Abstract
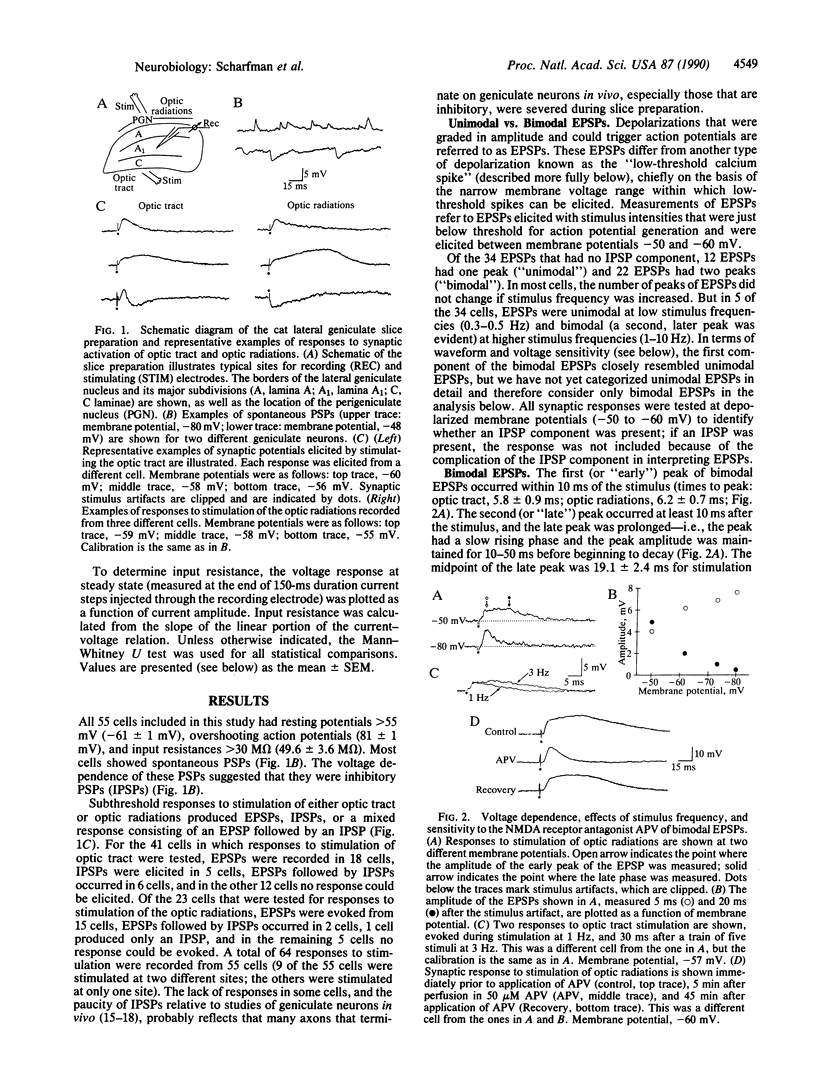
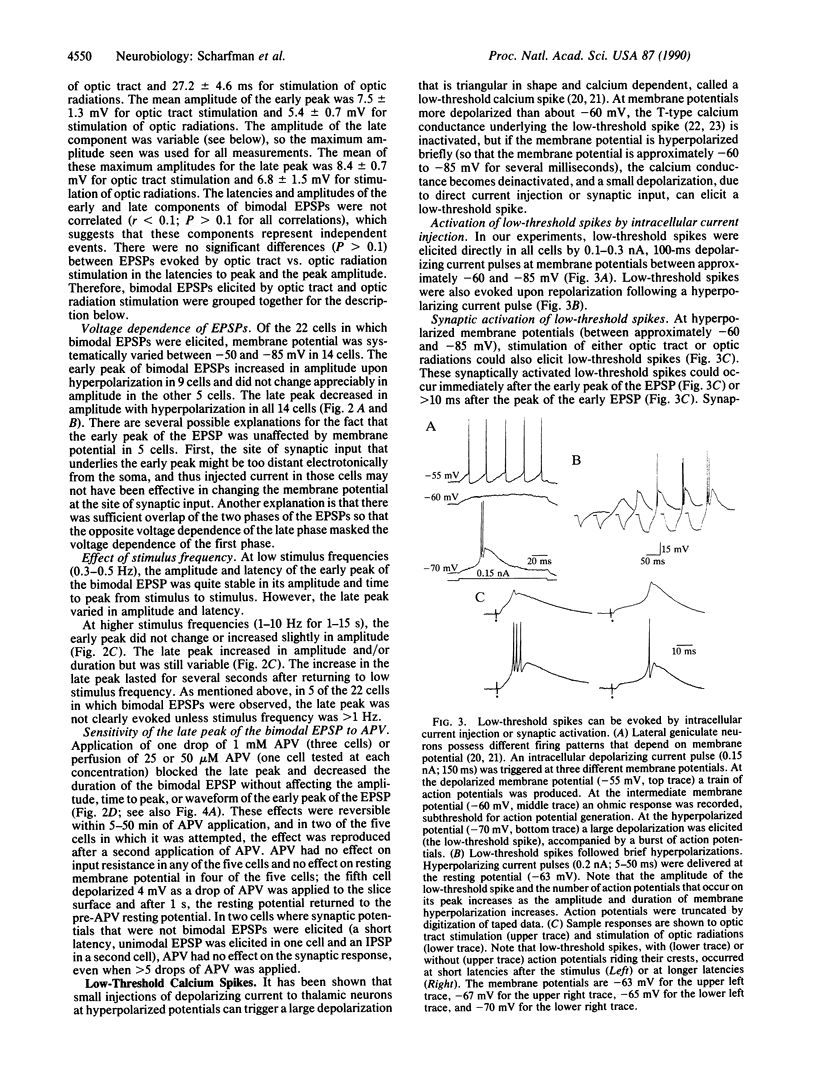
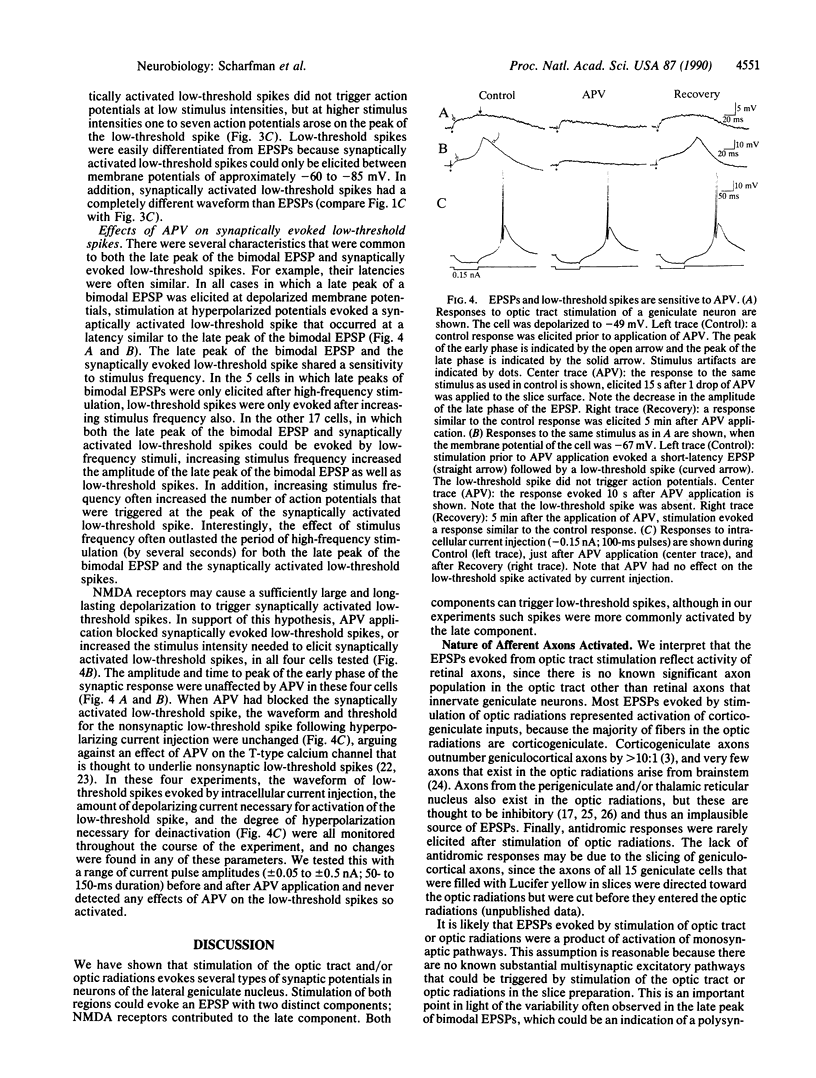
Neurons of the cat's dorsal lateral geniculate nucleus were recorded intracellularly to study the contribution of N-methyl-D-aspartate (NMDA) receptors to excitatory postsynaptic potentials (EPSPs) and low-threshold calcium spikes. EPSPs were evoked by stimulation of retinogeniculate axons in the optic tract and/or corticogeniculate axons in the optic radiations; EPSPs from both sources were similar. These EPSPs had one or two components, and the second component had several characteristics of NMDA receptor-mediated events. For example, EPSP amplitude decreased when neurons were hyperpolarized and increased when stimulus frequency was increased; these EPSPs could also be blocked reversibly by application of the selective NMDA receptor antagonist DL-2-amino-5-phosphonovaleric acid (APV). We also studied the influence of NMDA receptors on low-threshold calcium spikes, which are large, voltage- and calcium-dependent depolarizations that are often accompanied by high-frequency action potential discharge. APV blocked synaptically activated low-threshold calcium spikes, but APV had no effect on low-threshold calcium spikes that were elicited by current injection. Therefore, APV does not appear to have a direct effect on the T-type calcium channel that is involved in generation of low-threshold calcium spikes. The voltage and frequency dependence of the NMDA receptor-mediated component of the EPSPs, as well as its ability to trigger low-threshold calcium spikes, provide for complex signal processing in the lateral geniculate nucleus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bear M. F., Cooper L. N., Ebner F. F. A physiological basis for a theory of synapse modification. Science. 1987 Jul 3;237(4810):42–48. doi: 10.1126/science.3037696. [DOI] [PubMed] [Google Scholar]
- Bloomfield S. A., Sherman S. M. Postsynaptic potentials recorded in neurons of the cat's lateral geniculate nucleus following electrical stimulation of the optic chiasm. J Neurophysiol. 1988 Dec;60(6):1924–1945. doi: 10.1152/jn.1988.60.6.1924. [DOI] [PubMed] [Google Scholar]
- Cline H. T., Constantine-Paton M. NMDA receptor antagonists disrupt the retinotectal topographic map. Neuron. 1989 Oct;3(4):413–426. doi: 10.1016/0896-6273(89)90201-8. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J Physiol. 1988 May;399:301–312. doi: 10.1113/jphysiol.1988.sp017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Kelly J. S., Leresche N., Pirchio M. On the excitatory post-synaptic potential evoked by stimulation of the optic tract in the rat lateral geniculate nucleus. J Physiol. 1987 Mar;384:603–618. doi: 10.1113/jphysiol.1987.sp016472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Lightowler S., Pollard C. E. A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J Physiol. 1989 Jun;413:543–561. doi: 10.1113/jphysiol.1989.sp017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes M., Paradis M., Roy J. P., Steriade M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. J Neurophysiol. 1984 Jun;51(6):1196–1219. doi: 10.1152/jn.1984.51.6.1196. [DOI] [PubMed] [Google Scholar]
- Dubin M. W., Cleland B. G. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. J Neurophysiol. 1977 Mar;40(2):410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- Eysel U. T. Quantitative studies of intracellular postsynaptic potentials in the lateral geniculate nucleus of the cat with respect to optic tract stimulus response latencies. Exp Brain Res. 1976 Jul 28;25:469–486. doi: 10.1007/BF00239782. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J., Langmoen I. A. N-methyl-D-aspartate receptor antagonists reduce synaptic excitation in the hippocampus. J Neurosci. 1986 Jan;6(1):102–106. doi: 10.1523/JNEUROSCI.06-01-00102.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Cruz A., Pape H. C. Identification of two calcium currents in acutely dissociated neurons from the rat lateral geniculate nucleus. J Neurophysiol. 1989 Jun;61(6):1270–1283. doi: 10.1152/jn.1989.61.6.1270. [DOI] [PubMed] [Google Scholar]
- Hu B., Steriade M., Deschênes M. The effects of brainstem peribrachial stimulation on perigeniculate neurons: the blockage of spindle waves. Neuroscience. 1989;31(1):1–12. doi: 10.1016/0306-4522(89)90026-2. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kayama Y., Shimada S., Hishikawa Y., Ogawa T. Effects of stimulating the dorsal raphe nucleus of the rat on neuronal activity in the dorsal lateral geniculate nucleus. Brain Res. 1989 Jun 5;489(1):1–11. doi: 10.1016/0006-8993(89)90002-4. [DOI] [PubMed] [Google Scholar]
- Kemp J. A., Sillito A. M. The nature of the excitatory transmitter mediating X and Y cell inputs to the cat dorsal lateral geniculate nucleus. J Physiol. 1982 Feb;323:377–391. doi: 10.1113/jphysiol.1982.sp014078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström S. Synaptic organization of inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Brain Res. 1982 Feb 25;234(2):447–453. doi: 10.1016/0006-8993(82)90885-x. [DOI] [PubMed] [Google Scholar]
- Llinás R., Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982 Jun 3;297(5865):406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- Lo Fu-Sun, Murray Sherman S. Dependence of Retinogeniculate Transmission on Membrane Voltage in the Cat. Eur J Neurosci. 1989 May;1(3):204–209. doi: 10.1111/j.1460-9568.1989.tb00789.x. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol. 1987 Nov;392:147–165. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J Neurophysiol. 1988 Mar;59(3):978–996. doi: 10.1152/jn.1988.59.3.978. [DOI] [PubMed] [Google Scholar]
- Montero V. M., Singer W. Ultrastructure and synaptic relations of neural elements containing glutamic acid decarboxylase (GAD) in the perigeniculate nucleus of the cat. A light and electron microscopic immunocytochemical study. Exp Brain Res. 1984;56(1):115–125. doi: 10.1007/BF00237447. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Pape H. C., McCormick D. A. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989 Aug 31;340(6236):715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Singer W., Bedworth N. Inhibitory interaction between X and Y units in the cat lateral geniculate nucleus. Brain Res. 1973 Jan 30;49(2):291–307. doi: 10.1016/0006-8993(73)90424-1. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. A magnesium-sensitive post-synaptic potential in rat cerebral cortex resembles neuronal responses to N-methylaspartate. J Physiol. 1986 Jan;370:531–549. doi: 10.1113/jphysiol.1986.sp015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. M. Glycine modulation of the NMDA receptor/channel complex. Trends Neurosci. 1989 Sep;12(9):349–353. doi: 10.1016/0166-2236(89)90042-8. [DOI] [PubMed] [Google Scholar]
- Uhlrich D. J., Cucchiaro J. B., Sherman S. M. The projection of individual axons from the parabrachial region of the brain stem to the dorsal lateral geniculate nucleus in the cat. J Neurosci. 1988 Dec;8(12):4565–4575. doi: 10.1523/JNEUROSCI.08-12-04565.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



