Abstract
Oncolytic virus (OV) therapy utilizes replication-competent viruses to kill cancer cells, leaving non-malignant cells unharmed. With the first U.S. Food and Drug Administration-approved OV, dozens of clinical trials ongoing, and an abundance of translational research in the field, OV therapy is poised to be one of the leading treatments for cancer. A number of recombinant OVs expressing a transgene for p53 (TP53) or another p53 family member (TP63 or TP73) were engineered with the goal of generating more potent OVs that function synergistically with host immunity and/or other therapies to reduce or eliminate tumor burden. Such transgenes have proven effective at improving OV therapies, and basic research has shown mechanisms of p53-mediated enhancement of OV therapy, provided optimized p53 transgenes, explored drug-OV combinational treatments, and challenged canonical roles for p53 in virus-host interactions and tumor suppression. This review summarizes studies combining p53 gene therapy with replication-competent OV therapy, reviews preclinical and clinical studies with replication-deficient gene therapy vectors expressing p53 transgene, examines how wild-type p53 and p53 modifications affect OV replication and anti-tumor effects of OV therapy, and explores future directions for rational design of OV therapy combined with p53 gene therapy.
Keywords: oncolytic virus, virotherapy, tumor suppressor, TP53, tumor protein p53, p53, p63, p73, cancer gene therapy, combination treatment
Main Text
Oncolytic virus (OV) therapy utilizes replication-competent viruses to kill cancer cells, leaving non-malignant (“normal”) cells unharmed. The first correlative observations of tumor regression following viral infection were reported in the mid-1800s.1 In the last 20 years, the development of new genetic techniques has allowed for an explosion of preclinical and clinical research with almost every major group of animal virus being tested for OV efficacy against most cancer types. There are currently at least three OVs approved for clinical use, including the U.S. Food and Drug Administration-approved2 and The European Commission-approved3 talimogene laherparepvec (T-VEC; based on herpes simplex virus 1) for inoperable metastatic melanoma; Riga virus (RIGVIR; based on enteric cytopathic human orphan virus 7) for melanoma in Latvia, Georgia, and Armenia;4 and Gendicine and Oncorine (both based on adenovirus type 5) for head and neck squamous cell carcinoma in China.5
Rational OV development aims to generate ideal OVs that are: (1) highly attenuated in healthy tissues and safe in patients with weakened immune systems (oncoselectivity); (2) highly effective at infecting and killing cancer cells (oncotoxicity); (3) able to stimulate an adaptive immune response against cancer cells; and (4) resistant to premature clearance by the immune system during treatment. Despite encouraging results, OV monotherapy based exclusively on virus replication-induced oncolysis often does not demonstrate all of these desired qualities, especially when tested against virus-resistant malignancies. Today’s hurdles facing OV therapies remain the same as those described in early6, 7, 8 and recent reviews.9, 10 Several methods have been developed to increase the anti-cancer activities of OVs. Most commonly, OVs are engineered to express an exogenous transgene with anti-tumor activity and/or combined with standard treatments like radiotherapy or chemotherapy, or with small-molecule inhibitors of virus-host interactions. Here, we focus specifically on OVs engineered to encode a transgene for the tumor protein p53 (TP53). Since its discovery in 197911 and identification as a tumor suppressor in 1989,12 p53 has been extensively studied for its role in suppressing tumorigenesis and explored as a promising cancer therapeutic.
Serving as a tetrameric transcriptional factor,13, 14 wild-type (WT) p53 can activate or repress expression of genes involved in DNA damage, hypoxia, oncogene activation, apoptosis, senescence, cell cycle arrest, metabolism, necrosis, autophagy, and reactive oxygen species (ROS) accumulation.15, 16, 17 Importantly, WT p53 can exist in at least 12 isoforms, which are generated through different promoters, alternative splicing, and translation start sites.18 Although physical and functional interactions between these isoforms are not well understood, some of the them inhibit or stimulate biological activities of WT p53.18 Moreover, to be functional, p53 requires homotetramerization of four subunits of p53, and its activities are modified by more than 50 different posttranslational modifications, including phosphorylation, methylation, acetylation, glycosylation, ubiquitylation, sumoylation, neddylation, nitration, and poly-ribosylation.19 Additionally, the p53 tumor suppressor family includes two other members, tumor protein p63 (TP63) and tumor protein p73 (TP73), which share homology with p53 at the N-terminal transactivation domain, the central core domain for DNA binding, and the C-terminal oligomerization domain.20 However, unlike p53, p63 and p73 contain an additional Sterile Alpha Motif (SAM) domain, which plays a role in protein-protein interactions and in lipid binding.21, 22 Contrary to p53, the tetramerization domains of p63 and p73 proteins can lead to the formation of mixed heterotetramers consisting of p63 and p73, but not p53, subunits.23 Like p53, p63 and p73 are able to transactivate similar target genes including p21, BAX, and GADD45, as well as provoke cell cycle arrest and apoptosis through various mechanisms.24 In addition, p63 and p73 have the ability to trigger autophagy, senescence, differentiation, immune system activation, or angiogenesis regulation.25, 26, 27, 28, 29 A better understanding of the expression, modifications, and tumor suppressor functions of the known p53 isoforms and p63 and p73 family members is essential for the rational development of a p53-based gene therapy.
WT p53 is a key player in cancer development not only because of its normal functions as a powerful tumor suppressor, but also through its devastating roles once mutated, mostly via missense mutations (especially at hotspots V157, R158, R175, G245, R248, R249, and R273).30 Those mutations have two major consequences: First, they can cause the loss of function of normal p53 via several mechanisms. In particular, in the heterozygous form, many mutant p53 proteins show a dominant-negative effect via heterotetramerization with WT p53, preventing its normal checkpoint functions.31 Second, many p53 mutants acquire gain-of-function oncogenic activities, promoting cell survival, proliferation, invasion, migration, chemoresistance, tissue remodeling, chronic inflammation, as well as inactivation of p53 paralogs p63 and p73, which belong to the same p53 tumor suppressor family and are important tumor suppressors.32, 33 Not surprisingly, tumors depleted of the WT p53 gene retain the mutant form of the protein and thus gain a selective advantage.34 Importantly, although p53 mutations are present in approximately 50% of cancers, in almost all cases where WT p53 is retained, its tumor suppressor function is eliminated via direct binding of two main p53 binding protein groups: (1) cellular mouse double minute 2 homolog (MDM2) or transformed mouse 3T3 cell double minute 4 (MDM4: also known as MDMX),35, 36 or (2) proteins encoded by DNA viruses such as the E6 protein of high-risk human papillomavirus (HPV).37 Finally, mutations in the conformation-sensitive core domain of p53 induce the association of p53 with chaperone proteins such as heat shock protein 90 (Hsp90) forming a complex p53-Hsp90-MDM2 and provoking the MDM2 inhibition leading to the stabilization of p53 mutants.38
Various pharmaceutical approaches have been developed to restore the WT function of p53 mutants (using p53 reactivation and induction of massive apoptosis [PRIMA-1], SH group-targeting compound that induces massive apoptosis [STIMA-1], CP-31398, and other compounds) or to block interactions of WT p53 with MDM2/MDMX (using nutlin-3a, RG7112, CGM097, SAR405838, and other compounds).28, 29, 39, 40 Each of these approaches has its limitations. For example, it is unclear whether PRIMA-1 and similar compounds effectively target all mutant p53 variants and whether the tumor suppressor functions of p63 and p73 could be negatively affected by these drugs. Further, although MDM2/MDMX antagonists may be beneficial in cancers with WT p53 and high MDM2/MDMX expression, they are unlikely to be effective in tumors with a high prevalence of p53 mutations, where Hsp90 inhibits MDM2-mediated mutant p53 degradation.38 Moreover, because in the absence of this Hsp90 activity MDM2 is able to inactivate p53,38, 41, 42 the use of MDM2/MDMX antagonists in premalignant lesions may increase the number of p53 mutant forms and the risk of tumor progression. Indeed, long-term exposure to nutlin-3a promotes the emergence of p53 mutations.29, 30, 40, 43
Many of these challenges associated with p53 could be more effectively addressed through the development of approaches allowing successful delivery and expression of the WT p53 transgene in tumor cells. Unlike the pharmaceutical approaches described above, p53 gene therapy is expected to be effective independently of the p53 tumor status. The history of p53 as a cancer gene therapeutic has been reviewed.44 In brief, in the late 1980s and early 1990s it was known that treatment of cancer cells with WT p53 resulted in senescence45 or apoptosis46 depending on the cancer tested. Dr. Jack Roth (MD Anderson Cancer Center) was the first to successfully use p53 therapy in vivo in humans using replication-deficient retroviral vector-driven expression of human p53 against non-small cell lung carcinoma.47 The focus of the p53-based cancer gene therapy field then shifted to replication-deficient adenoviral vectors because of their low risk of integration into the host genome, the ability of the vectors to inhibit growth of many malignancies in vitro, and the ability to achieve cost-effective, large-scale good manufacturing practice (GMP) production.44 Since then, many clinical trials were conducted using different p53 expression replication-deficient viral vectors (discussed below), with thousands of patients receiving the therapies without significant adverse effects. This approach also had limited success.44 To date, no such therapeutic approach has been approved in the United States.
This review discusses the large number of preclinical studies combining the benefits of replication-competent OV therapy with p53 gene therapy in vitro and/or in vivo. First, we highlight how the expression of WT p53 transgenes improves OV therapy safety and oncoselectivity, increases oncotoxicity, and augments anti-tumor effects by promoting the stimulation of anti-cancer immune responses. We also review strategies to modify and improve p53 activities to counteract cancer cell resistance to p53 gene therapy. To facilitate comparisons, we organize OV-p53 viruses (WT or modified transgenes) (see Table 1) based on their modifications and the issues they address. Finally, we review preclinical and clinical studies (see Table 2) that utilized replication-deficient gene therapy vectors expressing a p53 transgene, with a focus on their prospective use in the context of OV therapy.
Table 1.
OVs Encoding p53 or p53 Family Members
| OV Name | Virus Genome Modifications | Transgene Location in Viral Genome | Transgene Modifications | Cancer Targeted | Toxicological Study | In Vitro Effects | In Vivo Tumor and Mice Models | Virus Dose (Total) and Injection Mode | In Vivo Effects | Therapeutic Combination | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenovirus SG600-p53 | E1a CR2-deleted 24 nt (nt 923–946); E1a under hTERT promoter; E1b under HRE cis-elements | TP53 between E1a and E1b | WT gene under CMV promoter | – | yes | – | – | 1–4 × 1011 VP/kg (i.v.) for safety pharmacology, 2.5 × 1013 VP/kg (i.m.) for acute toxicity test via one injection | ↑ safety ↑ toleration no adverse effects | – | 57 |
| Adenovirus SG600-p53 | E1a CR2-deleted 24 nt (nt 923–946); E1a under hTERT promoter; E1b under HRE cis-elements | TP53 between E1a and E1b | WT gene under CMV promoter | lung, liver, cervical, pancreas | no | ↑ oncoselectivity ↑ p53 expression ↑ cytotoxicity | lung subcutaneous xenograft (NCI-H1299) BALB/c nude mice | 5 × 108 to 2 × 109 PFU (i.t.) via five injections | ↓ tumor growth ↑ necrosis areas ↑ p53 level in cancer cells injected ↑ apoptosis | – | 168 |
| Adenovirus SG635-p53 | E1a CR2-deleted 24 nt (nt 923–946); E1a under hTERT promoter; E1b under HRE cis-elements Ad35 (shaft + knob) | TP53 between E1a and E1b | WT gene under CMV promoter | breast | no | ↑ infectivity ↑ viral replication ↑ p53 expression ↑ viral progeny production ↑ cytotoxicity | breast subcutaneous xenograft (Bcap-37) BALB/c nude mice | 1 × 109 PFU (i.t.) via five injections | ↑ cell growth inhibition ↑ necrosis area ↑ viral progeny production ↑ p53 level in cancer cells injected | – | 169 |
| Adenovirus AdSurp-p53 | E1a under Survivin promoter | TP53 upstream E1a | WT gene under CMV promoter | gallbladder, hepatic | no | ↑ cytotoxicity ↑ oncoselectivity ↑ p53 level ↑ viral proliferation | gallbladder subcutaneous xenograft (EH-GB1) BALB/c nude mice | 1 × 109 PFU (i.t.) via five injections | ↑ tumor growth inhibition ↑ p53 expression in cancer cells ↑ apoptosis ↑ necrosis area | – | 170 |
| Adenovirus AdCB016-mp53(268N) | E1a CR1-deleted (aa 27–80); E1a CR2-deleted 24 nt (aa 122–129) | TP53 in E3 | mutation (D268N) | cervical | no | ↑ oncoselectivity ↑ cytotoxicity ↑ resistance to HPV E6 ↑ p53 transactivation function in E6-positive cells | ND | ND | ND | – | 94 |
| Adenovirus OV.shHDAC1.p73 | E1a CR2-deleted 24 nt (nt 923–946); shHDAC1 between E4 and right terminal repeat | TP73 in fiber | WT gene | melanoma | no | ↑ cytotoxicity lifting of epigenetic blockage ↑ apoptosis ↑ autophagy no inhibition of viral replication ↑ viral progeny production | subcutaneous xenograft (SK-Mel-147) NMRI nude mice | 3 × 108 PFU (i.t.) via three injections | ↓ tumor growth no recurrence ↑ survival | shRNA against HDAC1 | 127 |
| Adenovirus Adp53rc | ADP deletion | TP53 in fiber; E3 deletion | WT gene | lung | no | no inhibition of viral replication ↑ viral spread ↑ p53 level exogenous p53 in the nucleus ↑ oncolytic activity ↑ apoptosis | – | – | – | – | 171 |
| Adenovirus Adp53W23S | ADP deletion; E3 deletion | TP53 in fiber | mutation (W23S) | lung, colorectal | no | no inhibition of viral replication ↑ viral spread ↑ resistance to E1b-55kD and MDM2 nuclear localization of p53 no export to cytosol ↑ p53 level ↑ p53 half-life (stability) ↑ cytotoxicity mildly decreased p53 transactivation | subcutaneous xenograft (A549) NCrNU-M nude mice | 1 × 109 PFU (i.t.) via one injection | Tumor size unaffected by p53 expression ↑ p53 expression in cancer cells from virus-infected areas p53 nuclear expression | – | 83 |
| Adenovirus AdΔ24-p53 | E1a CR2-deleted 24 nt (aa 122–129) | TP53 in E3 | WT gene | diverse human cancer cells | no | ↑ cytotoxicity ↑ early virus release oncolysis independent of E1a binding to pRb and independent of E3 functions | – | – | – | – | 74 |
| Adenovirus AdΔ24-p53 | E1a CR2-deleted 24 nt (aa 122–129) | TP53 in E3 | WT gene | neuroblastoma | no | ↑ cytotoxicity effect independent of endogenous p53 cellular status | subcutaneous xenografts (IGR-N91, IGR-NB8) SPF-Swiss athymic nude mice | 5 × 108 PFU (i.t.) via five injections | ↓ tumor growth ↑ cell lysis ↑ apoptosis ↑ fibrous fascicles ↑ necrosis areas | – | 76 |
| Adenovirus AdΔ24-p53 | E1a CR2-deleted 24 nt (aa 122–129) | TP53 in E3 | WT gene | glioma, glioblastoma | no | ↑ cytotoxicity effect independent of endogenous p53 cellular status replication in normal brain tissue ex vivo, but low amount of progeny virus produced | subcutaneous xenografts (IGRG88, IGRG121) SPF-Swiss athymic nude mice | 5 × 107 to 5 × 109 PFU (i.t.) via five injections | ↓ tumor growth ↑ mice survival no inhibition of viral replication ↑ apoptosis ↑ inflammatory cell infiltration (lymphocyte, macrophage) ↑ necrosis areas | – | 75 |
| Adenovirus AdΔ24-p53 | E1a CR2-deleted 24 nt (aa 122–129) | TP53 in E3 | WT gene | glioma | no | ↑ cytotoxicity with radiotherapy no change of viral replication with radiotherapy ↑ apoptosis with radiotherapy, radiotherapy does not increase p53 phosphorylation no increase of p53 on co-treatment outcome | subcutaneous xenograft (U-87MG) SPF-athymic nude mice | 1 × 109 PFU (i.t.) via three injections | ↓ tumor growth viral replication allowed no detection of exogenous p53 ↑ survival no benefits brought by exogenous p53 | radiotherapy | 106 |
| Adenovirus AdΔ24-p53(14/19) | E1a CR2-deleted 24 nt (aa 122–129) | TP53 in E3 | mutation (2 nt substitutions) T > A leading to L14Q; T > G leading to F19S | gastric, osteosarcoma, lung, ovarian, astrocytoma | no | ↑ cytotoxicity ↑ resistance to MDM2 ↑ p53 stability ↑ p53 half-life mutation provokes moderate loss of p53 transactivation function | – | – | – | – | 172 |
| Adenovirus S7605-11R-p53 | E1a under hTERT promoter; absence of E1b and its promoter too | TP53 in E3 | fused with 11R (polyarginine peptide), CMV promoter | gallbladder | no | ↑ cytotoxicity ↑ oncoselectivity | BALB/c nude mice (EH-GB2) | 1 × 109 PFU (i.t.) via five injections | ↓ cell growth ↑ necrotic foci ↑ p53 level in cells ↑ apoptosis | – | 99 |
| VSV-mp53 | WT | murine TP53 between G and L | WT gene | breast, melanoma | yes | WT M protein blocks host mRNA export ↑ oncoselectivity ↑ p53 level | TS/A-luc tumor cells (injected i.v.) in BALB/c mice and BALB/c nude mice | 5 × 107 or 5 × 108 PFU (i.v.) via one injection for toxicity test, increasing doses from 1 × 108 to 1 × 109 PFU (i.v. and i.n.) | ↑ virus attenuation in immunocompetent mice ↓ cell growth ↑ mice survival fatal toxicity after i.n. injection in nude mice | – | 62 |
| VSV-M(mut)-mp53 | M (matrix protein) mutations (aa 52–54) | murine TP53 between G and L | WT gene | breast, melanoma | yes | M(mut) allows host mRNA export ↑ oncoselectivity ↑ p53 level | TS/A-luc tumor cells (injected i.v.) in BALB/c mice and BALB/c nude mice | 5 × 107 or 5 × 108 PFU (i.v.) via one injection for toxicity test, increasing doses from 1 × 108 to 1 × 109 PFU (i.v. and i.n.) | ↑ virus attenuation in immunocompetent mice ↓ toxicity ↓ cell growth ↑ mice survival ↑ anti-tumor immunity (IFN, CD49b+ NK, CD8+ T responses) ↓ inflammatory cytokines (IL-6, IP-10) fatal toxicity after i.n. injection in nude mice | – | 62 |
| VSV-p53wt | M (matrix protein) mutation ΔM51 | TP53 between G and L | WT gene | pancreas | no | no inhibition of viral replication ↑ oncoselectivity ↓ IFN response (NF-κB inhibition) nuclear localization of exogenous p53 | – | – | – | – | 67 |
| VSV-p53CC | M (matrix protein) mutation ΔM51 | TP53 between G and L | chimeric p53CC | pancreas | no | no inhibition of viral replication ↑ oncoselectivity ↓ IFN response (NF-κB inhibition) counteract dominant-negative effects from endogenous mutant p53 nuclear localization of exogenous p53 | – | – | – | – | 67 |
| Newcastle disease virus rNDV-p53 | WT | TP53 between F and HN | WT gene | hepatoma | yes | ↓ cell growth ↑ exogenous p53 level no inhibition of viral replication with exogenous p53 ↑ early apoptosis (reduction of mitochondrial membrane potential) | subcutaneous xenograft (H22) ICR mice | 1 × 107 PFU (i.t.) via one injection 10 systemic injections for toxicity study | ↓ tumor growth ↑ mice survival ↑ apoptosis no toxicity and no serum chemistries changes | – | 61 |
Mode of injections: i.m., intramuscular; i.n., intranasally; i.t., intratumoral; i.v., intravenous. CMV, cytomegalovirus; CPU, colony-forming unit; ND, not determined; PFU, plaque-forming unit; VP, viral particles.
Table 2.
Clinical Trials with Replication-Deficient Viruses Encoding p53
| Trial ID | Phase | Cancer | Virus Name | Combination with Treatments/Vaccination | Patients (n) | Mode of Injection | Reported Results | Dose | References |
|---|---|---|---|---|---|---|---|---|---|
| I | lung NSCLC | adenovirus rAd/p53 (SCH58500) | – | 15 | i.t. | no toxicity (safe) transient gene transfer efficacy detection of specific WT p53 sequences local disease control no response at untreated tumor sites 2 patients with SDI 11 patients with PD | 1 × 107 to 1 × 1010 PFU (= 7.5 × 109 to 7.5 × 1012 VP) (dose escalation) | 128 | |
| I | advanced solid cancers | adenovirus Ad-p53 | hyperthermia | 15 | i.t. | no dose-limiting toxicity, safe and efficient four patients with PR two patients with CR eight patients with SDI one patient with PD | 1 × 1012 VP given four to eight times | 173 | |
| I | NSCLC | adenovirus Ad-p53 | – | 28 | i.t. | minimal toxicity presence of vector in 86% of patients apoptosis increased in 11 patients 2 patients with PR 16 patients with SDI 7 patients with PD | 1 × 106 PFU to 1 × 1011 PFU given monthly up to six times | 174 | |
| I/II | metastatic pancreatic cancer | adenovirus SCH58500 | gemcitabine | 3 | intra-arterial | transient toxicity one patient with transient SDI one patient with reduction of tumor burden | 2.5 × 1012 VP (four times), or 2.5 × 1012 VP (two times), or 7.5 × 1012 VP (one time) | 129 | |
| I | colorectal | adenovirus SCH58500 | – | 45 | hepatic arterial infusion | limited and transient inflammatory responses increase of total and neutralizing Ab virus delivery in tumor and liver apoptosis detected in tumor with an elevated nuclear expression of p53 | 7.5 × 109 to 7.5 × 1013 VP for single dose or 7.5 × 1011 to 2.5 × 1013 VP for multiples doses | 130 | |
| I | NSCLC | adenovirus Ad-p53 | cisplatin | 24 | i.t. | mild toxicity transgene expression detected in tumor apoptosis increased in 11 patients 2 patients with PR 17 patients with SDI 4 patients with PD | 1 × 106 to 1 × 1011 VP (dose escalation) | 175 | |
| I | glioma | adenovirus Ad-p53 (INGN 201) | – | 15 | i.t. | minimum of toxicity exogenous p53 detection in tumor cells in all patients apoptosis triggering lack of viral widespread no determination of response to Ad-p53 | 3 × 1010 to 3 × 1012 VP (dose escalation) | 131 | |
| II | squamous cell carcinoma of the oral cavity | adenovirus Ad5CMV-p53 (INGN 201) | cisplatin + radiotherapy | 13 | i.t. | excellent 1-year progression-free survival rate (92%) | 2.5 × 1012 VP (three times) | 132 | |
| II | breast cancer | adenovirus Ad5CMV-p53 | docetaxel and doxorubicin | 13 | i.t. | no systemic toxicity (safe) no CR increase in p53 mRNA local immunomodulator effects | 2.5 × 1012 VP (two times/cycle during six cycles) | 176 | |
| I | HNSCC | adenovirus Ad-p53 | – | 33 | i.t. | no toxicity p53 expression detected two patients with tumor regression six patients with SDI one patient with CR nine patients with PD | 1 × 106 to 1 × 1011 PFU (dose escalation) 3 × 1012 PFU/patients (total dose) | 177 | |
| I | bladder | adenovirus SCH58500 (rAd/p53) | – | 12 | i.t., intravesicular instillation | mild toxicity (safe) p53 transgene detection in tumor cells high transduction of adenovirus in tumor by intravesical instillation | 7.5 × 1011 to 7.5 × 1013 VP (one dose) | 133 | |
| I/II | ovarian | adenovirus rAd-p53 (SCH58500) | – | 41 | i.p. | no toxicity (safe) no severe side effects increase of serum adenoviral antibody titers effects virus tolerated detection of p53 transgene | 7.5 × 1010 to 7.5 × 1013 VP (single or multiple doses) | 134 | |
| II | NSCLC | adenovirus rAd/p53 (SCH58500) | chemotherapy (carboplatin + paclitaxel or cisplatin + vinorelbine) | 25 | i.t. | mild toxicity transgene detection in tumor no benefit of p53 viral therapy on lesions no benefit concerning the survival of patients | 7.5 × 1012 VP/cycle during three cycles | 135 | |
| II | lung | adenovirus Ad-p53 (INGN 201) | radiation | 19 | i.t. | tolerated treatment tumor regression at the primary injected tumor increase of BAK gene expression 1 patient with CR 11 patients with PR 3 patients with SDI 2 patients with PD | 3 × 1011 to 3 × 1012 VP (three times) | 136 | |
| I | lung | adenovirus Ad-p53 | – | 25 | bronchoalveolar lavage (BAL) | safe low side effects for 5 × 1011 VP 16 patients with SDI | 2 × 109 to 2 × 1012 VP (dose escalation) | 138 | |
| I | NSCLC | retrovirus having p53 transgene (ITRp53A) | – | 9 | i.t. | no toxic effects increase of apoptosis detection of vector p53 sequence | 5 × 107 CFU (single or multiple cycles) | 47 | |
| I/II | metastatic malignant liver tumors | adenovirus CMV-p53 | – | 19 | hepatic artery infusion | ND | 1 × 109 to 3 × 1011 PFU (dose escalation) | 178 | |
| I | ovarian | adenovirus Adp53 (INGN 201) | – | 17 | i.p. | no severe toxicities effects virus tolerated (no dose limited toxicities) four patients with DS five patients with PD | 3 × 1010 to 3 × 1012 VP (daily for 5 days every 3 weeks) | 137 | |
| – | NSCLC | adenovirus rAd-p53 | bronchial arterial infusion (BAI) | 58 | i.t. or bronchial arterial access | virus tolerated delay of the disease progression increase patient life quality no benefit on patient survival | 1 × 1012 to 4 × 1012 VP (four times) | 179 | |
| I | SCLC | adenovirus Ad-p53 | vaccination with dendritic cells infected by Ad-p53 | 29 | intradermal | 57.1% of patients present a p53-specific T cell response slight increase of anti-adenovirus antibodies better response to chemotherapy after vaccination one patient showed a clinical response to vaccination | ND | 139 | |
| NCT01191684 | I | refractory gastrointestinal cancers | poxvirus Modified Ankara virus (p53MVA) | vaccination | 12 | subcutaneous | virus tolerated no severe adverse events strong CD8+ T cell response against cancer cells expressing p53 | 1 × 108 to 5.6 × 108 PFU (dose escalation) | 147 |
| NCT00003147 | I | hepatocellular carcinoma | Ad5CMV-p53 | – | – | percutaneous injection | – | ND | |
| NCT02418988 | II | hepatocellular carcinoma | rAd-p53 | TACE (transarterial chemoembolization) | – | arterial injection | recruiting | ND | |
| NCT00902083 | IV | oral and maxillofacial tumors | rAd-p53 | chemotherapy/surgery | – | i.t. | – | 1 × 1012 VP | |
| NCT00902122 | IV | thyroid | rAd-p53 | radioactive iodine/ surgery | – | i.t. | – | 1 × 1012 VP | |
| NCT02429037 | II | head and neck | rAd-p53 | radio-chemotherapy (cisplatin/paclitaxel) | – | i.t. | – | 2 × 1012 VP | |
| NCT02429726 | II | malignant pleural effusion | rAd-p53 | cisplatin | – | intra-chest cavity infusion | – | 2 × 1012 VP | |
| NCT02275039 | I | recurrent ovarian cancer | poxvirus Modified Ankara virus (p53MVA) | vaccination + gemcitabine | – | subcutaneous | ongoing | ND | |
| NCT02432963 | I | solid tumors | poxvirus Modified Ankara virus (p53MVA) | vaccination + pembrolizumab | – | subcutaneous | ongoing | ND | |
| NCT00776295 | II | SCLC | adenovirus Ad-p53 | vaccination with dendritic cells infected by Ad-p53 and T lymphocytes | – | unknown | – | ND | |
| NCT00049218 | I/II | SCLC | adenovirus Ad-p53 | vaccination with dendritic cells infected by Ad-p53+ carboplatin or etoposide | – | subcutaneous | – | ND |
CR, complete response; i.p., intraperitoneal; i.t., intratumoral; mRNA, messenger RNA; NSCLC, non-small cell lung cancer; PD, progressive disease; PR, partial response; SCLC, small cell lung cancer; SDI, stable disease.
Arming Viruses with WT p53 Transgene to Improve OV Therapy
Improved Safety and Oncoselectivity
An ideal OV must be safe and oncoselective, meaning that the virus should replicate in cancer cells, but not in normal cells, and that any negative side effects should be limited. WT p53 endogenous gene expression was shown to inhibit replication of various viruses in normal cells in vitro and in vivo via enhanced type I interferon (IFN) antiviral signaling and apoptosis in infected cells.48, 49, 50, 51, 52, 53, 54, 55, 56 For example, in contrast with WT mice, p53−/− mice were highly susceptible to vesicular stomatitis virus (VSV; a rhabdovirus) and succumbed to VSV infection. Furthermore, a 100-fold increase in VSV load was observed in the sera of p53−/− mice compared with WT mice.49 Based on these studies, the addition of a p53 transgene to an OV genome may attenuate the OV in normal cells.
The conditionally replicative adenovirus (CRAd) encoding p53 (SG600-p53) has been subjected to a comprehensive safety study by Su et al.57 The CRAds are replication-competent adenoviruses that selectively replicate in tumor cells. The restriction of CRAd replication to tumor cells is generally based on a tumor-specific promoter controlling the expression of an essential early adenovirus gene or involves mutations in viral genes,44, 45, 58, 59 allowing CRAds to replicate in tumor cells, but not in normal cells.60 Su et al.57 investigated SG600-p53, where the viral E1a gene has a deletion of 24 nt and is controlled by the promoter of human telomerase reverse transcriptase (hTERT), the E1b promoter is replaced by a cis-element of five copies of hypoxia regulatory element (HRE), and the p53 transgene cassette was inserted between the E1a and E1b genes. Following intravenous (i.v.) administration of increasing virus doses in mice (1–4 × 1011 virus particles [VP]/kg) or in cats (2–8 × 1010 VP/kg), no toxic effect was observed (behavioral, nervous, cardiovascular, and respiratory systems were examined).57 Further, after the intramuscular (i.m.) administration of a very large virus dose (2.5 × 1013 VP/kg), mice survived without weight loss or signs of cyanosis, hyperspasmia, inflammation, or ulceration. Whether SG600-p53 could trigger anaphylaxis in guinea pigs following several intraperitoneal (i.p.) and i.v. injections also was tested. No animals died or presented signs of anaphylaxis. Repeated i.m. injections of SG600-p53 in rats (1–10 × 1011 VP/kg) and in cynomolgus monkeys (5–50 × 1010 VP/kg) were also shown to be safe.57
Other OVs armed with a p53 transgene were demonstrated to be safe in animals, including Newcastle disease virus (NDV; a paramyxovirus)61 and various recombinant VSVs.62 Toxicity studies in mice showed that after 10 systemic injections of rNDV-p53 (1 × 107 PFU each), the serum from mice showed no changes in serum creatinine, aspartate transaminases, alanine transaminases, or blood urea nitrogen levels, supporting the safety of that virus.61 However, neither the CRAd SG600-p5357 nor rNDV-p5361 studies investigated specific contributions of the p53 transgene to the safety of these viruses because they were not compared with control viruses. This issue was addressed in a VSV study comparing VSV-M(mut)-mp53 [an attenuated VSV-M(mut) virus encoding murine p53] against its parental virus VSV-M(mut).62 The study showed that following i.v. injection of 108 to 109 plaque-forming units (PFUs), 84% (6/7) of the mice treated with VSV-M(mut)-mp53 compared with 14% (1/7, one mouse died unrelated to virus-induced encephalitis) of the mice treated with VSV-M(mut) survived treatment, indicating that VSV-M(mut)-mp53 was safer than VSV-M(mut). This study utilized M(mut), a mutant matrix (M) protein gene of VSV used to improve the virus safety profile and maximize activities of the p53 transgene.62 Several well-studied VSV M mutations [such as a mutation or deletion of the methionine codon at position 5163, 64, 65 or mutation of residues 52–54 from DTY to AAA62 in the M(mut)] abrogate M protein’s ability to inhibit nuclear export of host mRNAs, including those for antiviral genes induced by VSV infection. Consequently, VSV recombinants bearing such M mutations cannot inhibit host gene expression and are highly attenuated in normal cell types, but they remain very effective in tumors, most of which show various defects in antiviral responses.66 The utilization of these mutant M proteins not only improves VSV oncoselectivity, but also allows for the expression of p53 target genes in cancer cells infected with VSV-M(mut)-mp53.
Although these studies demonstrated safety of OVs bearing p53 transgenes, over-attenuation of the OVs as a result of the WT p53 activities that stimulate antiviral signaling48, 49, 50, 51, 52, 53, 54, 55, 56 remained a concern. Indeed, such activities of virus-encoded p53 transgenes in cancer cells could reduce OV efficacy by decreasing virus oncoselectivity. To address this important concern, our recent study engineered and compared VSV recombinants either expressing WT human p53 fused to near-infrared fluorescent protein (eqFP650, herein called RFP) (“VSV-p53wt”) or expressing RFP only (“VSV”) for the abilities of these viruses to induce type I IFN signaling in several human pancreatic ductal adenocarcinoma (PDAC) cell lines.67 Surprisingly, and in contrast with the expected enhancement of antiviral signaling by p53, expression of genes associated with type I IFN signaling pathway was dramatically attenuated in p53 transgene-expressing cells. For example, although a 291-fold increase in IFN-β transcripts was detected in cells infected with the VSV recombinant that does not express p53, only a 25-fold increase in IFN-β transcripts was observed in cells infected with VSV-p53wt.67 These data suggest that OV-encoded p53 can simultaneously produce anti-cancer activities while assisting, rather than inhibiting, virus replication in cancer cells. Although the exact mechanism of this observation is unknown, our additional experiments suggested that p53 is likely to inhibit the type I IFN response via inhibition of nuclear factor κB (NF-κB), which is usually constitutively expressed in PDAC cancer cells and is crucial for early IFN-β expression and resistance to virus replication.68 The inhibition of the NF-κB pathway by p53 via several mechanisms has been shown, including prevention of NF-κB nuclear translocation through upregulation of NF-κB inhibitor, IκBα,69 competition between p53 and NF-κB for common transcription cofactors,70 or direct binding of p53 to NF-κB (RelA subunit p65) to prevent its binding to DNA.71 In addition, p53 also can decrease the IFN response through STAT1 inhibition, in particular by increasing the expression of the STAT1 inhibitor suppressor of cytokine signaling 1 (SOCS1).72 Importantly, our study also showed that recombinant VSVs encoding the p53 transgene were not able to inhibit antiviral signaling in non-malignant human pancreatic ductal cells, which retained their resistance to viral infection. The inability of p53 to inhibit OV replication in cancer cells also was demonstrated for adenoviruses expressing p53 in vitro.73, 74 One of these studies compared AdΔ24 and AdΔ24-p53 viruses.74 AdΔ24 carries a 24-bp deletion corresponding to aa 122–129 in the CR2 domain of E1A necessary for binding to the Rb protein, and it lacks the entire E3 region, whereas AdΔ24-p53 also encodes a p53 transgene in place of the deleted E3 region. In vivo, AdΔ24-p53 demonstrated the same rate of replication in subcutaneous xenograft glioma IGRG121 in athymic nude mice compared with the parental virus AdΔ24.75 Similarly, the inability of p53 to inhibit replication of AdΔ24-p53 was observed in IGR-NB8 and IGR-N91 neuroblastoma tumors.76
Improved Oncotoxicity
One of the major advantages of using p53-armed OVs is their enhanced oncotoxicity in vitro and in vivo. In this section, we focus on direct OV-mediated oncotoxicity following virus infection and replication in cancer cells, with cell death occurring independently of the activation of adaptive immunity against tumor. Such direct oncotoxicity is typically measured in vitro (where immune cells are naturally absent) or in vivo in athymic mice, typically by testing an OV against a panel of human cancer cell lines. Most studies examining the effects of WT p53 transgene expression on OV cytotoxicity have been conducted using CRAds. The original study analyzing the effect of human WT p53 on CRAd cytotoxicity was conducted using AdΔ24-p53.74 The addition of the p53 transgene resulted in virus cytotoxicity in vitro in 80% of tested cancer cells from different tissues, accelerating cell death by several days and increasing the early virus progeny release. The CRAd potency was 100-fold higher when p53 was expressed, regardless of the test cell endogenous p53 status (p53-WT, p53 null, p53-R248Q, or p53-R273H).74
The same CRAd AdΔ24-p53, compared with the parental AdΔ24, was more potent in killing malignant glioma cell lines75 and neuroblastoma cell lines.76 As in the previously described study,74 the increase of viral cytotoxicity was independent of the intracellular p53 status. Interestingly, the glioma xenograft-derived short-term cultured IGRG121 (primary multiforme glioblastoma) and IGRG88 (malignant oligodendroglioma) cell lines were both sensitive to CRAds in vitro but responded differently in vivo.75 Indeed, compared with injections of parental AdΔ24, intratumoral (i.t.) injections of AdΔ24-p53 into IGRG121 xenograft in athymic nude mice led to delayed tumor growth, tumor regression, and improved survival.75 Tumor mass showed increased necrosis, lymphatic infiltration, and apoptosis.75 In contrast, both AdΔ24-p53 and AdΔ24 were ineffective against IGRG88 in vivo. This study highlights certain limitations of p53-mediated OV therapy.
WT p53 transgenes also increased cytotoxicity of two other OVs, VSV and NDV.48, 54, 61, 67 In our recent study, VSV-p53 showed enhanced killing of the pancreatic cancer cell line SUIT2 compared with VSV not expressing p53 when these viruses were tested at a low MOI.67 rNDV-p53 encoding a p53 gene provoked stronger suppression of cell growth in HepG2 human hepatocellular carcinoma cells and improved early apoptosis by reducing mitochondrial membrane potential, compared with rNDV in vitro.61 In vivo, after i.t. injection, rNDV-p53 suppressed tumor growth in an H22 murine hepatoma cell xenograft model in nude mice more efficiently than rNDV, notably by stronger apoptosis induction. Importantly, rNDV-p53 also significantly improved the survival of mice.61
Stimulation of Anti-tumor Immunity
OV therapies aim not only for direct oncolysis of cancer cells following virus replication, but also stimulation of a host’s anti-cancer (innate and adaptive) immune responses. Various studies have shown that beneficial p53 functions include promotion of enhanced anti-tumor immunity, both innate and adaptive.77 Therefore, it was anticipated that p53 transgene expression would augment the anti-tumor immunity to help eliminate the tumor during OV treatment. When VSV-M(mut)-mp53 was assessed against B16 (F10) melanoma and TS/A breast cancer cells in vivo,62 i.v. injections of the virus (5–50 × 107 PFU) protected BALB/c immunocompetent mice from TS/A tumor by dramatically decreasing tumor burden and improving survival compared with control VSVs not containing the p53 transgene. Because the in vitro replication levels of VSV-M(mut)-mp53 and VSVs not containing the p53 transgene were similar in cancer cells, the in vivo tumor burden decrease could be attributed to VSV-encoded p53-improved anti-tumor immunity. Indeed, VSV-M(mut)-mp53 increased the number of CD8+ cells in the spleen, as well as the number of T cells secreting IFN-γ. Further, VSV-M(mut)-mp53 also increased the number of CD49b+ natural killer (NK) cells in the spleen and IFN-β level in the serum. Finally, VSV-M(mut)-mp53 reduced the production of inflammatory cytokines, such as interleukin (IL)-6 and IFN gamma-induced protein 10 (IP-10; promoters of angiogenesis and cell growth), facilitating tumor clearance.
An enhanced innate immune response against tumor cells was reported for AdΔ24-p53, which was efficient in vivo against different neuroblastoma xenografts (IGR-N91 and IGR-NB8) in athymic nude mice after i.t. injection by provoking the infiltration of inflammatory cells such as lymphocytes and macrophages into the tumor site.76
Modifying p53 Activities to Improve OV Therapy
OVs Encoding Modified p53 Transgenes
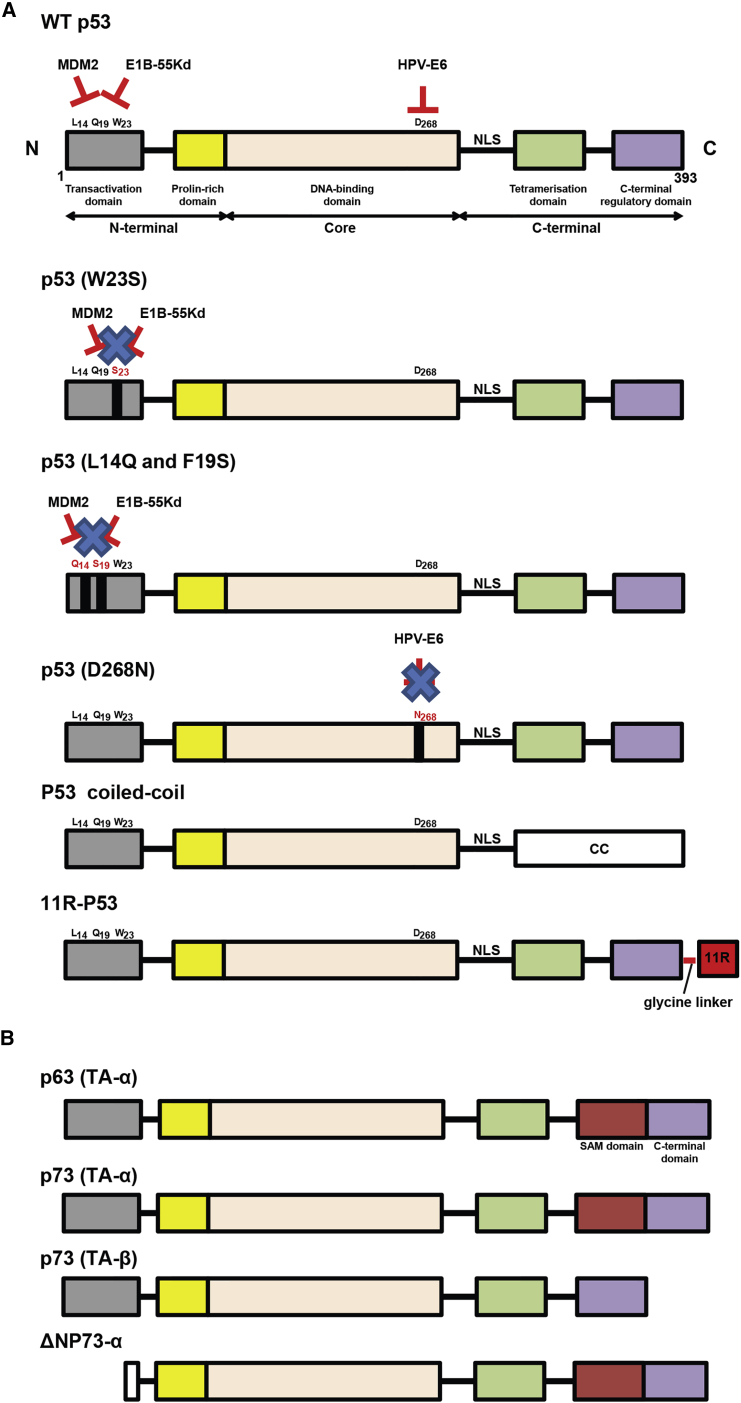
Many strategies have been developed to increase p53 efficacy and counteract resistance of cancer cells to p53 therapy to improve OV-p53 therapy. One of the most notable problems of any p53-based cancer gene therapy is the ability of many tumors to inhibit virus-encoded exogenous p53 activities by cellular proteins such as MDM2 or MDMX,35, 36 or viral proteins such as E6 of HPV.37 Various modifications of WT p53 have been engineered to prevent such inhibitions. For example, endogenous cellular p53 expression is controlled by a cellular E3 ubiquitin protein ligase, which regulates p53 expression through a negative feedback loop. When p53 is expressed, MDM2 binds to and ubiquitinates p53 to cause its degradation by the proteasome pathways.65, 66, 78, 79 Such MDM2 activity can inhibit not only endogenously expressed p53, but also virus-encoded exogenous p53. MDM2 is known to be overexpressed in many cancers like lung, glioma, myeloma, chronic lymphocytic leukemia, B cell lymphoma, and osteosarcomas cancer cells.80 In addition to MDM2, the adenoviral proteins E1b55K and E4orf6 assemble an E3 ubiquitin ligase complex together with cellular proteins Cullin 5 and Elongin B/C, and, similarly to MDM2, induce p53 degradation.68, 69, 81, 82 Thus, cancer cells, via MDM2 overexpression or when infected by E1b-55Kd-expressing adenoviruses, develop resistance to exogenous p53, decreasing the efficacy of p53-based gene therapy. To address this issue, Sauthoff et al.83 engineered an oncolytic adenovirus encoding a p53 transgene with amino acid (aa) 23 mutated from tryptophan to serine (W23S) (Figure 1). Although p53 interacts with MDM2 via aa 19, 23, and 26, the W23S change was sufficient to abrogate binding between both proteins.84 Importantly, the same mutation also prevented E1B-55Kd-mediated degradation of p53, but did not decrease the p53 capacity to transactivate its target genes such as p21, which is important for the promotion of the anti-tumor effects of p53. In addition, this mutation did not inhibit the infectivity of the oncolytic adenovirus.83 In agreement with the improved qualities of the p53-W23S protein, the adenovirus Adp53W23S was more efficient in vitro in several tested cell lines compared with the Adp53 (encodes WT p53) and Ad-co viruses (a control adenovirus not encoding p53). However, in an A549 lung tumor xenograft tumor model, tumor size was unaffected by p53 expression. It is likely that the failure in vivo was due to a possibility that p53-W23S, although resistant to MDM2 and E1B-55Kd, remained sensitive to the activities of two other adenoviral proteins, E1b-19Kd or E1a, both known to oppose p53 functions. Indeed, E1b-19Kd is known to be an inhibitor of p53-dependent apoptosis.72, 73, 85, 86 In addition, two cellular proteins, p300 or p400/TRAPP, are cofactors of p53 and also can be inhibited by E1a, leading to a loss of p53 function.87, 88, 89 Whether Adp53W23S was impeded by sensitivity of p53-W23S to those adenoviral proteins should be addressed in future studies with adenovirus-based OVs.
Figure 1.
Schematic Representation of the p53 Family Members Described in This Review
(A) Modifications of p53 to increase the WT p53 efficacy and counteract resistance of cancer cells to OV-p53 therapy. The different domains of WT p53 are shown. The amino acid residues substituted in some p53 mutants are shown in red, whereas the non-modified residues are indicated in black. (B) Schematic representation of p63 and p73 proteins, which share some domains with p53, but also contain the Sterile Alpha Motif (SAM) domain. The anti-apoptotic isoform of p73 (ΔTAp73) is overexpressed in metastatic melanomas, and it can oppose the proapoptotic functions of p53 and full-length p73. To counteract ΔTAp73, we used a transcriptionally active form of p73 (isoform TA-p73β). 11R, 11 polyarginine peptide; CC, coiled-coil; NLS, nuclear localization signal.
In addition to p53-W23S, other rationally designed p53 transgenes have been used in OVs. The AdΔ24-p53(14/19) encodes a p53 mutant with two aa substitutions (L14Q and F19S), which results in the resistance of p53 to MDM2-mediated degradation.76 AdΔ24-p53(14/19) was tested in different cancer cells known to overexpress MDM2, such as MNNG-HOS and MG-63 osteosarcoma, A2780 ovary carcinoma, MKN45 gastric adenocarcinoma, and SF763 astrocytoma cell lines. Compared with AdΔ24 and AdΔ24-p53, AdΔ24-p53(14/19) was 10 times more efficient in killing these cell lines, although p53(14/19) was less effective in transactivation of target genes, compared with WT p53.
Another challenge to p53-based gene therapy is the expression of p53 targeting viral oncoproteins in many cancer types, originated from previous viral infections, notably high-risk HPV-encoded E6 oncoprotein. The HPV E6 protein is known to promote cell proliferation by stimulating degradation of p53 via the formation of a trimeric complex comprising E6, p53, and the cellular ubiquitination enzyme E6-AP.90, 91, 92, 93 Such E6-expressing tumors are expected to be highly resistant to p53-based therapy. To prevent E6-mediated p53 inactivation, Heideman et al.94 used an oncolytic adenovirus AdCB016 encoding a mutant of murine p53 (mp53) called mp53(268N), where an aspartate residue was substituted by asparagine at the position 268 of p53 to evade E6. The data indicate that this mutation did not inhibit transcriptional activity of mp53(268N) but prevented its interaction with HPV E6 protein. The mp53(268N) had the same transactivation activities as WT p53 in the HPV-negative SaOs-2 cells, whereas mp53(268N)-mediated transactivation was 30-fold more efficient in the HPV-positive cells (SiHa, CaSki, HeLa cells, and HPV16 E6-expressing SaOs-2).94 It should be noted that the AdCB016-mp53(268N) virus, in addition to the successful expression of functional p53, exhibited preferential replication in HPV E6/E7-expressing keratinocytes compared with normal keratinocytes. This is due to two deletions in the adenoviral E1A protein, one in the CR2 domain to prevent sequestration of cellular retinoblastoma protein (pRb) from E2F by HPV E7, and another one in the CR1 domain to abolish E1A binding to p300 histone acetyl transferase (required for adenoviral replication), which can be functionally complemented by HPV E6. Therefore, AdCB016 can preferentially replicate in HPV E6/E7-expressing cells.95
Another challenge for p53 gene therapy is the expression of endogenous p53 dominant-negative mutants in many cancer types. Mutant p53 proteins show a dominant-negative effect via heterotetramerization with WT p53, preventing its normal checkpoint functions.31 Several approaches may be applicable to address this challenge, although most are not yet tested in the context of OV therapy. For example, Okal et al.96 engineered a chimeric p53-CC, which evades the dominant-negative activities of endogenously expressed mutant p53. Similarly to the C terminus of WT p53, the CC domain of the Bcr protein allows for formation of antiparallel tetramers, but unlike the C terminus of WT p53, the CC domain is not a binding site for endogenous mutant p53.96 As a result, the chimeric p53-CC protein was shown to evade endogenous dominant-negative p53 and restore p53 activity better than WT p53 in vitro.96 In our work, we adopted this p53 design to generate VSV expressing either WT p53 or p53-CC.67 The VSV-directed p53-CC was tested in several pancreatic cancer cell lines and demonstrated better transactivation activities compared with VSV-directed WT p53. Although both WT p53 and p53-CC inhibited type I IFN response in cancer cells, but not normal cells (likely via blocking the NF-κB pathway), the p53-CC transgene did so more effectively.67
Finally, an interesting approach was used by Takenobu et al.97 to increase the efficacy of the exogenous p53 by fusing it to a cell-penetrating peptide (CPP), 11R (11 polyarginine peptide). CPPs are used in gene therapy applications to facilitate cellular intake/uptake of various molecules (from small molecules and large fragments of DNA or even whole proteins) through endocytosis.98 The engineered adenovirus virus, SG7605-11R-p53, tested in gallbladder cancer cell lines in vitro and against EH-GB1 xenografts in vivo in mice, demonstrated that infection with p53-11R fusion improved the anti-tumor effect and prolonged survival, compared with control viruses.99 The mechanisms of this improvement are unclear. It is possible that after successful replication of SG7605-11R-p53 in infected cells, a large amount of p53-11R was produced, released, and, because of 11R, was able to enter uninfected cells to improve the bystander effect.
Combination of OV-p53 Transgene with Radiotherapy
Because p53 has been shown to enhance the effects of radiotherapy (radiosensitization),100, 101, 102, 103, 104, 105 Idema et al.106 tested the combination of OVs with radiotherapy against glioma cancer cells in vitro and in vivo. Either AdΔ24 or AdΔ24-p53 and radiotherapy increased anti-tumor efficacy compared with each single treatment, both in vitro and in vivo. In vitro, AdΔ24-p53 was more effective against glioma cells than the control AdΔ24, and the combination of radiotherapy with AdΔ24-p53 caused an increase in the percentage of apoptotic cells. In contrast, although OV and radiotherapy combinations increased anti-tumor efficacy compared with each single treatment in vivo, no differences between viruses could be seen. Although this study highlights that AdΔ24 or AdΔ24-p53 combined with radiotherapy can eradicate tumors, which would otherwise escape OV monotherapy, the authors concluded that this additive effect was not due to the p53 transgene expression.106
Lessons from Preclinical and Clinical Studies Using Replication-Deficient p53 Gene Therapy Vectors
Expressing Other p53 Family Members
Although several approaches to overcome the exogenous p53 inactivation in tumors were described above, another approach uses other members of the TP53 gene family, p63 (TP63/p51/p73L/p40) or p73 (TP73), each presenting unique advantages. For example, p63 and p73 can arrest the cell cycle in G1 phase via upregulation of p21 and p57/Kip2 transcripts. Moreover, p63 and p73 can trigger intrinsic apoptosis via the induction of Bax and PUMA proteins, leading to BAX translocation to the mitochondria, cytochrome c release, and a decrease in the mitochondrial membrane potential.107, 108, 109 Importantly, p73 and p63 can also induce cell death in a p53-independent manner through the upregulation of scotin, a trans-membrane protein that causes endoplasmic reticulum (ER) stress.98, 99, 109, 110 On the other hand, p63 and p73 also act on the extrinsic apoptosis pathway by inducing the expression of death receptors like TRAIL-R1/R2, TNF-R1, and FAS.96, 98, 100, 107, 109, 111 Thus, p63 or p73 therapy could complement the apoptosis triggered by the endogenous p53 or, in the case of p53-deficient cancer cells, allow the induction of apoptosis in a p53-independent manner. Kunisaki et al.112 showed that the overexpression of p53 and p63 by adenoviral transduction in EBC1 lung cancer cells led not only to an improved suppression of cell growth in vitro, but also in vivo via apoptosis induction. Unlike p53 and p73, p63 interacts very weakly with MDM2 and has strong anti-tumor activities even in cancer cells overexpressing MDM2.41 Also, the oncoproteins E6 and E1B-55Kd are not able to inactivate the p73 transgene encoded by an adenoviral vector, which is still active against cervical cancer cells overexpressing HPV E6.113, 114, 115 Similarly, Das et al.116, 117, 118 demonstrated that a replication-deficient adenovirus (Ad-p73) bearing a TP73 transgene showed stronger growth inhibition of HPV 16 E6-expressing cells, but also other human cancer cells via a cell cycle G1 phase arrest, and increased apoptosis, with an increased p21 expression noted after infection by Ad-p73.
In contrast with p53, which is often mutated and inactivated in human cancers, only a few mutations have been reported for p63 or p73.20 For example, the N-terminally truncated p73α (ΔNp73α, lacks its transactivation domain) is overexpressed in some cancers (e.g., breast, lung, neuroblastoma, vulval, and ovarian), and this mutant is associated with a poor prognosis in patients. Indeed, ΔNp73α cellular expression impairs the abilities of p53 and p73 to transactivate target genes, which leads to apoptosis resistance. This truncated form of the p73 protein has been assessed in immunotherapy to overcome immune tolerance and to develop an anti-tumor response. Hu et al.119 showed that immature dendritic cells transduced by a ΔNp73α recombinant adenovirus presented the tumor-associated antigen ΔNp73α to lymphocytes, which in vitro provoked a specific immune cytotoxic T lymphocyte (CTL) response against A549, K-562, and MCF7 cancer cells expressing high levels of ΔNp73α. Moreover, metastatic melanoma cells typically overexpress the p73 isoform ΔTA-p73, which is associated with impaired apoptosis and resistance of chemotherapy.120 To overcome the ΔTA-p73 effects, Tuve et al.121 infected melanoma cells with an adenovirus bearing the isoform TA-p73β gene capable of producing a transcriptionally active form of p73, which evaded the effects of the endogenous ΔTA-p73 effects in vitro and in vivo, and increased melanoma chemosensitivity through the induction of p21.
Combining p53-Based Gene Therapy with Histone Deacetylase Inhibitors
Histone deacetylase inhibitors (HDACi) are classified as chemotherapeutic agents showing high efficiency against various cancers. For example, HDACi could enhance gene therapy with transgenes encoding p53 family members. Generally, HDACi induce hyperacetylation of nucleosome core histones, which results in transcriptional activation and expression of genes that may inhibit tumor cell growth.122 Moreover, HDACi have been shown to induce acetylation of non-histone proteins, including p53,123 thus inducing anti-cancer activity in a histone-independent manner. Sasaki et al.124 investigated the combination treatment of HDACi FK228 (depsipeptide FR901228) with Ad-p53, Ad-p63, or Ad-p73 and determined that FK228 provoked hyperacetylation of the Ad-p53-encoded exogenous p53 protein. Acetylation of p53 was previously shown to be important for activation of p53-targeted gene expression and apoptosis induction.125 In addition, the same study showed that FK228 treatment increased the expression level of the exogenous p53 in MKN45 cells. The increases in p63 and p73 expression levels were also detected in the presence of FK228 for Ad-63 and Ad-73 vectors, respectively. Pre-treatment of MKN45 and SW480 cells with FK228 also greatly enhanced apoptosis and sensitized the cells to adenoviral infection. The increase in apoptosis resulted from a combination of FK228 with a recombinant adenovirus, which triggered BAX translocation from cytosol to the mitochondria. FK228 also increased expression levels of the coxsackievirus adenovirus receptor (CAR, often downregulated in cancer cells), leading to an enhanced infection. Furthermore, FK228 improved the effects of p53 and p63 transgenes (Ad-p73 was not tested) in vivo in MKN45 xenografts.124
Another HDACi, suberoylanilide hydroxamic acid (SAHA), also was shown to ameliorate adenovirus infection by increasing expression of CAR.126 Moreover, SAHA complemented the effects of adenoviral vectors bearing a p63 or a p73 transgene to kill head and neck squamous cell carcinoma (HNSCC) cell lines via apoptosis by an enhanced expression of p21 and cleaved-Poly(ADP-Ribose) Polymerase 1. In addition, the effect of SAHA was specific to cancer cells, showing no toxicity in normal fibroblasts.126
The approaches using HDACi described here have been tested mainly in the context of gene therapy using replication-deficient viral vectors. However, one study tested a replication-competent OV to genetically downregulate HDAC. In this study, Schipper et al.127 made an oncolytic adenovirus OV.shHDAC1.p73 encoding both the p73 transgene and small hairpin RNA (shRNA) against histone deacetylase 1 (shHDAC1), and they tested this virus in vitro and in vivo. The study showed that the presence of shHDAC1 and p73 enhances cell cytotoxicity by promoting apoptosis via caspase-3 cleavage and triggering autophagy in infected cells, which led to increased production of virus progeny in vitro in SK-Mel-147 and SK-Mel-103 cells. In vivo, i.t. injection of OV.shHDAC1.p73 (108 PFU) into subcutaneous tumors established from human SK-Mel-147 melanoma cells in nude mice showed a complete tumor regression within 17 days after treatment and showed an enhanced anti-tumor efficacy compared with the control viruses. The treatment with OV.shHDAC1.p73 also prolonged mouse survival, and no recurrence of tumors was observed within 16 weeks.127
Lessons from Clinical Trials Using Replication-Deficient p53 Vectors
Because numerous studies demonstrated an improved anti-tumor efficacy of viral vectors encoding a p53 transgene in preclinical models, different clinical trials were performed in cancer patients (summarized in Table 2). To the best of our knowledge, all clinical trials used replication-deficient viral vectors. The first phase I clinical trial was conducted with the retroviral vector (ITRp53A) against lung cancer. The retrovirus encoding WT p53 was injected i.t. (5 × 107 CFU) and was safe and well tolerated by patients. The p53 transgene expression directed by the retroviral vector was detectable in tumors and resulted in increased apoptosis.47 Nine patients were treated: three showed tumor growth stabilization and three showed minor tumor regression. Although these results seem promising, the safety limitations of the retroviral vector system shifted the focus of p53-based gene therapy to other vectors. Most of the subsequent trials used adenoviral vectors SCH58500 or INGN201 containing a WT p53 transgene.128, 129, 130, 131, 132, 133, 134, 135, 136, 137 Both SCH58500 (Schering-Plough) and INGN201 (also known as Advexin; Introgen Therapeutics) contain a WT p53 expression cassette in place of the Ad E1 region, with WT p53 gene under the control of the human cytomegalovirus promoter. These vectors were tested against various tumors such as lung, glioma, neurinoma, breast, pancreas, colorectal, bladder, ovarian, head and neck, thyroid, liver, skin, or liposarcoma and were delivered as a single agent or in combination with chemotherapy and/or radiotherapy (Table 2). Most of those clinical trials were phase I or II, but two phase IV trials are ongoing with the adenoviral vector rAd-p53 against oral-maxillofacial (NCT00902083) and thyroid (NCT00902122) tumors. Adenoviral vectors were injected at different doses depending on the administration route. The i.t. route was most commonly used with an OV dose ranging from 1 × 106 to 7.5 × 1012 VPs. Other injection routes were also utilized such as intra-arterial (7.5 × 109 to 7.5 × 1013 VPs) or i.p. (3 × 1010 to 7.5 × 1013 VPs). Regarding bladder and lung cancer, the adenoviral vectors were transmitted by intravesicular instillation (7.5 × 1011 to 7.5 × 1013 VPs)133 and bronchoalveolar lavage (2 × 109 to 2 × 1012 VPs),138 respectively. Finally, a recent clinical trial used percutaneous injections against hepatocellular carcinoma (NCT00003147). In most cases, the rAd-p53 vector was safe, well tolerated by patients, and lacked severe toxicities. However, some transient side effects like fever, leucopenia, nausea, and an increase of bilirubin were observed. Concerning the anti-tumor effects, the therapeutic vector reached the tumor site despite the presence of adenoviral neutralizing antibodies and promoted p53 transgene expression detectable in the nuclei of cancer cells, likely leading to increased apoptosis. Consequently, the majority of patients who received OV therapy displayed a regression of tumor mass or a transient stabilization of their disease.
Another strategy tested in clinical trials was to vaccinate cancer patients with dendritic cells isolated from the same patients and infected ex vivo by a recombinant virus encoding WT p53.139 The goal was to stimulate the adaptive immune system, specifically the CD8+ T lymphocytes, to provoke a specific anti-tumor response against tumor cells overexpressing high levels of p53. Indeed, unlike WT p53, which has a short half-life and is weakly expressed in normal cells, mutant p53 has a prolonged half-life and is overexpressed in cancer cells. Many studies show that such immunization of animals with WT p53 (using viral or non-viral delivery) results in a substantial CTL response against tumor cells not only expressing WT p53, but also mutant p53 genes, with no signs of autoimmune reactions to normal cells.140, 141, 142, 143, 144, 145, 146 Translating this approach to the clinic, we used dendritic cells infected by rAd-p53 in a phase I trial against small cell lung cancer (SCLC) via intradermal injections.139 In this trial, 57.1% of patients developed a p53-specific CTL response, and one patient showed a clinical response (a 60% decrease in the size of all of her measurable lesions) after vaccination. In addition, similar clinical trials (phase I/II) were recently performed on SCLC by combining vaccination of dendritic cells infected by rAd-p53 with T lymphocytes (NCT00776295) or with chemotherapy like carboplatin or etoposide (NCT00049218) in an attempt to improve the anti-tumor response.
The p53 vaccination strategy also was used in a phase I trial with a direct administration of a Modified Vaccinia Ankara virus (p53-MVA). p53-MVA is an attenuated vaccine strain of a poxvirus encoding WT p53.147 In this trial (NCT01191684), 1 × 108 to 5.6 × 108 PFU was administrated subcutaneously in 12 patients with refractory gastrointestinal cancers. The virus was well tolerated with no strong side effects, and a high CTL cell response was observed against tumor cells expressing p53. However, this effect was transient, suggesting that p53-MVA requires combination with immunomodulatory agents to deliver clinical benefit.147 Currently, two other phase I trials are planned for patients having solid tumors (NCT02432963) and recurrent ovarian cancers (NCT02275039) by combining p53-MVA vaccination and chemotherapy such as gemcitabine or pembrolizumab.
Conclusions
Important advances have been made in combining OV therapy with p53 gene therapy. Despite some interesting observations in replication-deficient viral vector clinical trials, the therapeutic effects of p53 gene therapy have been limited. Some of the failures may be attributed to the nature of gene therapy (use of replication-deficient viral vectors, biology of adenovirus-based vectors), rather than limitations of p53. By design, replication-deficient viral vectors are unable to spread within the tumor, and they exert their effects in proximity of the injection site. With regard to the adenoviruses (replication-deficient vectors or replication-competent OVs), efficacy is often limited by virus capture by the reticuloendothelial system (RES), notably by Kupffer cells (KCs) in the liver148, 149, 150, 151, 152 and by pre-existing serum antibodies.143, 144, 153, 154 Also, adenovirus infection can be restricted by the low expression of the CAR in many tumors.155, 156, 157, 158
In our opinion, replicating OV-p53 viruses based on other viruses (or adenoviruses with a modified tropism) could be more relevant in future clinical trials, because they may spread within the tumors amplifying p53 expression and increasing oncolysis. Moreover, replicating OVs could improve the anti-tumor response by the lysis of tumor cells to allow the release of numerous anti-tumor antigens into the tumor microenvironment. These antigens could be processed and potentially lead to sustainable adaptive immune responses. Finally, during in vivo treatment, OV-p53 could infect dendritic and T cells and could stimulate an adaptive immune response against cancer cells overexpressing mutant p53, essentially vaccinating patients. Thus, OV-p53 therapy can simultaneously achieve direct oncolysis and anti-tumor immunity (against several tumor-specific antigens, including mutant p53).
We think that several approaches with p53 transgenes successfully tested with replication-deficient vectors could be tested in the context of OV therapy. Most of the previous studies tested OVs that encode a major variant of the WT p53. As mentioned above, WT p53 has at least 12 isoforms, and they could be compared for the anti-tumor effects as OV-encoded transgenes. Moreover, WT p53 variant sequences could be used to generate p53 with posttranslational modifications that may increase efficacy. We envision a rationally designed p53 with a modified DNA binding domain to target and turn off cancer-specific gene regulatory sequences, like vi-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS), such that the therapy may slow cancer growth and spread. Additionally, p53 fusions with other proteins or protein domains could be tested.
Although most of the studies used human p53, comparison with animal orthologs likely would yield additional knowledge. Also, further investigations of OVs encoding p63 and p73 will improve OV-p53 therapy, and it will be useful to test whether their co-expression from the same OV could provide an additive therapeutic effect. Furthermore, it would be ideal if p53 transgene expression could be controlled with an on and off switch. Niopek et al.159 recently showed that the activity of p53 fused to a light-inducible nuclear export system (LEXY; based on a single, genetically encoded tag) can be controlled by light. This and other p53 variants could be tested in the context of OV in a variety of available in vitro and in vivo systems.
We anticipate that more studies will be conducted combining OV-p53 viruses with chemotherapy or small-molecule inhibitors targeting endogenously expressed mutant p53 and/or MDM2/4. Other therapeutic strategies could be developed to promote mutant p53 degradation. Indeed, we have already highlighted above that p53 mutants can be stabilized in cells notably via the formation of MDM2-Hsp90-p53 complex leading to MDM2 inhibition. However, to be functional this complex requires an interaction with HDAC6.160 This is why an OV bearing a p53 transgene and a shRNA sequence against HDAC6 could be useful to impair the complex MDM2-Hsp90-p53 formation and promote the degradation of p53 mutants by MDM2 or by other chaperone-associated E3 ubiquitin ligase such as CHIP.161, 162 OV-p53 also could be combined with other molecules capable of blocking the pathways regulated by mutant p53. For example, mutant p53 was shown to be responsible for abnormal architecture of breast tumors, and the use of statins demonstrated that this abnormality was a result of mutant p53-induced upregulation of the mevalonate (cholesterol synthesis) pathway in the tumor cells.163 This mechanism may explain why statins, which are well established in the clinic to treat hypercholesterolemia, have been shown to exhibit anti-cancer activities in several studies.33 Also, some studies demonstrated that p53 mutants can stimulate tumor growth via activation of several kinases, including epidermal growth factor receptor, a mesenchymal-epithelial transition kinase, as well as a mitogen-activated protein kinase.164, 165, 166, 167 Therefore, the use of statins or the inhibitors of these kinases in combination with OV encoding p53 could provide additional benefits.
Although speculative, the ideas mentioned above highlight the vast expanse of preclinical research yet to be explored. What is certain, however, is that the marriage of OVs and p53-based therapies is progressing and will lead to important clinical successes.
Acknowledgments
The authors are grateful to Dr. Didier Dréau, Sébastien Felt, Sara Seegers, Marina Baranova, and Connor Frasier for critical review of the manuscript. The authors apologize to those whose work has not been discussed because of space limitations. E.H. is supported by a postdoctoral fellowship (129351-PF-16-024-01-CSM) from the American Cancer Society. V.Z.G. is funded by grant 1R15CA195463 from the National Cancer Institute, NIH.
References
- 1.Kelly E., Russell S.J. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 2.Orloff M. Spotlight on talimogene laherparepvec for the treatment of melanoma lesions in the skin and lymph nodes. Oncolytic Virother. 2016;5:91–98. doi: 10.2147/OV.S99532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rehman H., Silk A.W., Kane M.P., Kaufman H.L. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Cancer. 2016;4:53. doi: 10.1186/s40425-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doniņa S., Strēle I., Proboka G., Auziņš J., Alberts P., Jonsson B., Venskus D., Muceniece A. Adapted ECHO-7 virus Rigvir immunotherapy (oncolytic virotherapy) prolongs survival in melanoma patients after surgical excision of the tumour in a retrospective study. Melanoma Res. 2015;25:421–426. doi: 10.1097/CMR.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garber K. China approves world’s first oncolytic virus therapy for cancer treatment. J. Natl. Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 6.Chiocca E.A. Guided genes for tumor warfare. Nat. Biotechnol. 2002;20:235–236. doi: 10.1038/nbt0302-235. [DOI] [PubMed] [Google Scholar]
- 7.Davis J.J., Fang B. Oncolytic virotherapy for cancer treatment: challenges and solutions. J. Gene Med. 2005;7:1380–1389. doi: 10.1002/jgm.800. [DOI] [PubMed] [Google Scholar]
- 8.Bell J.C., Lichty B., Stojdl D. Getting oncolytic virus therapies off the ground. Cancer Cell. 2003;4:7–11. doi: 10.1016/s1535-6108(03)00170-3. [DOI] [PubMed] [Google Scholar]
- 9.Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell J., Kirn D. Recent advances in the development of oncolytic viruses as cancer therapeutics. Foreword. Cytokine Growth Factor Rev. 2010;21:83–84. doi: 10.1016/j.cytogfr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Kress M., May E., Cassingena R., May P. Simian virus 40-transformed cells express new species of proteins precipitable by anti-simian virus 40 tumor serum. J. Virol. 1979;31:472–483. doi: 10.1128/jvi.31.2.472-483.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finlay C.A., Hinds P.W., Levine A.J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989;57:1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- 13.el-Deiry W.S., Kern S.E., Pietenpol J.A., Kinzler K.W., Vogelstein B. Definition of a consensus binding site for p53. Nat. Genet. 1992;1:45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- 14.Funk W.D., Pak D.T., Karas R.H., Wright W.E., Shay J.W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol. Cell. Biol. 1992;12:2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freed-Pastor W.A., Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26:1268–1286. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bieging K.T., Mello S.S., Attardi L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hager K.M., Gu W. Understanding the non-canonical pathways involved in p53-mediated tumor suppression. Carcinogenesis. 2014;35:740–746. doi: 10.1093/carcin/bgt487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surget S., Khoury M.P., Bourdon J.C. Uncovering the role of p53 splice variants in human malignancy: a clinical perspective. Onco Targets Ther. 2013;7:57–68. doi: 10.2147/OTT.S53876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai C., Gu W. p53 post-translational modification: deregulated in tumorigenesis. Trends Mol. Med. 2010;16:528–536. doi: 10.1016/j.molmed.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki Y., Oshima Y., Koyama R., Tamura M., Kashima L., Idogawa M., Yamashita T., Toyota M., Imai K., Shinomura Y., Tokino T. A novel approach to cancer treatment using structural hybrids of the p53 gene family. Cancer Gene Ther. 2012;19:749–756. doi: 10.1038/cgt.2012.51. [DOI] [PubMed] [Google Scholar]
- 21.Thanos C.D., Bowie J.U. p53 family members p63 and p73 are SAM domain-containing proteins. Protein Sci. 1999;8:1708–1710. doi: 10.1110/ps.8.8.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrera F.N., Poveda J.A., González-Ros J.M., Neira J.L. Binding of the C-terminal sterile alpha motif (SAM) domain of human p73 to lipid membranes. J. Biol. Chem. 2003;278:46878–46885. doi: 10.1074/jbc.M307846200. [DOI] [PubMed] [Google Scholar]
- 23.Joerger A.C., Rajagopalan S., Natan E., Veprintsev D.B., Robinson C.V., Fersht A.R. Structural evolution of p53, p63, and p73: implication for heterotetramer formation. Proc. Natl. Acad. Sci. USA. 2009;106:17705–17710. doi: 10.1073/pnas.0905867106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramadan S., Terrinoni A., Catani M.V., Sayan A.E., Knight R.A., Mueller M., Krammer P.H., Melino G., Candi E. p73 induces apoptosis by different mechanisms. Biochem. Biophys. Res. Commun. 2005;331:713–717. doi: 10.1016/j.bbrc.2005.03.156. [DOI] [PubMed] [Google Scholar]
- 25.Napoli M., Flores E.R. The family that eats together stays together: new p53 family transcriptional targets in autophagy. Genes Dev. 2013;27:971–974. doi: 10.1101/gad.219147.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian Y., Chen X. Senescence regulation by the p53 protein family. Methods Mol. Biol. 2013;965:37–61. doi: 10.1007/978-1-62703-239-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray-Zmijewski F., Lane D.P., Bourdon J.C. p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006;13:962–972. doi: 10.1038/sj.cdd.4401914. [DOI] [PubMed] [Google Scholar]
- 28.Martin A.G., Trama J., Crighton D., Ryan K.M., Fearnhead H.O. Activation of p73 and induction of Noxa by DNA damage requires NF-kappa B. Aging (Albany NY) 2009;1:335–349. doi: 10.18632/aging.100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farhang Ghahremani M., Goossens S., Haigh J.J. The p53 family and VEGF regulation: “It’s complicated”. Cell Cycle. 2013;12:1331–1332. doi: 10.4161/cc.24579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kandoth C., McLellan M.D., Vandin F., Ye K., Niu B., Lu C., Xie M., Zhang Q., McMichael J.F., Wyczalkowski M.A. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chène P. The role of tetramerization in p53 function. Oncogene. 2001;20:2611–2617. doi: 10.1038/sj.onc.1204373. [DOI] [PubMed] [Google Scholar]
- 32.Muller P.A., Vousden K.H. p53 mutations in cancer. Nat. Cell Biol. 2013;15:2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 33.Mantovani F., Walerych D., Sal G.D. Targeting mutant p53 in cancer: a long road to precision therapy. FEBS J. 2017;284:837–850. doi: 10.1111/febs.13948. [DOI] [PubMed] [Google Scholar]
- 34.Alexandrova E.M., Yallowitz A.R., Li D., Xu S., Schulz R., Proia D.A., Lozano G., Dobbelstein M., Moll U.M. Improving survival by exploiting tumour dependence on stabilized mutant p53 for treatment. Nature. 2015;523:352–356. doi: 10.1038/nature14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashcroft M., Vousden K.H. Regulation of p53 stability. Oncogene. 1999;18:7637–7643. doi: 10.1038/sj.onc.1203012. [DOI] [PubMed] [Google Scholar]
- 36.Gu J., Kawai H., Nie L., Kitao H., Wiederschain D., Jochemsen A.G., Parant J., Lozano G., Yuan Z.M. Mutual dependence of MDM2 and MDMX in their functional inactivation of p53. J. Biol. Chem. 2002;277:19251–19254. doi: 10.1074/jbc.C200150200. [DOI] [PubMed] [Google Scholar]
- 37.Hengstermann A., Linares L.K., Ciechanover A., Whitaker N.J., Scheffner M. Complete switch from Mdm2 to human papillomavirus E6-mediated degradation of p53 in cervical cancer cells. Proc. Natl. Acad. Sci. USA. 2001;98:1218–1223. doi: 10.1073/pnas.031470698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng Y., Chen L., Li C., Lu W., Chen J. Inhibition of MDM2 by hsp90 contributes to mutant p53 stabilization. J. Biol. Chem. 2001;276:40583–40590. doi: 10.1074/jbc.M102817200. [DOI] [PubMed] [Google Scholar]
- 39.Duffy M.J., Synnott N.C., McGowan P.M., Crown J., O’Connor D., Gallagher W.M. p53 as a target for the treatment of cancer. Cancer Treat. Rev. 2014;40:1153–1160. doi: 10.1016/j.ctrv.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Parrales A., Iwakuma T. Targeting oncogenic mutant p53 for cancer therapy. Front. Oncol. 2015;5:288. doi: 10.3389/fonc.2015.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stindt M.H., Muller P.A., Ludwig R.L., Kehrloesser S., Dötsch V., Vousden K.H. Functional interplay between MDM2, p63/p73 and mutant p53. Oncogene. 2015;34:4300–4310. doi: 10.1038/onc.2014.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller P.A., Vousden K.H. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014;25:304–317. doi: 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michaelis M., Rothweiler F., Barth S., Cinatl J., van Rikxoort M., Löschmann N., Voges Y., Breitling R., von Deimling A., Rödel F. Adaptation of cancer cells from different entities to the MDM2 inhibitor nutlin-3 results in the emergence of p53-mutated multi-drug-resistant cancer cells. Cell Death Dis. 2011;2:e243. doi: 10.1038/cddis.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lane D.P., Cheok C.F., Lain S. p53-based cancer therapy. Cold Spring Harb. Perspect. Biol. 2010;2:a001222. doi: 10.1101/cshperspect.a001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue W., Zender L., Miething C., Dickins R.A., Hernando E., Krizhanovsky V., Cordon-Cardo C., Lowe S.W. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ventura A., Kirsch D.G., McLaughlin M.E., Tuveson D.A., Grimm J., Lintault L., Newman J., Reczek E.E., Weissleder R., Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 47.Roth J.A., Nguyen D., Lawrence D.D., Kemp B.L., Carrasco C.H., Ferson D.Z., Hong W.K., Komaki R., Lee J.J., Nesbitt J.C. Retrovirus-mediated wild-type p53 gene transfer to tumors of patients with lung cancer. Nat. Med. 1996;2:985–991. doi: 10.1038/nm0996-985. [DOI] [PubMed] [Google Scholar]
- 48.Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991;352:345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- 49.Takaoka A., Hayakawa S., Yanai H., Stoiber D., Negishi H., Kikuchi H., Sasaki S., Imai K., Shibue T., Honda K., Taniguchi T. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 50.Lazo P.A., Santos C.R. Interference with p53 functions in human viral infections, a target for novel antiviral strategies? Rev. Med. Virol. 2011;21:285–300. doi: 10.1002/rmv.696. [DOI] [PubMed] [Google Scholar]
- 51.Muñoz-Fontela C., Macip S., Martínez-Sobrido L., Brown L., Ashour J., García-Sastre A., Lee S.W., Aaronson S.A. Transcriptional role of p53 in interferon-mediated antiviral immunity. J. Exp. Med. 2008;205:1929–1938. doi: 10.1084/jem.20080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muñoz-Fontela C., Pazos M., Delgado I., Murk W., Mungamuri S.K., Lee S.W., García-Sastre A., Moran T.M., Aaronson S.A. p53 serves as a host antiviral factor that enhances innate and adaptive immune responses to influenza A virus. J. Immunol. 2011;187:6428–6436. doi: 10.4049/jimmunol.1101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Munoz-Fontela C., Garcia M.A., Garcia-Cao I., Collado M., Arroyo J., Esteban M., Serrano M., Rivas C. Resistance to viral infection of super p53 mice. Oncogene. 2005;24:3059–3062. doi: 10.1038/sj.onc.1208477. [DOI] [PubMed] [Google Scholar]
- 54.He X.P., Su C.Q., Wang X.H., Pan X., Tu Z.X., Gong Y.F., Gao J., Liao Z., Jin J., Wu H.Y. E1B-55kD-deleted oncolytic adenovirus armed with canstatin gene yields an enhanced anti-tumor efficacy on pancreatic cancer. Cancer Lett. 2009;285:89–98. doi: 10.1016/j.canlet.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Shin-Ya M., Hirai H., Satoh E., Kishida T., Asada H., Aoki F., Tsukamoto M., Imanishi J., Mazda O. Intracellular interferon triggers Jak/Stat signaling cascade and induces p53-dependent antiviral protection. Biochem. Biophys. Res. Commun. 2005;329:1139–1146. doi: 10.1016/j.bbrc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 56.Dharel N., Kato N., Muroyama R., Taniguchi H., Otsuka M., Wang Y., Jazag A., Shao R.X., Chang J.H., Adler M.K. Potential contribution of tumor suppressor p53 in the host defense against hepatitis C virus. Hepatology. 2008;47:1136–1149. doi: 10.1002/hep.22176. [DOI] [PubMed] [Google Scholar]
- 57.Su C., Cao H., Tan S., Huang Y., Jia X., Jiang L., Wang K., Chen Y., Long J., Liu X. Toxicology profiles of a novel p53-armed replication-competent oncolytic adenovirus in rodents, felids, and nonhuman primates. Toxicol. Sci. 2008;106:242–250. doi: 10.1093/toxsci/kfn168. [DOI] [PubMed] [Google Scholar]
- 58.Alemany R. Cancer selective adenoviruses. Mol. Aspects Med. 2007;28:42–58. doi: 10.1016/j.mam.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Cervantes-García D., Ortiz-López R., Mayek-Pérez N., Rojas-Martínez A. Oncolytic virotherapy. Ann. Hepatol. 2008;7:34–45. [PubMed] [Google Scholar]
- 60.Rosewell Shaw A., Suzuki M. Recent advances in oncolytic adenovirus therapies for cancer. Curr. Opin. Virol. 2016;21:9–15. doi: 10.1016/j.coviro.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.An Y., Liu T., He J., Wu H., Chen R., Liu Y., Wu Y., Bai Y., Guo X., Zheng Q. Recombinant Newcastle disease virus expressing P53 demonstrates promising antitumor efficiency in hepatoma model. J. Biomed. Sci. 2016;23:55. doi: 10.1186/s12929-016-0273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heiber J.F., Barber G.N. Vesicular stomatitis virus expressing tumor suppressor p53 is a highly attenuated, potent oncolytic agent. J. Virol. 2011;85:10440–10450. doi: 10.1128/JVI.05408-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Black B.L., Rhodes R.B., McKenzie M., Lyles D.S. The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J. Virol. 1993;67:4814–4821. doi: 10.1128/jvi.67.8.4814-4821.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coulon P., Deutsch V., Lafay F., Martinet-Edelist C., Wyers F., Herman R.C., Flamand A. Genetic evidence for multiple functions of the matrix protein of vesicular stomatitis virus. J. Gen. Virol. 1990;71:991–996. doi: 10.1099/0022-1317-71-4-991. [DOI] [PubMed] [Google Scholar]
- 65.Stojdl D.F., Lichty B.D., tenOever B.R., Paterson J.M., Power A.T., Knowles S., Marius R., Reynard J., Poliquin L., Atkins H. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 66.Hastie E., Grdzelishvili V.Z. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J. Gen. Virol. 2012;93:2529–2545. doi: 10.1099/vir.0.046672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hastie E., Cataldi M., Steuerwald N., Grdzelishvili V.Z. An unexpected inhibition of antiviral signaling by virus-encoded tumor suppressor p53 in pancreatic cancer cells. Virology. 2015;483:126–140. doi: 10.1016/j.virol.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J., Basagoudanavar S.H., Wang X., Hopewell E., Albrecht R., García-Sastre A., Balachandran S., Beg A.A. NF-kappa B RelA subunit is crucial for early IFN-beta expression and resistance to RNA virus replication. J. Immunol. 2010;185:1720–1729. doi: 10.4049/jimmunol.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shao J., Fujiwara T., Kadowaki Y., Fukazawa T., Waku T., Itoshima T., Yamatsuji T., Nishizaki M., Roth J.A., Tanaka N. Overexpression of the wild-type p53 gene inhibits NF-kappaB activity and synergizes with aspirin to induce apoptosis in human colon cancer cells. Oncogene. 2000;19:726–736. doi: 10.1038/sj.onc.1203383. [DOI] [PubMed] [Google Scholar]
- 70.Culmsee C., Siewe J., Junker V., Retiounskaia M., Schwarz S., Camandola S., El-Metainy S., Behnke H., Mattson M.P., Krieglstein J. Reciprocal inhibition of p53 and nuclear factor-kappaB transcriptional activities determines cell survival or death in neurons. J. Neurosci. 2003;23:8586–8595. doi: 10.1523/JNEUROSCI.23-24-08586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawauchi K., Araki K., Tobiume K., Tanaka N. Activated p53 induces NF-kappaB DNA binding but suppresses its transcriptional activation. Biochem. Biophys. Res. Commun. 2008;372:137–141. doi: 10.1016/j.bbrc.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 72.Song M.M., Shuai K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J. Biol. Chem. 1998;273:35056–35062. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- 73.Haviv Y.S., Takayama K., Glasgow J.N., Blackwell J.L., Wang M., Lei X., Curiel D.T. A model system for the design of armed replicating adenoviruses using p53 as a candidate transgene. Mol. Cancer Ther. 2002;1:321–328. [PubMed] [Google Scholar]
- 74.van Beusechem V.W., van den Doel P.B., Grill J., Pinedo H.M., Gerritsen W.R. Conditionally replicative adenovirus expressing p53 exhibits enhanced oncolytic potency. Cancer Res. 2002;62:6165–6171. [PubMed] [Google Scholar]
- 75.Geoerger B., Vassal G., Opolon P., Dirven C.M., Morizet J., Laudani L., Grill J., Giaccone G., Vandertop W.P., Gerritsen W.R., van Beusechem V.W. Oncolytic activity of p53-expressing conditionally replicative adenovirus AdDelta24-p53 against human malignant glioma. Cancer Res. 2004;64:5753–5759. doi: 10.1158/0008-5472.CAN-04-0499. [DOI] [PubMed] [Google Scholar]
- 76.Geoerger B., van Beusechem V.W., Opolon P., Morizet J., Laudani L., Lecluse Y., Barrois M., Idema S., Grill J., Gerritsen W.R., Vassal G. Expression of p53, or targeting towards EGFR, enhances the oncolytic potency of conditionally replicative adenovirus against neuroblastoma. J. Gene Med. 2005;7:584–594. doi: 10.1002/jgm.703. [DOI] [PubMed] [Google Scholar]
- 77.Menendez D., Shatz M., Resnick M.A. Interactions between the tumor suppressor p53 and immune responses. Curr. Opin. Oncol. 2013;25:85–92. doi: 10.1097/CCO.0b013e32835b6386. [DOI] [PubMed] [Google Scholar]
- 78.Kubbutat M.H., Jones S.N., Vousden K.H. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 79.Haupt Y., Maya R., Kazaz A., Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 80.Momand J., Jung D., Wilczynski S., Niland J. The MDM2 gene amplification database. Nucleic Acids Res. 1998;26:3453–3459. doi: 10.1093/nar/26.15.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harada J.N., Shevchenko A., Shevchenko A., Pallas D.C., Berk A.J. Analysis of the adenovirus E1B-55K-anchored proteome reveals its link to ubiquitination machinery. J. Virol. 2002;76:9194–9206. doi: 10.1128/JVI.76.18.9194-9206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Querido E., Blanchette P., Yan Q., Kamura T., Morrison M., Boivin D., Kaelin W.G., Conaway R.C., Conaway J.W., Branton P.E. Degradation of p53 by adenovirus E4orf6 and E1B55K proteins occurs via a novel mechanism involving a Cullin-containing complex. Genes Dev. 2001;15:3104–3117. doi: 10.1101/gad.926401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sauthoff H., Pipiya T., Chen S., Heitner S., Cheng J., Huang Y.Q., Rom W.N., Hay J.G. Modification of the p53 transgene of a replication-competent adenovirus prevents mdm2- and E1b-55kD-mediated degradation of p53. Cancer Gene Ther. 2006;13:686–695. doi: 10.1038/sj.cgt.7700936. [DOI] [PubMed] [Google Scholar]
- 84.Lin J., Chen J., Elenbaas B., Levine A.J. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994;8:1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- 85.Thomas A., White E. Suppression of the p300-dependent mdm2 negative-feedback loop induces the p53 apoptotic function. Genes Dev. 1998;12:1975–1985. doi: 10.1101/gad.12.13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Debbas M., White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 87.Hobom U., Dobbelstein M. E1B-55-kilodalton protein is not required to block p53-induced transcription during adenovirus infection. J. Virol. 2004;78:7685–7697. doi: 10.1128/JVI.78.14.7685-7697.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scolnick D.M., Chehab N.H., Stavridi E.S., Lien M.C., Caruso L., Moran E., Berger S.L., Halazonetis T.D. CREB-binding protein and p300/CBP-associated factor are transcriptional coactivators of the p53 tumor suppressor protein. Cancer Res. 1997;57:3693–3696. [PubMed] [Google Scholar]
- 89.Ard P.G., Chatterjee C., Kunjibettu S., Adside L.R., Gralinski L.E., McMahon S.B. Transcriptional regulation of the mdm2 oncogene by p53 requires TRRAP acetyltransferase complexes. Mol. Cell. Biol. 2002;22:5650–5661. doi: 10.1128/MCB.22.16.5650-5661.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yim E.K., Park J.S. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res. Treat. 2005;37:319–324. doi: 10.4143/crt.2005.37.6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kobet E., Zeng X., Zhu Y., Keller D., Lu H. MDM2 inhibits p300-mediated p53 acetylation and activation by forming a ternary complex with the two proteins. Proc. Natl. Acad. Sci. USA. 2000;97:12547–12552. doi: 10.1073/pnas.97.23.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi D., Gu W. Dual roles of MDM2 in the regulation of p53: ubiquitination dependent and ubiquitination independent mechanisms of MDM2 repression of p53 activity. Genes Cancer. 2012;3:240–248. doi: 10.1177/1947601912455199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mantovani F., Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001;20:7874–7887. doi: 10.1038/sj.onc.1204869. [DOI] [PubMed] [Google Scholar]
- 94.Heideman D.A., Steenbergen R.D., van der Torre J., Scheffner M., Alemany R., Gerritsen W.R., Meijer C.J., Snijders P.J., van Beusechem V.W. Oncolytic adenovirus expressing a p53 variant resistant to degradation by HPV E6 protein exhibits potent and selective replication in cervical cancer. Mol. Ther. 2005;12:1083–1090. doi: 10.1016/j.ymthe.2005.06.443. [DOI] [PubMed] [Google Scholar]
- 95.Balagué C., Noya F., Alemany R., Chow L.T., Curiel D.T. Human papillomavirus E6E7-mediated adenovirus cell killing: selectivity of mutant adenovirus replication in organotypic cultures of human keratinocytes. J. Virol. 2001;75:7602–7611. doi: 10.1128/JVI.75.16.7602-7611.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Okal A., Mossalam M., Matissek K.J., Dixon A.S., Moos P.J., Lim C.S. A chimeric p53 evades mutant p53 transdominant inhibition in cancer cells. Mol. Pharm. 2013;10:3922–3933. doi: 10.1021/mp400379c. [DOI] [PubMed] [Google Scholar]
- 97.Takenobu T., Tomizawa K., Matsushita M., Li S.T., Moriwaki A., Lu Y.F., Matsui H. Development of p53 protein transduction therapy using membrane-permeable peptides and the application to oral cancer cells. Mol. Cancer Ther. 2002;1:1043–1049. [PubMed] [Google Scholar]
- 98.Bolhassani A., Jafarzade B.S., Mardani G. In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides. 2017;87:50–63. doi: 10.1016/j.peptides.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 99.Wang J., Yu Y., Yan Z., Hu Z., Li L., Li J., Jiang X., Qian Q. Anticancer activity of oncolytic adenoviruses carrying p53 is augmented by 11R in gallbladder cancer cell lines in vitro and in vivo. Oncol. Rep. 2013;30:833–841. doi: 10.3892/or.2013.2511. [DOI] [PubMed] [Google Scholar]
- 100.Cheng G., Kong D., Hou X., Liang B., He M., Liang N., Ma S., Liu X. The tumor suppressor, p53, contributes to radiosensitivity of lung cancer cells by regulating autophagy and apoptosis. Cancer Biother. Radiopharm. 2013;28:153–159. doi: 10.1089/cbr.2012.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee J.M., Bernstein A. p53 mutations increase resistance to ionizing radiation. Proc. Natl. Acad. Sci. USA. 1993;90:5742–5746. doi: 10.1073/pnas.90.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McIlwrath A.J., Vasey P.A., Ross G.M., Brown R. Cell cycle arrests and radiosensitivity of human tumor cell lines: dependence on wild-type p53 for radiosensitivity. Cancer Res. 1994;54:3718–3722. [PubMed] [Google Scholar]
- 103.Fan S., el-Deiry W.S., Bae I., Freeman J., Jondle D., Bhatia K., Fornace A.J., Jr., Magrath I., Kohn K.W., O’Connor P.M. p53 gene mutations are associated with decreased sensitivity of human lymphoma cells to DNA damaging agents. Cancer Res. 1994;54:5824–5830. [PubMed] [Google Scholar]
- 104.Gudkov A.V., Komarova E.A. The role of p53 in determining sensitivity to radiotherapy. Nat. Rev. Cancer. 2003;3:117–129. doi: 10.1038/nrc992. [DOI] [PubMed] [Google Scholar]
- 105.Fei P., El-Deiry W.S. P53 and radiation responses. Oncogene. 2003;22:5774–5783. doi: 10.1038/sj.onc.1206677. [DOI] [PubMed] [Google Scholar]
- 106.Idema S., Lamfers M.L., van Beusechem V.W., Noske D.P., Heukelom S., Moeniralm S., Gerritsen W.R., Vandertop W.P., Dirven C.M. AdDelta24 and the p53-expressing variant AdDelta24-p53 achieve potent anti-tumor activity in glioma when combined with radiotherapy. J. Gene Med. 2007;9:1046–1056. doi: 10.1002/jgm.1113. [DOI] [PubMed] [Google Scholar]
- 107.Gressner O., Schilling T., Lorenz K., Schulze Schleithoff E., Koch A., Schulze-Bergkamen H., Lena A.M., Candi E., Terrinoni A., Catani M.V. TAp63alpha induces apoptosis by activating signaling via death receptors and mitochondria. EMBO J. 2005;24:2458–2471. doi: 10.1038/sj.emboj.7600708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pietsch E.C., Murphy M.E. Low risk HPV-E6 traps p53 in the cytoplasm and induces p53-dependent apoptosis. Cancer Biol. Ther. 2008;7:1916–1918. doi: 10.4161/cbt.7.12.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allocati N., Di Ilio C., De Laurenzi V. p63/p73 in the control of cell cycle and cell death. Exp. Cell Res. 2012;318:1285–1290. doi: 10.1016/j.yexcr.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 110.Bourdon J.C., Renzing J., Robertson P.L., Fernandes K.N., Lane D.P. Scotin, a novel p53-inducible proapoptotic protein located in the ER and the nuclear membrane. J. Cell Biol. 2002;158:235–246. doi: 10.1083/jcb.200203006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schuster A., Schilling T., De Laurenzi V., Koch A.F., Seitz S., Staib F., Teufel A., Thorgeirsson S.S., Galle P., Melino G. ΔNp73β is oncogenic in hepatocellular carcinoma by blocking apoptosis signaling via death receptors and mitochondria. Cell Cycle. 2010;9:2629–2639. doi: 10.4161/cc.9.13.12110. [DOI] [PubMed] [Google Scholar]
- 112.Kunisaki R., Ikawa S., Maeda T., Nakazaki Y., Kurita R., Harata M., Shutoh Y., Bai Y.S., Soda Y., Tanabe T. p51/p63, a novel p53 homologue, potentiates p53 activity and is a human cancer gene therapy candidate. J. Gene Med. 2006;8:1121–1130. doi: 10.1002/jgm.945. [DOI] [PubMed] [Google Scholar]
- 113.Roth J., König C., Wienzek S., Weigel S., Ristea S., Dobbelstein M. Inactivation of p53 but not p73 by adenovirus type 5 E1B 55-kilodalton and E4 34-kilodalton oncoproteins. J. Virol. 1998;72:8510–8516. doi: 10.1128/jvi.72.11.8510-8516.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marin M.C., Jost C.A., Irwin M.S., DeCaprio J.A., Caput D., Kaelin W.G., Jr. Viral oncoproteins discriminate between p53 and the p53 homolog p73. Mol. Cell. Biol. 1998;18:6316–6324. doi: 10.1128/mcb.18.11.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dobbelstein M., Roth J. The large T antigen of simian virus 40 binds and inactivates p53 but not p73. J. Gen. Virol. 1998;79:3079–3083. doi: 10.1099/0022-1317-79-12-3079. [DOI] [PubMed] [Google Scholar]
- 116.Das S., El-Deiry W.S., Somasundaram K. Efficient growth inhibition of HPV 16 E6-expressing cells by an adenovirus-expressing p53 homologue p73beta. Oncogene. 2003;22:8394–8402. doi: 10.1038/sj.onc.1206908. [DOI] [PubMed] [Google Scholar]
- 117.Das S., Nama S., Antony S., Somasundaram K. p73 beta-expressing recombinant adenovirus: a potential anticancer agent. Cancer Gene Ther. 2005;12:417–426. doi: 10.1038/sj.cgt.7700803. [DOI] [PubMed] [Google Scholar]
- 118.Das S.C., Nayak D., Zhou Y., Pattnaik A.K. Visualization of intracellular transport of vesicular stomatitis virus nucleocapsids in living cells. J. Virol. 2006;80:6368–6377. doi: 10.1128/JVI.00211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu Y., He Y., Srivenugopal K.S., Fan S., Jiang Y. In vitro antitumor cytotoxic T lymphocyte response induced by dendritic cells transduced with DeltaNp73alpha recombinant adenovirus. Oncol. Rep. 2007;18:1085–1091. [PubMed] [Google Scholar]
- 120.Tuve S., Wagner S.N., Schittek B., Pützer B.M. Alterations of DeltaTA-p 73 splice transcripts during melanoma development and progression. Int. J. Cancer. 2004;108:162–166. doi: 10.1002/ijc.11552. [DOI] [PubMed] [Google Scholar]
- 121.Tuve S., Racek T., Niemetz A., Schultz J., Soengas M.S., Pützer B.M. Adenovirus-mediated TA-p73beta gene transfer increases chemosensitivity of human malignant melanomas. Apoptosis. 2006;11:235–243. doi: 10.1007/s10495-006-3407-0. [DOI] [PubMed] [Google Scholar]
- 122.Johnstone R.W. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 123.Gu W., Roeder R.G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 124.Sasaki Y., Negishi H., Idogawa M., Suzuki H., Mita H., Toyota M., Shinomura Y., Imai K., Tokino T. Histone deacetylase inhibitor FK228 enhances adenovirus-mediated p53 family gene therapy in cancer models. Mol. Cancer Ther. 2008;7:779–787. doi: 10.1158/1535-7163.MCT-07-0395. [DOI] [PubMed] [Google Scholar]
- 125.Barlev N.A., Liu L., Chehab N.H., Mansfield K., Harris K.G., Halazonetis T.D., Berger S.L. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell. 2001;8:1243–1254. doi: 10.1016/s1097-2765(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 126.Shim S.H., Lee C.T., Lee J.J., Kim S.Y., Hah J.H., Heo D.S., Sung M.W. A combination treatment with SAHA and ad-p63/p73 shows an enhanced anticancer effect in HNSCC. Tumour Biol. 2010;31:659–666. doi: 10.1007/s13277-010-0083-z. [DOI] [PubMed] [Google Scholar]
- 127.Schipper H., Alla V., Meier C., Nettelbeck D.M., Herchenröder O., Pützer B.M. Eradication of metastatic melanoma through cooperative expression of RNA-based HDAC1 inhibitor and p73 by oncolytic adenovirus. Oncotarget. 2014;5:5893–5907. doi: 10.18632/oncotarget.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schuler M., Rochlitz C., Horowitz J.A., Schlegel J., Perruchoud A.P., Kommoss F., Bolliger C.T., Kauczor H.U., Dalquen P., Fritz M.A. A phase I study of adenovirus-mediated wild-type p53 gene transfer in patients with advanced non-small cell lung cancer. Hum. Gene Ther. 1998;9:2075–2082. doi: 10.1089/hum.1998.9.14-2075. [DOI] [PubMed] [Google Scholar]
- 129.Wolkersdörfer G.W., Thiede C., Fischer R., Ehninger G., Haag C. Adenoviral p53 gene transfer and gemcitabine in three patients with liver metastases due to advanced pancreatic carcinoma. HPB (Oxford) 2007;9:16–25. doi: 10.1080/13651820600839555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Atencio I.A., Grace M., Bordens R., Fritz M., Horowitz J.A., Hutchins B., Indelicato S., Jacobs S., Kolz K., Maneval D. Biological activities of a recombinant adenovirus p53 (SCH 58500) administered by hepatic arterial infusion in a Phase 1 colorectal cancer trial. Cancer Gene Ther. 2006;13:169–181. doi: 10.1038/sj.cgt.7700870. [DOI] [PubMed] [Google Scholar]
- 131.Lang F.F., Bruner J.M., Fuller G.N., Aldape K., Prados M.D., Chang S., Berger M.S., McDermott M.W., Kunwar S.M., Junck L.R. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J. Clin. Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 132.Yoo G.H., Moon J., Leblanc M., Lonardo F., Urba S., Kim H., Hanna E., Tsue T., Valentino J., Ensley J., Wolf G. A phase 2 trial of surgery with perioperative INGN 201 (Ad5CMV-p53) gene therapy followed by chemoradiotherapy for advanced, resectable squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, and larynx: report of the Southwest Oncology Group. Arch. Otolaryngol. Head Neck Surg. 2009;135:869–874. doi: 10.1001/archoto.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kuball J., Wen S.F., Leissner J., Atkins D., Meinhardt P., Quijano E., Engler H., Hutchins B., Maneval D.C., Grace M.J. Successful adenovirus-mediated wild-type p53 gene transfer in patients with bladder cancer by intravesical vector instillation. J. Clin. Oncol. 2002;20:957–965. doi: 10.1200/JCO.2002.20.4.957. [DOI] [PubMed] [Google Scholar]
- 134.Buller R.E., Runnebaum I.B., Karlan B.Y., Horowitz J.A., Shahin M., Buekers T., Petrauskas S., Kreienberg R., Slamon D., Pegram M. A phase I/II trial of rAd/p53 (SCH 58500) gene replacement in recurrent ovarian cancer. Cancer Gene Ther. 2002;9:553–566. doi: 10.1038/sj.cgt.7700472. [DOI] [PubMed] [Google Scholar]
- 135.Schuler M., Herrmann R., De Greve J.L., Stewart A.K., Gatzemeier U., Stewart D.J., Laufman L., Gralla R., Kuball J., Buhl R. Adenovirus-mediated wild-type p53 gene transfer in patients receiving chemotherapy for advanced non-small-cell lung cancer: results of a multicenter phase II study. J. Clin. Oncol. 2001;19:1750–1758. doi: 10.1200/JCO.2001.19.6.1750. [DOI] [PubMed] [Google Scholar]
- 136.Swisher S.G., Roth J.A., Komaki R., Gu J., Lee J.J., Hicks M., Ro J.Y., Hong W.K., Merritt J.A., Ahrar K. Induction of p53-regulated genes and tumor regression in lung cancer patients after intratumoral delivery of adenoviral p53 (INGN 201) and radiation therapy. Clin. Cancer Res. 2003;9:93–101. [PubMed] [Google Scholar]
- 137.Wolf J.K., Bodurka D.C., Gano J.B., Deavers M., Ramondetta L., Ramirez P.T., Levenback C., Gershenson D.M. A phase I study of Adp53 (INGN 201; ADVEXIN) for patients with platinum- and paclitaxel-resistant epithelial ovarian cancer. Gynecol. Oncol. 2004;94:442–448. doi: 10.1016/j.ygyno.2004.05.041. [DOI] [PubMed] [Google Scholar]
- 138.Keedy V., Wang W., Schiller J., Chada S., Slovis B., Coffee K., Worrell J., Thet L.A., Johnson D.H., Carbone D.P. Phase I study of adenovirus p53 administered by bronchoalveolar lavage in patients with bronchioloalveolar cell lung carcinoma: ECOG 6597. J. Clin. Oncol. 2008;26:4166–4171. doi: 10.1200/JCO.2007.15.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Antonia S.J., Mirza N., Fricke I., Chiappori A., Thompson P., Williams N., Bepler G., Simon G., Janssen W., Lee J.H. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin. Cancer Res. 2006;12:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 140.Ishida T., Chada S., Stipanov M., Nadaf S., Ciernik F.I., Gabrilovich D.I., Carbone D.P. Dendritic cells transduced with wild-type p53 gene elicit potent anti-tumour immune responses. Clin. Exp. Immunol. 1999;117:244–251. doi: 10.1046/j.1365-2249.1999.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nikitina E.Y., Chada S., Muro-Cacho C., Fang B., Zhang R., Roth J.A., Gabrilovich D.I. An effective immunization and cancer treatment with activated dendritic cells transduced with full-length wild-type p53. Gene Ther. 2002;9:345–352. doi: 10.1038/sj.gt.3301670. [DOI] [PubMed] [Google Scholar]
- 142.Vierboom M.P., Nijman H.W., Offringa R., van der Voort E.I., van Hall T., van den Broek L., Fleuren G.J., Kenemans P., Kast W.M., Melief C.J. Tumor eradication by wild-type p53-specific cytotoxic T lymphocytes. J. Exp. Med. 1997;186:695–704. doi: 10.1084/jem.186.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zwaveling S., Vierboom M.P., Ferreira Mota S.C., Hendriks J.A., Ooms M.E., Sutmuller R.P., Franken K.L., Nijman H.W., Ossendorp F., Van Der Burg S.H. Antitumor efficacy of wild-type p53-specific CD4(+) T-helper cells. Cancer Res. 2002;62:6187–6193. [PubMed] [Google Scholar]
- 144.Parajuli P., Pisarev V., Sublet J., Steffel A., Varney M., Singh R., LaFace D., Talmadge J.E. Immunization with wild-type p53 gene sequences coadministered with Flt3 ligand induces an antigen-specific type 1 T-cell response. Cancer Res. 2001;61:8227–8234. [PubMed] [Google Scholar]
- 145.Hurpin C., Rotarioa C., Bisceglia H., Chevalier M., Tartaglia J., Erdile L. The mode of presentation and route of administration are critical for the induction of immune responses to p53 and antitumor immunity. Vaccine. 1998;16:208–215. doi: 10.1016/s0264-410x(97)00190-4. [DOI] [PubMed] [Google Scholar]
- 146.Roth J., Dittmer D., Rea D., Tartaglia J., Paoletti E., Levine A.J. p53 as a target for cancer vaccines: recombinant canarypox virus vectors expressing p53 protect mice against lethal tumor cell challenge. Proc. Natl. Acad. Sci. USA. 1996;93:4781–4786. doi: 10.1073/pnas.93.10.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hardwick N.R., Carroll M., Kaltcheva T., Qian D., Lim D., Leong L., Chu P., Kim J., Chao J., Fakih M. p53MVA therapy in patients with refractory gastrointestinal malignancies elevates p53-specific CD8+ T-cell responses. Clin. Cancer Res. 2014;20:4459–4470. doi: 10.1158/1078-0432.CCR-13-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tao N., Gao G.P., Parr M., Johnston J., Baradet T., Wilson J.M., Barsoum J., Fawell S.E. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol. Ther. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- 149.Alemany R., Suzuki K., Curiel D.T. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 2000;81:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- 150.Khare R., Chen C.Y., Weaver E.A., Barry M.A. Advances and future challenges in adenoviral vector pharmacology and targeting. Curr. Gene Ther. 2011;11:241–258. doi: 10.2174/156652311796150363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hofherr S.E., Adams K.E., Chen C.Y., May S., Weaver E.A., Barry M.A. Real-time dynamic imaging of virus distribution in vivo. PLoS ONE. 2011;6:e17076. doi: 10.1371/journal.pone.0017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wolff G., Worgall S., van Rooijen N., Song W.R., Harvey B.G., Crystal R.G. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J. Virol. 1997;71:624–629. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bessis N., GarciaCozar F.J., Boissier M.C. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11(Suppl 1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 154.Gahéry-Ségard H., Farace F., Godfrin D., Gaston J., Lengagne R., Tursz T., Boulanger P., Guillet J.G. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J. Virol. 1998;72:2388–2397. doi: 10.1128/jvi.72.3.2388-2397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Okegawa T., Pong R.C., Li Y., Bergelson J.M., Sagalowsky A.I., Hsieh J.T. The mechanism of the growth-inhibitory effect of coxsackie and adenovirus receptor (CAR) on human bladder cancer: a functional analysis of car protein structure. Cancer Res. 2001;61:6592–6600. [PubMed] [Google Scholar]
- 156.Haviv Y.S., Blackwell J.L., Kanerva A., Nagi P., Krasnykh V., Dmitriev I., Wang M., Naito S., Lei X., Hemminki A. Adenoviral gene therapy for renal cancer requires retargeting to alternative cellular receptors. Cancer Res. 2002;62:4273–4281. [PubMed] [Google Scholar]
- 157.Raki M., Kanerva A., Ristimaki A., Desmond R.A., Chen D.T., Ranki T., Sarkioja M., Kangasniemi L., Hemminki A. Combination of gemcitabine and Ad5/3-Delta24, a tropism modified conditionally replicating adenovirus, for the treatment of ovarian cancer. Gene Ther. 2005;12:1198–1205. doi: 10.1038/sj.gt.3302517. [DOI] [PubMed] [Google Scholar]
- 158.Tyler M.A., Ulasov I.V., Borovjagin A., Sonabend A.M., Khramtsov A., Han Y., Dent P., Fisher P.B., Curiel D.T., Lesniak M.S. Enhanced transduction of malignant glioma with a double targeted Ad5/3-RGD fiber-modified adenovirus. Mol. Cancer Ther. 2006;5:2408–2416. doi: 10.1158/1535-7163.MCT-06-0187. [DOI] [PubMed] [Google Scholar]
- 159.Niopek D., Wehler P., Roensch J., Eils R., Di Ventura B. Optogenetic control of nuclear protein export. Nat. Commun. 2016;7:10624. doi: 10.1038/ncomms10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li D., Marchenko N.D., Schulz R., Fischer V., Velasco-Hernandez T., Talos F., Moll U.M. Functional inactivation of endogenous MDM2 and CHIP by HSP90 causes aberrant stabilization of mutant p53 in human cancer cells. Mol. Cancer Res. 2011;9:577–588. doi: 10.1158/1541-7786.MCR-10-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Esser C., Scheffner M., Höhfeld J. The chaperone-associated ubiquitin ligase CHIP is able to target p53 for proteasomal degradation. J. Biol. Chem. 2005;280:27443–27448. doi: 10.1074/jbc.M501574200. [DOI] [PubMed] [Google Scholar]
- 162.Lukashchuk N., Vousden K.H. Ubiquitination and degradation of mutant p53. Mol. Cell. Biol. 2007;27:8284–8295. doi: 10.1128/MCB.00050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Freed-Pastor W.A., Mizuno H., Zhao X., Langerød A., Moon S.H., Rodriguez-Barrueco R., Barsotti A., Chicas A., Li W., Polotskaia A. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148:244–258. doi: 10.1016/j.cell.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Adorno M., Cordenonsi M., Montagner M., Dupont S., Wong C., Hann B., Solari A., Bobisse S., Rondina M.B., Guzzardo V. A mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 165.Muller P.A., Caswell P.T., Doyle B., Iwanicki M.P., Tan E.H., Karim S., Lukashchuk N., Gillespie D.A., Ludwig R.L., Gosselin P. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009;139:1327–1341. doi: 10.1016/j.cell.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 166.Sauer L., Gitenay D., Vo C., Baron V.T. Mutant p53 initiates a feedback loop that involves Egr-1/EGF receptor/ERK in prostate cancer cells. Oncogene. 2010;29:2628–2637. doi: 10.1038/onc.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang W., Cheng B., Miao L., Mei Y., Wu M. Mutant p53-R273H gains new function in sustained activation of EGFR signaling via suppressing miR-27a expression. Cell Death Dis. 2013;4:e574. doi: 10.1038/cddis.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang X., Su C., Cao H., Li K., Chen J., Jiang L., Zhang Q., Wu X., Jia X., Liu Y. A novel triple-regulated oncolytic adenovirus carrying p53 gene exerts potent antitumor efficacy on common human solid cancers. Mol. Cancer Ther. 2008;7:1598–1603. doi: 10.1158/1535-7163.MCT-07-2429. [DOI] [PubMed] [Google Scholar]
- 169.He X., Liu J., Yang C., Su C., Zhou C., Zhang Q., Li L., Wu H., Liu X., Wu M., Qian Q. 5/35 fiber-modified conditionally replicative adenovirus armed with p53 shows increased tumor-suppressing capacity to breast cancer cells. Hum. Gene Ther. 2011;22:283–292. doi: 10.1089/hum.2010.058. [DOI] [PubMed] [Google Scholar]
- 170.Liu C., Sun B., An N., Tan W., Cao L., Luo X., Yu Y., Feng F., Li B., Wu M. Inhibitory effect of Survivin promoter-regulated oncolytic adenovirus carrying P53 gene against gallbladder cancer. Mol. Oncol. 2011;5:545–554. doi: 10.1016/j.molonc.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sauthoff H., Pipiya T., Heitner S., Chen S., Norman R.G., Rom W.N., Hay J.G. Late expression of p53 from a replicating adenovirus improves tumor cell killing and is more tumor cell specific than expression of the adenoviral death protein. Hum. Gene Ther. 2002;13:1859–1871. doi: 10.1089/104303402760372954. [DOI] [PubMed] [Google Scholar]
- 172.van Beusechem V.W., van den Doel P.B., Gerritsen W.R. Conditionally replicative adenovirus expressing degradation-resistant p53 for enhanced oncolysis of human cancer cells overexpressing murine double minute 2. Mol. Cancer Ther. 2005;4:1013–1018. doi: 10.1158/1535-7163.MCT-05-0010. [DOI] [PubMed] [Google Scholar]
- 173.Zhang S., Xu G., Liu C., Xiao S., Sun Y., Su X., Cai Y., Li D., Xu B. Clinical study of recombinant adenovirus-p53 (Adp53) combined with hyperthermia in advanced cancer (a report of 15 cases) Int. J. Hyperthermia. 2005;21:631–636. doi: 10.1080/02656730500147868. [DOI] [PubMed] [Google Scholar]
- 174.Swisher S.G., Roth J.A., Nemunaitis J., Lawrence D.D., Kemp B.L., Carrasco C.H., Connors D.G., El-Naggar A.K., Fossella F., Glisson B.S. Adenovirus-mediated p53 gene transfer in advanced non-small-cell lung cancer. J. Natl. Cancer Inst. 1999;91:763–771. doi: 10.1093/jnci/91.9.763. [DOI] [PubMed] [Google Scholar]
- 175.Nemunaitis J., Swisher S.G., Timmons T., Connors D., Mack M., Doerksen L., Weill D., Wait J., Lawrence D.D., Kemp B.L. Adenovirus-mediated p53 gene transfer in sequence with cisplatin to tumors of patients with non-small-cell lung cancer. J. Clin. Oncol. 2000;18:609–622. doi: 10.1200/JCO.2000.18.3.609. [DOI] [PubMed] [Google Scholar]
- 176.Cristofanilli M., Krishnamurthy S., Guerra L., Broglio K., Arun B., Booser D.J., Menander K., Van Wart Hood J., Valero V., Hortobagyi G.N. A nonreplicating adenoviral vector that contains the wild-type p53 transgene combined with chemotherapy for primary breast cancer: safety, efficacy, and biologic activity of a novel gene-therapy approach. Cancer. 2006;107:935–944. doi: 10.1002/cncr.22080. [DOI] [PubMed] [Google Scholar]
- 177.Clayman G.L., el-Naggar A.K., Lippman S.M., Henderson Y.C., Frederick M., Merritt J.A., Zumstein L.A., Timmons T.M., Liu T.J., Ginsberg L. Adenovirus-mediated p53 gene transfer in patients with advanced recurrent head and neck squamous cell carcinoma. J. Clin. Oncol. 1998;16:2221–2232. doi: 10.1200/JCO.1998.16.6.2221. [DOI] [PubMed] [Google Scholar]
- 178.Habib N.A., Hodgson H.J., Lemoine N., Pignatelli M. A phase I/II study of hepatic artery infusion with wtp53-CMV-Ad in metastatic malignant liver tumours. Hum. Gene Ther. 1999;10:2019–2034. doi: 10.1089/10430349950017383. [DOI] [PubMed] [Google Scholar]
- 179.Guan Y.S., Liu Y., Zou Q., He Q., La Z., Yang L., Hu Y. Adenovirus-mediated wild-type p53 gene transfer in combination with bronchial arterial infusion for treatment of advanced non-small-cell lung cancer, one year follow-up. J. Zhejiang Univ. Sci. B. 2009;10:331–340. doi: 10.1631/jzus.B0820248. [DOI] [PMC free article] [PubMed] [Google Scholar]