Abstract
The conditionally replicating oncolytic adenovirus Delta24-RGD (Ad) is currently under investigation in clinical trials for glioblastoma, including in combination with temozolomide (TMZ), the standard chemotherapy for this tumor. Previously, we showed that the efficacy of Delta24-RGD in a murine model is primarily dependent on the virus-induced anti-tumor immune response. As observed with most chemotherapies, TMZ has pronounced immune-modulating effects. Here, we studied the combined effects of these treatments in a murine glioma model. In vitro, we observed a synergistic activity between Delta24-RGD and TMZ. In vivo, C57BL/6 mice bearing intracranial GL261 tumors were treated with TMZ for 5 days either prior to intratumoral Delta24-RGD injection (TMZ/Ad) or post virus injection (Ad/TMZ). Notably, the Ad/TMZ regimen led to similar tumoral CD8+ T cell influx as the virus-only treatment, but increased the ability of CD8+ T cells to specifically recognize the tumor cells. This was accompanied by improved survival. The TMZ/Ad regimen also improved survival significantly compared to controls, but not compared to virus alone. In this group, the influx of dendritic cells is impaired, followed by a significantly lower number of tumor-infiltrating CD8+ T cells and no recognition of tumor cells. Depletion of either CD4+ T cells or CD8+ T cells impaired the efficacy of Delta24-RGD, underscoring the role of these cells in therapeutic activity of the virus. Overall, we show that the addition of TMZ to Delta24-RGD treatment leads to a significant increase in survival and that the order of sequence of these treatments affects the CD8+T cell anti-tumor activity.
Keywords: glioma, oncolytic virotherapy, adenovirus, immune response, temozolomide, mouse model
Introduction
Glioblastoma (GBM) is both the most common and most malignant of all brain tumors in adults.1 Despite current standard treatment by surgical resection, radiation therapy, and chemotherapy, the prognosis remains dismal.1 The median survival time of GBM patients at the time of diagnosis is only 14.6 months,2 highlighting the necessity of novel therapies. Delta24-RGD, an oncolytic adenovirus, has shown promising preclinical efficacy and is currently under investigation in clinical phase I/II trials in recurrent GBM patients.3 This virus selectively replicates in tumor cells harboring a mutation in the retinoblastoma (Rb) tumor suppressor gene or pathway but not in normal healthy cells.4 After replication, the produced viral particles are released from the host cell by lysis and spread to neighboring tumor cells.
Recently, a clinical trial combining the chemotherapeutic agent temozolomide (TMZ) with Delta24-RGD was initiated.5 Previously, TMZ was shown to synergize with Delta24-RGD in human U87MG glioma cells by disabling the cellular DNA repair machinery and improving the survival of mice bearing U87 xenografts.6 In an immune-competent mouse model, we have previously shown that the therapeutic efficacy of Delta24-RGD is primarily dependent on an anti-tumor immune response, most likely executed by CD4+ or CD8+ T cells.7, 8 On the one hand, TMZ induces lymphopenia,9 which has been reported to be particularly pronounced with respect to CD4+ cells, CD8+ T cells, and natural killer (NK) cells.10 On the other hand, there are also reports that demonstrate that immune suppression is lowered by TMZ by decreasing the numbers of peripheral regulatory T cells (Tregs),11 which could be favorable in the combined treatment setting. Also, it has been postulated that TMZ increases tumor antigen presentation to immune cells by inducing autophagic tumor cell death rather than apoptosis or necrosis, therewith facilitating the involvement of antigen-presenting cells, such as dendritic cells (DCs), and improved generation and localization of anti-tumor CD8+ T cells.12, 13, 14 We hypothesized that these differential effects of TMZ depend on the timing of chemotherapy in relation to oncolytic virus treatment, which would be in line with observations from immunotherapy studies using adenoviral vectors and/or immune-stimulatory cytokines.15, 16 To test this hypothesis, we studied the effects of the combination and sequence of TMZ and Delta24-RGD treatment on immune response and therapeutic outcome in an immune-competent mouse model for glioma.
Results
TMZ and Delta24-RGD Act Synergistically In Vitro
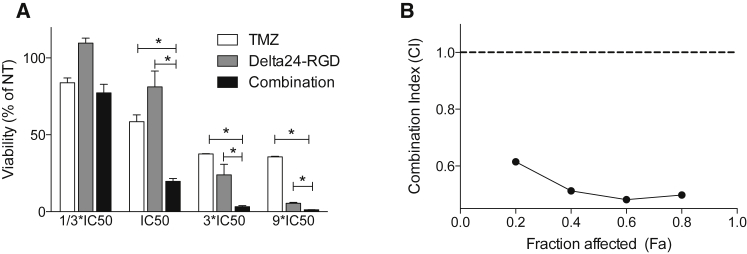
To evaluate the cytotoxicity and interaction of TMZ and Delta24-RGD (Ad) on murine glioma GL261 cells, synergy between the two treatment modalities was assessed in vitro by the Chou-Talalay method.17 Three-fold serial dilutions of Ad and TMZ, with several concentration points above and below the half maximal inhibitory concentration (IC50) for these agents, were added simultaneously to GL261 cells, and cell viability was measured after 5 days (Figure 1A). The combination reduced viability compared to the mono-treatments (all p < 0.05), and the calculated combination index (CI) also showed a synergistic effect (CI < 1) between the two treatments (Figure 1B).
Figure 1.
Synergy between TMZ and Delta24-RGD
(A) Viability of TMZ- (white bars), Delta24-RGD- (gray bars), and combination- (black bars) treated GL261 cells in triplicate was measured after 5 days. Dosages were chosen around the predetermined IC50. Viability, determined by the presence of ATP, is expressed as a percentage of non-treated cells. Error bars indicate SD; *p < 0.05. (B) Using the Chou-Talalay formulas, combination index (CI) was calculated. A CI <1 indicates synergy.
Combined Delta24-RGD and TMZ Treatment Induces Apoptosis and Autophagic Flux
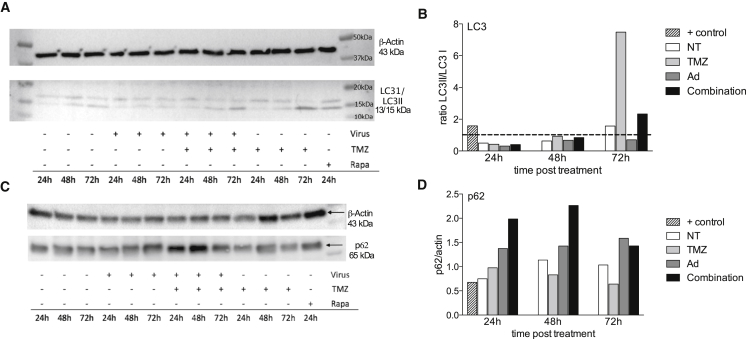
Multiple types of induced cell death have been described for both TMZ and Delta24-RGD, where autophagic cell death and apoptosis are the most important mechanisms.12 To evaluate the effect of combination therapy on these two types of cell death, a western blot analysis for LC3-I/LC3-II and p62 expression was performed, as conversion of LC3-I to LC3-II and consumption of p62 have been suggested to provide indicators of autophagic activity. Apoptosis was assessed by the caspase 3/7 assay.
Induction of the LC3-II protein was most profound in the TMZ-treated cells and combination-treated cells (Figure 2A). When quantified, a ratio >1 between LC3-II and LC3-I is considered indicative of active autophagy.18 Whereas Delta24-RGD did not induce LC3 conversion in GL261 cells, TMZ induced an almost 8-fold increase in the LC3-II/LC3-I ratio by 72 hr (Figure 2B, line indicates 1). The combination treatment induced an intermediate response. p62 accumulated in virus-treated cells and, in particular, in the combination-treated cells up to 48 hr and decreased again by 72 hr (Figures 2C and 2D).
Figure 2.
Combined Treatment Induces Autophagy
(A and B) The ratio between LC3-II and LC3-I was determined by western blotting (A) and quantified using Image Lab 4.1 software (Biorad) (B). A ratio between LC3-II and LC3-I >1 indicates active autophagy, as shown by the rapamycin-treated positive control (striped bar) at 24 hr. TMZ- (light gray bars) treated cells show a high ratio after 72 hr, while Delta24-RGD (dark gray bars) does not induce autophagy. Non-treated (NT) cells were used as a control (white bars). In the combination-treated cells (black bars), autophagy is induced at 72 hr, albeit at a lower level compared to TMZ. (C and D) The accumulation of p62 was also determined by western blotting as a measure of autophagy (C) and the intensity was corrected by the intensity levels of actin (D).
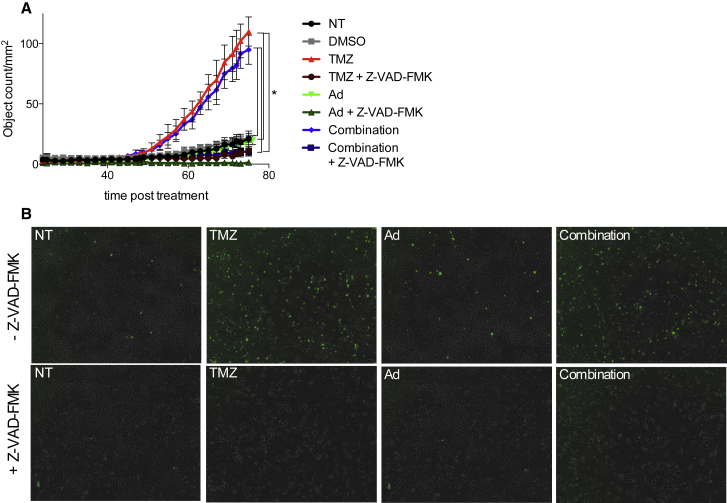
Analysis of caspase 3/7 activity in Delta24-RGD-, TMZ-, and combination-treated treated GL261 cells clearly revealed the induction of apoptosis in the murine glioma cells after TMZ treatment (Figures 3A and 3C, p < 0.001). This activation is not hampered when the two treatments are combined (Figure 3A, non-treated [NT] versus combination, p < 0.001). Interestingly, addition of the pan-caspase inhibitor Z-VAD-FMK to TMZ or combination-treated cells inhibited the cleavage of caspase 3/7 (p < 0.001; Figure 3A). Pictures show comparable morphology of the cells in each of the different treatment groups with or without Z-VAD-FMK (Figure 3B). Overall, our data suggest that both autophagic cell death and apoptosis are induced in GL261 cells by combining TMZ and Delta24-RGD treatment.
Figure 3.
Combined Treatment Induces Caspase 3/7
(A and B) The induction of caspase 3/7, as a measure of apoptosis (A) (error bars indicate SD), was quantified by counting the GFP-positive cells in triplicate (B), which represent cells with active caspase 3/7. After 72 hr, significantly more caspase 3/7-positive cells are detected in the TMZ and combined treated cells (p < 0.05). Z-VAD-FMK, a pan-caspase inhibitor, blocked the induction of caspase 3/7 in TMZ and combined treated cells (p < 0.05).
Combining TMZ and Delta24-RGD Improves Survival of Glioma-Bearing Mice
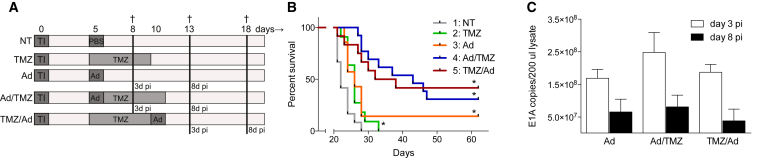
To assess the effects of combined treatment on survival, GL261 tumor-bearing mice were treated with TMZ, Delta24-RGD, or a combination schedule. As pre- (TMZ/Ad) or post- (Ad/TMZ) treatment with TMZ could influence the therapeutic activity of the virus, both treatment schedules were tested. Details of the experimental design are depicted in Figure 4A. TMZ delayed tumor growth for approximately 4 days (green, p = 0.01, Figure 4B) and Delta24-RGD improved survival compared to the NT mice, leading to long-term survival (>120 days) in 14% of animals (orange, p = 0.0281). The two combination regimens (blue [Ad/TMZ] and red [TMZ/Ad]) extended median survival compared to the NT mice (both p < 0.0001, with 21 and 13 days, respectively). Furthermore, the Ad/TMZ regimen (blue) improved survival significantly compared to both TMZ and Delta24-RGD alone (p < 0.0001 and p = 0.0353, respectively), whereas the TMZ/Ad regimen (red) improved survival significantly compared to TMZ (p = 0.0045), but not to Delta24RGD (p = 0.1043).
Figure 4.
The Combination of TMZ and Delta24-RGD Significantly Improves Survival of Glioma-Bearing Mice
(A) Schematic drawing of treatment schedules. At day 0, GL261 tumor cells were injected (tumor inoculation [TI]), and, after 5 days, treatment was initiated according to schedule. Mice were followed for survival or sacrificed (†) at earlier indicated time points. (B) Results of two combined survival studies with control non-treated mice (NT, gray line, n = 12), TMZ-treated mice (green, n = 11), Delta24-RGD-treated mice (orange, n = 7), post-treatment schedule (Ad/TMZ, blue, n = 13) and pre-treatment schedule (TMZ/Ad), red, n = 13). All treatment groups significantly prolong survival compared to the controls (log rank test, *p < 0.05). The Ad/TMZ group is also significantly different from TMZ alone or Delta24-RGD alone (p < 0.0001 and p = 0.0353, respectively). (C) To determine tumoral viral titers, E1A copies were measured by PCR in brain lysate 3 days (white bars) and 8 days (black bars) post injection (pi). There is no significant difference between the treatment groups (p > 0.05). Error bars indicate SD.
The intratumoral viral load of the different treatment groups was assessed over time by quantifying the viral E1A DNA content in the tumor-bearing hemispheres. At 3 and 8 days post virus injection, the number of E1A copies was similar in the three treatment groups, although the overall E1A copy numbers on day 8 were lower than on day 3 (Figure 4C).
TMZ Prior to Delta24-RGD Treatment Abrogates Intratumoral Accumulation of CD8+ T Cells
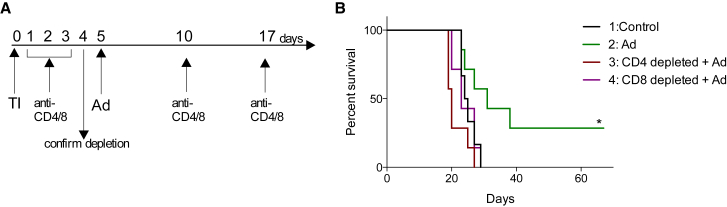
We previously showed that Delta24-RGD elicits an anti-tumor immune response, which is, at least in part, responsible for the therapeutic effect of the virus.7, 8 To assess whether CD4+ or CD8+ T cells are the effector cells in this response, anti-CD4 and anti-CD8 antibodies were used to deplete these cells in virus-treated mice. Delta24-RGD treatment led to 35% long-term survivors (p < 0.05 NT versus Ad), while concomitant depletion of CD4+ or CD8+ cells completely abolished the therapeutic effect of the virus (Figure 5, p = 0.0066 and p = 0.0265, respectively), highlighting the importance of both subtypes of T-lymphocytes for the efficacy of Delta24-RGD treatment.
Figure 5.
Overall Survival of CD4- and CD8-Depleted Delta24-RGD-Treated Mice Is Impaired
(A) Schematic drawing of treatment schedules. TI, tumor inoculation. (B) Survival analysis for control non-treated mice (n = 6, black line), Delta24-RGD-treated mice (n = 7, green line), CD4-depleted Delta24-RGD-treated mice (n = 7, red line), and CD8-depleted Delta24-RGD-treated mice (n = 7, purple line). Survival of Delta24-RGD-treated mice is significantly different from control, CD4-depleted or CD8-depleted mice (log rank test, p = 0.0456, p = 0.0066 and p = 0.0265, respectively). *p < 0.05.
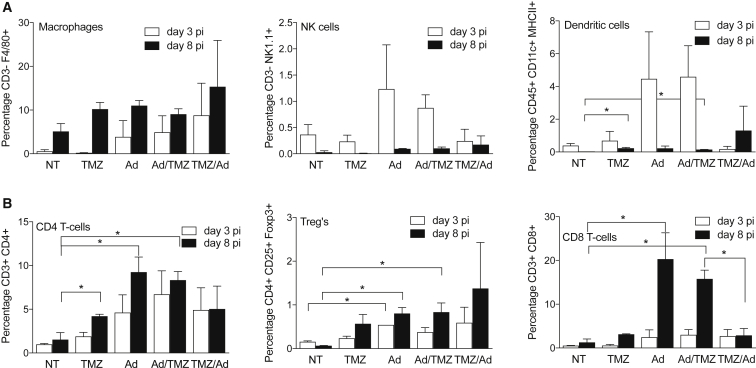
To examine whether co-treatment with TMZ affects this response, we mapped the local immune environment during TMZ, Delta24-RGD, and the combination treatments. Brains were harvested at 3 and 8 days post-treatment to analyze brain-infiltrating leukocytes by flow cytometry (Figure 6). An increase in infiltrating macrophages (CD3−F4/80+) in the tumor-bearing hemisphere between day 3 and day 8 was found in all conditions, including the PBS-treated control mice. On day 3, these numbers were slightly higher in the mice treated with Delta24-RGD or combination therapy compared to PBS or TMZ monotherapy; however, these differences did not reach statistical significance (Figure 6A, left graph). The relative numbers of NK cells (CD3− NK1.1+; Figure 6A, middle graph) and DCs (CD45+ CD11c+ MHCII+, right graph) were lower compared to macrophages. Percentages of NK cells and DCs were markedly increased at day 3 post-treatment in the virus (Ad) group and the post-treatment (Ad/TMZ) group, whereas the pre-treatment (TMZ/Ad) group revealed virtually no NK cell or DC infiltration at this time point. At day 8, NK cell and DC levels were normalized to the levels in control mice in all treatment groups.
Figure 6.
Intratumoral Influx of CD8 T cells Is Enhanced in Ad/TMZ, but Not TMZ/Ad, Treatment
(A and B) Influxed intratumoral immune cells (n = 2 for mono-treatments, n = 3 for combined treatments), both from the innate (A) and adaptive (B) arm of the immune system, were characterized and quantified using flow cytometry analysis 3 (white bars) and 8 (black bars) days post viral injection. Error bars indicate SD; *p < 0.05. (A) For the innate arm, macrophages (% CD3− F4/80+ cells of total events, left graph), natural killer cells (NK) (CD3− NK1.1+, middle graph), and dendritic cells (DC) (CD45+ CD11c+ MHCII+, right graph) were measured (B) For the adaptive arm, the amounts CD4+ T cells (CD3+ CD4+, left graph), regulatory T cells (Treg, CD4+ CD25+ FoxP3+, middle graph), and CD8+ T cells (CD3+ CD8+, right graph) were assessed.
A significant increase in CD4+ T cells (CD3+ CD4+) was detected 8 days post-treatment in the TMZ, Ad, and Ad/TMZ groups (Figure 6B, left graph, p < 0.05), but not in the TMZ/Ad group. The number of Tregs (CD4+ CD25+FoxP3+) was generally low; however, increased numbers were present after 8 days in the Ad and Ad/TMZ post-treatment groups (middle graph, p < 0.05). The most pronounced effect of the different treatment schedules was found in the CD8+ T cell population (CD3+ CD8+; Figure 6B, right graph). These numbers increased to approximately 10-fold on day 8 in the Ad and Ad/TMZ groups, whereas numbers remained near baseline in the TMZ/Ad group (p < 0.05). Therefore, it appears that pre-treatment of mice with TMZ reduces the virus-induced early influx of DCs and NK cells and the subsequent influx of CD4+ and, in particular, CD8+ T cells.
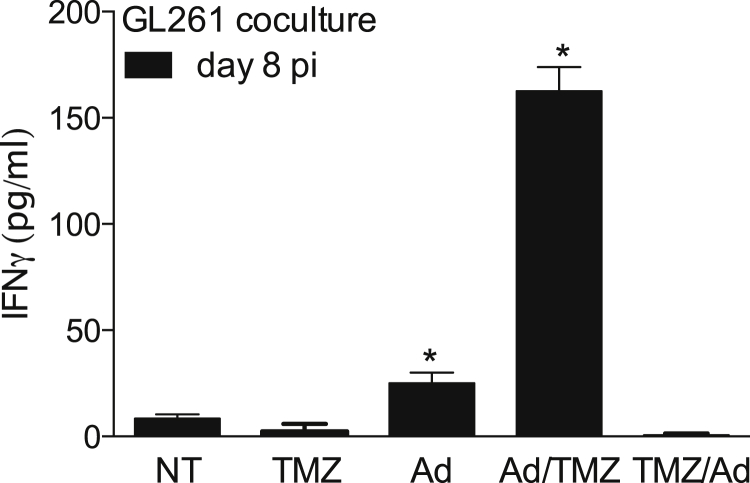
Ad/TMZ, but Not TMZ/Ad, Regimen Results in Enhanced Recognition of Glioma Cells by CD8+ T Cells
Whether the sequence of TMZ and Delta24-RGD treatment affects the specific anti-tumor response was evaluated in splenocytes derived 8 days post virus injection (Figure 7). Splenocytes from Delta24-RGD-treated mice produced more interferon γ (IFN-γ) compared to splenocytes from NT mice when cocultured with GL261 cells (NT versus Ad, white bars, p = 0.0059). Strikingly, splenocytes from the post-treatment regimen (Ad/TMZ) produced 6-fold more IFN-γ than Ad-only treated mice (NT versus Ad/TMZ p < 0.0001, Ad versus Ad/TMZ p < 0.0001). In contrast, splenocytes from TMZ/Ad-treated mice did not recognize GL261 cells, further supporting the view that TMZ pre-treatment abrogates virus-induced immune response to the tumor.
Figure 7.
Ad/TMZ, but Not TMZ/Ad, Treatment In Vivo Induces Enhanced Recognition of Glioma Cells by Peripheral T Cells
Splenocytes derived from non-treated (NT), Delta24-RGD (Ad), post-treated (Ad/TMZ), and pre-treated (TMZ/Ad) mice were cocultured with GL261 cells ex vivo, and supernatants were assessed for IFN-γ in triplicate as a measure of antigen recognition. Splenocytes from virus and post-treated mice, not pre-treated mice, produced significantly more IFN-γ 8 days after viral treatment (pi, black bars, *p < 0.05). Error bars indicate SD.
Discussion
For further clinical development of the oncolytic adenovirus Delta24-RGD for glioma, evaluation of combination treatment with current standard therapies is warranted. It was previously shown in a glioma xenograft model that TMZ acts synergistically with the virus and that this effect is mainly based on virus-mediated interference with DNA repair genes: in particular, MGMT.6 However, more recent data point toward a major contribution of the immune system in Delta24-RGD therapy, and combined therapy with immune suppressive agents, such as TMZ, may undermine this response.7, 8 On the other hand, TMZ may also enhance the anti-tumor response (e.g., by decreasing peripheral Tregs or increasing tumor-antigen presentation).11, 12 To gain insight into these interactions, we assessed the effects of the combination of Delta24-RGD with TMZ given either prior to injection or post virus injection in an immune-competent mouse model.
Combining TMZ with Delta24-RGD significantly improved the survival of glioma-bearing mice compared to controls in both combination groups, yielding 30%–50% long-term survivors (Figure 4). Only the Ad/TMZ combination regimen improved survival significantly compared to both mono-treatment groups. Analysis of the intracranial tumors of the virus-treated mice revealed similar amounts of virus in the combination groups versus Delta24-RGD-treated mice, suggesting that improved efficacy was not due to enhanced intratumoral virus replication. It cannot be excluded, however, that retrieved viral amounts were affected by differences in tumor size at the time of virus injection as a result of the timing of TMZ therapy. Moreover, we have previously shown that the Delta24-RGD lytic cycle is attenuated in murine cells, which corresponds with decreasing viral amounts between days 3 and 8 in all three groups.7
To gain insight into the mechanisms underlying the in vivo synergistic activity of Delta24-RGD and TMZ, several experiments were performed. Synergy between the two treatment modalities with respect to oncolysis was demonstrated in vitro by Chou-Talalay analysis17 (Figure 1). One explanation for the synergy is that Delta24-RGD silences the MGMT promoter and/or interacts with other DNA repair genes,6 thereby enhancing TMZ efficacy. Indeed, GL261 tumors have been reported to weakly express MGMT19 in vivo, although we could not confirm this in vitro (not shown). We cannot exclude, however, that other DNA repair enzymes are affected by Delta24-RGD infection.
Both autophagy and apoptosis are reported to play a role in Delta24-RGD- and TMZ-induced cell death.20, 21 Surprisingly, we found that autophagy, as measured by the conversion of the protein LC3-I to LC3-II, is not induced above control levels by Delta24-RGD in GL261 cells (Figure 2). Addition of TMZ to the viral treatment did increase the ratio of LC3-II/LC3-I, but not to the extent induced by TMZ monotherapy. Interestingly, whereas a decrease in p62 is expected during autophagy, we observed accumulation of p62 after virus treatment, which was further enhanced in combination with TMZ. This may suggest that the increased autophagic flux, which is noted by the LC3 turnover, is hampered in the degradation phase. Others have reported Delta24-RGD to preferentially induce autophagy over cell lysis in tumor cells during the final stages of the replication cycle.8, 21 This discrepancy with our results may possibly be explained by species-related differences in response to the virus and the attenuated replication cycle of Delta24-RGD in the murine GL261 cells may be associated with a different mechanism of cell death induction.7 Apoptosis, as measured by caspase 3/7 activation, is also induced in TMZ-treated cells over a 3-day period, while Ad alone does not increase apoptosis. The combination treatment also results in high levels of apoptosis. Caspase cleavage, however, does not always lead to apoptotic cell death.22 Indeed, the pan-caspase inhibitor Z-VAD-FMK reduced expression of caspase 3/7 (Figure 3) without inducing cytotoxicity (results not shown). Autophagy, initially perceived as a cell survival mechanism, is associated with immunogenic cell death, whereas apoptosis generally is not. The two cell death-associated mechanisms are, however, not mutually exclusive.23 Furthermore, pathogen-induced cell death is, by definition, an immunogenic form of cell death, as is shown by the release of HMGB1 after other oncolytic adenovirus infections.12, 24, 25 Overall, we observed that TMZ effectively induces autophagic flux and apoptosis and that the combination of both treatments ensures that both processes are activated in the infected cells. It cannot be excluded that other forms of cell death (e.g., necrosis, anoikis, and non-apoptotic programmed cell death), which were not investigated in this study, may also contribute to the synergistic cell killing by Delta24-RGD and TMZ.26
In addition to the cell-killing properties of the two treatments, the interaction with the immune system also is anticipated to affect the therapeutic outcome. We previously demonstrated that Delta24-RGD activity in this model is primarily driven by an anti-tumor response.7, 8 Here we show, in a depletion experiment, that both CD4+ T cells and CD8+ T cells are required for this anti-tumor response (Figure 5). TMZ has been shown to change leukocyte proportions by inducing a transient lymphopenia of CD4+ T cells and NK cells and by increasing numbers of Tregs without affecting monocytes.10 It is believed that the repopulating immune cells experience less competition for antigens,27 resulting in increased numbers of effector T cells specific for tumor and/or virus. Adding TMZ to Delta24-RGD treatment may, therefore, enhance the anti-tumor immune response. On the other hand, the TMZ-induced lymphopenia could hamper the therapeutic efficacy of the virus by suppressing an anti-tumor immune reaction due to the increased numbers of Tregs and insufficient T effector cell activation and expansion. The splenocyte assay revealed that T cells do recognize the tumor cells in the post-treatment group, and, in fact, are even more pronounced than in mice treated with Delta24-RGD only (Figure 7), which suggests that they might have a higher frequency. Interestingly, this reaction was not detected in mice from the pre-treatment group, underscoring that the order in which TMZ and Delta24-RGD therapy is administered is of paramount importance in the generation of virus-induced anti-tumor immunity. When interpreting these results, the timing of TMZ administration and the analyzed time points in relation to the number of days after viral treatment should be kept in mind.
Differences in tumoral immune cell influx between the two combination regimens were also observed (Figure 6). In general, viral treatment induces chemotaxis signaling, leading to the influx of macrophages, NK cells, DCs, and CD4+ and CD8+ T cells with a more rapid infiltration of the tumor by macrophages than by CD8+ T cells.7 Strikingly, DC infiltration is completely abrogated in mice receiving the TMZ/Ad pre-treatment schedule, but not in mice receiving the Ad/TMZ post-treatment schedule. This is subsequently reflected in a diminished influx of CD4+ and, in particular, CD8+ T cells in TMZ/Ad-treated mice, which is an important finding as CD8+ T cells are required for the therapeutic efficacy of Delta24-RGD (Figure 5). Together, the immune cell influx data are congruent with the results of the splenocyte assay, showing that the pre-treatment (TMZ/Ad) schedule abrogates immune cell influx and generation of a tumor-specific T cell response. The similar survival benefit in both combination treatment groups may be a consequence of two differential mechanisms: the direct cytotoxic/oncolytic effects of TMZ and Delta24-RGD versus an induced anti-tumor immune response. The lack of immune cell influx in the pre-treatment group may have led to improved and prolonged oncolytic activity of the virus in these mice because the virus is cleared less rapidly. Despite this, and based on the immune cell influx and splenocyte data, we recommend the post-treatment sequence (Ad/TMZ) for further (clinical) evaluation.
In conclusion, in an immune-competent setting, the oncolytic virus Delta24-RGD administered prior to TMZ significantly prolongs survival without hampering the anti-tumor immune response, thereby highlighting the potency of this combination strategy over the use of single agents.
Materials and Methods
Cell Culture
Both the murine glioma cell line GL261, obtained from National Cancer Institute (NCI) Tumor Repository and the human lung carcinoma cell line A549 (ATCC) were cultured in DMEM (Life Technologies) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (both Life Technologies).
Viruses and Chemicals
The human Delta24-RGD virus was previously described by Suzuki et al.28, and viral stocks were produced as described previously.29 For purification, the AdEasy Virus Purification Kit (Stratagene) was used, and titration of the new virus batches was done on A549 cells using the Adeno-X Rapid Titer Kit (Clontech Laboratories). TMZ (kindly provided by Sun Pharmaceutical Industries) was dissolved in DMSO (Sigma-Aldrich) for in vitro experiments and further diluted in cell culture medium. To control for DMSO-induced cytotoxicity, the highest percentage of DMSO was taken as a control. For the in vivo study, TMZ was dissolved in PBS (Life Technologies).
In Vivo Experiments
All animal experiments described in this paper have been conducted according to Dutch guidelines for animal experimentation. All animal experiments were reviewed by and performed with approval of the Erasmus MC Animal Ethics Committee (DEC, protocol numbers EMC3045 and EMC3069) of the Erasmus Medical Center. The mice were housed in individually ventilated cages with sterile bedding, water, and rodent chow. Female C57BL/6 mice (6–9 weeks old, Harlan) were stereotactically injected with 5 × 104 GL261 cells 3 mm deep in the right hemisphere (2.2 mm lateral, 0.5 mm anterior of bregma) as described previously.30 Treatment for the combination of Delta24-RGD with TMZ started after 5 days with the following regimens: PBS as a control, TMZ alone (7.5 mg/kg/day, administered intraperitoneally for 5 days), Delta24-RGD alone (8.7 × 108 IU/mice, intratumorally), pre-treatment with TMZ followed by Delta24-RGD, and Delta24-RGD followed by post-treatment with TMZ. For the CD4 and CD8 depletion experiment, animals received 0.2 mg per mouse anti-CD4 (Clone GK1.5, Bio X Cell) or 0.2 mg per mouse anti-CD8 (Clone 53-6.72, Bio X Cell) on days 1, 2, and 3. On day 4, depletion of CD4+ or CD8+ was confirmed in the blood of all mice using fluorescence-activated cell sorting (FACS) analysis (results not shown). At day 5, mice received Delta24-RGD (3.5 × 107 IU/mice, intratumorally). Injection of anti-CD4 and anti-CD8 was repeated every 7 days. Mice were followed for survival and sacrificed upon more than 20% weight loss or appearance of neurological symptoms. Results of two separate Delta24-RGD + TMZ combination experiments were combined. For flow cytometry analysis, qPCR, and the splenocyte assay, mice of the combination study were sacrificed at earlier time points (3 and 8 days after the start of the treatment schedule), and brains and spleens were harvested.
Analysis of the In Vivo Viral Load by qPCR
To compare the in vivo viral load between the different treatment schedules, five cryosections of 10 μM were cut from the tumor-containing hemisphere near the injection site. A homogeneous solution was made by crushing the tissue in RPMI medium (Life Technologies) with a micro tissue homogenizer. Nucleic acids were extracted using the MagNA Pure LC Total Nucleic Acid Isolation Kit (Roche Molecular Systems). The DNA concentration of each sample was measured using the Nanodrop machine (Thermo Scientific) and was not significantly different between the treatment groups (results not shown). Amplification was performed using the 2x Taqman Universal Mastermix (Life Technologies), and primers add24-RGDfwd (5′-acactaaacggtacacaggaaacag-3′) and add24-RGDrev (5′-gccagaccagtcccatgaaa-3′) in the presence of FAM-BHQ labeled probe add24-RGD (5′-ccgcggagactgtttctgccca-3′). Real time PCR amplification was measured on a Lightcycler 480 (Roche Molecular Systems). In parallel, a calibration curve of a Delta24-RGD stock with a known viral particle titer was run.
Chou-Talalay Assay
Drug synergy can be calculated by using the Chou-Talalay equations.17 GL261 cells were plated at 1,000 cells/well in a 96-well plate (Greiner Bio One) and, after 24 hr, cells were treated in triplicate with either TMZ or Delta24-RGD alone or in combination. Concentrations varied between 1/27*IC50 and 9*IC50, based on predetermined IC50 values of 100 μM TMZ and MOI 170 Delta24-RGD. On day 5, viability was determined using the Cell Titer Glo Assay (Promega). Dose response curves were fitted, and the CI was calculated. A CI >1 is considered antagonistic, CI = 1 additive, while synergy is indicated by CIs of <1.
Caspase 3/7 Assay
GL261 cells/well (8,000/well) were seeded in a clear-bottom black 96-well plate (Greiner Bio One). The following day, cells were treated with TMZ (100 μM), Delta24-RGD (100 MOI), or the combination in triplicate. Untreated cells and DMSO- (0.1%) treated cells served as negative controls. The CellPlayer Caspase 3/7 reagent (Essen Bioscience) was added to the wells according to the manufacturer’s instructions. The pan-caspase inhibitor Z-VAD-FMK (MBL International) was added at a concentration of 20 μM. Fluorescent signals from caspase 3/7-positive cells were measured every 3 hr using IncuCyte (Essen Bioscience). The object count (GFP-positive cells) was determined using the IncuCyte Software (Essen Bioscience). After 72 hr, cell viability was determined using the Cell Titer Glo Assay (Promega).
LC3/p62 Western Blotting
T75 flasks of GL261 cells were treated with 100 μM TMZ, 100 MOI Delta24-RGD, or both and harvested 24 hr, 48 hr, and 72 hr post-treatment. GL261 cells treated with 1 μM Rapamycin (Cayman Chemical) for 24 hr served as positive control, and NT GL261 cells harvested at the same time points served as negative control. Protein fractions were collected with use of RIPA Buffer (Sigma-Aldrich). Bradford Assay (Pierce) was performed to determine protein concentrations, and 1,000 μg of protein was loaded onto precast 4%–15% SDS-PAGE gel (Bio-Rad). The gel was run at 150V for 45 min before being transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore) at 380 mA/85V on ice for 1 hr. The membrane was washed with Tris-buffered saline-Tween 20 (TBST), blocked for 30 min at room temperature and incubated with primary antibodies against murine β-actin (1:10,000, Millipore), murine LC3-I/LC3-II (1:375, Cell Signaling Technology), and murine p62 (1:500, Abcam) in 0.2% TBST/5% BSA at 4°C overnight. After washing, the membrane was incubated with polyclonal anti-rabbit-horseradish peroxidase (HRP) or anti-mouse-HRP (both 1:2,000, DAKO) in 2.5% milk in TBST for 1 hr at room temperature. After final washing, the membrane was incubated with enhanced chemiluminescence (ECL) solution (Pierce) and scanned using the Chemidoc Imager (Bio-Rad). Analysis was performed using Image Lab 4.1 software (Bio-Rad).
Detection of Glioma-Reactive Splenocytic T Cells
T cell reactivity in spleens was monitored as described by Pouw and colleagues.31 In short, splenocytes were pooled per treatment group and maintained in complete mouse medium containing RPMI 1640 with 25 mM HEPES and L-glutamine (Life Technologies) supplemented with 10% FCS, 1% penicillin/streptomycin, 1% non-essential amino acids (Lonza), 1 mM sodium pyruvate (Life Technologies), and 50 μM β-mercaptoethanol (Sigma-Aldrich). The splenocytes were stimulated with concanavalin A (2.5 μg/mL, Sigma-Aldrich) and 100 U/mL human recombinant interleukin (IL)-2 (Proleukin, Chiron) for 48 hr. To assess IFN-γ production, 106 stimulated splenocytes were subsequently co-cultured with 104 GL261 cells for 24 hr. Supernatants were harvested, and the level IFN-γ was determined with OptEIA ELISA kit II (BD Biosciences).
Flow Cytometry Analysis of Tumor-Infiltrating Leukocytes
Mice (n = 2 for mono-treatment, n = 3 for combination treatments) were sacrificed, and tumor-containing hemispheres were mechanically dissociated using a 40-μm nylon cell strainer (Corning). Leukocyte fractions from these suspensions were obtained by 30%–70% Percoll density gradient centrifugation (GE Healthcare Life Sciences) and stained with CD3, F4/80, and NK1.1 (all eBioscience), CD3, CD4, CD8, CD11c, CD25, CD45, and I-E[k]/MHCII (all BD Biosciences). For the intracellular FoxP3 staining, the FoxP3 staining set (eBioscience) was used according to the manufacturer’s instructions. Cells were acquired on a FACSAriall (BD Biosciences), and data were analyzed with Infinicyt software (Cytognos). Prior to gating of the different cell populations, debris was gated out using FSC-A and FSC-H parameters. Per staining tube, all time-points were analyzed together by file merging and consequent gating.
Statistical Analysis
The students’t test was used for significance testing of in vitro experiments. For the survival curve analysis, the log rank test was performed. p values < 0.05 were considered significant and are indicated with asterisks in the graphs. All tests were done using GraphPad Prism (GraphPad Software).
Author Contributions
A.K., W.v.d.B, R.D, J.d.V., and M.L.M.L. designed the experiments, analyzed data, and drafted and finalized the manuscript. A.K., W.v.d.B., E.S.H., Z.B, C.B.-M., J.J.K., and S.D.P. developed protocols and conducted in vitro and in vivo experiments. S.L, C.M.F.D, and M.L.M.L. oversaw all aspects of the studies and analysis. All authors were involved in critical review of the manuscript.
Conflicts of Interest
C.M.F.D. and M.L.M.L. have consulted for DNAtrix Therapeutics, Inc., for which Erasmus MC received compensation. No other relationships or activities exist that could be perceived to have influenced the submitted work. The other authors declare no potential conflict of interest.
Acknowledgments
This study was supported by a grant from The Dutch Cancer Society (KWF Kankerbestrijding; 2006-3696).
References
- 1.Wen P.Y., Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.DNAtrix, Inc. https://clinicaltrials.gov/ct2/show/NCT00805376?term=delta24rgd&rank=3.
- 4.Fueyo J., Alemany R., Gomez-Manzano C., Fuller G.N., Khan A., Conrad C.A., Liu T.J., Jiang H., Lemoine M.G., Suzuki K. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J. Natl. Cancer Inst. 2003;95:652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 5.Tejada, S. https://clinicaltrials.gov/ct2/show/NCT01956734?term=delta24rgd&rank=4.
- 6.Alonso M.M., Gomez-Manzano C., Bekele B.N., Yung W.K., Fueyo J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 2007;67:11499–11504. doi: 10.1158/0008-5472.CAN-07-5312. [DOI] [PubMed] [Google Scholar]
- 7.Kleijn A., Kloezeman J., Treffers-Westerlaken E., Fulci G., Leenstra S., Dirven C., Debets R., Lamfers M. The in vivo therapeutic efficacy of the oncolytic adenovirus Delta24-RGD is mediated by tumor-specific immunity. PLoS ONE. 2014;9:e97495. doi: 10.1371/journal.pone.0097495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang H., Clise-Dwyer K., Ruisaard K.E., Fan X., Tian W., Gumin J., Lamfers M.L., Kleijn A., Lang F.F., Yung W.K. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS ONE. 2014;9:e97407. doi: 10.1371/journal.pone.0097407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvino E., Pepponi R., Pagani E., Lacal P.M., Nunziata C., Bonmassar E., D’Atri S. O(6)-benzylguanine enhances the in vitro immunotoxic activity of temozolomide on natural or antigen-dependent immunity. J. Pharmacol. Exp. Ther. 1999;291:1292–1300. [PubMed] [Google Scholar]
- 10.Fadul C.E., Fisher J.L., Gui J., Hampton T.H., Côté A.L., Ernstoff M.S. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro-oncol. 2011;13:393–400. doi: 10.1093/neuonc/noq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su Y.B., Sohn S., Krown S.E., Livingston P.O., Wolchok J.D., Quinn C., Williams L., Foster T., Sepkowitz K.A., Chapman P.B. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J. Clin. Oncol. 2004;22:610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 12.Liikanen I., Ahtiainen L., Hirvinen M.L., Bramante S., Cerullo V., Nokisalmi P., Hemminki O., Diaconu I., Pesonen S., Koski A. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther. 2013;21:1212–1223. doi: 10.1038/mt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martins I., Michaud M., Sukkurwala A.Q., Adjemian S., Ma Y., Shen S., Kepp O., Menger L., Vacchelli E., Galluzzi L. Premortem autophagy determines the immunogenicity of chemotherapy-induced cancer cell death. Autophagy. 2012;8:413–415. doi: 10.4161/auto.19009. [DOI] [PubMed] [Google Scholar]
- 14.Michaud M., Martins I., Sukkurwala A.Q., Adjemian S., Ma Y., Pellegatti P., Shen S., Kepp O., Scoazec M., Mignot G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 15.Candolfi M., Yagiz K., Wibowo M., Ahlzadeh G.E., Puntel M., Ghiasi H., Kamran N., Paran C., Lowenstein P.R., Castro M.G. Temozolomide does not impair gene therapy-mediated antitumor immunity in syngeneic brain tumor models. Clin Cancer Res. 2014;20:1555–1565. doi: 10.1158/1078-0432.CCR-13-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Gast G.C., Batchelor D., Kersten M.J., Vyth-Dreese F.A., Sein J., van de Kasteele W.F., Nooijen W.J., Nieweg O.E., de Waal M.A., Boogerd W. Temozolomide followed by combined immunotherapy with GM-CSF, low-dose IL2 and IFN alpha in patients with metastatic melanoma. Br. J. Cancer. 2003;88:175–180. doi: 10.1038/sj.bjc.6600717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou T.C., Talalay P. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur J Biochem. 1981;115:207–216. doi: 10.1111/j.1432-1033.1981.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 18.Kimura S., Fujita N., Noda T., Yoshimori T. Monitoring autophagy in mammalian cultured cells through the dynamics of LC3. Methods Enzymol. 2009;452:1–12. doi: 10.1016/S0076-6879(08)03601-X. [DOI] [PubMed] [Google Scholar]
- 19.Zhu T., Shen Y., Tang Q., Chen L., Gao H., Zhu J. BCNU/PLGA microspheres: a promising strategy for the treatment of gliomas in mice. Chin J Cancer Res. 2014;26:81–88. doi: 10.3978/j.issn.1000-9604.2014.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knizhnik A.V., Roos W.P., Nikolova T., Quiros S., Tomaszowski K.H., Christmann M., Kaina B. Survival and death strategies in glioma cells: autophagy, senescence and apoptosis triggered by a single type of temozolomide-induced DNA damage. PLoS ONE. 2013;8:e55665. doi: 10.1371/journal.pone.0055665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito H., Aoki H., Kühnel F., Kondo Y., Kubicka S., Wirth T., Iwado E., Iwamaru A., Fujiwara K., Hess K.R. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J. Natl. Cancer Inst. 2006;98:625–636. doi: 10.1093/jnci/djj161. [DOI] [PubMed] [Google Scholar]
- 22.Whilding L.M., Archibald K.M., Kulbe H., Balkwill F.R., Öberg D., McNeish I.A. Vaccinia virus induces programmed necrosis in ovarian cancer cells. Mol Ther. 2013;21:2074–2086. doi: 10.1038/mt.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiuri M.C., Zalckvar E., Kimchi A., Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 24.Kroemer G., Galluzzi L., Kepp O., Zitvogel L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 25.Angelova A.L., Grekova S.P., Heller A., Kuhlmann O., Soyka E., Giese T., Aprahamian M., Bour G., Rüffer S., Cziepluch C. Complementary induction of immunogenic cell death by oncolytic parvovirus H-1PV and gemcitabine in pancreatic cancer. J. Virol. 2014;88:5263–5276. doi: 10.1128/JVI.03688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotchkiss R.S., Strasser A., McDunn J.E., Swanson P.E. Cell death. N. Engl. J. Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heimberger A.B., Sun W., Hussain S.F., Dey M., Crutcher L., Aldape K., Gilbert M., Hassenbusch S.J., Sawaya R., Schmittling B. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro-oncol. 2008;10:98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki K., Fueyo J., Krasnykh V., Reynolds P.N., Curiel D.T., Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res. 2001;7:120–126. [PubMed] [Google Scholar]
- 29.Lamfers M.L., Grill J., Dirven C.M., Van Beusechem V.W., Geoerger B., Van Den Berg J., Alemany R., Fueyo J., Curiel D.T., Vassal G. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62:5736–5742. [PubMed] [Google Scholar]
- 30.Lamfers M.L.M., Fulci G., Gianni D., Tang Y., Kurozumi K., Kaur B., Moeniralm S., Saeki Y., Carette J.E., Weissleder R. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Mol. Ther. 2006;14:779–788. doi: 10.1016/j.ymthe.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pouw N., Treffers-Westerlaken E., Kraan J., Wittink F., ten Hagen T., Verweij J., Debets R. Combination of IL-21 and IL-15 enhances tumour-specific cytotoxicity and cytokine production of TCR-transduced primary T cells. Cancer Immunol. Immunother. 2010;59:921–931. doi: 10.1007/s00262-010-0818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]