Abstract
Ex vivo transduction of human CD34+ hematopoietic stem/progenitor cells (hCD34+ HSPCs) and T lymphocytes is a key process that requires high efficiency and low toxicity to achieve effective clinical results. So far, several enhancers have been used to improve this process. Among them, Retronectin highly meliorates VSV-G and RD114-TR pseudotyped lentiviral vector delivery in hCD34+ HSPCs and T lymphocytes. However, Retronectin is expensive and requires pre-coating of culture dishes or bags before cell seeding, resulting in a cumbersome procedure. Recently, an alternative transduction adjuvant has been developed, named Vectofusin-1, whose effect has been demonstrated on gene delivery to cell lines and primary hCD34+ HSPCs by lentiviral vectors pseudotyped with different envelope glycoproteins. In this study, we have focused our analysis on the effect of Vectofusin-1 on the transduction of hCD34+ HSPCs and T lymphocytes by using mostly RD114-TR pseudotyped lentivectors and clinical transduction protocols. Here, we have proved that Vectofusin-1 reproducibly enhances gene delivery to hCD34+ HSPCs and activated T cells without cell toxicity and with efficacy comparable to that of Retronectin. The use of Vectofusin-1 will therefore help to shorten and simplify clinical cell manipulation, especially if automated systems are planned for transducing large-scale clinical lots.
Keywords: lentiviral vector, transduction, HSC, T lymphocytes, additive, peptide
Introduction
Manipulation of human CD34+ (hCD34+) hematopoietic stem/progenitor cells (HSPCs) and T lymphocytes is a key process that dramatically influences the outcome of ex vivo gene therapy applications. In this context, MolMed has set up specific clinical protocols for transducing these human cell types with both lentiviral (LV) and gamma retroviral (RV) vectors.1, 2, 3, 4 In both systems, CD34+ HSPCs and T lymphocytes are genetically modified in the presence of fibronectin fragment FN CH-296, named Retronectin, which is a recombinant polypeptide that facilitates co-localization of vectors and cells expressing VLA-4 or VLA-5 integrins.5 Although this peptide is very efficient in enhancing vector transduction, it is also expensive and requires pre-coating of cell culture dishes or bags before cell seeding, rendering the transduction process rather complicated and tedious.6, 7
Vectofusin-1 is a new histidine-rich cationic amphipathic short peptide derived from the LAH4 peptide family,8 which promotes HSPC transduction with various retroviral and lentiviral pseudotypes.9 Vectofusin-1 is a soluble additive that acts at the entry step by promoting adhesion and fusion between vector and cellular membranes.9, 10 Its efficacy was previously demonstrated on transduction of hCD34+ HSPCs with GALV-TR, RD114-TR, MLV-A, and VSV-G pseudotyped LVs. In contrast, its effect is negligible or even absent on transduction of T cells with VSV-G pseudotyped LVs.11
RD114-TR chimeric envelope, derived from feline endogenous leukemia retrovirus, represents an interesting molecule to pseudotype LVs. Its intrinsic characteristics, i.e., lack of cellular toxicity under constitutive expression and high stability at room temperature after freezing-and-thawing cycles and after vector concentration, make its use suitable for both ex vivo and in vivo gene delivery approaches.12, 13 On this basis, we exploited these unique features of RD114-TR to develop MolMed’s proprietary RD-MolPack technology for stable and continuous production of both second- and third-generation LVs.14, 15
Here, we have further characterized the effect of Vectofusin-1 on transduction of human CD34+ HSPCs and primary T cells using clinical processes validated with VSV-G LVs, but not yet with RD114-TR LVs, in which Retronectin is used as the gold standard. We have also compared in T cells the action of Vectofusin-1 with that of other enhancers, i.e., polybrene and protamine sulfate, which are cationic additives that function by neutralizing membrane charges and promoting virus aggregation.16, 17
Results
Effect of Vectofusin-1 on the Transduction of Human CD34+ Cells by LVs Pseudotyped with Either VSV-G or RD114-TR Envelope
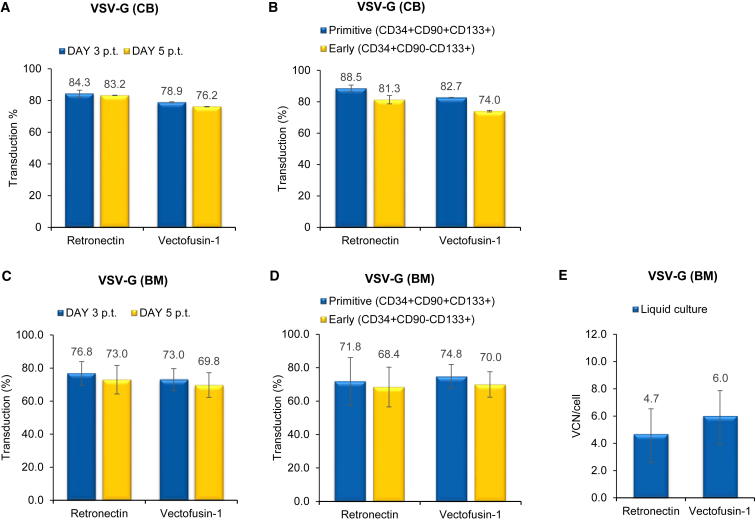
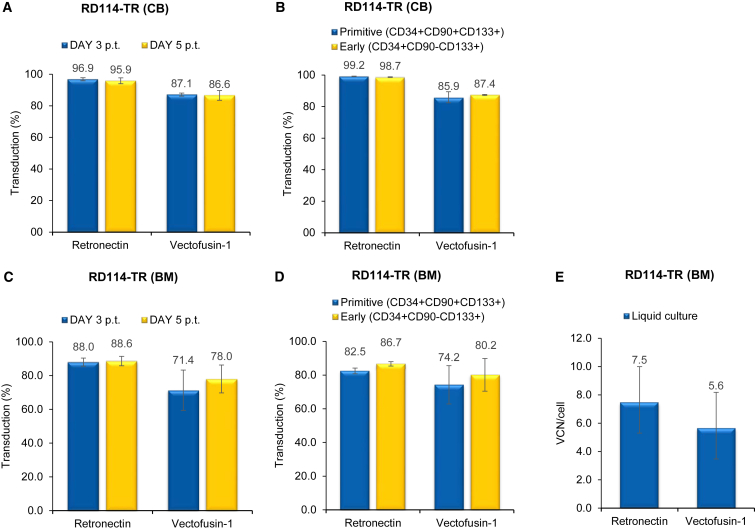
We compared the effect of Vectofusin-1 and Retronectin by transducing human cord blood (CB)- and bone marrow (BM)-derived CD34+ HSPC cells derived from one and three different healthy donors, respectively, with two types of self-inactivating (SIN) LVs expressing GFP transgene. The first was a highly purified VSV-G LV, obtained by large-scale transient transfection and subsequent anion exchange and gel filtration chromatography. The second was an RD114-TR LV produced by RD3-MolPack-GFP stable producer cells and concentrated 100-fold by ultracentrifugation.15 We initially applied a clinical validated protocol of transduction1, 2 used for VSV-G LV consisting of MOI 160 for CB-derived CD34+ HSPCs and MOI 200 for BM-derived CD34+ HSPCs (two hits of 80 and 100 each, respectively), with the exception that 96-well plates were used instead of bags. RD114-TR LVs were used at MOI 8 (two hits of four each) based on our previous observations.8, 9, 14 CD34+ HSPCs were activated for 24 hr with a cocktail of cytokines (stem cell factor [SCF], FMS-like tyrosine kinase 3 ligand [FLT-3L], thrombopoietin [TPO], and interleukin 3 [IL-3]) and then transduced with either Retronectin or Vectofusin-1. Retronectin was pre-coated in 96-well plates for 2 hr, whereas Vectofusin-1 was mixed with LVs just before transduction. We observed that Vectofusin-1 was as efficient as Retronectin in promoting a high level of gene transfer in hCD34+ HSPCs. The level of transduction either on total CD34+ HSPC populations after 3 and 5 days (Figures 1A, 1C, 2A, and 2C) or on primitive (CD34+CD133+CD90+) or early progenitor (CD34+CD133+CD90−) subpopulations after 3 days (Figures 1B and 2B) was comparable for both enhancers (n.s., p > 0.05).
Figure 1.
Comparison of the Effect of Vectofusin-1 versus That of Retronectin on Transduction of VSV-G LVs in CD34+ HSPCs
(A) CB-derived human CD34+ cells were transduced with purified VSV-G-pseudotyped SIN-GFP LVs at MOI 160 (two hits at MOI 80). GFP expression was analyzed 3 and 5 days post transduction (p.t.) by FACS analysis. Values are the mean ± SEM of n = 3 independent transductions. (B) Percentage of GFP+/primitive (CD34+CD90+CD133+) and early (CD34+CD90−CD133+) progenitor cells 3 days p.t. evaluated by FACS analysis. Values are the mean ± SEM of n = 3 independent transductions. (C) FACS analysis of GFP expression analyzed 3 and 5 days after transduction of BM-derived human CD34+ HSPCs transduced with VSV-G pseudotyped SIN-GFP LVs at MOI 200 (two hits at MOI 100). Values are the mean ± SEM of n = 4 independent transductions. (D) FACS analysis of GFP+ expression in primitive (CD34+CD90+CD133+) and early (CD34+CD90−CD133+) progenitor cells 3 days after transduction. Values are the mean ± SEM of n = 4 independent transductions. (E) Quantification of SIN-GFP VCN/cell calculated on liquid culture. Each sample was evaluated by Q-PCR in triplicate.
Figure 2.
Comparison of the Effect of Vectofusin-1 versus That of Retronectin on Transduction Efficiency of RD3-MolPack-GFP LV in CD34+ HSPCs
(A) CB-derived human CD34+ cells were transduced with unpurified RD114-TR-pseudotyped SIN-GFP LVs at MOI 8 (two hits at MOI 4), and GFP expression was analyzed 3 and 5 days after transduction by FACS analysis. Values are the mean ± SEM of n = 3 independent transductions. (B) Percentage of GFP+/primitive (CD34+CD133+CD90+) and early (CD34+CD133+CD90−) progenitor cells 3 days after transduction evaluated by FACS analysis. Values are the mean ± SEM of n = 3 independent transductions. (C) BM-derived human CD34+ cells were transduced with unpurified RD114-TR pseudotyped SIN-GFP LVs at MOI 8 (two hits at MOI 4), and GFP expression was analyzed 3 and 5 days after transduction by FACS analysis. Values are the mean ± SEM of n = 4 independent transductions. (D) Percentage of GFP+/primitive (CD34+CD90+CD133+) and early (CD34+CD90−CD133+) progenitor cells 3 days after transduction evaluated by FACS analysis. Values are the mean ± SEM of n = 4 independent transductions. (E) Quantification of SIN-GFP VCN/cell calculated on liquid culture. Each sample was evaluated by Q-PCR in triplicate.
We also evaluated cell toxicity secondary to transduction and differentiation potential of CD34+ HSPCs by carrying out an in vitro colony-forming cell (CFC) assay. Cells exposed to Vectofusin-1 did not display toxicity in terms of colony growth (Table S1). We observed a slight increase in the number of colonies in only CB-derived cells transduced with VSV-G pseudotyped LV, whereas in all other conditions and in BM-derived cells, no difference was relevant (Table S1). Furthermore, the average vector copy number (VCN), measured by quantitative PCR (Q-PCR), in cells kept in liquid culture for 14 days was comparable in samples transduced with Vectofusin or Retronectin (Figures 1E and 2E). Taken together, these results indicate that the ability of Vectofusin-1 to enhance hCD34+ HSPCs transduction is similar to that of Retronectin.
Effect of Vectofusin-1 on the Transduction of Human T Lymphocytes by LVs Pseudotyped with RD114-TR Envelope
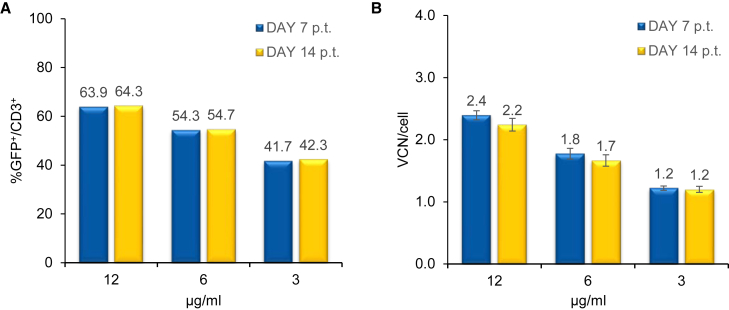
Next, we investigated the efficiency of Vectofusin-1 in promoting ex vivo transduction of human activated T cells with LV batches obtained by RD3-MolPack-GFP producer cells.15 Primary T cells were activated for 48 hr by either CD3/CD28 Dynabeads or interleukin (IL)-7 and IL-15 cytokine stimulation or by OKT-3 and IL-2 activation. Based on the knowledge that concentrations of Vectofusin-1 above 15 μg/mL can lead to substantial cell death,9 we tested three concentrations below this value to define the optimal amount of Vectofusin-1 necessary for efficient gene transfer into T lymphocytes. LV supernatants (MOI = 1) were mixed with 3, 6, and 12 μg/mL of Vectofusin-1 and immediately added to cell suspension. 18 hours after transduction, cell suspension was diluted from 1 × 106 cells/mL to 3 × 105 cells/mL and cultivated until the transduction level was monitored by FACS analysis. The optimal concentration of Vectofusin-1 was defined as 12 μg/mL in terms of both GFP positivity (Figure 3A) and VCN detected into target cells (Figure 3B). This amount of Vectofusin-1 is consistent with that used on hCD34+ HSPCs.9
Figure 3.
Determination of the Optimal Concentration of Vectofusin-1 for T Cell Transduction
(A) Percentage of CD3+/GFP+ cells was evaluated 7 and 14 days p.t. at MOI 1 by FACS analysis. (B) SIN-GFP VCN/cell was evaluated by Q-PCR in triplicate.
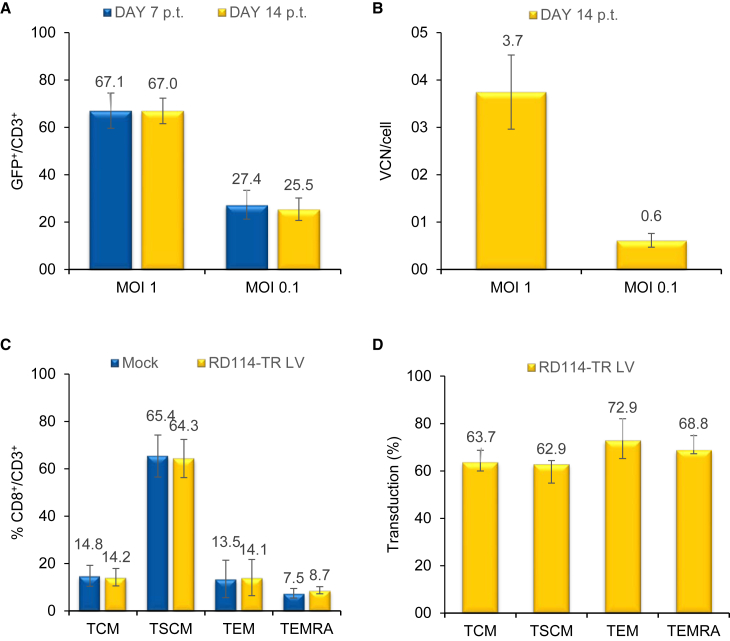
We then assessed the activity of Vectofusin-1 on T cell transduction using a different MOI of RD114-TR pseudotyped LVs. 14 days after transduction, the mean percentage of GFP+/CD3+ cells was 67% at MOI 1 and 25% at MOI 0.1, and the average VCN/cell was 3.7 and 0.6, respectively (n = 3) (Figures 4A and 4B). Importantly, Vectofusin-1 did not affect the T cell differentiation phenotype, which was similar to that of mock-transduced cells (Figure 4C; n.s., p > 0.05). Furthermore, CD3+CD8+ T cell memory subpopulations, T Central Memory (TCM, CD45RA−CD62L+), T Stem Cell Memory (TSCM, CD45RA+CD62L+CD95+), T Effector Memory (TEM, CD45RA−CD62L−), and T Effector Memory RA+ (TEMRA, CD45RA+CD62L−) were transduced with comparable efficiencies (64%, 63%, 73%, and 69%, respectively) (Figure 4D, n.s., p > 0.05). Moreover, the addition of Vectofusin-1 did not alter cellular viability, expansion, and the CD3+ percentage (Table 1).
Figure 4.
Transduction Efficiency and Memory Phenotype of T Cells Transduced with RD3-MolPack-GFP LVs in the Presence of Vectofusin-1
(A) T lymphocytes were transduced with RD114-TR-pseudotyped SIN-GFP LVs (MOI 1 and 0.1) in the presence of 12 μg/mL Vectofusin-1, and the transduction efficiency was analyzed after 7 and 14 days by FACS analysis. (B) SIN-GFP VCN/cell was quantified 14 days p.t. by Q-PCR. Values in (A) and (B) are the mean ± SEM of n = 7 (MOI 1) and n = 4 (MOI 0.1) independent transductions. (C) Relative frequency of CD3+CD8+ TCM (CD45RA−CD62L+), TSCM (CD45RA+CD62L+CD95+), TEM (CD45RA−CD62L−), and TEMRA (CD45RA+CD62L−) 1 day p.t. in mock-transduced cells versus RD114-TR-pseudotyped SIN-GFP LV-transduced cells (MOI 1) in the presence of 12 μg/mL Vectofusin-1. (D) Percentage of GFP+ cells among TCM, TSCM, TEM, and TEMRA subtypes. Values in (C) and (D) are the mean ± SEM of n = 3 independent transductions.
Table 1.
Lack of Toxicity of Vectofusin-1 on T Cells Transduced with RD3-MolPack LV
| Nonea | Vectofusin-1a | |
|---|---|---|
| Cell growth fold increase (day 16 versus 0) | 106.9 ± 20.9 | 101.2 ± 16.74 |
| Cell viability (%) | >98 | >98 |
| CD3+ | 99.6 ± 0.08 | 99.5 ± 0.06 |
Values are the mean ± SEM of three independent transductions calculated at 16 days post transduction.
MOI = 1
Comparison of the Effect of Vectofusin-1 with Other Transduction Enhancers in T Lymphocytes
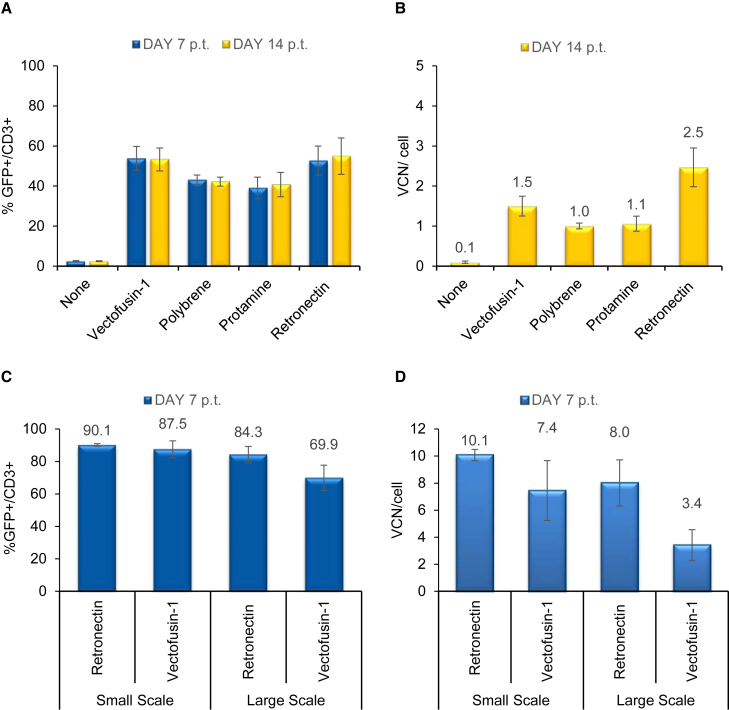
We next compared the effect of Vectofusin-1 with that of other culture additives, i.e., Retronectin, polybrene, and protamine sulfate, on T lymphocytes transduced with RD3-MolPack-GFP LVs. Vectofusin-1 showed a similar adjuvant capability as polybrene, Retronectin, and protamine sulfate when RD114-TR pseudotyped LV was used at MOI 1. 7 days after transduction, the percentage of CD3+/GFP+ was 53%, 42%, 41%, and 55%, respectively (Figure 5A; Vectofusin-1 versus protamine, p < 0.05). Gene transfer efficiency 7 and 14 days after transduction was equal when the same experimental protocol was used (Figure 5A). The VCN calculated 14 days after transduction at MOI 1 was comparable in cells treated with either Vectofusin-1 or other adjuvants (Figure 5B, n.s., p > 0.05).
Figure 5.
Comparison of the Effect of Vectofusin-1 versus Different Enhancers in a Static Small- and Large-Scale Transduction Protocol Using RD3-MolPack-GFP LV in T Cells
(A) T cells were transduced with RD114-TR pseudotyped SIN-GFP LVs (MOI 1) in the absence (none) or in the presence of either soluble Vectofusin-1 (12 μg/mL), polybrene (8 μg/mL), protamine sulfate (4 μg/mL), or Retronectin-coated dishes (1.2 μg/cm2) (n = 3). GFP expression in CD3+ T cells was evaluated 7 and 14 days p.t. (B) Average VCN/cell, quantified by Q-PCR, was evaluated 14 days p.t. in T cells transduced at MOI 1. Transduction efficiency (C) and VCN (D) of T cells transduced with RD114-TR-pseudotyped SIN-GFP LVs (MOI = 1) in a 24-well plate or bag with Vectofusin-1 or Retronectin (n = 3).
We next tested Vectofusin-1 in a large-scale T lymphocyte transduction protocol developed by MolMed SpA for the retroviral-vector-based TK008 clinical trial (NCT00914628) and applied it to RD114-TR LV transduction. Primary T cells were activated for 48 hr by OKT-3 and IL-2 and were then transduced in either small- or large-scale settings. Comparison between small- (24-well plate) and large-scale (cell culture bag) transduction showed that Vectofusin-1 enhances T lymphocyte transduction in both scales (87.5% ± 5.2 in small scale versus 69.9 ± 7.9 in large scale, n.s. p > 0.05) (Figure 5C), even if integration efficiency is slightly lower in large scale (n.s. p > 0.05) (Figure 5D).
In addition, we evaluated the effect of spinoculation, which consists of the centrifugal inoculation of vectors into target cells, on the enhancer effect of Vectofusin-1 in comparison to that of polybrene. We carried out transduction of activated primary T cells with RD3-MolPack-GFP LVs at MOI 0.1 and 1 in the presence of either Vectofusin-1 or polybrene by static or centrifugal vector inoculation. Of interest, we observed that spinoculation did not augment the percentage of Vectofusin-1-treated GFP+/CD3+ cells; rather, it slightly decreased both the GFP MFI and SIN-GFP VCN. In contrast, as expected, in the presence of polybrene, both transduction efficiency (% of GFP+/CD3+ cells and MFI of GFP expression) and VCN were positively affected by spinoculation (Table 2). Altogether, these data indicate that Vectofusin-1 is a key adjuvant for clinical transduction protocols, in which spinoculation is usually not applied.
Table 2.
Effect of Spinoculation on Transduction Efficiency
| Ratio Spin/No Spin | Polybrene (n = 2) | Vectofusin-1 (n =3) |
|---|---|---|
| GFP+/CD3+ cells (%) | 1.81 ± 0.18 | 0.91 ± 0.04 |
| MFI GFP+ cells | 1.25 ± 0.02 | 0.86 ± 0.09 |
| VCN/cell | 2.52 ± 0.06 | 0.68 ± 0.09 |
Values are the mean ± SEM of n = 2 or n = 3 independent transductions calculated at 14 days post transduction.
Discussion
To date, most clinical gene therapy protocols involving hCD34+ HSPCs use the VSV-G envelope and include Retronectin as adjuvant to facilitate the interaction between vectors and cells. In this context, Vectofusin-1 has been previously proven to be as efficient as other additives in enhancing transduction of hCD34+ HSPCs with LVs pseudotyped with either VSV-G or other envelopes, i.e., GALV-TR, RD114-TR, and MLV-A.9 In this study, we have confirmed that in hCD34+ HSPCs, Vectofusin-1 is as effective as Retronectin in enhancing transduction of both VSV-G and RD114-TR pseudotyped LVs, without showing any sign of toxicity using a clinical transduction protocol.
Vectofusin-1 shows several advantages as compared to Retronectin. First, Vectofusin-1 can be added directly to the transduction mixture, therefore avoiding the pre-coating step that is indeed required for Retronectin. Second, Vectofusin-1 is also efficacious in closed bags following a static transduction process, which is normally used in a clinical protocol. Third, water solubility of Vectofusin-1 makes its manufacturing easier, opening up the possibility to a fully automated transduction protocol in good manufacturing practice (GMP) conditions. Automation of transduction is, in fact, mandatory when a large number of patients are treated, e.g., in immunotherapy approaches for hematologic malignancies.18, 19 For instance, the EF-C peptide, another transduction enhancer, has to be dissolved in DMSO, which, although used, is not preferable to water for clinical settings.20
Moreover, Vectofusin-1 significantly improves gene delivery in activated human T cells with RD114-TR pseudotyped LVs. Gene transfer level in terms of both GFP expression and integrated VCN is higher with Vectofusin-1 as compared to conditions in the absence of any adjuvant. In addition, the presence of Vectofusin-1 did not alter viability, expansion, and phenotype of T cells, therefore indicating lack of toxicity of the compound on these cells.
Like Retronectin, other cationic additives, such as polybrene and protamine sulfate, enhance transduction efficiency by promoting vector adhesion to the plasma membrane of target cells via neutralization of membrane charges. Similarly, it was recently shown that Vectofusin-1 acts on cell adhesion and the cell-vector membranes fusion step.9, 10
Here, we have demonstrated that Vectofusin-1 displays a similar adjuvant capability as Retronectin, polybrene, and protamine sulfate in T lymphocyte transduction protocols.
In conclusion, Vectofusin-1, being able to promote hCD34+ HSPC and T cell gene transfer without toxicity, needs consideration as an excellent reagent for LV transduction of primary cells in simple and safe large-scale clinical processes.
Materials and Methods
Cells and Reagents
CB-derived human CD34+ HSPCs were either isolated from umbilical cord blood (UCB) of healthy donors (Ospedale San Raffaele, OSR, prot. n. 347/DG, RD-MolPack 13/07/09) or purchased from Lonza Group as purified cells. BM-derived human CD34+ HSPCs were purchased from Lonza Group as purified cells. To isolate mononucleated cells, CB samples were centrifuged on a Ficoll-Hypaque gradient (Lymphoprep, StemCell Technologies). CD34+ HSPCs were then purified from the mononucleated cells by positive selection using the CD34 MicroBeads Kit and MiniMACS Separator Columns (Miltenyi Biotec GmbH). CD34+ HSPCs purity was >97%, as established by FACS analysis (FACS Canto II, BD Bioscience) using anti-CD34-phycoerythrin (PE) antibody (BD PharMingen) and the FACS Diva software (BD Bioscience).
Human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of healthy donors (Ospedale San Raffaele, OSR) after centrifugation on a Ficoll-Hypaque gradient (Lymphoprep, StemCell Technologies) and then frozen in aliquots of 5 × 107 cells/vial.
Vectofusin-1 was produced by standard fluorenyl-methyloxy-carbonyl (Fmoc) chloride solid-phase peptide synthesis, purified by preparative reverse phase high-performance liquid chromatography (HPLC), and analyzed by HPLC and mass spectrometry (Genecust). Retronectin was obtained from Takara Bio, and polybrene and protamine sulfate were obtained from Sigma Aldrich.
Clonogenic Assay
Colony forming cell (CFC) assay was performed at the end of the transduction process by seeding 500 cells/dish in quadruplicate in MethoCult 35-mm dishes (Stem Cell Technologies) for each condition. 14 days after transduction, total colonies were counted.
LV Production, Concentration, and Titration
Different lots of LV, pseudotyped with either VSV-G or RD114-TR envelope, were produced by transient and stable RD-MolPack technology, respectively. The characteristics of each lot are reported in Table S2.
Human CD34+ HSPC Transduction
The validated clinical protocol for Retronectin-mediated transduction of hCD34+ HSPCs was adapted to small scale as follows. Cells were seeded at 1 × 106 cells/mL on Retronectin-coated 96-well plates (1.2 μg/cm2) and activated for 24 hr in CellGro medium containing SCF (300 ng/mL), FLT-3L (300 ng/mL), TPO (100 ng/mL), and IL-3 (60 ng/mL) (medium and cytokines were purchased from CellGenix). Activated cells were then transduced with a first hit of LVs at the indicated MOI. After 14 hr, fresh complete medium was replaced and cells were incubated at 37°C in 5% CO2 for a 10-hr resting phase. Then, cells underwent a second hit of transduction at the indicated MOI in the same conditions of the first one.
Vectofusin-1-based transduction protocol was performed by pre-activating CD34+ HSPCs in standard 96-well plates for 24 hr, as described for the Retronectin-mediated transduction. The next day, cells were incubated with fresh complete medium and LV (first hit, MOI indicated in each experiment), which was previously mixed for a maximum of 15 min with Vectofusin-1 (12 μg/mL). 14 hours after incubation, cells were treated as described for the Retronectin protocol (resting phase and second hit of transduction). A liquid culture was kept for 14 days after transduction to evaluate the VCN by Q-PCR.
Human T Lymphocyte Transduction
PBMCs were activated for 48 hr with either the Dynabeads Human T-Expander CD3/CD28 (Thermo Fisher) or OKT-3 (Miltenyi Biotec) in RPMI medium 10% fetal calf serum (FCS) containing IL-7 and IL-15 (5 ng/mL each) (Miltenyi Biotec) or IL-2 (600 IU/mL) (Novartis), respectively. A Retronectin-based protocol was carried out by pre-coating Retronectin for 2 hr in 96-well plates (1.2 μg/cm2) and then pre-incubating LVs on the plate at 37°C in 5% CO2 for 1 hr. Cells were then seeded at a 1 × 106 cells/mL density, and, after overnight incubation at 37°C in 5% CO2, transduction was stopped by the addition of fresh complete medium. Where indicated, Vectofusin-1 (12 μg/mL concentration, unless otherwise indicated) was mixed with LVs for a maximum of 15 min before incubation with cells. In selected experiments, cells were transduced in the presence of polybrene (8 μg/mL) or protamine sulfate (4 μg/mL). For large-scale experiments, PBMCs were activated for 48 hr with OKT-3 in X-VIVO 15 medium and 3% human plasma (Plasmasafe, Kedrion) containing IL-2 (600 IU/mL). 20 million activated cells were seeded in VueLife Cell Culture Bags (CellGenix) at a density of 1 × 106 cells/mL and transduced with LVs pre-mixed with Vectofusin-1 (12 μg/mL).
VCN Determination by Q-PCR
SIN-GFP VCN was measured by Q-PCR using the ABI Prism 7900HT FAST Real-Time PCR system (Applied Biosystems) and SDS 2.3 software (Applied Biosystems). DNA was extracted using lysis buffer (10 mM Tris-HCl pH 8, 0.1% lauryl ether) and proteinase K (100 μg/mL). Each sample was run in triplicates. The standard amplification curve was obtained by serial dilutions of cellular lysate of CEM A3.01 cells carrying one copy of the pCCL-sin.PPT.hPGK.GFP.WPRE transfer vector, which was kindly provided by L. Naldini (TIGET, OSR). The curve covers the range from 7.5 × 104 to 4.8 copies (R2 > 0.99, slope range from −3.7 to −3.2). The sequences of primer and probe sets are reported in Table S2. The conditions of the Q-PCR were the following: 2 min at 50°C and 15 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C.
Flow Cytometry Analysis
The phenotype and efficiency of transduction of hCD34+ HSPCs and T lymphocytes were analyzed by Flow Cytometry (FACS Canto II Instrument and DIVA software). hCD34+ HSPCs were stained with PE-conjugated anti-CD90 antibody (BD PharMingen), VioBlue-conjugated anti-CD34 antibody (Miltenyi Biotec), and antigen-presenting cell (APC)-conjugated anti-CD133 antibody (Miltenyi Biotec).
PBMCs were stained with anti-CD3 monoclonal antibody before and after activation (Miltenyi Biotec). In selected experiments, T cell memory phenotype was detected by staining with monoclonal antibodies specific for CD3 (VioGreen-conjugated), CD8 (APC-Vio770-conjugated), CD45RA (PE-Vio770-conjugated), CD62L (VioBlue-conjugated), and CD95 (APC-conjugated) (Miltenyi Biotec). For both cell types, cell mortality and transduction efficiency were evaluated by 7-AAD labeling (BD PharMingen) and by the level of GFP expression, respectively.
Statistical Analysis
Mean values ± SEM are given, unless otherwise stated. The values were compared by using the paired Student t test. p ≤ 0.05 is considered significant.
Author Contributions
Conceptualization, C. Bovolenta, D.F., and A.G.; Investigation, C.P., V.M., C.S., S.C., E.G., and S.B.; Writing – Original Draft, C. Bovolenta, and C.P.; Writing – Review & Editing, C. Bovolenta, D.F., C.P., and A.G.; Funding Acquisition, C. Bordignon; Supervision, C. Bovolenta, G.P.R., and C. Bordignon.
Conflicts of Interest
C.P., V.M., C.S., S.C., E.G., M.P., S.B., C. Bordignon, G.P.R., and C. Bovolenta are employees of MolMed S.p.A.
Acknowledgments
We wish to thank Ian Johnston (Miltenyi Biotec) for providing us Vectofusin and Christophe Poquet (Genethon) for discussion of the results.
Footnotes
Supplemental Information includes two tables and can be found with this article online at http://dx.doi.org/10.1016/j.omtm.2017.02.003.
Supplemental Information
References
- 1.Aiuti A., Biasco L., Scaramuzza S., Ferrua F., Cicalese M.P., Baricordi C., Dionisio F., Calabria A., Giannelli S., Castiello M.C. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 3.Bonini C., Ferrari G., Verzeletti S., Servida P., Zappone E., Ruggieri L., Ponzoni M., Rossini S., Mavilio F., Traversari C. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 4.Ciceri F., Bonini C., Stanghellini M.T., Bondanza A., Traversari C., Salomoni M., Turchetto L., Colombi S., Bernardi M., Peccatori J. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 5.Murray L., Luens K., Tushinski R., Jin L., Burton M., Chen J., Forestell S., Hill B. Optimization of retroviral gene transduction of mobilized primitive hematopoietic progenitors by using thrombopoietin, Flt3, and Kit ligands and RetroNectin culture. Hum. Gene Ther. 1999;10:1743–1752. doi: 10.1089/10430349950017428. [DOI] [PubMed] [Google Scholar]
- 6.Lee J., Sadelain M., Brentjens R. Retroviral transduction of murine primary T lymphocytes. Methods Mol. Biol. 2009;506:83–96. doi: 10.1007/978-1-59745-409-4_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millington M., Arndt A., Boyd M., Applegate T., Shen S. Towards a clinically relevant lentiviral transduction protocol for primary human CD34 hematopoietic stem/progenitor cells. PLoS ONE. 2009;4:e6461. doi: 10.1371/journal.pone.0006461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenard D., Genries S., Scherman D., Galy A., Martin S., Kichler A. Infectivity enhancement of different HIV-1-based lentiviral pseudotypes in presence of the cationic amphipathic peptide LAH4-L1. J. Virol. Methods. 2013;189:375–378. doi: 10.1016/j.jviromet.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Fenard D., Ingrao D., Seye A., Buisset J., Genries S., Martin S., Kichler A., Galy A. Vectofusin-1, a new viral entry enhancer, strongly promotes lentiviral transduction of human hematopoietic stem cells. Mol. Ther. Nucleic Acids. 2013;2:e90. doi: 10.1038/mtna.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingrao D., Majdoul S., Seye A.K., Galy A., Fenard D. Concurrent measures of fusion and transduction efficiency of primary CD34+ cells with human immunodeficiency virus 1-based lentiviral vectors reveal different effects of transduction enhancers. Hum. Gene Ther. Methods. 2014;25:48–56. doi: 10.1089/hgtb.2013.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majdoul S., Seye A.K., Kichler A., Holic N., Galy A., Bechinger B., Fenard D. Molecular determinants of Vectofusin-1 and its derivatives for the enhancement of lentivirally mediated gene transfer into hematopoietic stem/progenitor cells. J. Biol. Chem. 2016;291:2161–2169. doi: 10.1074/jbc.M115.675033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandrin V., Boson B., Salmon P., Gay W., Nègre D., Le Grand R., Trono D., Cosset F.L. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood. 2002;100:823–832. doi: 10.1182/blood-2001-11-0042. [DOI] [PubMed] [Google Scholar]
- 13.Strang B.L., Ikeda Y., Cosset F.L., Collins M.K., Takeuchi Y. Characterization of HIV-1 vectors with gammaretrovirus envelope glycoproteins produced from stable packaging cells. Gene Ther. 2004;11:591–598. doi: 10.1038/sj.gt.3302189. [DOI] [PubMed] [Google Scholar]
- 14.Stornaiuolo A., Piovani B.M., Bossi S., Zucchelli E., Corna S., Salvatori F., Mavilio F., Bordignon C., Rizzardi G.P., Bovolenta C. RD2-MolPack-Chim3, a packaging cell line for stable production of lentiviral vectors for anti-HIV gene therapy. Hum. Gene Ther. Methods. 2013;24:228–240. doi: 10.1089/hgtb.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marin V., Stornaiuolo A., Piovan C., Corna S., Bossi S., Pema M., Giuliani E., Scavullo C., Zucchelli E., Bordignon C. RD-MolPack technology for the constitutive production of self-inactivating lentiviral vectors pseudotyped with the nontoxic RD114-TR envelope. Mol. Ther. Methods Clin. Dev. 2016;3:16033. doi: 10.1038/mtm.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costello E., Munoz M., Buetti E., Meylan P.R., Diggelmann H., Thali M. Gene transfer into stimulated and unstimulated T lymphocytes by HIV-1-derived lentiviral vectors. Gene Ther. 2000;7:596–604. doi: 10.1038/sj.gt.3301135. [DOI] [PubMed] [Google Scholar]
- 17.Cornetta K., Anderson W.F. Protamine sulfate as an effective alternative to polybrene in retroviral-mediated gene-transfer: implications for human gene therapy. J. Virol. Methods. 1989;23:187–194. doi: 10.1016/0166-0934(89)90132-8. [DOI] [PubMed] [Google Scholar]
- 18.Rambaldi A., Biagi E., Bonini C., Biondi A., Introna M. Cell-based strategies to manage leukemia relapse: efficacy and feasibility of immunotherapy approaches. Leukemia. 2015;29:1–10. doi: 10.1038/leu.2014.189. [DOI] [PubMed] [Google Scholar]
- 19.Cieri N., Mastaglio S., Oliveira G., Casucci M., Bondanza A., Bonini C. Adoptive immunotherapy with genetically modified lymphocytes in allogeneic stem cell transplantation. Immunol. Rev. 2014;257:165–180. doi: 10.1111/imr.12130. [DOI] [PubMed] [Google Scholar]
- 20.Yolamanova M., Meier C., Shaytan A.K., Vas V., Bertoncini C.W., Arnold F., Zirafi O., Usmani S.M., Müller J.A., Sauter D. Peptide nanofibrils boost retroviral gene transfer and provide a rapid means for concentrating viruses. Nat. Nanotechnol. 2013;8:130–136. doi: 10.1038/nnano.2012.248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.