Abstract
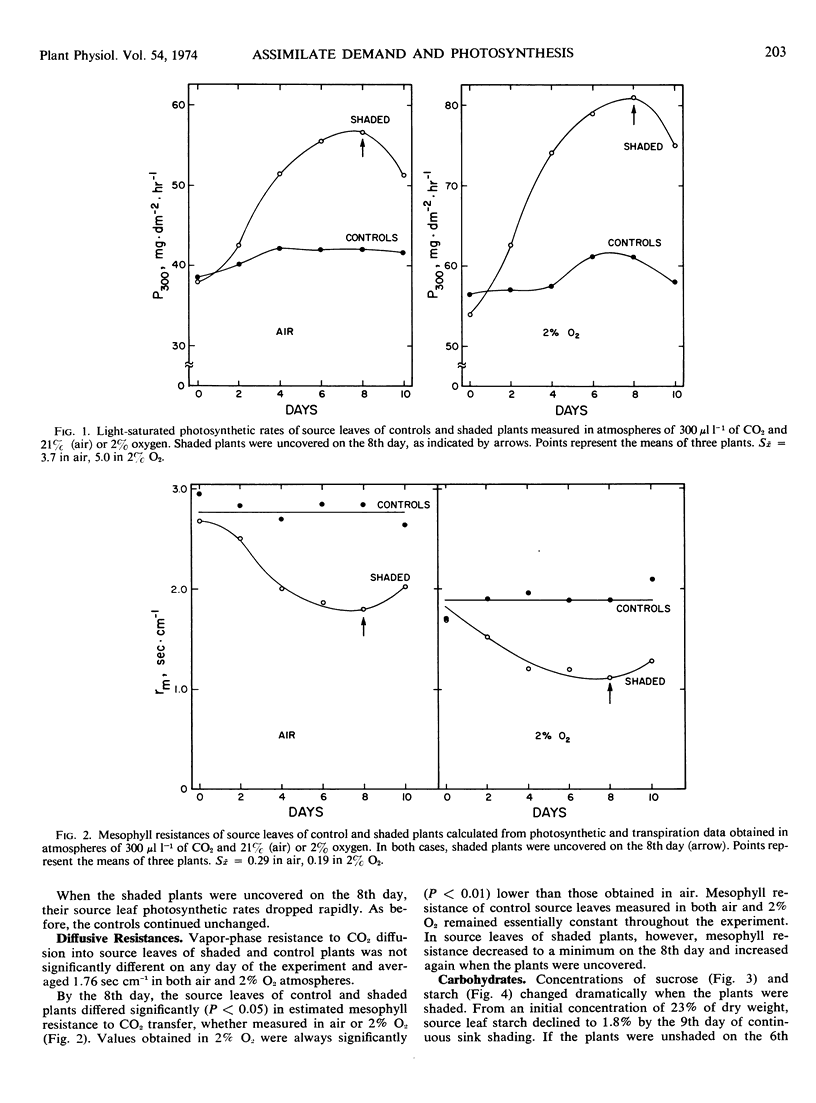
Rates of net photosynthesis and translocation, CO2 diffusive resistances, levels of carbohydrates, total protein, chlorophyll, and inorganic phosphate, and ribulose 1,5-diphosphate carboxylase activity were measured in soybean (Glycine max L. Merrill) leaves to ascertain the effect of altered assimilate demand. To increase assimilate demand, the pods, stems, and all but one leaf (the “source leaf”) of potted plants were completely shaded for 6 or 8 days and the responses of the illuminated source leaf were monitored. Rate of net photosynthesis in the source leaf of the shaded plants was found to increase curvilinearly to a maximum on the 8th day. The source leaf of the control plants (no sink shading) maintained a constant photosynthetic rate during this period. Vapor-phase resistance to CO2 diffusion did not vary with treatment, but mesophyll (liquid phase) resistance was significantly lower in the source leaf of the shaded plants.
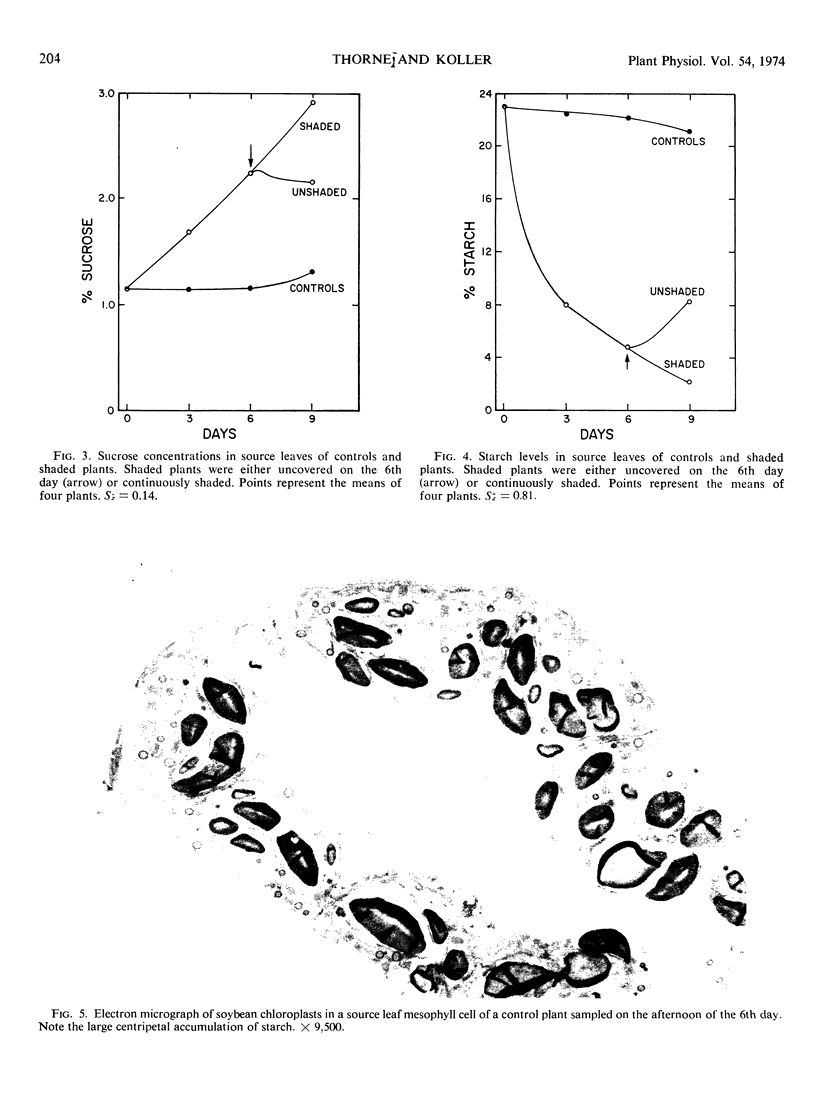
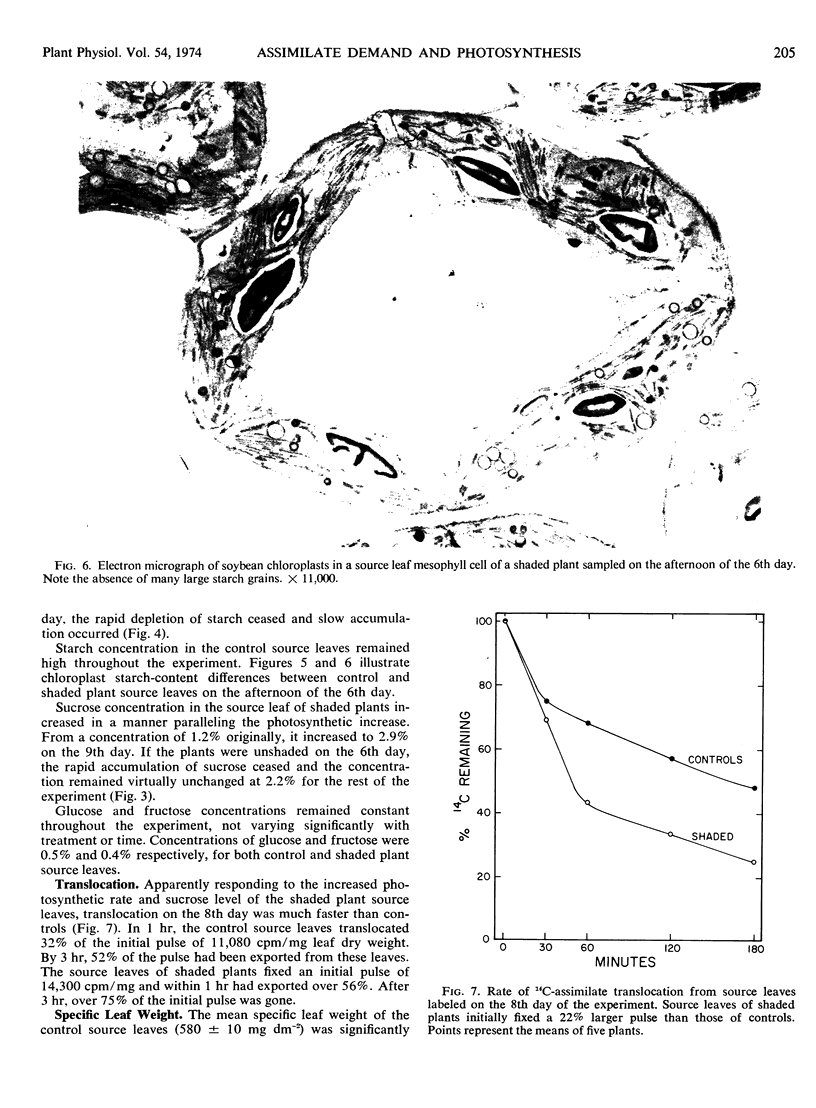
Starch concentration in the source leaf of shaded plants decreased more than 10-fold during the 8-day shading period. In this same period, sucrose concentration rose nearly 3-fold. Conversely, in the source leaf of the unshaded plants, starch concentration remained high (23% of leaf dry weight) and sucrose concentration remained very low (1.2%). When measured on the 8th day of treatment, translocation rate, ribulose 1,5-diphosphate carboxylase activity, and inorganic phosphate concentration were found to be significantly higher in the source leaf of the shaded plants than in the control source leaf.
When shaded plants were again illuminated, all measured response trends in the source leaf were reversed. These data indicate that assimilate demand has a marked influence on source-leaf photosynthesis and carbohydrate formation and export.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Giaquinta R. T., Sovonick S. A., Fellows R. J. Solute distribution in sugar beet leaves in relation to Phloem loading and translocation. Plant Physiol. 1973 Dec;52(6):585–589. doi: 10.1104/pp.52.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. R., Hodges T. K. Phosphorus metabolism of germinating oat seeds. Plant Physiol. 1966 Nov;41(9):1459–1464. doi: 10.1104/pp.41.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartt C. E., Kortschak H. P. Sugar Gradients and Translocation of Sucrose in Detached Blades of Sugarcane. Plant Physiol. 1964 May;39(3):460–474. doi: 10.1104/pp.39.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller H. R., Thorne J. H. An Apparatus to Produce Gas Mixtures with Controlled CO(2), O(2), and Water Vapor Concentrations. Plant Physiol. 1974 Jan;53(1):11–13. doi: 10.1104/pp.53.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Sanwal G. G., Greenberg E., Hardie J., Cameron E. C., Preiss J. Regulation of starch biosynthesis in plant leaves: activation and inhibition of ADPglucose pyrophosphorylase. Plant Physiol. 1968 Mar;43(3):417–427. doi: 10.1104/pp.43.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treharne K. J., Stoddart J. L., Pughe J., Paranjothy K., Wareing P. F. Effects of gibberellin and cytokinins on the activity of photosynthetic enzymes and plastid ribosomal RNA synthesis in Phaseolus vulgaris L. Nature. 1970 Oct 10;228(5267):129–131. doi: 10.1038/228129a0. [DOI] [PubMed] [Google Scholar]
- Upmeyer D. J., Koller H. R. Diurnal trends in net photosynthetic rate and carbohydrate levels of soybean leaves. Plant Physiol. 1973 May;51(5):871–874. doi: 10.1104/pp.51.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing P. F., Khalifa M. M., Treharne K. J. Rate-limiting processes in photosynthesis at saturating light intensities. Nature. 1968 Nov 2;220(5166):453–457. doi: 10.1038/220453a0. [DOI] [PubMed] [Google Scholar]