Abstract
Enadenotucirev is an oncolytic group B adenovirus identified by a process of bio-selection for the ability to selectively propagate in and rapidly kill carcinoma cells. It is resistant to inactivation by human blood components, potentially enabling intravenous dosing in patients with metastatic cancer. However, there are no known permissive animal models described for group B adenoviruses that could facilitate a conventional approach to preclinical safety studies. In this manuscript, we describe our tailored preclinical strategy designed to evaluate the key biological properties of enadenotucirev. As enadenotucirev does not replicate in animal cells, a panel of primary human cells was used to evaluate enadenotucirev replication selectivity in vitro, demonstrating that virus genome levels were >100-fold lower in normal cells relative to tumor cells. Acute intravenous tolerability in mice was used to assess virus particle-mediated toxicology and effects on innate immunity. These studies showed that particle toxicity could be ameliorated by dose fractionation, using an initial dose of virus to condition the host such that cytokine responses to subsequent doses were significantly attenuated. This, in turn, supported the initiation of a phase I intravenous clinical trial with a starting dose of 1 × 1010 virus particles given on days 1, 3, and 5.
Keywords: enadenotucirev, adenovirus, oncolytic virotherapy, preclinical model, mouse model
Introduction
Translating complex biological drug candidates from the laboratory to a clinical trial can be challenging, particularly when species specificity limits the relevance of animal models. Here, we describe the approach taken for the preclinical development of enadenotucirev, a human-specific oncolytic virus.
Enadenotucirev (formerly known as ColoAd1) is a chimeric Ad11p/Ad3 adenovirus identified by bio-selection from a library of adenovirus serotypes enriched for recombinants, for the ability to replicate and exit rapidly from human colorectal tumor cells.1 In contrast to wild-type viruses that have evolved for a defined pathogen-host relationship, bio-selection can potentially identify virus properties that are optimized for growth in transformed cells. Enadenotucirev is active against a broad range of human carcinoma cell lines, achieving lysis more rapidly than the wild-type adenoviruses Ad11p, Ad3, or Ad5, while activity in normal cells or normal tissue samples is attenuated.1 One particular advantage of enadenotucirev is that the capsid is entirely derived from Ad11p, for which the prevalence of neutralizing antibodies is generally lower in humans relative to other serotypes.2 We have already shown that clinically relevant particle concentrations of enadenotucirev can infect tumor cells in the presence of whole human blood, presenting a realistic opportunity for systemic delivery.3 Additionally, the primary receptor for Ad11, CD46, is often reported to be upregulated in human tumors4, 5 and distributed all over the surface of cancer cells6 relative to the modest and basolateral expression in normal epithelial tissue,7 also making it more accessible in a cancer setting.8
As an adenovirus, enadenotucirev has many features desirable for a product candidate, including the ability to manufacture consistent batches of high-titer particles, a high fidelity double-strand proofreading polymerase, and a robust non-enveloped protein capsid providing good stability under a range of conditions.
Following systemic delivery, oncolytic viruses can potentially cause two types of toxicity in patients. One relates to the direct inflammatory effects of the input virus particles (particle toxicity), while the other relates to the potential for viral replication and activity in non-target tissues (replication toxicity). Particle toxicity is an anticipated and dose-dependent, but transient, effect that typically presents with flu-like signs and symptoms over a 24–72 hr period. Broadly comparable acute signs and symptoms have been observed in clinical trials with a range of different virus classes, including Ad5,9 vaccinia virus,5 and reovirus10 and is probably due to particles interacting with scavenging receptors on innate immune cells. For adenoviruses, this has been studied in some detail, with liver Kupffer cells appearing to be largely responsible for these events.11 Since particle scavenging by the innate immune system is a relatively conserved function, simple rodent models can be quite effective in predicting the basic aspects of particle-based circulation kinetics and inflammation.
In contrast, the potential to measure replication toxicities of viruses in animals is limited, since most viruses are species-specific to some degree. This is particularly relevant for enadenotucirev, since Ad11p (the closest wild-type variant of enadenotucirev) has no known animal host. Given that off-target replication toxicity in human cells is a potential clinical risk, we reasoned that only studies in human cells would provide reliable and meaningful preclinical data on potential replication toxicity in humans. In contrast, attempts to try and create an artificial animal model would always carry significant doubt with respect to clinical translation.
Due to the unique nature of enadenotucirev, we proceeded to design a set of preclinical studies using a combination of murine in vivo experiments, where relevant, to determine acute particle responses and in vitro safety studies for replication selectivity. The development of this approach involved early engagement with regulators and clinical advisors and facilitated the initiation of a first-in-human, phase 1 dose-escalation clinical trial by intravenous administration (ClinicalTrials.gov Identifier: NCT02028442).
Results
Selectivity and Activity of Enadenotucirev
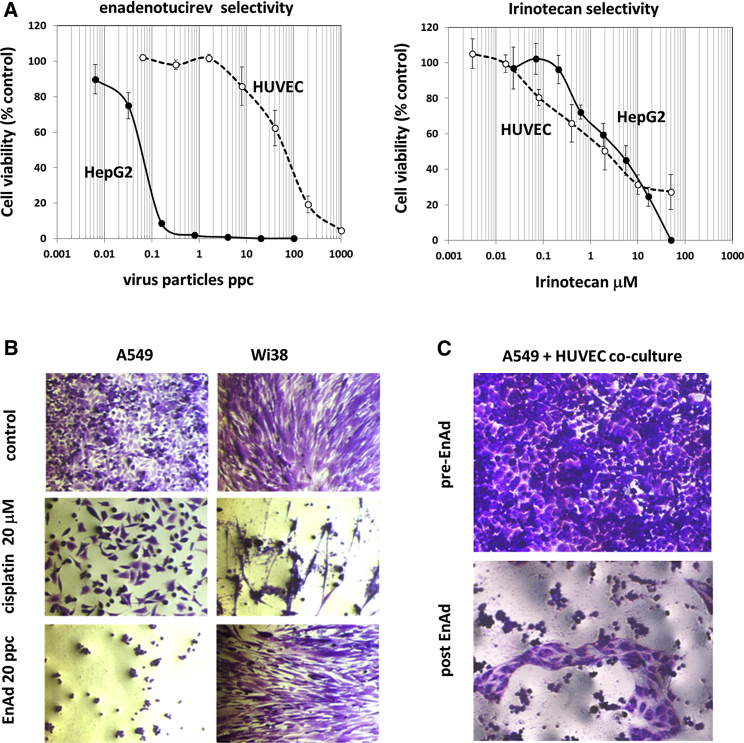
In standard in vitro cytotoxicity assays, enadenotucirev shows greater lytic potency in human tumor cells relative to replicating human endothelial cells or fibroblasts and a greater therapeutic index than wild-type viruses.1 In contrast, many established anticancer cytotoxic agents show only limited selectivity for cancer cells compared to replicating normal cells. We compared enadenotucirev with the topoisomerase-1 inhibitor irinotecan and showed that, whereas enadenotucirev displayed approximately 1,000-fold selectivity for killing HepG2 hepatocellular carcinoma cells compared to proliferating human umbilical vein endothelial cells (HUVEC), irinotecan showed no selectivity at all (Figure 1A). Similarly, concentrations of cisplatin required to kill 50% of A549 lung carcinoma cells also killed a high proportion of Wi38 fibroblasts, whereas complete lysis of A549 cells could be achieved by enadenotucirev without obvious impact on fibroblasts (Figure 1B). When A549 and HUVECs were cultured together, selectivity of enadenotucirev for A549 cells was clearly apparent by light microscopy (Figure 1C). These data demonstrate that, in principle, enadenotucirev should have fewer off-target cytotoxic events compared to standard, broad-spectrum chemotherapy drugs currently used in cancer therapy.
Figure 1.
Assessment of Enadenotucirev Selectivity in Tissue Culture
(A) Proliferating HepG2 (hepatoma) and HUVEC were exposed to a range of enadenotucirev or irinotecan concentrations. After 5 days, cytotoxicity was determined using an MTS viability assay. Data represent mean values ± SD (n = 3). (B) Proliferating A549 cells and fibroblasts (Wi38) were exposed to 20 particles per cell of enadenotucirev or 20 mM cisplatin. (C) A 1:1 mixed culture of A549 and HUVEC exposed to enadenotucirev shows selective cytotoxicity for A549 cells, leaving visibly intact regions of HUVEC.
Activity in Drug-Resistant Cells and Stem Cell-like Populations
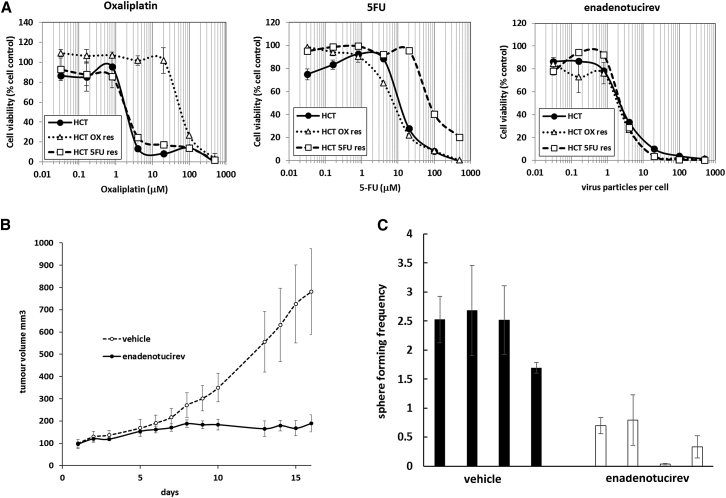
Direct cell killing through lysis rather than through intrinsic cellular pathways could provide an opportunity to treat cells resistant to traditional chemotherapy agents. Indeed, the cytotoxicity of enadenotucirev in HCT116 colon carcinoma cells resistant to 5-fluorouracil (5-FU) or oxaliplatin was very similar to those for parental HCT116 cells (Figure 2A). Cancer stem cells, which have a tumor repopulating phenotype, are also thought to be inherently resistant to some forms of chemotherapy,12 but not necessarily to virotherapy.13 To quantify the impact of enadenotucirev on this subpopulation, we used a sphere-forming assay following a subcutaneous tumor growth experiment. Subcutaneous HCT116 cell xenograft tumors were dosed intratumorally with either saline or 5 × 109 virus particles (vp) of enadenotucirev. Virus treatment significantly inhibited tumor growth in this model (Figure 2B). At the end of the study, when the control group reached a predetermined humane tumor burden endpoint, cells were isolated from the tumors and cultured under sphere-forming conditions to determine the relative portions of cells with a stem-like phenotype. The enadenotucirev-treated group had a lower tumor burden and significantly fewer spheres were recovered, indicating that cells with a stem-like phenotype were sensitive to lysis (Figure 2C). These data, together with previously published studies1 and observations of stability in whole human blood,3 show that enadenotucirev has significant promise as a cancer-targeted therapeutic agent and supported its progression into human trials.
Figure 2.
Activity of Enadenotucirev in Drug-Resistant Cells and Cells with a Sphere-Forming Phenotype
(A) HCT116 cells or variants resistant to 5-FU or oxaliplatin (OX) were exposed to titrations of enadenotucirev or cytotoxic drugs and assessed for cell viability after 5 days. The data represent mean values ± SD (n = 3). (B) CD-1 nude mice bearing subcutaneous HCT116 tumors were dosed intratumorally twice with 5 × 109 particles of enadenotucirev in 10 μL saline or vehicle control on days 0, 2, and 4. The tumor volume was measured five times per week. The data represent 95% confidence intervals (1.96 × SEM, n = 10). (C) At 21 days post-treatment, tumors from four randomly selected mice were resected, dissociated, and cultured ex vivo for 7 days. For each mouse, spheres of ≥50 μm were counted. The data represent mean frequency of spheres per 100 recovered cells ±SD (n = 3).
Lack of Permissive Animal Species for Generating Replication Selectivity Data
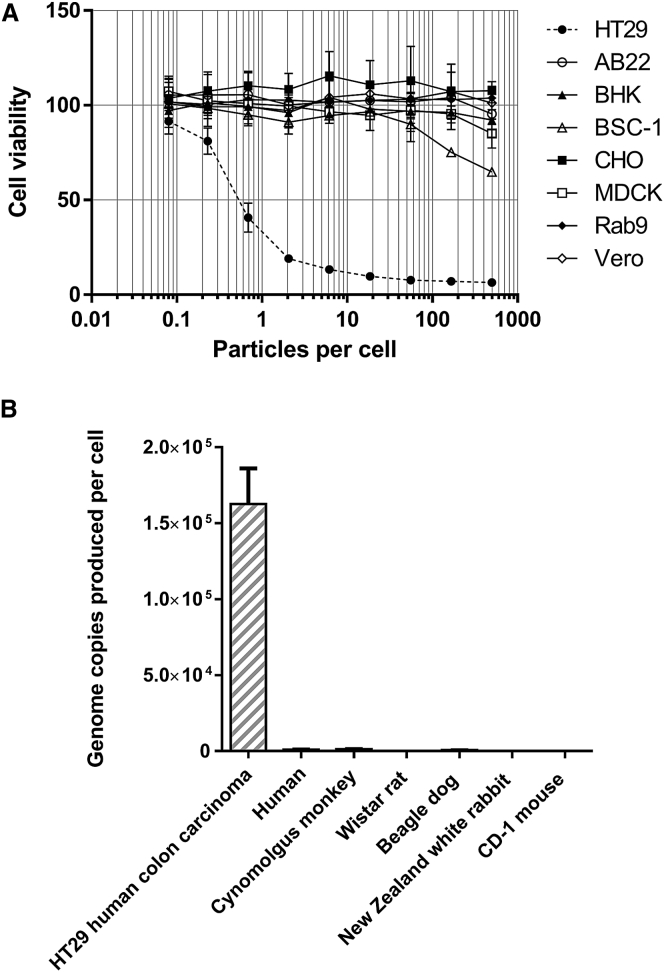
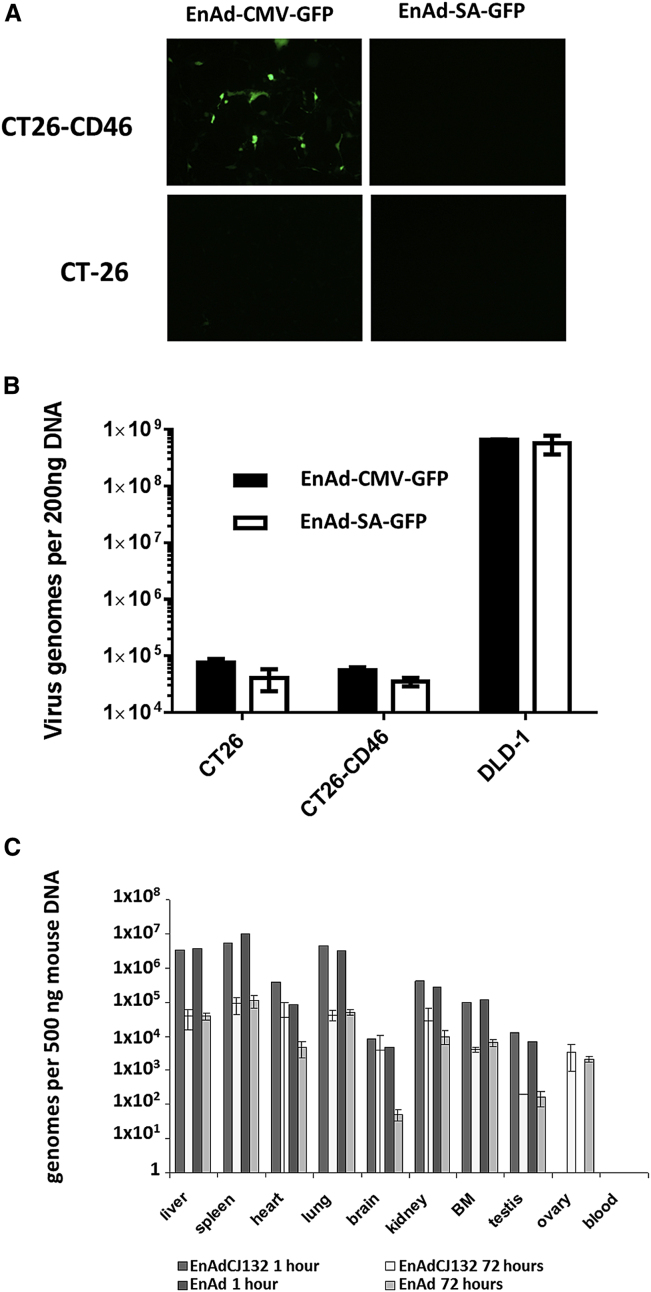
For many experimental agents, animal models can provide a useful insight into potential drug behavior in humans. However, there are no known animal hosts for group B adenoviruses. We found that enadenotucirev was inactive in cell lines from a range of non-human species (Figure 3A). Even Vero cells (African green monkey kidney epithelial cells), which express primate CD46 and are able to support measles virus infection,14 were not sensitive to enadenotucirev. We also evaluated virus activity in primary hepatocytes and found that enadenotucirev was unable to replicate in hepatocytes from any species tested, including human, cynomolgus monkey, and beagle dog (Figure 3B). Wild-type Ad11 was also unable to grow efficiently in primary non-human hepatocytes (data not shown). The introduction of human CD46 into non-permissive murine CT26 adenocarcinoma cells, which do not naturally express CD46, using a lentivirus enabled transgene expression from an enadenotucirev virus expressing GFP under the control of a CMV promotor, but not when GFP was placed under control of the viral major late promoter/splice acceptor (SA) that is only active late in the virus life cycle (Figure 4A).15 Similarly, no virus genome amplification was detected by qPCR. These data suggest the virus can reach the nucleus of these CD46-expressing murine cells and perhaps express some early genes, but encounters a block prior to genome replication (Figure 4B). Furthermore, a pilot study using CD46 transgenic (CD46tg) mice also showed no evidence of virus replication. C57BL/6 hCD46tg mice were injected intravenously with 1 × 1011 vp of enadenotucirev or a replication-defective variant, enadenotucirev-CJ132. Biodistribution and cytokine expression was determined at 1 and 72 hr post-administration. The greatest numbers of viral genomes at 1 hr post-administration were recovered from the spleen, liver, and lungs (Figure 4C). Tissue levels may, to some extent, reflect virus associated with leukocytes, but overall, the pattern is broadly consistent with results reported for Ad5 administered intravenously in mice.16 Degradation of genomes was rapid, with copy numbers in most organs falling by two orders of magnitude over 72 hr. This result is consistent with the hypothesis that the majority of particles are taken up by macrophages. There was no appreciable difference in the biodistribution between replication-competent and -incompetent enadenotucirev, confirming that mice are not a permissive host for group B viruses, even when they express human CD46. We observed a transient drop in white blood cell count in both enadenotucirev and enadenotucirev-CJ132 groups at 24 hr that began to recover by 72 hr (Table S1). This could be due to either direct interaction of peripheral blood monocytes with virus particles or a response to Kupffer cell-produced cytokines, mediating a transient activation and marginalization. Platelet counts were generally lower for the virus treatment groups, while changes in erythrocytes and hematocrit were minor. Transaminase levels increased slightly, but not to the extent previously reported with group C adenoviruses and consistent with experience using Ad11 capsid studies published previously.17 Transient increases in levels of the chemokines TNFα, IFNα, MCP-1, and IL-6 were also observed, but were not significantly different between the groups with a small number of animals (Table S1).
Figure 3.
Enadenotucirev Does Not Show Appreciable Replication in Animal Cell Lines or Primary Cells
(A) AB22 (murine lung mesothelioma), BHK (baby hamster kidney fibroblast), CHO (Chinese hamster ovary epithelial), MDCK (cocker spaniel kidney epithelial), Rab9 (New Zealand white rabbit epithelial), Vero and BSC-1 (African green monkey kidney epithelial), and HT29 (human colorectal carcinoma) cells were exposed to a range of enadenotucirev concentrations. At 72 hr post infection, cell viability was measured by MTS assay. Data represent mean values ± SD (n = 3). (B) HT29 cells or a range of primary human or animal hepatocytes were exposed to enadenotucirev at ten particles per cell. At 72 hr post infection, cell monolayers were harvested, lysed, and viral genomes were quantified by qPCR. The data represent mean ± SD (n = 3).
Figure 4.
Expression of Human CD46 in Murine Cells Facilitates Infections, but Is Not Sufficient to Permit Replication of Enadenotucirev
(A) CT26 murine adenocarcinoma cells stably expressing human CD46 were infected with enadenotucirev variants expressing GFP under the control of either the CMV promoter (EnAd-CMV-GFP) or the replication-dependent splice acceptor (EnAd-SA-GFP). The cells were observed using fluorescent microscopy. CT26 parental cells were used as a negative control and permissive DLD human adenocarcinoma cells as a positive control. (B) Virus genome levels were measured 5 days post-infection by qPCR. Data represent mean values ± SD (n = 3). (C) CD46 transgenic mice were injected intravenously with 1 × 1011 particles of enadenotucirev (EnAd, n = 5); replication-incompetent enadenotucirev (EnAdCJ132, n = 4); or saline (n = 3) in a small pilot study. Virus genome biodistribution by qPCR was performed 1 (n = 1) and 72 hr (EnAd, n = 4 and EnAdCJ132, n = 3) post-virus administration. BM = bone marrow. Data represent mean values ± SD (n = 3).
Overall, high doses of enadenotucirev were well-tolerated in CD46-expressing mice and, with the exception of lower transaminase levels, comparable with results using group C adenoviruses. The lack of any appreciable signs of virus genome amplification suggests that replication fails to proceed in the nucleus of animal cells. Animal models are therefore unlikely to provide meaningful insight into replication-related toxicities. Accordingly, we proceeded to explore replication selectivity in a panel of human primary cells cultured in vitro, while using animal data to identify acute toxicology and particle kinetics.
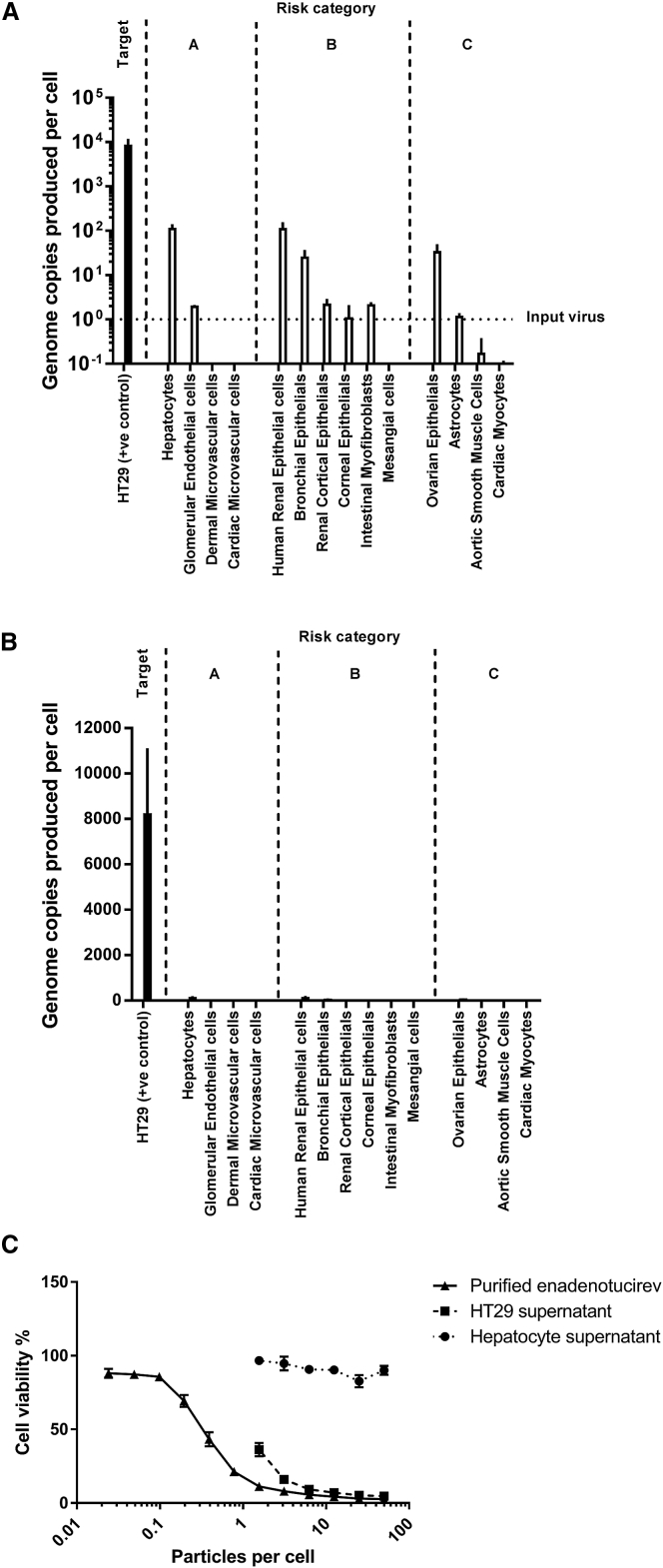
Use of a Panel of Normal Human Cells from Different Tissues to Assess Enadenotucirev Replication-Related Toxicity In Vitro
The absence of a permissive, immunocompetent animal model for enadenotucirev makes it difficult to identify any potential sites of clinical toxicity. Rather than performing long-term studies in animal models of questionable relevance, we chose to evaluate enadenotucirev activity in a broad range of normal cells isolated from different human tissues. We took a risk-based approach and categorized normal cells into three groups. The first group consisted of those cell types likely to be exposed to the highest number of virus particles following intravenous delivery, including endothelial cells and hepatocytes (Figures 5A and 5B, “risk group A”). The second group included cells from tissues that may not directly come into contact with systemically administered virus particles due to the endothelial barrier, but which have been associated with natural infections from external adenovirus exposure, such as the lungs, kidney, and colon (group B). The third group included cells not known to be exposed to adenoviruses, but were commercially available and added to the panel for completeness (group C).
Figure 5.
Genome Amplification on a Range of Normal Primary Cells
Primary cells or HT29 positive control (colorectal carcinoma cells) were exposed to one particle per cell of enadenotucirev. (A and B) After 72 hr, the number of genomes recovered in the supernatant was determined by qPCR (A, log10 scale and B, linear scale). Data represent mean values ± SD (n = 3). The genome amplification in normal cells was attenuated by at least 100-fold relative to the tumor cell line. In eight of 14 normal cell lines, no genomes were detected above the input amount. The relatively small number of genomes produced in normal hepatocytes in tissue culture were apparently inactive in a subsequent cytotoxicity assay. (C) Progeny genomes from HT29 cells or hepatocytes were normalized and reapplied to a fresh monolayer of HT29 cells. Relative to caesium chloride-purified virus, HT29 cells released infectious progeny, as measured by qPCR, whereas progeny genomes released from hepatocytes were not cytotoxic, suggesting that production of infectious particles remained incomplete. Data represent mean values ± SD (n = 3).
To preserve a normal phenotype during our studies, primary cells were infected in basal medium without growth factors and used without passaging. Following recovery from liquid nitrogen, all cells were assessed for viability before exposure to enadenotucirev for 72 hr. Genome copy numbers in cells or supernatants were determined by qPCR (Figures 5A and 5B). Relative to HT29 cells, normal cells supported much lower levels of replication, with progeny genome copy numbers at least 100-fold lower and many cells not showing appreciable levels above the input virus genome concentration (Figures 5A and 5B). However, genome amplification alone may not necessarily be a good surrogate for the complete replication cycle, which also requires functional assembly of infectious virus particles. When comparing supernatants recovered from hepatocytes to those from HT29 cells, they appear to be largely inactive when evaluated in a subsequent cytotoxicity assay, indicating that complete infectious particles had not been generated to a detectable level by hepatocytes (Figure 5C).
Formal Preclinical Replication Selectivity Studies in a GLP/GMP Compliant Laboratory
To support a clinical trial application, the in vitro selectivity study in normal cells was transferred to a Medicines and Healthcare Products Regulatory Agency-audited, GLP/GMP-compliant laboratory. During assay development, the following changes were introduced relative to the study outlined in Figure 5: (1) the HT29 cell line was expanded and archived in a cell bank to ensure consistency of results; (2) the readout for the cytotoxicity assay was established as a binary outcome, with significant cytotoxicity recorded as positive (as opposed to a relative numerical value); (3) supernatants from all cells were transferred into a secondary cytotoxicity assay to determine the potency of any virus particles produced by normal human cells; and (4) peripheral blood mononuclear cells (PBMCs) and CD34+ cells were added to the panel. The results of this study are summarized in Table 1. Consistent with the first study, genome amplification in normal cells was attenuated by at least two orders of magnitude. Relative to the first study, there are some minor differences in the low levels of genomes produced by normal cells, but this is not unexpected given that different primary cell lots were used.
Table 1.
Genome Amplification and Assessment of Productive Virus Yield from a Panel of Normal Human Cells
| Genomes/cell (Pellet) | % of Control | Genomes/cell (Supernatant) | % of Control | Primary Cytotoxicity | Secondary Cytotoxicity | |
|---|---|---|---|---|---|---|
| HT29 (+ve control) | 62,603.7 | 100 | 3,432.9 | 100 | yes | yes |
| Hepatocytes | 1.4 | 0.0021 | 0.2 | 0.01 | no | no |
| Glomerular endothelial cells | 0.1 | 0.0002 | 1.0 | 0.03 | no | no |
| Dermal microvascular cells | 0.2 | 0.0003 | 1.2 | 0.003 | no | no |
| Cardiac microvascular cells | 0.1 | 0.0002 | 1.0 | 0.003 | no | no |
| Corneal epithelial | 501.8 | 0.7889 | 56.5 | 1.65 | yes | no |
| Bronchial epithelial | 109.5 | 0.1722 | 23.1 | 0.67 | no | no |
| Renal cortical epithelial | 7.4 | 0.0116 | 2.9 | 0.09 | no | no |
| Mesangial cells | 0.2 | 0.0004 | 0.8 | 0.002 | no | no |
| Intestinal myofibroblasts | 0.2 | 0.0003 | 1.2 | 0.03 | no | no |
| Ovarian epithelial | 475.7 | 0.7479 | 79.9 | 2.33 | yes | no |
| Astrocytes | 0.1 | 0.0002 | 0.7 | 0.02 | no | no |
| Aortic smooth muscle cells | 0.2 | 0.0003 | 0.8 | 0.02 | no | no |
| Cardiac myocytes | 0.3 | 0.0004 | 0.6 | 0.02 | no | no |
| Renal proximal | 169.6 | 0.2666 | 3.6 | 0.10 | no | no |
| CD34+ | 0.3 | 0.0005 | 2.3 | 0.07 | no | no |
| PBMCs | 0.3 | 0.0004 | 0.8 | 0.02 | no | no |
Some particle cytotoxicity was observed in the primary cell viability (MTS) assay, but none was observed in the secondary assay, indicating that infectious virus particles are not efficiently produced by these non-transformed cells. Overall, these data indicate that enadenotucirev is selective for tumor cells and has minimal or no activity in normal primary cells.
Pharmacokinetics in Mice
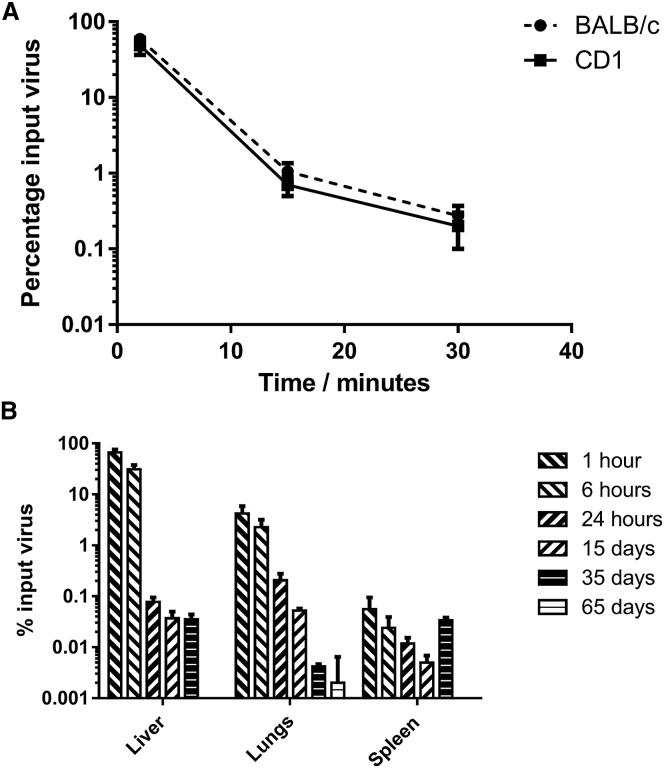
Particulate material circulating in the blood stream is typically cleared by particle scavenging cells of the reticuloendothelial system, particularly Kupffer cells in the liver.18 The liver has been implicated as the main organ for clearance by studies that demonstrate that isolation of the organ from the blood circulation results in extended particle circulation.19 Kinetic studies in CD-1 and BALB/c mice confirmed that enadenotucirev behaves in the expected manner, in line with other adenovirus studies in mice18 and is cleared with a half-life of approximately 2 min following intravenous injection (Figure 6A).
Figure 6.
Blood and Tissue Clearance Kinetics in Mice
(A) CD-1 or BALB/c mice were dosed intravenously with 5 × 109 particles of EnAd. Blood was sampled at 2, 15, and 30 min post-dosing and analyzed for enadenotucirev genomes by qPCR (scaled from 20 μL sample to total circulation volume based on 2 mL blood volume). The data represent mean ± SD (n = 3). (B) CD-1 mice were dosed as in (A) and then killed at various time points post dosing. The total genomes per organ were measured by qPCR. The data represent mean ± SD (n = 3).
Tissue Clearance in Mice
To determine the rate of clearance from murine tissues, enadenotucirev genomes were quantified in the liver, spleen, and lungs, the organs accounting for the greatest proportion of viral burden after intravenous injection in mice. After 65 days, the number of viral genomes fell below the limit of detection by qPCR (Figure 6B). At 35 days, there appeared to be a slight increase in copy number in the spleen, although the actual numbers were very low by this time point and any small changes may reflect assay variation or a redistribution of macrophages with partially degraded virus from other tissues (concurrently a loss in signal was seen in the lung). It is important to consider that the qPCR method applied here measures only a small 246-base pair fragment rather than whole and potentially infectious genomes. No infectious virions were recovered from mice when samples from day 15 were evaluated by plaque assay (data not shown). Given the lack of replication in mouse models, long-term biodistribution and shedding studies will have little clinical predictive value. However, short-term distribution data, particularly to the liver, agrees with the established primary mechanism of acute toxicity and clearance.
Single Fractionated-Dose Study in Male and Female CD-1 Mice
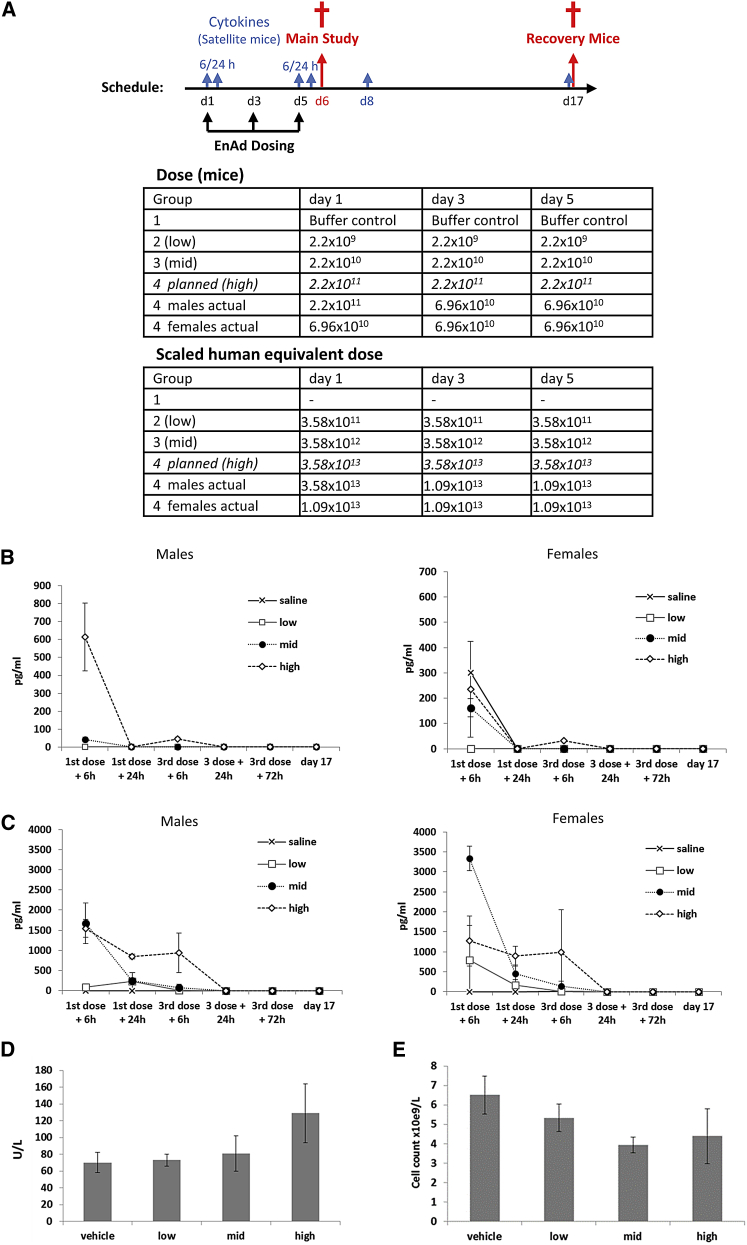
To determine a safe starting dose for clinical trials, a formal GLP toxicology study was carried out by Charles River Laboratories using CD-1 mice. The study was designed to assess acute particle toxicity over a period of 17 days including hematological measurements, blood chemistry, and cytokines. Mice were randomized into dosing cohorts, each with 25 male and 25 female mice of which eight mice were evaluated at day 6 and another eight at day 17. The remaining nine mice were assigned to satellite groups that were assessed for cytokines. The highest planned virus dose was 2.2 × 1011 vp in 100 μL, the maximum feasible dose based on the proposed concentration of clinical grade virus stock. The control group received virus storage buffer at a concentration equivalent to that of the virus stock. A multidose schedule for intravenous delivery (days 1, 3, and 5) was selected to reflect the proposed clinical trial protocol (outlined in Figure 7A).
Figure 7.
GLP Acute Toxicology Study in CD-1 Nude Mice
(A) Dosing schedule and groups for acute toxicology study in CD-1 nude mice and the scaled human equivalent doses. Mice were randomized into dosing cohorts each with 25 male and 25 female mice of which eight were evaluated at days 6, 8, and 17. The remaining nine mice were placed in satellite groups and used to assess cytokine levels at each additional time point (n = 3 per time point). The body surface area-based allometric scaling calculations43, 44, 45 were determined according to FDA guidelines. The highest planned dose was not tolerated in two of the male mice and dosing was decreased by approximately half a log for subsequent doses and all female mice. (B and C) IL-6 (B) and MCP-1 (C) levels were determined by flow cytometry using a Cytokine Bead Array Kit. Data represent mean values ± SD (n = 3). (D and E) On day 6, liver alanine transaminase levels (D) and total white blood counts (E) were measured. Data represent mean values ± SD (n = 3).
No significant clinical signs were observed in male or female mice in groups 1, 2, or 3. In group 4, two of 25 male mice did not tolerate the higher dose and a further six showed visual signs of acute toxicity, such as piloerection and lethargy, but fully recovered by day 2. Although these six mice appeared perfectly healthy, no further dosing was permitted under local Institutional Animal Care guidance and the mice were subsequently euthanized. All subsequent doses in the remaining 17 group 4 males were reduced; females received all three treatments at the modified dose (Figure 7A). Further dosing on days 3 and 5 did not result in any clinical signs, with the exception of piloerection in one group 4 male.
Minor changes in body weight were observed on day 1 following initial dosing, with males in groups 3 and 4 losing 2.3% and 5.7% of body weight and females in groups 1–4 losing 1.1%, 2.6%, 4.4%, and 6.5%, respectively. There were no similar losses following subsequent dosing and no changes in food consumption were observed during the study.
Haematological evaluation on day 6 showed decreases in leukocyte populations and increases in reticulocytes (Figure 7B). These changes were relatively minor and all had returned to the normal range by day 17. Plasma alanine aminotransferase and aspartate aminotransferase were elevated by approximately 2-fold on day 6 in males in group 4, but returned to the normal range by day 17 (Figure 7C). No other changes were observed in serum chemistry markers. There was a transient cytokine response following the first dose of virus, with MCP-1 and IL-6 being raised in groups 3 and 4 (Figures 7B and 7C). Levels of these cytokines normalized despite subsequent dosing, with most animals having little or no increase in MCP-1 or IL-6 following the third virus dose on day 4. These data indicate that the first systemic exposure to the high number of virus particles may “condition” the host liver for an attenuated response to subsequent exposures. Other cytokines such as IL-12 and IL-4 were low, with no consistent elevation at the time points studied (data not shown).
At necropsy on day 6, enlarged spleens were reported in half of the animals in group 4 only, which correlated with significant dose-related increases in spleen weight in all treatment groups. No enlarged spleens were seen on day 17, and spleen weights had decreased, but were still significantly increased in groups 3 and 4 relative to vehicle controls; group 2 spleen weights did not differ from controls on day 17. No other gross pathology or organ weight findings were reported at either day 6 or 17. Mild treatment-related changes were seen in the spleen of some animals in groups 3 and 4 only, including increased cellularity, lymphocytolysis (white pulp), and lymphoid follicular hyperplasia. This would be consistent with an expected immune response to the administered virus particles. No evidence of irritation was seen at the injection sites.
Overall, administration was well-tolerated up to a dose of 6.9 × 1010 vp (human equivalent dose, 1.09 × 1013 vp), with reversible signs relating to an acute innate immune response to input particles. The “no observed adverse dose effect level” was 2.2 × 109 vp (human equivalent, 3.58 × 1011 vp), sufficient to initiate a clinical trial with a starting dose of 1 × 1010 vp per administration.
Discussion
Translation of oncolytic viruses from laboratory studies to clinical investigation can be challenging due to the lack of an informative preclinical animal model system. Here, we employed a set of tailored studies to characterize the potential side effects of enadenotucirev ahead of a phase 1 clinical trial. In mice, systemic administration of virus particles showed an expected, dose-dependent acute response. Of particular interest is the apparent influence of the initial virus dose on “conditioning” the acute response with the effect of allowing subsequent doses to be administered with no further side effects. Previous studies in mice have shown that inactivation of Kupffer cells by predosing with adenovirus particles, or by alternative means such as clodronate liposomes, can improve delivery and tolerability of a subsequent dose of virus.20, 21, 22, 23 The ability to manage potential toxicities by dose scheduling in this way was anticipated in the clinical trial design, and subsequent patient data have supported the concept of the first dose conditioning effect (Calvo et al., 2014, J. Clin. Oncol., abstract).
The circulation kinetics and biodistribution data suggest that capture by hepatic or other phagocytes is the primary mechanism for clearance of virus particles from the plasma circulation. However, consistent with other published studies,17 liver tolerability of enadenotucirev was good, with only minor changes in serum transaminase levels, even in CD46 transgenic mice. In contrast, Ad5 vectors (including replication-incompetent vectors) can induce substantial hepatic toxicity in mice at high doses.17, 24 Outside the liver, spleen, and bone marrow, most normal tissues are not expected to be exposed to high concentrations of intravenously delivered virus particles due to their size and inability to pass through tight junctions between endothelial cells. This was nicely illustrated by Ye et al.,19 who showed that liver bypass surgery in mice allowed virus particles to circulate in the bloodstream without substantial clearance. In tumors, the relatively leaky vasculature25 provides the opportunity for particles to escape the blood stream and accumulate in the stroma. In comparison, low molecular weight chemotherapy drugs that are small enough to diffuse out of the blood stream do not have this physical aspect of size-based cancer selectivity.
When performing kinetic studies, it has to be recognized that there are substantial differences in virus behavior in murine and human blood.3, 26 We have previously shown that the Ad11 capsid has specific advantages in retaining viability in the presence of blood or serum at clinically relevant concentrations, opening up the potential for systemic delivery.3 In contrast, alternative therapeutic virus classes, including vaccinia virus, HSV-1, reovirus, and measles have all been shown to be neutralized in human blood through either antibody-dependent or -independent neutralization,27 in agreement with our own unpublished findings. Gaining a better understanding of systemic delivery was thus a key objective in the preclinical evaluation of enadenotucirev.
It was not possible to perform animal studies to examine off-target replication due to the species-specific nature of group B adenoviruses. In contrast to Ad5, where semi-permissive models are available,28 in part due to the expression of CAR receptors in animals, enadenotucirev will not replicate even following provision of the correct receptor, as mice do not naturally express a CD46 molecule. Higher primates do express CD46 on all nucleated cells, but, unlike humans, expression also occurs on erythrocytes.29 Given that the presence or absence of adenovirus primary receptors on the surface of erythrocytes substantially changes virus circulation kinetics and bioavailability,30 primates would be unlikely to accurately model intravenous delivery in humans. Furthermore, Vero cells expressing CD46 are insensitive to enadenotucirev replication, suggesting that animal cells are unable to support the virus life-cycle even when the virus is able to complete the initial stages of infection.
In cell culture studies, enadenotucirev demonstrated a wide therapeutic index in favor of cancer cell lines over normal cells. Wild-type Ad11 is relatively inactive in non-epithelial cells, and this provides an underlying level of target selectivity for an intravenous delivery route, where endothelial cells and blood cells will typically encounter the highest concentration of particles. Enadenotucirev also lacks E4orf4, a multifunctional protein that deregulates AMPK in normal cells. Deletions in E4orf4 have been shown to attenuate oncolytic virus activity in normal cells without impact on tumor cell lines.31
In addition, enadenotucirev has also lost several E3 proteins that are implicated in avoiding CD8 and NK cell-mediated clearance in normal tissue.32 This deletion should add a further level of selectivity in vivo, although this is difficult to demonstrate given the lack of permissive immune competent animal models. The main receptors for enadenotucirev, CD46 and DSG-2, are not thought to be primary determinants of selectivity, although it is helpful that both tend to be upregulated on tumor cells.4, 5, 33, 34, 35 This contrasts to the receptor for group C adenoviruses, CAR, which tends to be downregulated in cancer.36 Accordingly, several groups have augmented group C adenoviruses with group B fibers to enhance targeting of cancer cells and improve safety.2, 37
Taken together, these data suggest that enadenotucirev should have a good safety profile in humans. The acute particle effects are an expected toxicity, but potentially manageable by dose scheduling or anti-inflammatory agents, whereas off-target replication appears to be of lower risk, although this remains a consideration for a cautious first study in humans. The risk to individuals from natural infections with wild-type Ad11 is not thought to be severe and symptomatic infections are rare and typically present as self-limiting infections of the urinary tract.38, 39, 40 More severe complications can sometimes arise from otherwise harmless pathogens when subjects are heavily immune suppressed. In the case of adenoviruses, problems can occur in young patients undergoing severe immunosuppression during hematopoietic stem cell transfer, but these patients usually respond to treatment with the antiviral cidofovir. Cancer patients considered suitable for oncolytic virotherapy would not be so heavily immune-compromised, but, as a further layer of safety, we have demonstrated that cidofovir is also likely to be effective against enadenotucirev.41
The preclinical data package outlined in this report was sufficient to initiate the clinical safety and tolerability study with a starting dose of 1 × 1010 vp (ClinicalTrials.gov Identifier: NCT02028442). Early indications are consistent with laboratory studies, with patients experiencing dose-dependent, but reversible, side effects related to the administered particles and no indications of any off-target infection (Calvo et al., 2014, Ann. Oncol., abstract). Early data also suggest that live viruses can be found in the blood stream and delivered to tumor nodules,42 again in line with in vitro studies.3 These findings support the approach of tailoring a preclinical testing package to a given product, with a careful choice of informative models that can interrogate the important biological properties and predict toxicities, to facilitate efficient and ethical translation to clinical studies.
Materials and Methods
Cell Lines, Viruses, and Reagents
All cell lines were obtained from the American Type Culture Collection with the exception of the 5-FU- and oxaliplatin-resistant HCT116 cell lines, which were gifts from Professor Patrick Johnston (Queen’s University, Belfast, Northern Ireland, UK). Cell lines were maintained in DMEM (PAA), with the exception of the drug-resistant HCT116 cells, which were maintained in McCoy’s 5A medium. All media were supplemented with 5 mM L-glutamine, 2 mM sodium pyruvate (PAA), and 10% fetal calf serum (FCS, PAA), which was reduced to 2% for cell viability assays. All cell lines were periodically checked for mycoplasma contamination using the Lonza MycoAlert Test Kit (Lonza). Primary cells, purchased from Lonza, were seeded for cell viability assays directly from frozen vials without propagation. Assays using primary cells were carried out in cell-specific basal Clonetics medium (Lonza) supplemented with 5 mM L-glutamine, 2 mM sodium pyruvate, and 10% FCS. Enadenotucirev and its derivatives were propagated in 293 cells, purified by double caesium chloride gradient with an interim Benzonase step, and stored in 10 mM HEPES buffered saline solution (pH 7.8) supplemented with 10% glycerol (Sigma). We obtained 5-FU from Hospira UK and oxaliplatin from Sigma. For the GLP in vivo and in vitro toxicology studies, the pilot batch of enadenotucirev made and purified as for the clinical material by Finvector Vision Therapies was used.
CT26 Cells Expressing CD46
A CT26-derived cell line stably and constitutively expressing human CD46 was constructed using a lentiviral vector. RNA was extracted from 1 × 107 DLD-1 human colon adenocarcinoma cells using the RNeasy Mini Kit (Sigma) according to manufacturer’s instructions (QIAGEN). Total RNA was converted to cDNA using the QuantiTect Reverse Transcription Kit according to manufacturer’s instructions (QIAGEN). The coding sequence for the human CD46 gene was amplified by PCR using primers CD46-lenti F (CAGGTACCATGGAGCCTCCCGGC) and CD46-lenti R (CTCTAGACTATTCAGCCTCTCTGCTCTG). The resulting fragment was ligated into pSF-Lenti (Oxford Genetics) using KpnI and XbaI. The transgene was packaged into VSVG-pseudotyped lentiviruses by transfecting HEK293T cells with 2.13 μg pSF-Lenti-CD46, 0.64 μg pSF-CMV-VSVG, 2.13 μg pSF-CMV-HIV-Rev, and 5.1 μg pSF-CMV-HIV PolGag (Oxford Genetics) in a 10 cm tissue culture dish using Lipofectamine 2000 (Thermo Fisher Scientific) according to manufacturer’s instructions. At 2 days post transfection, supernatant was harvested, centrifuged at 400 g for 5 min, and syringe filtered using a 0.45 μm filter. Cleared supernatant was added to CT26 cells seeded in a 12-well plate in 5-fold serial dilution steps. At 3 days post infection, the medium was changed to selection medium containing 10 μg/mL puromycin. Single colonies were isolated by limiting dilution in selection medium, which were then tested for CD46 expression by flow cytometry using a PE-conjugated αCD46 antibody (1:200, clone: TRA-2-10, BioLegend).
Cell Viability Assay
Cells were seeded at cell-specific densities (between 5,000 and 30,000 cells per well) in 96-well plates. Primary cells were seeded in collagen-I-coated plates (Lonza). Cells were seeded in media as described above at 100 μL per well 24 hr before exposure to virus or cytotoxic drugs. Media was aspirated before adding test substance or control in a 100 μL volume and incubating plates at 37°C/5% CO2. At assay-specific time points, cells were washed twice with PBS and exposed to 100 μl of DMEM without phenol red (PAA) plus 20 μL of MTS reagent for 1 hr at 37°C. Absorbance was recorded by microplate reader (Wallac Victor 1420, Perkin Elmer) at a wavelength of 490 nm. Mock-treated cells served as negative controls and were used to establish 100% survival, while medium alone with MTS reagent served as 0% survival.
Crystal Violet Staining
Where samples were stained with crystal violet, supernatants containing MTS reagent were carefully aspirated and the remaining cell monolayer was fixed in 4% buffered PFA solution for 10 min. The monolayer was then stained in 0.1% crystal violet solution for 30 min, washed with water, and air-dried. Wells were observed under a Zeiss Axiovert 25 Inverted Light/fluorescence Microscope (Zeiss), the images recorded using a Nikon DS-U2 camera (Nikon), and processed using NIS-element AR 3.00 software.
Where samples were obtained for qPCR analysis, additional wells were seeded and harvested before the addition of MTS reagent. Supernatants were removed, the monolayer gently washed with 100 μL 10% FCS in DMEM, and the wash pooled with the supernatant fraction before centrifugation at 300 g. The supernatant fraction was then collected before snap freezing in liquid nitrogen and storing at −80°C. The cell fraction was obtained by adding trypsin to the monolayer before adding 100 μL 10% FCS in DMEM and centrifuging at 300 g. The supernatant was discarded and the pellet resuspended in 200 μL 10% FCS in DMEM. The cell fraction was then combined with any residual cells removed by centrifugation from the supernatant fraction.
Quantitative PCR
Adenoviral genomes were quantified from extracted DNA samples by quantitative PCR in an ABI 7000 StepOnePlus Sequence Detection System. The following probe and primers were used to target the hexon gene of enadenotucirev: forward primer 5′ TACATGCACATCGCCGGA 3′, reverse primer 5′ CGGGCGAACTGCACCA 3′, and probe [6FAM] CCGGACTCAGGTACTCCGAAGCATCCT-[TAM]. The reaction mixture (total volume, 25 μL) contained qPCR BIO probe mix HI ROX (PCR Biosystems), 5 μL of DNA, 1 μmol/L forward/reverse primers and 100 nmol/L probe. Thermocycling parameters were as follows: 2 min at 50°C, 15 min at 95°C, followed by 40 cycles at 95°C for 30 s, and 60°C for 2 min. Data were analyzed using the accompanying software. Test samples were compared to standards containing known quantities of enadenotucirev DNA genomes, which were extracted from the same batch of virus as used in the study in question.
In Vivo Studies
All animal studies were carried out in accordance with national animal welfare regulations and approved by local ethical review. All animals were held in individually ventilated cages (IVCs) in specific pathogen-free (SPF) barrier units and allowed to acclimatize for 1 week prior to any procedures being carried out.
Biodistribution and Pharmacokinetics in CD-1 Mice
CD-1 or BALB/c female mice (Charles River) were dosed intravenously with 5 × 109 particles of enadenotucirev. There were 20 μL blood samples that were taken at 2, 15, and 30 min post dosing, each of which were added to 180 μl PBS, mixed, and frozen. For biodistribution, additional groups of CD-1 mice were dosed as described and then killed at 1, 6, and 24 hr, 15, 35, and 65 days after dosing at which point livers, spleens, lungs, ovaries, kidneys, and hearts were resected and snap frozen in liquid nitrogen before being stored at −80°C. Samples were later DNA extracted and analyzed by qPCR as described previously. Tissue samples were homogenized in 1× reporter lysis buffer (Promega) using a hand homogenizer before extraction.
GLP Preclinical Tolerability Study in CD-1 Mice
Male and female CD-1 mice aged 6–7 weeks old were dosed intravenously with enadenotucirev or vehicle control on days 0, 2, and 4. Groups of mice were killed and organs harvested for histology and histopathology 6 and 17 days after the first dose. Blood samples for cytokine analysis were taken 6 and 24 hr after the first and final dose, and a final blood sample was taken 72 hr after the final dose. Blood samples for clinical chemistry and hematology were taken just prior to necropsy. Cytokines were quantified by flow cytometry using a Cytokine Bead Array Kit (Becton Dickinson).
Biodistribution and Tolerability in CD46 Transgenic Mice
CD46 transgenic C57BL/6 mice, MCP8B (C57-CD46) previously described by Kemper et al.,34 were injected intravenously with 1 × 1011 particles of enadenotucirev, replication-incompetent control enadenotucirev-CJ132, or vehicle control. Animals were killed 1 and 72 hr post-dosing for collection of tissue samples from liver, spleen, heart, lung, brain, kidney, blood, bone marrow (femur), and testis or ovary. Samples were later DNA extracted and analyzed by qPCR as described previously. To assess the potential toxicity of enadenotucirev administration, additional cohorts of mice were dosed identically and blood samples were taken prior to dosing and 24 and 72 hr post-dosing for hematology, alanine aminotransferase, and cytokine analysis. Cytokines were quantified from serum by flow cytometry using a Cytokine Bead Array Kit (Becton Dickinson).
HCT116 Subcutaneous Model
HCT116 cells (5 × 106 cells, ATCC) were implanted into the flank of CD-1 nude female mice (Harlan) and were grouped once average tumor volume reached approximately 100 mm3. Mice were dosed intratumorally with 10 μL containing 5 × 109 particles of enadenotucirev or vehicle control in two spatially separate locations. Tumor volume was measured five times per week until the study end.
Cancer Stem Cell Sphere Assay from HCT116 Tumors
Tumors were removed from animals under aseptic conditions and stored in holding media (DMEM supplemented with 20 mM HEPES, 2 mM L-Glutamine, 100 U/mL penicillin/streptomycin, 50 μg/mL gentamicin, and 2.5 μg/mL amphotericin B [PAA]) at 4°C. Each tumor was then cut into small pieces, first with a scalpel, and then using a mechanical tissue chopper cutting at 100 μm intervals. The fragments from each tumor were incubated overnight at room temperature with agitation in 20 mL of a collagenase/dispase mixture (holding media further supplemented with 150 U/mL Collagenase XI, 0.25 μ/mL dispase 1 [Sigma], and 1% FCS). The following morning the suspensions were centrifuged at 800 rpm for 3 min and the supernatants discarded. Trypsin (10 mL) was added to each cell pellet and the cells incubated at 37°C for 20 min. The cells were then triturated using a 10 mL syringe and 21-gauge needle. FCS (5 mL) and DMEM (15 mL) were added to each suspension, which were passed through a 100 μm and then a 40 μm cell strainer. The filtrate was centrifuged at 1,000 rpm for 5 min and the supernatant discarded. There was 1 mL sphere medium (DMEM/F12, 100 U/mL penicillin/streptomycin, 1× B27 supplement [Invitrogen], 20 ng/mL epidermal growth factor, 5 μg/mL insulin, and 500 ng/mL hydrocortisone [Sigma]) that was added to the cell pellet and the suspension triturated using a 1 mL syringe and 25-gauge needle. Poly-HEMA was dissolved in 95% ethanol, added to 6-well plates, and the ethanol allowed to evaporate off overnight. Tumor cells were seeded into 6-well plates precoated with poly-HEMA at a density of 500 cells/cm2, three wells per tumor in sphere media. Cells were cultured for 7 days without medium replenishment. All spheres over 50 μm in diameter were manually counted using an Olympus CKX41 inverted microscope with a fitted graticule. The assay was carried out with the scientist blinded to the identity of the wells to ensure an unbiased sphere count. Sphere-forming efficiency was determined by dividing the number of spheres per well by the number of cells originally plated per well and multiplying this number by 100.
Author Contributions
S.I., L.W.S., J.B., and K.F. designed the project. S.I., Y.D., M.B., J.L., M.R.D., and S.A. performed the experiments, and collected and analyzed the data. S.I., J.L., L.W.S., and K.F. drafted the manuscript. B.C., A.L., and T.H. were involved in critical revision of the manuscript. All author approved the final version of the manuscript.
Conflicts of Interest
S.I., S.A., B.C., J.B., and K.F. are employees of PsiOxus Therapeutics Ltd. T.H. and L.W.S. hold equity in PsiOxus Therapeutics Ltd. M.B. and T.H. are employees of Bayer Healthcare. Y.D., J.L., M.R.D. (University of Oxford), and A.L. (University of Washington) declare no potential conflict of interest.
Acknowledgments
This research was supported by PsiOxus Therapeutics, Cancer Research UK grant C552/A17720, and Schering AG (now Bayer Healthcare).
Footnotes
Supplemental Information includes one table and can be found with this article online at http://dx.doi.org/10.1016/j.omto.2017.03.003.
Supplemental Information
References
- 1.Kuhn I., Harden P., Bauzon M., Chartier C., Nye J., Thorne S., Reid T., Ni S., Lieber A., Fisher K. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS ONE. 2008;3:e2409. doi: 10.1371/journal.pone.0002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogels R., Zuijdgeest D., van Rijnsoever R., Hartkoorn E., Damen I., de Béthune M.-P., Kostense S., Penders G., Helmus N., Koudstaal W. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Y., Seymour L., Fisher K. Activity of a group B oncolytic adenovirus (ColoAd1) in whole human blood. Gene Ther. 2014;21:440–443. doi: 10.1038/gt.2014.2. [DOI] [PubMed] [Google Scholar]
- 4.Thorsteinsson L., O’Dowd G.M., Harrington P.M., Johnson P.M. The complement regulatory proteins CD46 and CD59, but not CD55, are highly expressed by glandular epithelium of human breast and colorectal tumour tissues. APMIS. 1998;106:869–878. doi: 10.1111/j.1699-0463.1998.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 5.Shang Y., Chai N., Gu Y., Ding L., Yang Y., Zhou J., Ren G., Hao X., Fan D., Wu K., Nie Y. Systematic immunohistochemical analysis of the expression of CD46, CD55, and CD59 in colon cancer. Arch. Pathol. Lab. Med. 2014;138:910–919. doi: 10.5858/arpa.2013-0064-OA. [DOI] [PubMed] [Google Scholar]
- 6.Ravindranath N.M.H., Shuler C. Expression of complement restriction factors (CD46, CD55 & CD59) in head and neck squamous cell carcinomas. J. Oral Pathol. Med. 2006;35:560–567. doi: 10.1111/j.1600-0714.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 7.Maisner A., Zimmer G., Liszewski M.K., Lublin D.M., Atkinson J.P., Herrler G. Membrane cofactor protein (CD46) is a basolateral protein that is not endocytosed. Importance of the tetrapeptide FTSL at the carboxyl terminus. J. Biol. Chem. 1997;272:20793–20799. doi: 10.1074/jbc.272.33.20793. [DOI] [PubMed] [Google Scholar]
- 8.Chia S.-L., Lei J., Ferguson D.J.P., Dyer A., Fisher K.D., Seymour L.W. Group B adenovirus enadenotucirev infects polarised colorectal cancer cells efficiently from the basolateral surface expected to be encountered during intravenous delivery to treat disseminated cancer. Virology. 2017;505:162–171. doi: 10.1016/j.virol.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Reid T., Warren R., Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther. 2002;9:979–986. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris D.G., Feng X., DiFrancesco L.M., Fonseca K., Forsyth P.A., Paterson A.H., Coffey M.C., Thompson B. REO-001: A phase I trial of percutaneous intralesional administration of reovirus type 3 dearing (Reolysin®) in patients with advanced solid tumors. Invest. New Drugs. 2013;31:696–706. doi: 10.1007/s10637-012-9865-z. [DOI] [PubMed] [Google Scholar]
- 11.Alzuguren P., Hervas-Stubbs S., Gonzalez-Aseguinolaza G., Poutou J., Fortes P., Mancheno U., Bunuales M., Olagüe C., Razquin N., Van Rooijen N. Transient depletion of specific immune cell populations to improve adenovirus-mediated transgene expression in the liver. Liver Int. 2015;35:1274–1289. doi: 10.1111/liv.12571. [DOI] [PubMed] [Google Scholar]
- 12.Vidal S.J., Rodriguez-Bravo V., Galsky M., Cordon-Cardo C., Domingo-Domenech J. Targeting cancer stem cells to suppress acquired chemotherapy resistance. Oncogene. 2014;33:4451–4463. doi: 10.1038/onc.2013.411. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson M., Guse K., Bauerschmitz G., Virkkunen P., Tarkkanen M., Tanner M., Hakkarainen T., Kanerva A., Desmond R.A., Pesonen S., Hemminki A. Oncolytic adenoviruses kill breast cancer initiating CD44+CD24-/low cells. Mol. Ther. 2007;15:2088–2093. doi: 10.1038/sj.mt.6300300. [DOI] [PubMed] [Google Scholar]
- 14.Manchester M., Eto D.S., Valsamakis A., Liton P.B., Fernandez-Muñoz R., Rota P.A., Bellini W.J., Forthal D.N., Oldstone M.B. Clinical isolates of measles virus use CD46 as a cellular receptor. J. Virol. 2000;74:3967–3974. doi: 10.1128/jvi.74.9.3967-3974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen-Durr P., Boeuf H., Kédinger C. Replication-induced stimulation of the major late promoter of adenovirus is correlated to the binding of a factor to sequences in the first intron. Nucleic Acids Res. 1988;16:3771–3786. doi: 10.1093/nar/16.9.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waddington S.N., McVey J.H., Bhella D., Parker A.L., Barker K., Atoda H., Pink R., Buckley S.M., Greig J.A., Denby L. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Shashkova E.V., May S.M., Barry M.A. Characterization of human adenovirus serotypes 5, 6, 11, and 35 as anticancer agents. Virology. 2009;394:311–320. doi: 10.1016/j.virol.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alemany R., Suzuki K., Curiel D.T. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 2000;81:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- 19.Ye X., Jerebtsova M., Ray P.E. Liver bypass significantly increases the transduction efficiency of recombinant adenoviral vectors in the lung, intestine, and kidney. Hum. Gene Ther. 2000;11:621–627. doi: 10.1089/10430340050015806. [DOI] [PubMed] [Google Scholar]
- 20.Manickan E., Smith J.S., Tian J., Eggerman T.L., Lozier J.N., Muller J., Byrnes A.P. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol. Ther. 2006;13:108–117. doi: 10.1016/j.ymthe.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Green N.K., Hale A., Cawood R., Illingworth S., Herbert C., Hermiston T., Subr V., Ulbrich K., van Rooijen N., Seymour L.W., Fisher K.D. Tropism ablation and stealthing of oncolytic adenovirus enhances systemic delivery to tumors and improves virotherapy of cancer. Nanomedicine (Lond.) 2012;7:1683–1695. doi: 10.2217/nnm.12.50. [DOI] [PubMed] [Google Scholar]
- 22.Shashkova E.V., Doronin K., Senac J.S., Barry M.A. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 2008;68:5896–5904. doi: 10.1158/0008-5472.CAN-08-0488. [DOI] [PubMed] [Google Scholar]
- 23.Kuzmin A.I., Finegold M.J., Eisensmith R.C. Macrophage depletion increases the safety, efficacy and persistence of adenovirus-mediated gene transfer in vivo. Gene Ther. 1997;4:309–316. doi: 10.1038/sj.gt.3300377. [DOI] [PubMed] [Google Scholar]
- 24.Lieber A., He C.Y., Meuse L., Schowalter D., Kirillova I., Winther B., Kay M.A. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dvorak H.F., Nagy J.A., Dvorak J.T., Dvorak A.M. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am. J. Pathol. 1988;133:95–109. [PMC free article] [PubMed] [Google Scholar]
- 26.Lyons M., Onion D., Green N.K., Aslan K., Rajaratnam R., Bazan-Peregrino M., Phipps S., Hale S., Mautner V., Seymour L.W., Fisher K.D. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol. Ther. 2006;14:118–128. doi: 10.1016/j.ymthe.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Evgin L., Acuna S.A., Tanese de Souza C., Marguerie M., Lemay C.G., Ilkow C.S., Findlay C.S., Falls T., Parato K.A., Hanwell D. Complement inhibition prevents oncolytic vaccinia virus neutralization in immune humans and cynomolgus macaques. Mol. Ther. 2015;23:1066–1076. doi: 10.1038/mt.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas M.A., Spencer J.F., La Regina M.C., Dhar D., Tollefson A.E., Toth K., Wold W.S. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 29.Shayakhmetov D.M., Papayannopoulou T., Stamatoyannopoulos G., Lieber A. Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. J. Virol. 2000;74:2567–2583. doi: 10.1128/jvi.74.6.2567-2583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlisle R.C., Di Y., Cerny A.M., Sonnen A.F.-P., Sim R.B., Green N.K., Subr V., Ulbrich K., Gilbert R.J., Fisher K.D. Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1. Blood. 2009;113:1909–1918. doi: 10.1182/blood-2008-09-178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Shea C., Klupsch K., Choi S., Bagus B., Soria C., Shen J., McCormick F., Stokoe D. Adenoviral proteins mimic nutrient/growth signals to activate the mTOR pathway for viral replication. EMBO J. 2005;24:1211–1221. doi: 10.1038/sj.emboj.7600597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McSharry B.P., Burgert H.-G., Owen D.P., Stanton R.J., Prod’homme V., Sester M., Koebernick K., Groh V., Spies T., Cox S. Adenovirus E3/19K promotes evasion of NK cell recognition by intracellular sequestration of the NKG2D ligands major histocompatibility complex class I chain-related proteins A and B. J. Virol. 2008;82:4585–4594. doi: 10.1128/JVI.02251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surowiak P., Materna V., Maciejczyk A., Kaplenko I., Spaczynski M., Dietel M., Lage H., Zabel M. CD46 expression is indicative of shorter revival-free survival for ovarian cancer patients. Anticancer Res. 2006;26(6C):4943–4948. [PubMed] [Google Scholar]
- 34.Kemper C., Leung M., Stephensen C.B., Pinkert C.A., Liszewski M.K., Cattaneo R., Atkinson J.P. Membrane cofactor protein (MCP; CD46) expression in transgenic mice. Clin. Exp. Immunol. 2001;124:180–189. doi: 10.1046/j.1365-2249.2001.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beyer I., van Rensburg R., Lieber A. Overcoming physical barriers in cancer therapy. Tissue Barriers. 2013;1:e23647. doi: 10.4161/tisb.23647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stecker K., Vieth M., Koschel A., Wiedenmann B., Röcken C., Anders M. Impact of the coxsackievirus and adenovirus receptor on the adenoma-carcinoma sequence of colon cancer. Br. J. Cancer. 2011;104:1426–1433. doi: 10.1038/bjc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni S., Gaggar A., Di Paolo N., Li Z.Y., Liu Y., Strauss R., Sova P., Morihara J., Feng Q., Kiviat N. Evaluation of adenovirus vectors containing serotype 35 fibers for tumor targeting. Cancer Gene Ther. 2006;13:1072–1081. doi: 10.1038/sj.cgt.7700981. [DOI] [PubMed] [Google Scholar]
- 38.Numazaki Y., Shigeta S., Kumasaka T., Miyazawa T., Yamanaka M., Yano N., Takai S., Ishida N. Acute hemorrhagic cystitis in children. Isolation of adenovirus type II. N. Engl. J. Med. 1968;278:700–704. doi: 10.1056/NEJM196803282781303. [DOI] [PubMed] [Google Scholar]
- 39.Yusuf U., Hale G.A., Carr J., Gu Z., Benaim E., Woodard P., Kasow K.A., Horwitz E.M., Leung W., Srivastava D.K. Cidofovir for the treatment of adenoviral infection in pediatric hematopoietic stem cell transplant patients. Transplantation. 2006;81:1398–1404. doi: 10.1097/01.tp.0000209195.95115.8e. [DOI] [PubMed] [Google Scholar]
- 40.Neofytos D., Ojha A., Mookerjee B., Wagner J., Filicko J., Ferber A., Dessain S., Grosso D., Brunner J., Flomenberg N., Flomenberg P. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biol. Blood Marrow Transplant. 2007;13:74–81. doi: 10.1016/j.bbmt.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 41.Bauzon M., Jin F., Kretschmer P., Hermiston T. In vitro analysis of cidofovir and genetically engineered TK expression as potential approaches for the intervention of ColoAd1-based treatment of cancer. Gene Ther. 2009;16:1169–1174. doi: 10.1038/gt.2009.69. [DOI] [PubMed] [Google Scholar]
- 42.Boni V., La Portilla F.D., Cubillo A., Gil-Martin M., Calvo E., Salazar R., Santos C., Sanchez-Gastaldo,Prados S., Sanjuan X. 1068P A phase 1 mechanism of action study of intra-tumoural (IT) or intravenous (IV) administration of enadenotucirev, an oncolytic Ad11/Ad3 chimeric group B adenovirus in colon cancer patients undergoing resection of primary tumour. Ann. Oncol. 2014;25 abstract iv368–iv368. [Google Scholar]
- 43.Freireich E.J., Gehan E.A., Rall D.P., Schmidt L.H., Skipper H.E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother. Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- 44.Pilaro A.M., Serabian M.A. Preclinical development strategies for novel gene therapeutic products. Toxicol. Pathol. 1999;27:4–7. doi: 10.1177/019262339902700102. [DOI] [PubMed] [Google Scholar]
- 45.Sharma V., McNeill J.H. To scale or not to scale: the principles of dose extrapolation. Br. J. Pharmacol. 2009;157:907–921. doi: 10.1111/j.1476-5381.2009.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.