and right hindlimb were shown by cytology, histology and culture to be caused by Prototheca wickerhamii. These lesions failed to improve in response to systemic therapy treatment with itraconazole, but excision by sharp en bloc resection with follow-up oral itraconazole therapy proved curative for one lesion, although the other lesion recurred, necessitating a second surgery.
Relevance and novel information
This is only the second reported case of feline protothecosis from Australia and the first case that has been cultured and identified to the species level. Also of great interest was the presence of multiple papillomavirus-associated neoplastic lesions, which may have afforded a portal of entry for the algal pathogen and the cat’s positive FIV status; the latter might have impacted on both viral and algal pathogenesis by effects on immunocompetence.
Introduction
Prototheca species are currently classified as an achlorophyllic algae, possibly related to Chlorella species. They are ubiquitous in the environment and have the potential to cause disease in mammalian hosts, including humans.1 The first recorded Prototheca species infection in a human patient was a focal infection in the foot of a rice worker in Sierra Leone.2 Most human infections are focal, caused by Prototheca wickerhamii, and probably secondary to local traumatic inoculation of propagules into subcutaneous tissues, including the olecranon bursa. Disseminated infections can occur, however, and comprise perhaps 20% of human cases, typically occurring in immunocompromised individuals. Generally, these disseminated infections are secondary to infection with Prototheca zopfii genotype 2.1
The first recorded case in the veterinary literature was a case of bovine mastitis in 1952, reported by Lerche.3 Subsequent work has provided detailed information about the epidemiology of this important cause of environmentally associated mastitis, often as case clusters, characteristically in certain geographical regions and associated with deficient husbandry practices,4 as the problem results from ascending infection from the environment via the teat canal. Protothecosis in other domestic animal species is uncommon.5,6 It is most often recorded in dogs as a disseminated infection following primary infection of the colon (often secondary to a predisposing condition such as granulomatous colitis due to adherent invasive Escherichia coli in Boxer dogs and French Bulldogs)7–9 and generally carries a poor prognosis, unless infections are detected while the infection is restricted to the colon, prior to haematogenous dissemination.10
Disease in cats appears to be extremely rare, with only six cases reported to date. Five of these describe a single focal skin lesion (four caused by P wickerhamii and one secondary to P zopfi genotype 2),11–15 presumably of similar pathogenesis to those seen in humans where penetrating injury (usually a cat scratch) introduces infectious propagules from some environmental reservoir. In the single case report with multifocal lesions secondary to P wickerhammii,16 penetrating local injury would also be suspected, as all lesions were peripheral with no evidence of systemic invasion.
Here we report a case in which a cat developed protothecal lesions on two separate paws; the lesions failed to respond to monotherapy with itraconazole, so aggressive surgical excision followed by further itraconazole was undertaken (Figure 1). As the cat was infected with feline immunodeficiency virus (FIV) and also had multiple papillomavirus-associated skin lesions, a possible defect in the immune system may have been important in allowing the algal infection to develop.
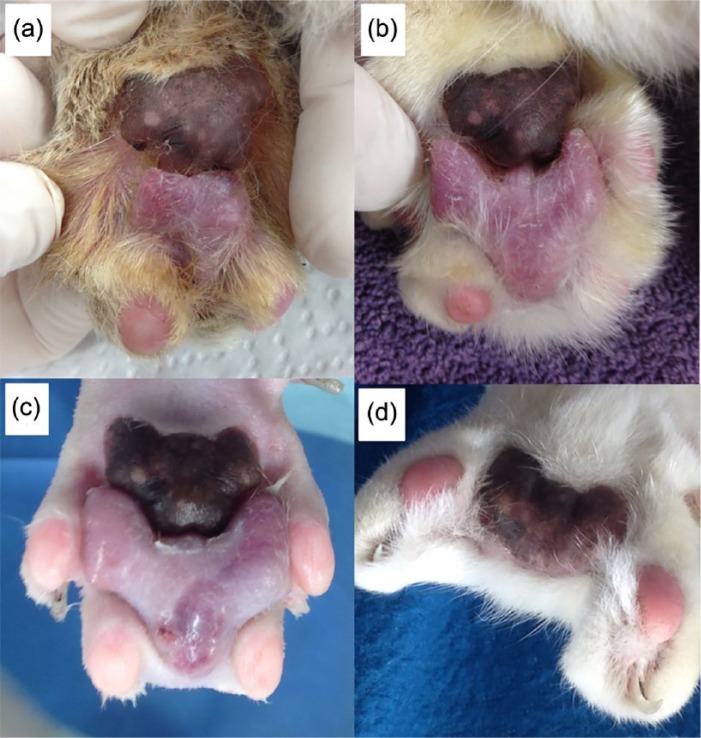
Figure 1.
Protothecal lesion left fore: progressive increase in size over 5 months, from (a) when first noticed, through to (b) and then (c), the day of surgery. (d) At 3 months after surgery, the main pad remains, and there is a space where the lesion and the two toes were removed
Case description
In August 2014, a 16-year-old, castrated male, domestic shorthair cat was presented with multiple skin lesions and weight loss. On physical examination, a heart murmur was heard on auscultation, accompanied by moderate weight loss (8.5 to 6.2 kg over 11 months, without dieting). Skin lesions included a slightly raised plaque (1 cm diameter) on the skin of the dorsal midline between the scapulae and similar smaller masses on the forehead, left medial periorbital skin and the cranial edge of the right pinna.
The cat was reported to eat well but not to be ravenous. Previous haematological and serum and biochemical tests results from a routine veterinary visit 1 month prior showed a very minimal increase in globulins, mild lymphopenia and total thyroxine concentration within the reference interval (RI). No treatment or further investigations were carried out at that time.
At re-examination on October 2014, the skin lesions persisted and mild weight loss had continued (6.1 kg). Additionally, there was an ulcerated lesion on the metatarsal pad of the left pelvic limb, accompanied by bilateral enlargement of both popliteal lymph nodes. Meloxicam orally and topical local chloramphenicol/corticosteroid (Chloroptson; Ceva) ointment were prescribed by the primary veterinarian.
The cat re-presented 8 months later (June 2015) with continuing weight loss (now 5.8 kg). The cat had developed a new cutaneous mass between the metacarpal pad and digital pads of the left thoracic limb (Figure 1a). The ulcerated lesion on the left hindpad was largely unchanged. A punch biopsy (Keys skin biopsy device; 6 mm diameter) of the left forelimb lesion and an excisional biopsy of the entire mass in the dorsal cervical region were performed (http://www.gosh.nhs.uk/health-professionals/clinical-guidelines/skin-biopsy-punch-method) after in-house haematology and biochemistry testing were reported as normal.
Histological examination of the skin biopsy from the dorsum showed multiple anatomising, densely packed trabeculae of dysplastic basaloid epidermal cells infiltrating from the ulcerated surface into the dermis. Cells contained vesicular nuclei displaying moderate anisokaryosis, prominent nucleoli, with 2–3 mitoses per high power field. Nuclei, especially in expanded areas in the epithelium of the superficial portion of the follicle, displayed elongated nuclei orientated in a slanted fashion (‘windrowing’). Squamous cell carcinoma was diagnosed but with features suggesting the lesion may have arisen within a Bowenoid in situ carcinoma (Figure 2).
Figure 2.
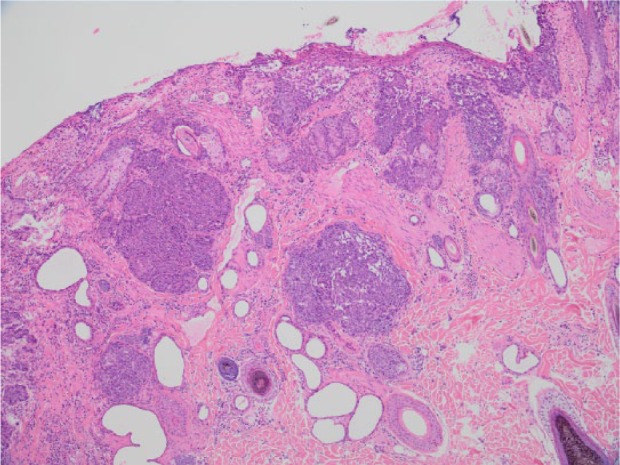
Squamous cell carcinoma that likely arose within a Bowenoid in situ carcinoma: multiple densely packed trabecular of dysplastic epidermal/basaloid like cells infiltrate from an ulcerated surface into the dermis
Microscopic evaluation of the left forelimb biopsy revealed multifocal to coalescing dermal and subcutaneous infiltrates of macrophages (some multi-nucleate) with lesser numbers of neutrophils. Most macrophages contained variable numbers of lightly staining 3–10 µm diameter spherical bodies. These were positive with both periodic acid–Schiff and silver staining (Figures 3 and 4). Thus, a diagnosis of pyogranulomatous pododermatitis with large numbers of intralesional bodies consistent with Prototheca species was made. The size of the spherical bodies and their shape and morphology was most consistent with P wickerhamii.
Figure 3.
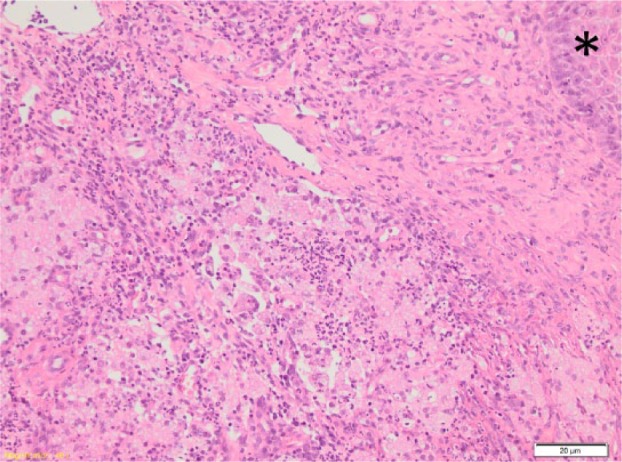
Left fore biopsy: the epidermis is indicated by an asterisk. Multiple-to-coalescing infiltrates of macrophages and neutrophils within the dermis
Figure 4.
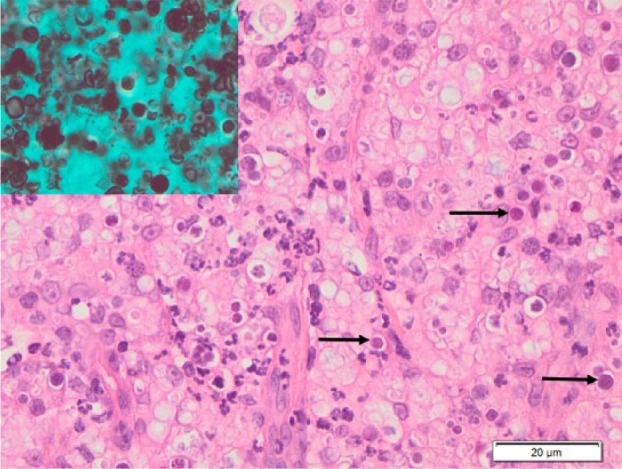
High power view of lesion shown in Figure 3. The arrows indicate some of the spherical bodies within macrophages, and the inset shows staining of these bodies with Grocott’s methenamine silver stain
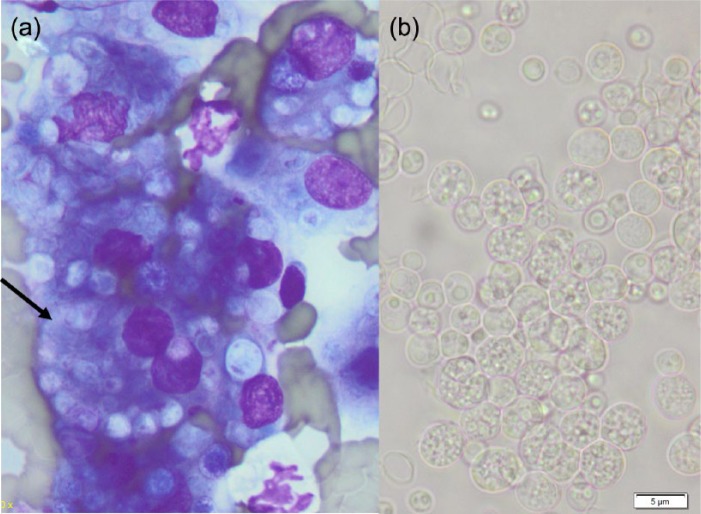
Subsequent cytological examination of a fine-needle aspirate (FNA) made from the left forelimb mass, undertaken to improve the morphological detail of the organisms, showed large numbers of macrophages (some multi-nucleate) that contained moderate to sometimes large numbers of thinly encapsulated, spherical, granular organisms (3–10 µm diameter), some of which contained smaller internal spherical structures (Figure 5a). Subsequently, a further FNA was obtained, sprayed into sterile saline and forwarded to the laboratory for culture. Incubation on sheep blood agar at room temperature and at 37 oC produced a moderate pure growth of 2 mm diameter white shiny colonies within 3 days. When examined microscopically, the colonies contained large numbers of spherical, thinly encapsulated spore-like structures (2–7 µm diameter) (Figure 5b). Some contained a granular internal structure, although most contained 3–10 smaller daughter cells (1–3 µm in diameter) that were themselves thinly encapsulated (sporangia). In the background, small numbers of ruptured spores were evident. Microscopic morphology, cultural characteristics and failure to metabolise trehalose positively identified the organism as P wickerhamii. Identification using MALDI-TOF mass spectrometry was also attempted, but was only able to identify the organism as Prototheca species, although this technique has been useful for species-level identification.17 However, DNA sequence analysis of the D1/D2 region of the 28S (large subunit) ribosomal DNA gene, using published primers and standard sequencing methodology, confirmed the morphological and biochemical identification of the organism as P wickerhamii.18
Figure 5.
(a) Fine-needle aspirate preparation stained with Diff-Quik of left fore lesion showing macrophages, some multinucleate (arrow) containing large numbers of spherical algal bodies. (b) Unstained suspension of a cultured colony of Prototheca wickerhamii organisms. Note spherical spores containing variable numbers of sporangia
Serial haematology and biochemistry testing was suggested to the owner, and two complete blood counts (CBCs) and serum biochemical examinations, 1 month apart, revealed borderline mature neutropenia (neutrophils 2.2 and 2.5 × 109/l, respectively; RI 2.5–12.5 × 109/l), mild-to-moderate lymphopenia (0.5 and 0.7 × 109/l, respectively; RI 1.5–7 × 109/l), accompanied by a mild-to-moderate hyperglobulinemia (58 and 62 g/l, respectively; RI 28–48 g/l). Total thyroxine concentration of plasma was within the RI on both occasions (19 and 33 nmol/l, respectively; RI 10–48 nmol/l). The cat was also FIV antibody ELISA-positive and feline leukaemia virus antigen ELISA-negative. Liver enzyme activities were always within RIs. The animal was placed on a course of cefovecin injections, once fortnightly for 12 weeks.
One month after biopsy a further lesion developed on the right hindpaw, on the medial aspect of the skin near digit four. FNA biopsy and culture revealed identical findings to that seen in the left thoracic limb, confirming a second separate focus of pyogranulomatous pododermatitis secondary to P wickerhamii infection. Cytology of the previously noted ulcerated left hind metatarsal pad lesion revealed a suppurative pododermatitis with mixed bacteria and no evidence of protothecal infection.
Empirical treatment of the left fore and right hind Prototheca-positive lesions commenced with oral itraconazole (Sporanox; Janssen). Initially, 100 mg total dose (one capsule) was administered once daily (with food), which was reduced to every second day after the animal became lethargic. Despite monotherapy with this triazole antifungal, both algal lesions continued to expand, especially the left forelimb lesion (Figure 1). A decision was therefore made to try to eliminate the infections by complete surgical excision of all infected tissues (October 2015) using an oncological-like approach using sharp dissection with en bloc resection of infected tissues, performed by a visiting referral surgeon. The multiple other skin lesions noted previously (that had continued to develop) were also excised at the same time.
At surgery, an amorphous hairless and expansile mass (1 cm diameter) was present medial to digit four of the right hindpaw. It was excised with margins of 3 mm by amputation of the digit through the centre of the first phalangeal bone (P1). A fusion podioplasty between digits three and five was performed to prevent digit five from deviating laterally. On the left forepaw, an amorphous mass (2.2 cm diameter) was present with a broad base of attachment to the metacarpal pad, expanding cranially between digits three and four (Figure 1c). This lesion was removed by excising the cranial 4 mm aspect of the metacarpal pad, as well as digits two and three through the metacarpophalangeal joints. The distal epiphyses of metacarpal bones two and three were removed with Rongeurs to allow the skin of the dorsum of the paw to be directly opposed to the remaining pad without undue tension over bony prominences (Figure 1d).
Histological examination of resected tissues revealed likely complete removal of the protothecal pyogranulomatous mass (ie, clean surgical margins) from the right hind lesion; however, protothecal organisms could be seen within 2 mm of one surgical margin from the left forelimb lesion, making complete excision of this lesion less confident. Multiple papillomatosis lesions were also excised from the skin of the left hindlimb, the right pinna, the right hindpaw, the lower lip and the dorsal skin between the scapulae and examined histologically. All were identified as Bowenoid carcinoma in situ. DNA was extracted from formalin-fixed paraffin-embedded tissue shavings from each of the five lesions diagnosed as Bowenoid carcinoma in situ, as previously described.19 To detect the presence of Felis catus papillomavirus type 2 (FcaPV-2) DNA within all primers specific for this PV type were used as previously described.19–22 A feline squamous cell carcinoma known to contain FcaPV-2 DNA was used as a positive control while no template DNA was added to the negative controls. FcaPV-2 DNA sequences were amplified from the positive control sample and all skin lesions from this patient but not from the negative control sample.
Itraconazole (100 mg every second day) was continued. Unfortunately, despite this, a recurrence of the lesion on the right hindlimb was confirmed cytologically 8 months after the first surgery and the lesion was surgically removed. Histological examination of resected tissues confirmed pyogranulomatous pododermatitis with intralesional protothecal organisms. There was no recurrence of the left forepaw lesion.
Two months later the animal developed ascites, shown to be a borderline modified transudate (protein 22 g/l), containing low numbers of red blood cells. A CBC revealed persistence of the mild neutropenia and lymphopenia noted previously and the development of a mild anaemia (packed cell volume 22 l/l; RI 24–35 l/l). Owing to the animal’s deteriorating condition, elective euthanasia was undertaken. A limited necropsy examination was performed and a number of organs examined histologically (liver, kidney, spleen, lymph node, pancreas, lung and small intestine). Significant findings were confined to the liver, which showed a moderate diffuse periacinar fibroplasia and hepatocyte atrophy with dilated portal lymphatics.
Discussion
Most previous cases of feline protothecosis have been focal pyogranulomatous dermal and/or subcutaneous lesions with large numbers of intralesional algae.11–15 Only one case was multifocal.16 None had evidence of widespread dissemination, although one case did have evidence of infection in the regional draining lymph node.11 The naso-ocular skin of the cat’s head has been affected most commonly, with the location of lesions being mist consistent with a primary cat scratch injury,23 although distal limb and tail involvement have also been documented. Most cats have been male, consistent with the contribution of fighting and cat scratches to the aetiology of many cases, while there is a wide age distribution (range 3–15 years). There was no overt evidence of immune suppression in any of these previous feline reports, except for a moderate neutropenia in one case.16 None of the cats were positive for retrovirus infections, although not all were tested.
In the case reported here, the organism was identified as P wickerhamii, which was the cause of all previous cases that were cultured except one.14 This represents the second case in which there were multifocal lesions with accompanying neutropenia,16 and in our cat the lesions were restricted to the skin and subcutis of the distal limb, an area where friction and maceration are to be expected. Neutrophils represent an important component of the innate response to saprophytic organisms induced into the subcutis, so the neutropenia might have played a role in terms of predisposing to infection. This cat is the first to be FIV coinfected and the first with concurrent multiple papillomavirus-associated skin neoplasms, some of which may have facilitated penetration of the infectious propagules into host tissues.
Traumatic introduction of the organism remains the most likely explanation for the aetiopathogenesis of this infection, as in previous feline cases, and is also likely the cause in focal human cases,1 as well as bovine mastitis cases where the trauma affects pendulous teats combined with poor environmental hygiene.4
The lesion in this case failed to respond to systemic treatment with a single fungistatic triazole agent (itraconazole), which is not a surprise as this infection is with algae rather than a true fungus. Ergosterol is far less important as a structural component of algal cell walls than fungal cell wall. Indeed, it is unclear whether the use of this agent impacted at all on the progress of the infection. Such a poor response is consistent with similar cases that have been reported in the feline literature. Perhaps co-administration of terbinafine, amphotericin B (systemically or intralesionally) or all three might have represented a more aggressive and effective approach to the medical management of this patient. In human patients, using a combination of itraconazole and amphotericin B is usually more effective,24,25 but use of amphotericin B in cats requires twice-weekly subcutaneous infusions, although intralesional therapy is also possible.26 Indeed, one could make a strong case that early surgical intervention while lesions are small represents the most cost-effective therapy for most feline patients and the cornerstone for therapy with the goal of curative intent, although consolidation therapy itraconazole and/or amphotericin B may still be important to prevent recurrence of infection due to persistence of algal elements in portions of the wound margin. Resolution was achieved for one lesion here with aggressive oncological-like surgery that required removal of adjacent digits to achieve satisfactory gross surgical margins of 4 mm (see Figure 1d). This could be difficult in other locations (eg, lesion on the naso-ocular region, nasal planum). This case also demonstrates the usefulness of FNA cytology, which can facilitate early identification of the characteristic morphology of the organism and subsequent culture and species identification.
The liver changes detected at necropsy examination were responsible for the development of a modified transudate in the abdominal cavity, and the changes within the liver suggest they have developed over a period of weeks rather than months. The actual cause of the hepatopathy is unknown, perhaps in part because a full necropsy was not permitted. Itraconazole can have toxic effects on hepatocytes,27 although there were never any elevations in alanine transferase activity (a hepatocyte cytosolic-specific enzyme in cats) on repeated sampling of this cat before and during treatment.
Conclusions
P wickerhamii is a rare cause of pyogranulomatous dermatitis in felines, but diagnosis is facilitated by cytological examination. Lesions may be treated successfully with full surgical excision, if location and size of lesion allow. This case report also suggests that immune suppression and pre-existing ulcerative lesions may contribute to local infection in some cats.
Acknowledgments
Thank you to the Gribbles Veterinary Pathology Company for support in active pursuit of this investigation, and especially Christina Wilkinson for microbiological expertise. Richard Malik is supported by the Valentine Charlton Bequest of the Centre for Veterinary Education of the University of Sydney.
Footnotes
Accepted: 19 December 2016
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Pathological investigation was largely funded by Gribbles Veterinary Pathology.
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev 2007; 20: 230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davies R, Spencer H, Wakelin P. A case of human protothecosis. Trans R Soc Trop Med Hyg 1964; 58: 448–451. [DOI] [PubMed] [Google Scholar]
- 3. Lerche M. Eine durch Algen (Prototheca) hervorgerufene Mastitis der Kuh. Berl Munch Tierarztl Wochenschr 1952; 4: 64–69. [Google Scholar]
- 4. Ricchi M, De Cicco C, Buzzini P, et al. First outbreak of bovine mastitis caused by Prototheca blaschkeae. Vet Microbiol 2013; 162: 997–999. [DOI] [PubMed] [Google Scholar]
- 5. Camboim EK, Garino FJ, Dantas AF, et al. Protothecosis by Prototheca wickerhamii in goats. Mycoses 2011; 54: e196–200. [DOI] [PubMed] [Google Scholar]
- 6. Ikeda T, Gouma M. Protothecosis in animals [article in Japanese]. Juui Rinnsho Hifuka 2002; 3: 23–32. [Google Scholar]
- 7. Migaki G, Font RL, Sauer RM, et al. Canine protothecosis: review of the literature and report of an additional case. J Am Vet Med Assoc 1982; 181: 794–797. [PubMed] [Google Scholar]
- 8. Simpson KW, Dogan B, Rishniw M, et al. Adherent and invasive Escherichia coli is associated with granulomatous colitis in Boxer dogs. Infect Immun 2006; 74: 4778–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stenner VJ, Mackay B, King T, et al. Protothecosis in 17 Australian dogs and a review of the canine literature. Med Mycol 2007; 45: 249–266. [DOI] [PubMed] [Google Scholar]
- 10. Pressler BM, Gookin JL, Sykes JE, et al. Urinary tract manifestations of protothecosis in dogs. J Vet Intern Med 2005; 19: 115–119. [DOI] [PubMed] [Google Scholar]
- 11. Coloe PJ, Allison JF. Protothecosis in a cat. J Am Vet Med Assoc 1982; 180: 78–79. [PubMed] [Google Scholar]
- 12. Dillberger JE, Homer B, Daubert D, et al. Protothecosis in two cats. J Am Vet Med Assoc 1988; 192: 1557–1559. [PubMed] [Google Scholar]
- 13. Finnie JW, Coloe PJ. Cutaneous protothecosis in a cat. Aust Vet J 1981; 57: 307–308. [DOI] [PubMed] [Google Scholar]
- 14. Huth N, Wenkel RF, Roschanski N, et al. Prototheca zopfii genotype 2-induced nasal dermatitis in a cat. J Comp Pathol 2015; 152: 287–290. [DOI] [PubMed] [Google Scholar]
- 15. Kaplan W, Chandler FW, Holzinger EA, et al. Protothecosis in a cat: first recorded case. Sabouraudia 1976; 14: 281–286. [DOI] [PubMed] [Google Scholar]
- 16. Endo S, Sekiguchi M, Kishimoto Y, et al. The first case of feline Prototheca wickerhamii infection in Japan. J Vet Med Sci 2010; 72: 1351–1353. [DOI] [PubMed] [Google Scholar]
- 17. Ahrholdt J, Murugaiyan J, Straubinger RK, et al. Epidemiological analysis of worldwide bovine, canine and human clinical Prototheca isolates by PCR genotyping and MALDI-TOF mass spectrometry proteomic phenotyping. Med Mycol 2012; 50: 234–243. [DOI] [PubMed] [Google Scholar]
- 18. White T, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications. Innis M, Gelfand D, Sninsky J, et al. (eds). New York: Academic Press, 1990, pp 315–322. [Google Scholar]
- 19. Munday JS, Kiupel M, French AF, et al. Amplification of papillomaviral DNA sequences from a high proportion of feline cutaneous in situ and invasive squamous cell carcinomas using a nested polymerase chain reaction. Vet Dermatol 2008; 19: 259–263. [DOI] [PubMed] [Google Scholar]
- 20. Munday JS, Kiupel M. Papillomavirus-associated cutaneous neoplasia in mammals. Vet Pathol 2010; 47: 254–264. [DOI] [PubMed] [Google Scholar]
- 21. Munday JS, Kiupel M, French AF, et al. Detection of papillomaviral sequences in feline Bowenoid in situ carcinoma using consensus primers. Vet Dermatol 2007; 18: 241–245. [DOI] [PubMed] [Google Scholar]
- 22. Munday JS, Peters-Kennedy J. Consistent detection of Felis domesticus papillomavirus 2 DNA sequences within feline viral plaques. J Vet Diagn Invest 2010; 22: 946–949. [DOI] [PubMed] [Google Scholar]
- 23. Malik R, Vogelnest L, O’Brien CR, et al. Infections and some other conditions affecting the skin and subcutis of the naso-ocular region of cats – clinical experience 1987–2003. J Feline Med Surg 2004; 6: 383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayhall CG, Miller CW, Eisen AZ, et al. Cutaneous protothecosis. Successful treatment with amphotericin B. Arch Dermatol 1976; 112: 1749–1752. [DOI] [PubMed] [Google Scholar]
- 25. McMullan B, Pollett S, Biswas C, et al. Successful treatment of cutaneous protothecosis with liposomal amphotericin and oral itraconazole. Med Mycol Case Rep 2016; 12: 21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gremião I, Schubach T, Pereira S, et al. Treatment of refractory feline sporotrichosis with a combination of intralesional amphotericin B and oral itraconazole. Aust Vet J 2011; 89: 346–351. [DOI] [PubMed] [Google Scholar]
- 27. Somchit N, Norshahida AR, Hasiah AH, et al. Hepatotoxicity induced by antifungal drugs itraconazole and fluconazole in rats: a comparative in vivo study. Hum Exp Toxicol 2004; 23: 519–525. [DOI] [PubMed] [Google Scholar]