Abstract
Several lysosomal enzymes currently used for enzyme replacement therapy in patients with lysosomal storage diseases contain very low levels of mannose 6-phosphate, limiting their uptake via mannose 6-phosphate receptors on the surface of the deficient cells. These enzymes are produced at high levels by mammalian cells and depend on endogenous GlcNAc-1-phosphotransferase α/β precursor to phosphorylate the mannose residues on their glycan chains. We show that co-expression of an engineered truncated GlcNAc-1-phosphotransferase α/β precursor and the lysosomal enzyme of interest in the producing cells resulted in markedly increased phosphorylation and cellular uptake of the secreted lysosomal enzyme. This method also results in the production of highly phosphorylated acid β-glucocerebrosidase, a lysosomal enzyme that normally has just trace amounts of this modification.
Keywords: GlcNAc-1-phosphotransferase, lysosomal enzyme, lysosomal storage disorders, mannose 6-phosphate, enzyme replacement therapy
Introduction
Enzyme replacement therapy (ERT) is currently the major form of treatment for a number of lysosomal storage diseases, although its efficacy varies among the individual disorders.1 Most of these inherited disorders arise from the lack of activity of a single lysosomal enzyme, which leads to the accumulation of the material normally degraded by the enzyme. The build-up of the storage material in the lysosome eventually results in cell and organ dysfunction. The goal of ERT is to introduce sufficient amounts of normal enzyme into the lysosomes of the deficient cells to clear the storage material and restore lysosome function. This form of therapy was first used in patients with type 1 Gaucher disease, who lack acid β-glucocerebrosidase (GBA) activity and accumulate glucosylceramide primarily in macrophage type cells.2 The replacement enzyme, containing N-linked glycans with terminal mannose residues, is infused intravenously and taken up by macrophages via cell surface mannose receptors. The endocytosed enzyme is then transported via endosomes to lysosomes, where it functions with good clinical results in this disorder.3
Because most cell types lack mannose receptors, the replacement enzymes used to treat lysosomal storage disorders that involve cell types other than macrophages use binding to the cation-independent mannose 6-phosphate receptor (CI-MPR) at the cell surface for subsequent delivery to lysosomes. In this regard, studies of a mouse model of Gaucher disease have produced evidence for the involvement of multiple cell lineages other than macrophages in the pathophysiology of this disorder, particularly osteoblasts, dendritic cells, and T cells.4 Therefore, a therapy that included these cell types may be of clinical benefit in this disorder.
The enzymes used for ERT are purified from the secretions of mammalian cells, mostly Chinese hamster ovary cells, engineered to produce high levels of the enzyme of interest. The feasibility of this approach is dependent upon the ability of the endogenous GlcNAc-1-phosphotransferase to phosphorylate mannose residues of the N-glycans of the expressed lysosomal enzyme. Some of the replacement enzymes produced by this technique are highly phosphorylated and bind well to the CI-MPR. Others, however, are poorly phosphorylated, limiting their effectiveness in ERT. This includes the Pompe disease enzyme (acid α-glucosidase [GAA]) and the alpha-mannosidosis enzyme (lysosomal acid α-mannosidase [LAMAN]).5, 6 In addition, the GBA currently used in the treatment of Gaucher disease contains a very low level of Man-6-P.7
We considered two possibilities for why GAA and LAMAN are poorly phosphorylated. First, the activity of the endogenous GlcNAc-1-phosphotransferase in the producing cells may be insufficient to effectively phosphorylate the high levels of these enzymes being synthesized. If this were the case, co-transfection of GlcNAc-1-phosphotransferase with the lysosomal enzyme would be expected to enhance phosphorylation. One potential limitation to this approach is that overexpression of wild-type (WT) GlcNAc-1-phosphotransferase in mammalian cells can result in a portion of the expressed enzyme remaining inactive because of lack of proteolytic cleavage by the Site-1 protease (S1P), which is essential for catalytic activation of the protein.8 A second possibility is that these two lysosomal enzymes are poor substrates for WT GlcNAc-1-phosphotransferase compared with other lysosomal enzymes. Evidence that this is the case with GAA has been presented.9 GBA differs from the other lysosomal enzymes in that it is known to be transported to lysosomes by a Man-6-P-independent pathway.10 This fact, plus its very low content of Man-6-P, has led to the conclusion that it is not a substrate for GlcNAc-1-phosphotransferase.
During the course of studies to understand the roles of the various domains of the α/β subunits of GlcNAc-1-phosphotransferase, we engineered a modified enzyme that bypasses the requirement for proteolytic cleavage, is highly expressed and has good activity toward a panel of endogenous lysosomal enzymes. We then compared the ability of the WT and modified GlcNAc-1-phosphotransferase to act on a number of poorly phosphorylated lysosomal enzymes when co-transfected into cells. Our findings document that it is possible to substantially enhance the phosphorylation of lysosomal enzymes, including GBA, that are poor substrates for GlcNAc-1-phosphotransferase using our modified GlcNAc-1-phosphotransferase. Acid hydrolases synthesized by this procedure display increased binding to Man-6-P receptors and enhanced uptake by target cells compared with the enzymes produced by the current procedure.
Results
Generation of a Highly Active Variant of GlcNAc-1-Phosphotransferase
GlcNAc-1-phosphotransferase is an α2β2γ2 hexamer encoded by two genes (GNPTAB and GNPTG). We have reported that the α/β subunits are able to phosphorylate most lysosomal enzymes in the absence of γ.11 Therefore, we used a construct encoding only the α/β precursor as the starting source of mannose phosphorylating activity. Proteolytic cleavage of this precursor at K928 to give rise to the α and β subunits is mediated by the Site-1 protease in the Golgi, and this process is essential for catalytic competency of the protein.8, 9 The α subunit contains two Notch modules and a DNA methyltransferase-associated protein (DMAP) interaction domain that mediate the specific recognition of protein determinants on the lysosomal enzyme substrates (Figure 1A).12 In addition, there are four so-called spacer domains in the α/β precursor whose functions are beginning to be elucidated. Thus, we have reported that spacer-1 (residues 86–322) determines the site of cleavage of the α/β precursor and serves to limit the phosphorylation of non-lysosomal glycoproteins,13 whereas spacer-2 contains the γ-subunit binding site.12, 14 Besides these domains, the α and β subunits also harbor four Stealth domains that together form the catalytic core of the protein. The Stealth domains of all eukaryotic GlcNAc-1-phosphotransferases are highly conserved and resemble sequences within bacterial proteins that encode sugar-phosphate transferases involved in cell wall polysaccharide biosynthesis.15 In contrast to the mammalian enzymes, however, the bacterial proteins such as the N. meningitidis GlcNAc-1-phosphotransferase (Figure S1) lack the other domains, with no evidence that proteolytic cleavage is necessary for catalytic activation.16 Thus, we asked if it was possible to engineer a human GlcNAc-1-phosphotransferase that is not cleaved but retained high catalytic activity toward the N-linked glycans of lysosomal enzymes.
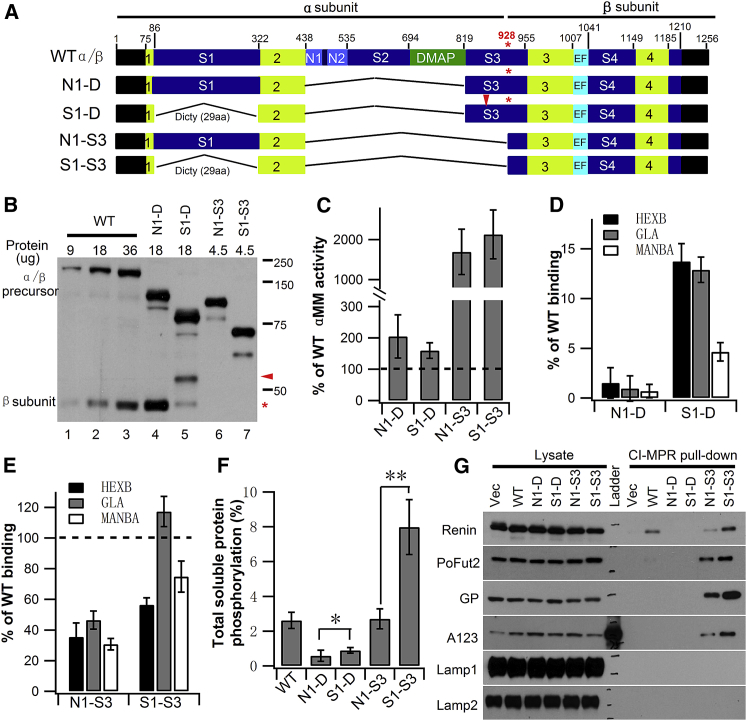
Figure 1.
Expression of a Minimal GlcNAc-1-Phosphotransferase and Analysis of Enzyme Activity
(A) Schematic of WT and the various α/β precursor deletion constructs expressed in GNPTAB−/− HeLa cells. The four regions in lime comprise the catalytic Stealth domain, while the two Notch modules (N1 and N2) and the DNA methyl-transferase-associated protein (DMAP) interaction domain are involved in lysosomal enzyme recognition. In S1-D and S1-S3, the 236 aa human spacer-1 (S1) sequence was replaced with 29 aa of the D. discoideum sequence, in addition to removal of N1 through most of spacer-3 (S3) up to the K928 cleavage site (asterisk). (B) Immunoblot of WT α/β precursor and the deletion mutants expressed in GNPTAB−/− HeLa cells. The indicated amount of each cell extract was loaded, and the α/β precursor and β subunits were detected with an anti-V5 antibody. (C) Catalytic activity of WT α/β precursor and the deletion mutants toward αMM using equal amounts of whole-cell extracts. The vector-only transfected GNPTAB−/− HeLa cell extract served as a control, and WT value was set to 100% after subtraction of vector-only background. (D and E) Transfection of GNPTAB−/− HeLa cells with either WT α/β precursor or the various deletion mutant cDNAs. The degree of phosphorylation mediated WT or mutant proteins was determined by binding of three endogenous lysosomal enzymes to CI-MPR-affinity beads. Bound material was assayed for activity, and values obtained with cells transfected with WT α/β are set to 100%. (F) Mannose phosphorylation of total soluble proteins was determined by transfecting GNPTAB−/− HeLa cells with WT α/β precursor or the indicated deletion mutant cDNAs, followed by [2-3H]mannose labeling. Values shown are calculated as the percentage of counts recovered with the CI-MPR affinity beads as a fraction of the total counts in the phosphotungstic acid precipitate. The background value of 0.8 ± 0.3% was subtracted to yield the final depicted vales. *p < 0.05, **p < 0.01. (G) Immunoblot analysis of GNPTAB−/− HeLa cells co-transfected with the expression plasmids for the indicated proteins along with empty vector, WT α/β precursor, or the indicated deletion mutant cDNAs. Cell lysates were incubated with CI-MPR-affinity beads, and the binding of the various proteins was determined by probing the blots with the following antibodies: anti-HA for Renin, anti-myc for PoFut2, anti-Strep tag for the vWF A1A2A3 domains, and antibodies generated against the native protein for GP, Lamp1, and Lamp2. Error bars represent mean ± SD.
We have previously reported that a construct (N1-D; Figure 1A) lacking the region from the Notch1 module to the end of DMAP (residues 438–819) is well expressed and has good catalytic activity toward the simple sugar α-methylmannoside (αMM) but is unable to phosphorylate lysosomal enzymes.12 When deletion of this region was combined with removal of spacer-1 (construct S1-D), the expressed protein (Figure 1B, lane 5) had similar activity as N1-D toward αMM but slightly greater phosphorylation activity toward the lysosomal enzyme panel (Figures 1C and 1D), despite only a small amount of the β subunit product resulting from this construct (Figure 1B, compare lanes 4 and 5). This is in agreement with our recent study that upon removal of spacer-1, the uncleaved α/β precursor retains some catalytic activity.13 To determine if it was possible to bypass the requirement for cleavage altogether, construct N1-S3 was made, which extended the deletion from Notch1 up to the Site-1 protease cleavage site in spacer-3 (residues 438–928). This construct, which remained a single-chain molecule, was very highly expressed (Figure 1B, lane 6), properly localized to the Golgi (Figure S2), and was 17-fold more active toward αMM than observed with WT α/β (Figure 1C). However, it only phosphorylated the panel of lysosomal enzymes about 30%–40% as well as the WT transferase (Figure 1E). We also tested the ability of N1-S3 and WT enzyme to phosphorylate the total cellular pool of soluble glycoproteins, which would include proteins other than lysosomal enzymes. For this experiment, cells transfected with either the WT or the modified construct were labeled with [2-3H]mannose for 2 hr. The cells were then lysed, and following removal of the membrane fraction by high-speed centrifugation, the supernatants were incubated with CI-MPR beads to bind the Man-6-P-containing proteins, and the percentage of total counts bound was determined. As shown in Figure 1F, both constructs phosphorylated about 2.6% of the total soluble glycoprotein pool. Because N1-S3 had less activity toward lysosomal enzymes than WT, this result indicated that it acted upon non-lysosomal glycoproteins to a greater extent than does the WT enzymes.
The final construct (S1-S3) had spacer-1 deleted along with the region from Notch1 to the cleavage site in spacer-3 (Figure 1A). This construct, like the N1-S3, was expressed at a much higher level than the WT construct (Figure 1B, lane 7), localized to the Golgi (Figure S2), and displayed a 21-fold increase in catalytic activity toward αMM over WT α/β (Figure 1C). It also phosphorylated the lysosomal enzyme panel better than observed with N1-S3, ranging from 56%–117% of WT level (Figure 1E). Strikingly, we noted that S1-S3 phosphorylated the total pool of cellular soluble glycoproteins 3-fold greater than N1-S3 and the WT enzyme, consistent with increased phosphorylation of lysosomal enzymes that are poorly phosphorylated by the WT enzyme as well as an enhanced ability to phosphorylate non-lysosomal glycoproteins (Figure 1F). To test the latter possibility, we determined the ability of the various constructs to phosphorylate a panel of non-lysosomal glycoproteins, two of which were known to be weak substrates of WT GlcNAc-1-phosphotransferase, namely, Renin and protein O-fucosyltransferase 2 (PoFut2).17, 18 Phosphorylation was determined by binding to CI-MPR beads followed by western blotting to detect the proteins. As shown in Figure 1G, the S1-S3 construct phosphorylated Renin, PoFut2, glycopepsinogen (GP), and the von Willebrand factor (vWF) A1A2A3 domain, whereas the WT construct only phosphorylated Renin weakly in this assay. The N1-S3 construct acted on these proteins as well but to a lesser degree than S1-S3. None of these constructs phosphorylated the membrane glycoproteins, Lamp1 and Lamp2.
In an attempt to further reduce human GlcNAc-1-phosphotransferase to resemble the N. meningitidis enzyme (Figure S1), a series of deletions within spacer-4 were generated. However, none of these mutants displayed enzyme activity toward αMM (data not shown).
Co-transfection of GlcNAc-1-Phosphotransferase with Lysosomal Enzymes Enhances Phosphorylation
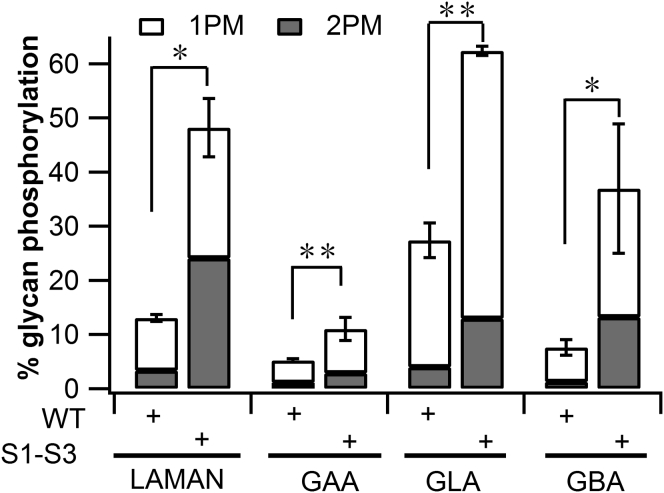
On the basis of the above findings, we selected construct S1-S3 along with the WT α/β precursor construct to co-transfect with plasmids encoding the three poorly phosphorylated lysosomal enzymes (LAMAN, GAA, and GBA) and GLA as a control for a well-phosphorylated enzyme. In the initial experiment, the constructs were co-expressed in GNPTAB−/− HeLa cells, followed by incubation with [2-3H]mannose to label the N-linked glycans of the lysosomal enzymes. The media was collected and the secreted proteins were immunoprecipitated and analyzed for their content of glycans containing one or two Man-6-P residues. As shown in Figure 2, the S1-S3 construct produced 2.1- to 4.9-fold greater phosphorylation of the glycans present on the panel of lysosomal enzymes that were co-expressed with this truncated α/β precursor relative to WT.
Figure 2.
Phosphorylation of Lysosomal Enzymes by S1-S3
GNPTAB−/− HeLa cells were co-transfected with either WT or S1-S3 α/β precursor cDNAs, along with expression plasmids for four lysosomal enzymes. Forty-eight hours post-transfection, cells were labeled for 2 hr with [2-3H]-mannose, followed by immunoprecipitation of the proteins secreted into the media and determination of the percentage N-glycans containing one (1PM) or two (2PM) Man-6-P residues. The absolute values of the percentage glycans phosphorylated are shown. *p < 0.05, **p < 0.01. Error bars represent mean ± SD.
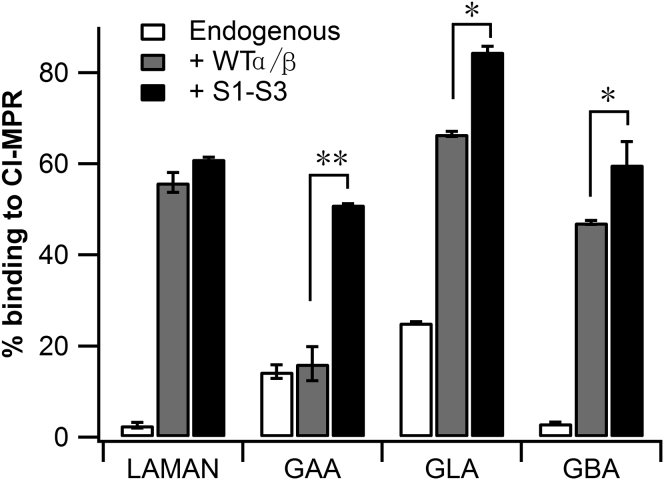
Next we co-transfected either Expi293 cells or mouse D9 L cells (lacking the CI-MPR) with the same plasmids and collected the media after 48–72 hr to use as a source of secreted lysosomal enzymes for receptor binding and cell uptake experiments. As a control for phosphorylation mediated by the endogenous GlcNAc-1-phosphotransferase, cells were transfected with plasmids encoding the lysosomal enzymes but not with the cDNAs for the α/β precursors. A representative Coomassie blue-stained SDS gel of the media from Expi293 cells is shown in Figure S3. In all instances, the enzyme secreted by cells co-transfected with the S1-S3 construct bound to the CI-MPR-beads to a much greater extent than observed with the lysosomal enzymes expressed alone in the cells (Figure 3). This is indicative of enhanced phosphorylation. Further, with the exception of LAMAN, co-transfection with S1-S3 resulted in significantly greater lysosomal enzyme binding to the CI-MPR-beads than obtained with the WT enzyme.
Figure 3.
Binding of Expressed Enzymes to CI-MPR
Expi293 cells or mouse D9 L-cells were co-transfected with expression plasmids for the indicated lysosomal enzymes along with empty vector, WT α/β precursor, or the S1-S3 mutant cDNA. D9 L-cells were used for generating GAA, while Expi293 cells were used for the other lysosomal enzymes. Forty-eight to 72 hr post-transfection, the media was collected, and aliquots were incubated with CI-MPR-beads to bind the phosphorylated lysosomal enzymes. The percentage of each lysosomal enzyme that bound to the CI-MPR-beads was determined as described in the Experimental Procedures. Values obtained with cells transfected with the empty vector (“endogenous”) represent the activity of the endogenous GlcNAc-1-phosphotransferase. *p < 0.05, **p < 0.01.
The impact of the increased Man-6-P content of the various lysosomal enzymes on their uptake by HeLa cells is shown in Table 1. In all four instances, the enzymes secreted by cells co-transfected with the plasmid encoding the truncated α/β precursor were internalized many fold better than enzyme secreted by cells using only the endogenous GlcNAc-1-phosphotransferase. Similar results were obtained with co-transfection of the WT α/β precursor with the exception of GAA, consistent the CI-MPR-bead binding results. Most of the uptake was blocked by the presence of 5 mM Man-6-P in the media, showing that the uptake is mediated by the CI-MPR. The results with LAMAN were particularly striking, with Man-6-P-inhibitable uptake being stimulated by 130- to 153-fold. It is also notable that the GBA was internalized to a great extent in a Man-6-P-dependent manner.
Table 1.
Cell Uptake of Secreted Lysosomal Enzymes Phosphorylated by Endogenous GlcNAc-1-Phosphotransferase or Overexpressed WT Enzyme or the S1-S3 Mutant
| Enzyme | PTase | Total Units Added | Man-6-P | % Uptakea |
Fold Increase | |
|---|---|---|---|---|---|---|
| M6PR Dependent | ||||||
| LAMAN | endogenous | 670 | − | 0.76 ± 0.23 | 0.14 | |
| + | 0.62 ± 0.27 | |||||
| WTα/β | 359 | − | 24.70 ± 1.34 | 21.5 | 153 | |
| + | 3.20 ± 0.77 | |||||
| S1-S3 | 530 | − | 22.46 ± 4.07 | 18.2 | 130 | |
| + | 4.30 ± 1.19 | |||||
| GAA | endogenous | 5.7 | − | 4.05 ± 0.46 | 3.8 | |
| + | 0.22 ± 0.42 | |||||
| WTα/β | 3.8 | − | 3.31 ± 1.13 | 2.9 | 0.8 | |
| + | 0.44 ± 1.16 | |||||
| S1-S3 | 3.2 | − | 12.79 ± 0.90 | 9.8 | 2.6 | |
| + | 2.99 ± 1.59 | |||||
| GBA | endogenous | 2.7 | − | 10.74 ± 1.94 | 5.1 | |
| + | 5.65 ± 1.51 | |||||
| WTα/β | 2.3 | − | 46.73 ± 4.00 | 37.4 | 7.3 | |
| + | 9.37 ± 1.26 | |||||
| S1-S3 | 2.8 | − | 49.74 ± 11.53 | 41.1 | 8.0 | |
| + | 8.67 ± 4.21 | |||||
| GLA | endogenous | 194 | − | 2.72 ± 0.91 | 1.7 | |
| + | 1.00 ± 0.11 | |||||
| WTα/β | 141 | − | 9.53 ± 0.11 | 8.2 | 4.8 | |
| + | 1.30 ± 0.13 | |||||
| S1-S3 | 237 | − | 7.01 ± 0.69 | 6.1 | 3.5 | |
| + | 0.95 ± 0.11 | |||||
One unit = 1 nmol 4-methylumbelliferone released per hour. Uptake experiments were performed in either the absence or the presence of 5 mM Man-6-P.
Percentage uptake per 200 μg cell protein in 24 hr.
Discussion
The findings presented here establish that phosphorylation of expressed lysosomal enzymes can be substantially increased by co-transfection with an engineered truncated α/β precursor of GlcNAc-1-phosphotransferase and, in some instances, with the WT α/β precursor. Importantly, the γ subunit, encoded by a different gene, is not required for this effect. This simplifies construction of the producing cells. The enhanced phosphorylation of the lysosomal enzymes increases their binding to the CI-MPR and uptake by cells. This effect even occurs with lysosomal enzymes such as GLA that are well phosphorylated by the endogenous GlcNAc-1-phosphotransferase. But most important is the finding that this method enhances the phosphorylation and uptake of human LAMAN and GAA, two lysosomal enzymes that are poorly phosphorylated by endogenous GlcNAc-1-phosphotransferase. In addition, the production of GBA containing high levels of Man-6-P offers the opportunity to restore enzyme activity to cell types in patients with Gaucher disease who lack the mannose receptor. This is of particular interest in light of the reported findings in a mouse model of Gaucher disease that cell types other than macrophages are dysfunctional in this disorder.4 The availability of highly phosphorylated GBA could potentially provide additional benefit to the current therapy that is targeted specifically to macrophages.
In conclusion, the methods described here have the potential to significantly improve the effectiveness of lysosomal enzymes in ERT. In addition to providing better cell uptake, these preparations may allow lower doses to be administered to patients, perhaps at less frequent intervals. The method should be applicable to the production of phosphorylated lysosomal enzymes for other lysosomal storage diseases that may be amenable to ERT.
Materials and Methods
Cell Lines
Expi293 cells (Life Technologies) were grown in suspension in Expi293 expression medium (Life Technologies). The GNPTAB−/− HeLa cell line has been described in detail elsewhere.12 Parental and GNPTAB−/− HeLa cells were maintained as a monolayer in DMEM (Life Technologies) containing 0.11 g/L sodium pyruvate and 4.5 g/L glucose, supplemented with 10% (vol/vol) fetal bovine serum (FBS) (Atlanta Biologicals), 100,000 U/L penicillin, 100 mg/L streptomycin (Life Technologies), and 2 mM L-glutamine (Life Technologies). The CI-MPR negative mouse D9 L-cell line has been described.19 D9 L- cells were maintained as a monolayer in α-MEM (Life Technologies) containing 100,000 U/L penicillin and 100 mg/L streptomycin (Life Technologies).
DNA Constructs
Human GNPTAB-V5/His 8 in pcDNA6 and the N1-D deletion construct has been described.12 Constructs S1-D and S1-S3 were generated in two steps as follows: in the first step, the human spacer-1 sequence was replaced with D. discoideum spacer-1 using a 0.5 kb gBlocks gene fragment (IDT) in a two-stage overlap extension PCR (OE-PCR). Nucleotides corresponding to amino acids 438–819 or 438–928 were subsequently removed in the second step to generate S1-D and S1-S3, respectively. The construct N1-S3 was generated by deleting nucleotides encoding amino acids 438–928 from the WT construct. The LAMAN-myc-Flag cDNA was purchased from Origene, while the GAA cDNA was a kind gift of Eline van Meel (Leiden University). The GLA and GBA cDNAs were generously provided by Amicus Therapeutics. A C-terminal myc-tag was appended to the GBA and GAA c-DNA. The PoFut2-myc cDNA was a gift from Robert Haltiwanger (University of Georgia. Athens, GA). Renin-HA cDNA was purchased from Addgene (Cambridge, MA), while the plasmid, vWF-A1A2A3-Strep-pCDNA6, was kindly provided by Joshua Muia and J. Evan Sadler (Washington University School of Medicine, St. Louis, MO).
Phosphotransferase Assay
Constructs encoding the WT and S1-S3 α/β precursors were expressed in GNPTAB−/− HeLa by transfection with Lipofectatime 3000 (Life Technologies) according to the manufacturer’s protocol. Forty-eight hours post-transfection, cells in six-well plates were harvested and lysed in 250 μL of buffer A (25 mM Tris-Cl [pH 7.2], 150 mM NaCl, 1% Triton X-100, and protease inhibitor cocktail). Either10 μL (WT) or 2 μL (S1-S3) of cell extract was incubated in buffer B (50 mM Tris-Cl [pH 7.4], 10 mM MgCl2, 10 mM MnCl2, 2 mg/mL BSA, 2 mM ATP) in the presence of 75 μM UDP-GlcNAc, 1 μCi UDP-[3H]GlcNAc, and 100 mM α-MM in a final volume of 50 μL for 1 hr at 37°C. The reactions were stopped by the addition of 1 mL of 2 mM EDTA [pH 8.0], and the samples were subjected to QAE-Sephadex chromatography as previously described.11
Western Blotting
Proteins resolved by SDS-PAGE under reducing conditions were transferred to nitrocellulose membrane and detected with antibodies as described in the figure legends. The indicated amounts of whole-cell extracts were loaded on the gels.
Immunofluorescence Microscopy
To visualize the subcellular localization of WT α/β and the S1-S3 mutant, the constructs were transfected into GNPTAB−/− HeLa cells using Lipofectamine 3000 according to the manufacturer’s protocol. Twenty-four hours post-transfection, the cells were fixed with 4% formaldehyde (Sigma-Aldrich) and the α/β subunits were detected with mouse anti-V5 monoclonal antibody (Life Technologies). The Golgi marker GOLPH4 was detected with rabbit anti-GOLPH4 polyclonal antibody (Abcam). The processed cells were mounted in ProLong Gold antifade mounting medium (Life Technologies), and the images were acquired with an LSM880 confocal microscope (Carl Zeiss). Images were analyzed using ImageJ software (Fiji).
[2-3H]Mannose Labeling Experiments for Lysosomal Enzymes
Labeling experiments were performed with transfected GNPTAB−/− HeLa cells as follows: 48 hr post-transfection, cells in 60 mm tissue culture plates were incubated with 50–150 μCi of [2-3H]mannose (Perkin Elmer) for 2 hr, followed by the addition of complete medium containing 5 mM glucose, 5 mM mannose, and 10 mM NH4Cl to stop mannose uptake and induce secretion. The cells were incubated for an additional 3 hr before the medium was collected for analysis. Acid hydrolases secreted into the media were immunoprecipitated, and oligosaccharides isolated and analyzed essentially as described in detail previously.20 Because the LAMAN, GAA, and GBA cDNAs contained a C-terminal myc-tag, 20 μL anti-myc monoclonal antibody (Santa Cruz Biotechnology) was pre-bound to 100 μL Protein G-agarose-PLUS beads (Santa Cruz Biotechnology) prior to immunoprecipitation of these lysosomal hydrolases from the media. In the case of GLA, the secreted enzyme was immunoprecipitated with Protein G-agarose-PLUS beads pre-bound to anti-α-Gal antibody (Amicus Therapeutics). Immunoprecipitated material was treated with Endo H (NEB) and filtered with Ultracel-10K (EMD Millipore). The filtrate containing the released neutral and phosphorylated high mannose glycans was treated with mild acid to remove any N-acetylglucosamine residues still attached to the phosphate moieties and applied to a QAE-column matrix to separate the oligosaccharides bearing zero, one or two Man-6-P residues. The retentate containing Endo H-resistant complex oligosaccharides was treated with Pronase (Roche Diagnostics) and fractionated on ConA-Sepharose 4B (GE Healthcare). The [2-3H]-mannose content of the various fractions was determined, and the percentage phosphorylation was calculated as described.20
[2-3H]Mannose Labeling Experiments for Total Soluble Glycoproteins
Labeling experiments were performed with transfected GNPTAB−/− HeLa cells as follows: 48 hr post-transfection, cells in six-well plates were incubated with 10 μCi of [2-3H]mannose (Perkin Elmer) for 2 hr. Following the 2 hr pulse, cells were rinsed twice with PBS and harvested, then resuspended in detergent-free buffer containing 25 mM Tris-Cl (pH 7.2) and 150 mM NaCl at 4°C with a protease inhibitor cocktail (Life Technologies). Cell were lysed by sonication, then subjected to ultracentrifugation at 100,000 × g for 1 hr to separate the membrane proteins from the soluble fraction. One hundred microliters of the soluble fraction was then incubated with purified CI-MPR that was covalently conjugated to Cyanogen bromide-activated-Sepharose 4B in order to pellet the mannose-phosphorylated glycoproteins, while 10 μL of the soluble fraction was precipitated by 1.5% phosphotungstic acid to obtain total [2-3H]mannose label incorporation into the soluble proteins. This method allowed the accurate quantification of all the mannose-labeled glycoproteins that were phosphorylated by either WT or mutant GlcNAc-1-PT.
Enzyme Production
Mouse D9 L-Cells were co-transfected with GAA cDNA, along with empty vector, WT α/β precursor, or S1-S3 mutant. Expi293 cells were co-transfected with LAMAN, GBA, or GLA cDNAs, along with either empty vector, WT α/β precursor, or S1-S3 mutant. The media was harvested after 2–3 days. For the production of GBA, 10 μM of compound AT3375 (Amicus Therapeutics) was added to the media to stabilize the secreted enzyme.
CI-MPR Affinity Chromatography and Lysosomal Enzyme Assays
Soluble bovine CI-MPR was purified from FBS and covalently conjugated to Cyanogen bromide-activated-Sepharose 4B (Sigma-Aldrich) as described.21 Aliquots of media from 2- to 3-day transfected Expi293 cells or mouse D9 L-cells were diluted with buffer A (25 mM Tris-Cl [pH 7.2], 150 mM NaCl, and 1% Triton X-100) and incubated with the CI-MPR beads at 4°C for 1 hr to bind the phosphorylated lysosomal enzymes. The beads were then sedimented, washed with buffer A, and assayed for lysosomal enzyme activity as described.12, 22, 23 The amount of the starting enzyme recovered on the beads was calculated.
Cell Uptake of Lysosomal Enzymes
Parental HeLa cells were plated on a 12-well plate at approximately 80% density 1 day prior to the cell uptake experiment. Aliquots of media containing each enzyme from the producing cells were added to the parental HeLa cells in a final volume of 500 μL. For competition experiments, Man-6-P was added to a final concentration of 5 mM. The cells were incubated with the media for 24 hr, and then the cells and media were collected separately. Cells were rinsed twice with PBS and then lysed in buffer A. The media and cell extracts were centrifuged at 20,000 × g, and the supernatants were assayed for their content of lysosomal enzyme activity.
Author Contributions
L.L., W.-S.L., B.D., and S.K. designed experiments. L.L. and W.-S.L. carried out experiments. L.L., W.-S.L., B.D., and S.K. analyzed data. B.D. and S.K. drafted the manuscript, and all authors contributed to editing it to produce the final version.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by National Institutes of Health grant CA-008759 and the Yash Gandhi Foundation.
Footnotes
Supplemental Information includes three figures and can be found with this article online at http://dx.doi.org/10.1016/j.omtm.2017.03.006.
Supplemental Information
References
- 1.Lachmann R.H. Enzyme replacement therapy for lysosomal storage diseases. Curr. Opin. Pediatr. 2011;23:588–593. doi: 10.1097/MOP.0b013e32834c20d9. [DOI] [PubMed] [Google Scholar]
- 2.Barton N.W., Brady R.O., Dambrosia J.M., Di Bisceglie A.M., Doppelt S.H., Hill S.C., Mankin H.J., Murray G.J., Parker R.I., Argoff C.E. Replacement therapy for inherited enzyme deficiency--macrophage-targeted glucocerebrosidase for Gaucher’s disease. N. Engl. J. Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- 3.Weinreb N.J., Goldblatt J., Villalobos J., Charrow J., Cole J.A., Kerstenetzky M., vom Dahl S., Hollak C. Long-term clinical outcomes in type 1 Gaucher disease following 10 years of imiglucerase treatment. J. Inherit. Metab. Dis. 2013;36:543–553. doi: 10.1007/s10545-012-9528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mistry P.K., Liu J., Yang M., Nottoli T., McGrath J., Jain D., Zhang K., Keutzer J., Chuang W.L., Mehal W.Z. Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc. Natl. Acad. Sci. USA. 2010;107:19473–19478. doi: 10.1073/pnas.1003308107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McVie-Wylie A.J., Lee K.L., Qiu H., Jin X., Do H., Gotschall R., Thurberg B.L., Rogers C., Raben N., O’Callaghan M. Biochemical and pharmacological characterization of different recombinant acid alpha-glucosidase preparations evaluated for the treatment of Pompe disease. Mol. Genet. Metab. 2008;94:448–455. doi: 10.1016/j.ymgme.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roces D.P., Lüllmann-Rauch R., Peng J., Balducci C., Andersson C., Tollersrud O., Fogh J., Orlacchio A., Beccari T., Saftig P., von Figura K. Efficacy of enzyme replacement therapy in alpha-mannosidosis mice: a preclinical animal study. Hum. Mol. Genet. 2004;13:1979–1988. doi: 10.1093/hmg/ddh220. [DOI] [PubMed] [Google Scholar]
- 7.Van Patten S.M., Hughes H., Huff M.R., Piepenhagen P.A., Waire J., Qiu H., Ganesa C., Reczek D., Ward P.V., Kutzko J.P., Edmunds T. Effect of mannose chain length on targeting of glucocerebrosidase for enzyme replacement therapy of Gaucher disease. Glycobiology. 2007;17:467–478. doi: 10.1093/glycob/cwm008. [DOI] [PubMed] [Google Scholar]
- 8.Marschner K., Kollmann K., Schweizer M., Braulke T., Pohl S. A key enzyme in the biogenesis of lysosomes is a protease that regulates cholesterol metabolism. Science. 2011;333:87–90. doi: 10.1126/science.1205677. [DOI] [PubMed] [Google Scholar]
- 9.Kudo M., Canfield W.M. Structural requirements for efficient processing and activation of recombinant human UDP-N-acetylglucosamine:lysosomal-enzyme-N-acetylglucosamine-1-phosphotransferase. J. Biol. Chem. 2006;281:11761–11768. doi: 10.1074/jbc.M513717200. [DOI] [PubMed] [Google Scholar]
- 10.Reczek D., Schwake M., Schröder J., Hughes H., Blanz J., Jin X., Brondyk W., Van Patten S., Edmunds T., Saftig P. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131:770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Qian Y., Lee I., Lee W.S., Qian M., Kudo M., Canfield W.M., Lobel P., Kornfeld S. Functions of the alpha, beta, and gamma subunits of UDP-GlcNAc:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase. J. Biol. Chem. 2010;285:3360–3370. doi: 10.1074/jbc.M109.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Meel E., Lee W.S., Liu L., Qian Y., Doray B., Kornfeld S. Multiple domains of GlcNAc-1-phosphotransferase mediate recognition of lysosomal enzymes. J. Biol. Chem. 2016;291:8295–8307. doi: 10.1074/jbc.M116.714568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L., Lee W.S., Doray B., Kornfeld S. Role of spacer-1 in the maturation and function of GlcNAc-1-phosphotransferase. FEBS Lett. 2017;591:47–55. doi: 10.1002/1873-3468.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velho R.V., De Pace R., Tidow H., Braulke T., Pohl S. Identification of the interaction domains between α- and γ-subunits of GlcNAc-1-phosphotransferase. FEBS Lett. 2016;590:4287–4295. doi: 10.1002/1873-3468.12456. [DOI] [PubMed] [Google Scholar]
- 15.Sperisen P., Schmid C.D., Bucher P., Zilian O. Stealth proteins: in silico identification of a novel protein family rendering bacterial pathogens invisible to host immune defense. PLoS Comput. Biol. 2005;1:e63. doi: 10.1371/journal.pcbi.0010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litschko C., Romano M.R., Pinto V., Claus H., Vogel U., Berti F., Gerardy-Schahn R., Fiebig T. The capsule polymerase CslB of Neisseria meningitidis serogroup L catalyzes the synthesis of a complex trimeric repeating unit comprising glycosidic and phosphodiester linkages. J. Biol. Chem. 2015;290:24355–24366. doi: 10.1074/jbc.M115.678094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faust P.L., Chirgwin J.M., Kornfeld S. Renin, a secretory glycoprotein, acquires phosphomannosyl residues. J. Cell Biol. 1987;105:1947–1955. doi: 10.1083/jcb.105.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sleat D.E., Zheng H., Qian M., Lobel P. Identification of sites of mannose 6-phosphorylation on lysosomal proteins. Mol. Cell. Proteomics. 2006;5:686–701. doi: 10.1074/mcp.M500343-MCP200. [DOI] [PubMed] [Google Scholar]
- 19.Gabel C.A., Foster S.A. Lysosomal enzyme trafficking in mannose 6-phosphate receptor-positive mouse L-cells: demonstration of a steady state accumulation of phosphorylated acid hydrolases. J. Cell Biol. 1986;102:943–950. doi: 10.1083/jcb.102.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dustin M.L., Baranski T.J., Sampath D., Kornfeld S. A novel mutagenesis strategy identifies distantly spaced amino acid sequences that are required for the phosphorylation of both the oligosaccharides of procathepsin D by N-acetylglucosamine 1-phosphotransferase. J. Biol. Chem. 1995;270:170–179. doi: 10.1074/jbc.270.1.170. [DOI] [PubMed] [Google Scholar]
- 21.Valenzano K.J., Remmler J., Lobel P. Soluble insulin-like growth factor II/mannose 6-phosphate receptor carries multiple high molecular weight forms of insulin-like growth factor II in fetal bovine serum. J. Biol. Chem. 1995;270:16441–16448. doi: 10.1074/jbc.270.27.16441. [DOI] [PubMed] [Google Scholar]
- 22.Steet R.A., Chung S., Wustman B., Powe A., Do H., Kornfeld S.A. The iminosugar isofagomine increases the activity of N370S mutant acid beta-glucosidase in Gaucher fibroblasts by several mechanisms. Proc. Natl. Acad. Sci. USA. 2006;103:13813–13818. doi: 10.1073/pnas.0605928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oude Elferink R.P., Brouwer-Kelder E.M., Surya I., Strijland A., Kroos M., Reuser A.J., Tager J.M. Isolation and characterization of a precursor form of lysosomal alpha-glucosidase from human urine. Eur. J. Biochem. 1984;139:489–495. doi: 10.1111/j.1432-1033.1984.tb08032.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.