Abstract
In experiment 1, juvenile sea urchins (n = 80, 0.088 ± 0.001 g wet weight and 5.72 ± 0.04 mm diameter) were held individually and fed ad libitum one of three semi-purified formulated diets (n = 16 individuals treatment-1). In the diets, protein was held constant (310g kg-1 dry, as fed) and carbohydrate level varied (190, 260, or 380 g kg-1 dry, as fed). Wet weights were measured every 2 weeks. Total wet weight gain was inversely proportional to dietary carbohydrate level and energy content of the respective diet. In experiment 2, sea urchins (5.60 ± 0.48g wet weight, n= 40) fed 190 g kg-1 carbohydrate consumed significantly more dry feed than those fed 260 g kg-1, but not more than those fed 380 g kg-1 carbohydrate. Based on differential feed intake rates, sea urchins that consumed more feed also consumed higher levels of protein and had the highest weight gain. Consequently, protein content and/or protein: energy ratio may be important in determining feed utilization and growth among sea urchins in this study. The average digestible energy intake was approximately 70 kcal kg-1 body weight day-1, suggesting daily caloric intake of juvenile Lytechinus variegatus is lower than in shrimp and fish.
Keywords: Sea urchin, carbohydrate level, Lytechinus variegatus, nutrition, growth, production
Introduction
Sea urchins are an important seafood product in many parts of the world, particularly Asia and Europe. Historically, sea urchins have been harvested from natural habitats for their roe. High demand for roe, ease of capture and long recruitment time have resulted in overfishing of wild populations (Sloan 1985; Keesing and Hall 1998; Lesser and Walker 1998; Andrews et al. 2002; Robinson 2004). Depletion of wild populations could be ecologically detrimental. Therefore, promoting the development of sea urchin aquaculture is desirable (Lesser and Walker 1998; Keesing and Hall 1998; Andrews et al. 2002; Robinson 2004; Kelly 2005; Cook and Kelly 2007; Eddy et al. 2010; Humphries et al. 2012; Rahman et al. 2014). For the development of a successful commercial sea urchin aquaculture industry, a nutritionally-complete formulated feed will be required (Watts et al. 2013).
Protein is an important macro-nutrient that provides essential amino acids for growth, maintenance, and reproduction among sea urchins (Hammer et al. 2004; Hammer et al. 2006; 2012; Heflin et al. 2012; Heflin 2015). However, protein is a costly feed ingredient and inclusion of excess dietary protein in formulated feed should be avoided. Furthermore, utilization of protein as an energy source when protein is limiting in the diet could reduce the amount allocated to other physiological processes associated with growth and/or reproduction.
Carbohydrate is the macro-nutrient that is most commonly oxidized and utilized as an energy source by herbivores and many omnivores (Morris 1991). Many carbohydrases that oxidize complex polysaccharides into functional monosaccharides have been identified in the sea urchin gut (Lawrence et al. 2013). Carbohydrates, usually in the form of glycogen, are stored by the nutritive phagocytes in the gonad (Pearse & Cameron 1991; Marsh et al. 2013; Hammer et al. 2006) and may provide energy for gametogenesis (Pearse & Cameron 1991; Marsh et al. 2013). Commercially, carbohydrate content may also affect gonad color and taste (Pearce et al. 2002; Unuma 2002).
In this study, sea urchins were fed three different semi-purified, cold-extruded formulated diets containing low, medium and high carbohydrate levels (190, 260, and 380 g kg-1 dry matter, as fed) at a fixed protein level of 310 g kg-1 crude protein, determined previously to support maximal weight gain in small L. variegatus (Hammer et al. 2004; Hammer et al. 2012; Heflin 2015). We evaluated the effect of dietary carbohydrate level on food and energy intake in relation to outcomes associated with growth.
Materials and Methods
Collection and Culture
Adult Lytechinus variegatus were collected from Saint Joseph Bay, Florida (30° N, 85.5°W) in May 2005, and transported to the University of Alabama at Birmingham in aerated coolers. ea urchins were induced to spawn by injecting (27 G ½ needle with 1cc syringe) 1 mL of 0.1 M acetylcholine solution in autoclaved artificial seawater (Instant Ocean Sea Salt®, Blacksburg, VA, USA, 32 ± 1 g L-1) through the peristomial membrane into the coelomic cavity (modified from Hinegardner 1969). Eggs were collected separately from 4 females and combined. Sperm was collected dry from 3 males and combined and diluted in artificial seawater. Ova were fertilized with the dilute sperm mixture. Developing embryos were transferred to one of 8 larval rearing jars containing 4 L of conditioned (previously exposed to adults) synthetic seawater (Instant Ocean®, Blacksburg, VA, USA) and 2 L of unconditioned (freshly made) synthetic seawater. Developing larvae were maintained at a concentration of 2 to 3 larvae mL-1 and were fed twice daily (in the morning and in the afternoon) a mixed unicellular algal diet at a rate of ca. 3,000 cells mL-1 of Isochrysis galbana, Rhodomonas salina and Dunaliella tertiolecta. Larval jars were cleaned once daily (rinsed and scrubbed), and water was exchanged at a rate of ca. 10% volume per day. Rearing temperature was held at 25°C and photo-period was maintained on a 12:12 light: dark cycle. Sea urchins began to settle and metamorphose ca. 14 days after spawning. As they metamorphosed, juvenile sea urchins were collected and transferred to 80 L aquaria, where they were reared on cultures of the benthic diatom Amphora helenensis until ca. 1-1.5 mm diameter. As juveniles reached ca. 1- 1.5 mm, they were transferred to 20 L aquaria and were fed a live, mixed-taxa green algal biofilm (MTAB) reported previously to support growth and survivorship in juveniles (Taylor et al. 2009). During this stage individuals were maintained on a constant photoperiod (12 hr.: 12 hr., light: dark) and water temperature maintained at 24 ± 1° C.
Experiment 1: Growth Trial
A large population of juveniles were maintained en masse (ca. 3 months) until reaching ca. 4.9-6.8 mm diameter and 0.07 – 0.123 g wet weight, at which time they were randomly assigned to one of three feeding treatments (described below). Individuals of this size class are sensitive to collection and handling; consequently, individuals that died within one week of stocking were replaced (<5% mortality).
For the feed trials, sea urchins were reared in two linked, rectangular fiberglass raceways (400 L) with recirculating synthetic saltwater (Taylor et al. 2009). Sea urchins were placed in individual cylindrical enclosure constructed of 3 mm polypropylene open mesh (7.5 cm diameter × 30 cm height) secured by plastic cable ties. Mesh enclosures were fitted into PVC couplings and elevated 0.5 cm above the bottom of the tank using plastic spacers that allowed for water circulation beneath the enclosures. The depth of the saltwater in the raceways was maintained from 10 to 15 cm throughout the study, increasing in depth as sea urchins increased in size. Salinities were checked and adjusted daily by adding filtered (reverse osmosis) water. Every two weeks, sea urchins were randomly redistributed and relocated throughout the raceways to eliminate bias due to cage positioning or current, and the cages were scrubbed weekly to ensure that there was minimal bacterial growth on the cages that could contribute to the nutrition of the sea urchins. Fresh food was provided daily and uneaten diet and feces were removed by siphon every two days. Water quality parameters were tested weekly using a LaMotte's saltwater test kit and maintained within optimal range acceptable for sea urchins (pH= 8.2, ammonia and nitrites < 0.5 mg L-1, nitrates < 10 mg L-1, alkalinity > 150 g L-1) (Basuyaux & Mathieu 1999).
Diets and Diet Preparation
Individuals were randomly assigned and fed ad libitum one of three diets for eight weeks (n=16 sea urchins treatment-1). These diets contained the same level of protein (310 g kg-1 dry, as fed) and carbohydrate level was adjusted to 190, 260, or 380 g kg-1 of dry weight. The diets were prepared from a base feed containing semi-purified and purified ingredients (approximately 280 g kg-1 marine source ingredients, 346 g kg-1 plant source ingredients, 65 g kg-1 crude fat, 11 g kg-1 carotenoids, 7.0 g kg-1 vitamin premix, 189 g kg-1 mineral premix, 102 g kg-1 binder-antifungal-antioxidant). Carbohydrate levels were adjusted using wheat starch and acid-washed diatomaceous earth. As a consequence Dry ingredients were blended in a twin-shell dry blender (Patterson-Kelley Co., East Stroudsburg, PA, USA) for 10 minutes and then mixed in a Hobart Mixer (Model A-200, Hobart Corporation, Troy, OH, USA) for 40 minutes. Deionized water (500 mL kg-1 dry mix) was then added to the dry ingredients and mixed an additional 10 minutes to achieve a mash consistency appropriate for extrusion. Extrusion was accomplished using a meat chopper attachment (Model A-200, Hobart Corporation, Troy, OH, USA) fitted with a 4.8 mm die. Moist diet strands were dried on wire racks in a forced air oven at 35° C to a moisture content of 8-10% and refrigerated at 4° C until used. Proximate analyses of diets were performed by Eurofins, Memphis, TN, USA (Table 1). Percent crude protein was determined by AOAC Method 990.3 (1990); FP-528 Nitrogen/Protein Determination; Leco Corporation, St. Joseph, MI, USA. Fat content was determined by acid hydrolysis, carbohydrate content was determined by subtraction, and energy content was determined by micro-bomb calorimetry (Parr Instrument Company, Moline, IL, USA).
Table 1.
Determined proximate nutrient composition (g kg-1) of the three semi-purified formulated diets (190, 260, or 380 g kg-1 carbohydrate and 310 g kg-1 protein). Values are expressed as dry weight. Carbohydrate values were determined by subtraction.
| Diets | |||
|---|---|---|---|
|
| |||
| 190 g kg-1 | 260 g kg-1 | 380 g kg-1 | |
| Crude Protein | 315.9 | 312.9 | 317.1 |
| Crude Fat | 64.7 | 60.0 | 67.7 |
| Carbohydrate | 188.9 | 261.5 | 384.7 |
| Crude Fiber | 33.8 | 32.0 | 16.7 |
| Ash | 396.6 | 333.7 | 213.9 |
| Moisture | 91.7 | 102.1 | 111.5 |
| Caloric Value (kcal g-1) | 3.07 | 3.28 | 3.82 |
| Protein: Energy Ratio (mg kcal-1) | 104 | 96 | 82 |
Growth
Wet weight and test diameter of each individual sea urchin was measured at the beginning of the study and every 2 weeks thereafter. For wet weights, individuals were blotted dry on a paper towel to remove excess water and weighed to the nearest mg. To acquire test diameters (mm), individuals were photographed out of water with a reference ruler using a Nikon® (Tokyo, Japan) digital camera. Images were analyzed using Optimus 6.51 imaging software (Media Cybernetics, L.P., Warrendale, PA, USA). Individual specific growth rates (% body weight gain day-1, SGR) were calculated using the equation:
A repeated measures analysis using The Mixed Procedure (PROC MIXED) was used to determine significance of the dependent variables, wet weights and diameters. An ANOVA with a Tukey's adjustment for multiple comparisons was used to determine significance of SGR. To analyze the scatter plot of diameter versus wet weight data for the diets, a log transform was applied to linearize the data. An ANCOVA, with diameter and wet weight as the covariates, was performed and a comparison of estimated marginal means separated by a Tukey's test at the mean diameter or wet weight of all treatments (19.0 mm and 2.67 g, respectively) was used to determine significant differences and interaction between the variables. All growth data were analyzed using the statistic software SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA). All values were considered significant when p < 0.05.
Organ Analyses
Sea urchins were dissected at the end of 8 weeks. Gonad, lantern, and test were blotted on a paper towel and weighed to the nearest mg. The gut (esophagus, stomach, and intestine combined) was removed, rinsed to remove any food pellets, blotted on a paper towel, and weighed. Percent moisture content of each organ was calculated as:
To determine dry organ weights, organs were dried in a convection oven at 60° C, for 48 hrs. or until constant dry weight. Wet and dry skeletal components, including test and lantern, were analyzed by ANCOVA using sea urchin test diameter as the covariate. Treatments were compared at the average test diameter of all sea urchins (19.0 mm). Gut wet and dry weights were analyzed by ANCOVA using the sea urchin final wet and total dry weights as the covariates, respectively. Treatments were compared at the average final wet and dry weights of all sea urchins (2.67 g and 0.60 g, respectively). Gonad wet and dry data were not normal and were determined to have unequal variance. Therefore, a non-parametric Kruskal-Wallis Mean Rank Test was performed. To determine differences in moisture content, an ANOVA was performed and data were separated by a Tukey's HSD adjustment. All dissection data were analyzed using the statistic software SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA). All values were considered significant when P < 0.05.
Experiment 2: Diet Intake and Digestibility
In experiment 1, individuals were initially very small and feed intake could not be accurately determined. Consequently, a larger cohort of individuals growing concurrently with individuals in experiment 1 were evaluated for feed intake metrics. This second cohort of cultured sea urchins (initial wet weight = 5.60 ± 0.48g, n= 30) was held in individual enclosures in the linked raceway system. All sea urchins were fed individually a reference diet (Hammer et al. 2012; 310 g kg-1 protein) (approximately 25 g kg-1 body weight day-1) for 7 days to standardize nutritional intake of a formulated diet. On day 8, sea urchins were randomly assigned to one of the three experimental diets (190, 260, or 380 g kg-1 carbohydrate; n = 10 treatment-1). Sea urchins were then fed the respective experimental diet (approximately 25 g kg-1 body weight g day-1) for an additional 7 days to provide experience with these experimental diets. On day 15, sea urchins were weighed (there were no significant differences in wet weights among treatments) and fed ad libitum a pre-weighed ration of the respective formulated diet once daily. For the next 12 days, uneaten food and fecal material were collected daily and rinsed quickly with freshwater to remove salts. On day 27, the last day of the feed intake study, sea urchins were weighed. Uneaten food and feces were dried in a convection oven at 60° C for 48 hrs. or until constant dry weight and were combined for each individual over the 12 day period.
The amount of dry diet consumed day-1 (mg day-1) was calculated using the formula:
The amount of energy consumed day-1 (cal day-1) was determined by the formula:
The g kg-1 digestible energy of the diets was determined using the formula:
The amount of digestible energy consumed day-1 was determined using the formula:
The apparent dry matter digestibility (ADMD) of the four formulated diets was determined by gravimetric collection. The collected feces were used to determine ADMD of each of the diets. The ADMD of the formulated diets were calculated using the formula:
All feed intake and digestibility data were analyzed using ANOVA with a Student-Newman-Keul's test for multiple comparisons. One sea urchin fed the 190 g kg-1 carbohydrate diet did not consume the diet (as confirmed by direct observation) and did not gain a significant amount of weight over the feed intake study. Data for this individual were excluded using Cook's Distances and Leverage Values. All data were analyzed using the statistic software SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA). All values were considered significant when P < 0.05.
Results
Survival and Water Quality
Survival was 100% in sea urchins fed the experimental formulated diets. The observed values for ammonia, nitrite, nitrate, temperature, salinity and pH remained within acceptable levels throughout the study (Basuyaux & Mathieu 1999).
Experiment 1: Growth Trial
At week eight, sea urchins fed the 190 g kg-1 carbohydrate diet weighed significantly more than sea urchins fed the 380 g kg-1 carbohydrate diet (p = 0.03, Fig. 1) but did not weigh more than sea urchins fed the 260 g kg-1 carbohydrate diet. Sea urchins fed the 190 g kg-1 carbohydrate diet had significantly larger test diameters than sea urchins fed the 380 g kg-1 carbohydrate diet at weeks six and eight (p < 0.02, Fig. 2). No significant difference in test diameter was detected between sea urchins fed the 190 g kg-1 carbohydrate diet and the 260 g kg-1 carbohydrate diet.
Figure 1.
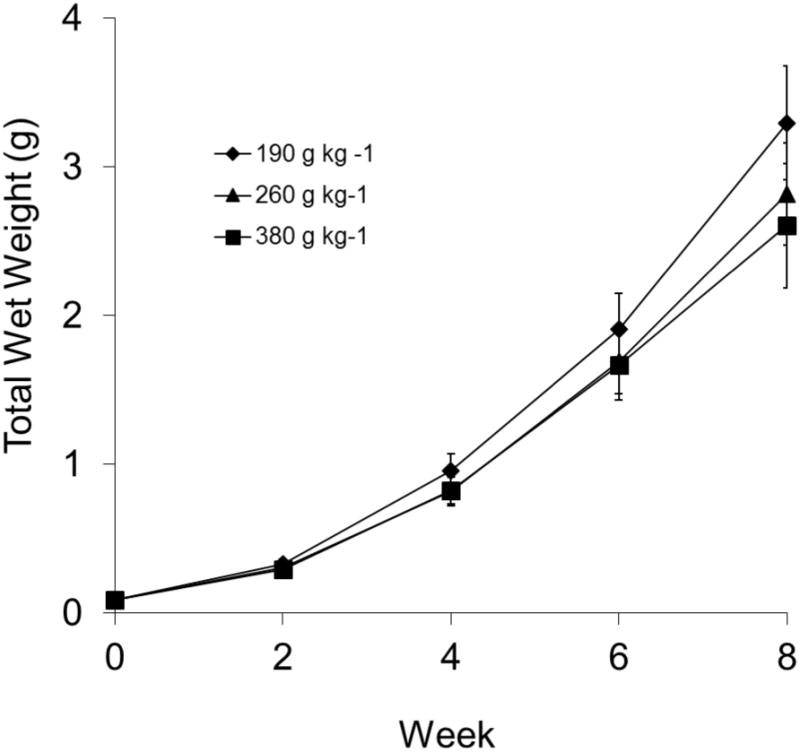
Total wet weight (g) of juvenile sea urchins, Lytechinus variegatus, fed one of three semi-purified, formulated diets (190, 260, or 380 g kg-1 carbohydrate and 310 g kg-1 protein). Values represent means ± SEM (n = 16 sea urchins treatment-1). Wet weight was significantly higher in those fed 190 g kg-1 carbohydrate than fed 380 g kg-1 carbohydrate (P < 0.05), but not different from those fed 260 g kg-1 carbohydrate (P > 0.05).
Figure 2.
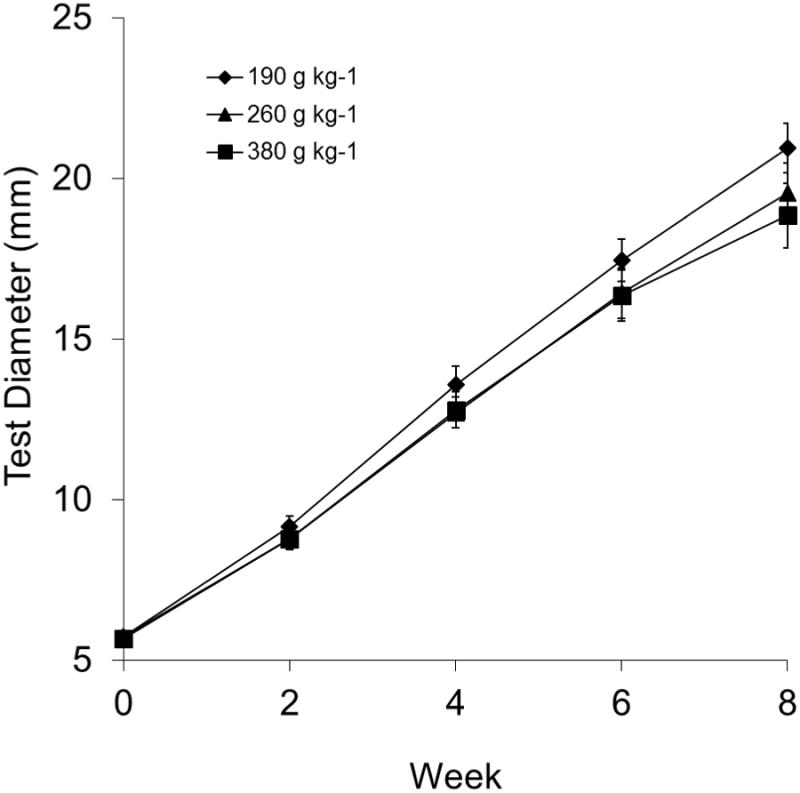
Test diameter (mm) of juvenile sea urchins, Lytechinus variegatus, fed one of three semi-purified, formulated diets (190, 260, or 380 g kg-1 carbohydrate and 310 g kg-1 protein). Values represent means ± SEM (N = 16 sea urchins treatment-1). Test diameter was significantly higher in those fed 190 g kg-1 carbohydrate than fed 380 g kg-1 carbohydrate (P < 0.05), but not different from those fed 260 g kg-1 carbohydrate (P > 0.05).
Specific growth rates (SGR) ranged from 8.3 to 9.1 for all treatments during the first 2 weeks of the study (Fig. 3). The SGR decreased in all treatments with time, and there were no significant differences among diet treatments over the study period.
Figure 3.
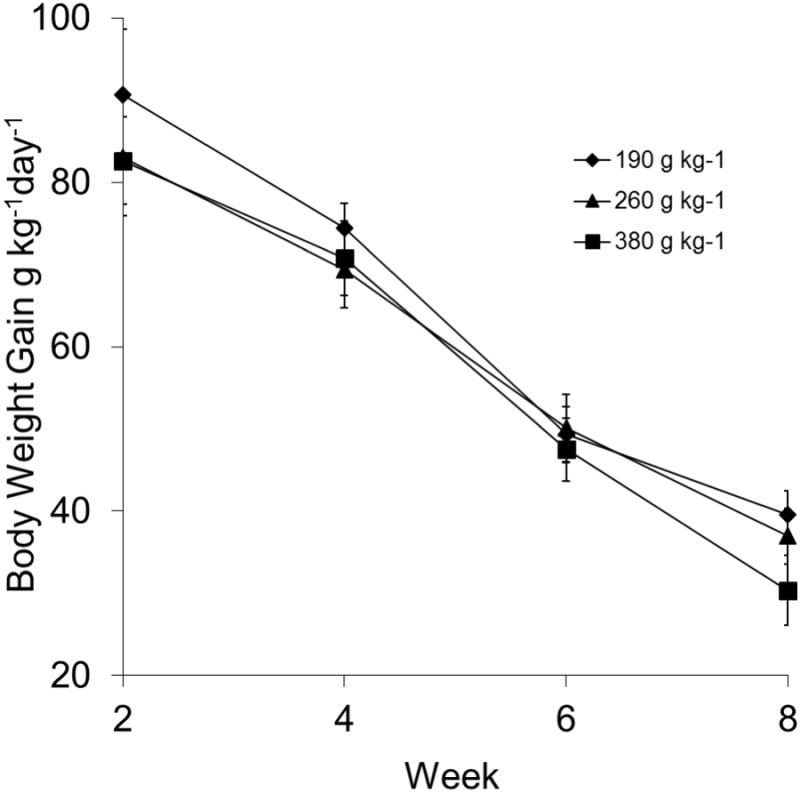
Specific growth rates (g kg-1 body weight gain day-1) calculated in 2-wk intervals of juvenile sea urchins, Lytechinus variegatus, fed one of three semi-purified, formulated diets (190, 260, or 380 g kg-1 carbohydrate and 310 g kg-1 protein). Values represent means ± SEM (n = 16 sea urchins treatment-1).
For all treatments combined, the relationship between diameter (mm) and wet weight (g) was best described by the equation y = 0.0006× 2.86, R2 = 0.99 (Fig. 4). Linear regression of the log transformed values for all treatments over the 8-wk study period indicates a strong relationship between diameter and wet weight described by the equation y = 2.84× − 3.21, R2 = 0.99 (Fig. 5). No significant differences were observed among the treatments.
Figure 4.
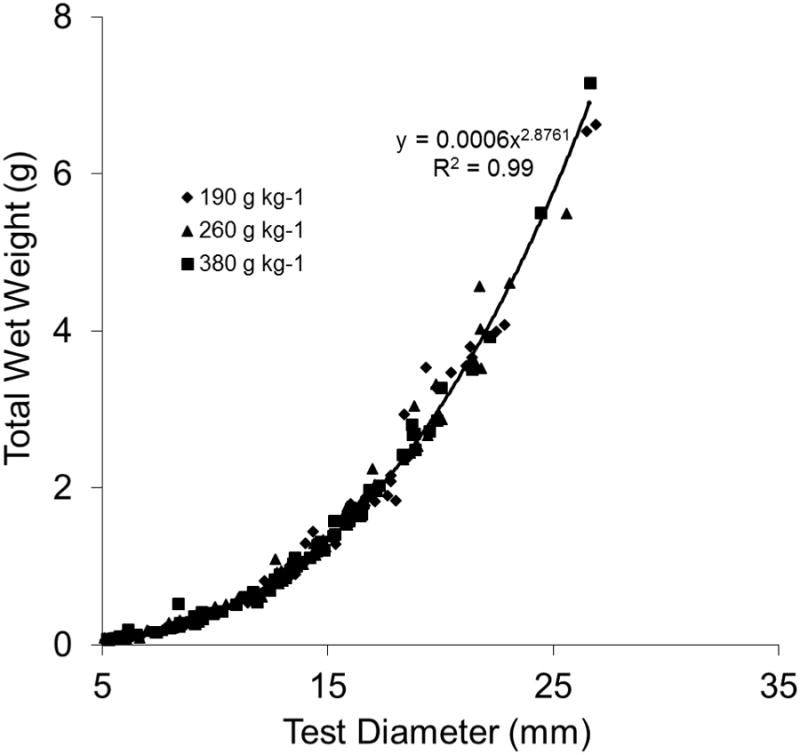
Relationship of diameter (mm) to total wet weight (g) of juvenile sea urchins, Lytechinus variegatus, fed one of three semi-purified, formulated diets (190, 260, or 380 g kg-1 carbohydrate and 310 g kg-1 protein). Values represent means ± SE (n = 16 sea urchins treatment-1).
Figure 5.
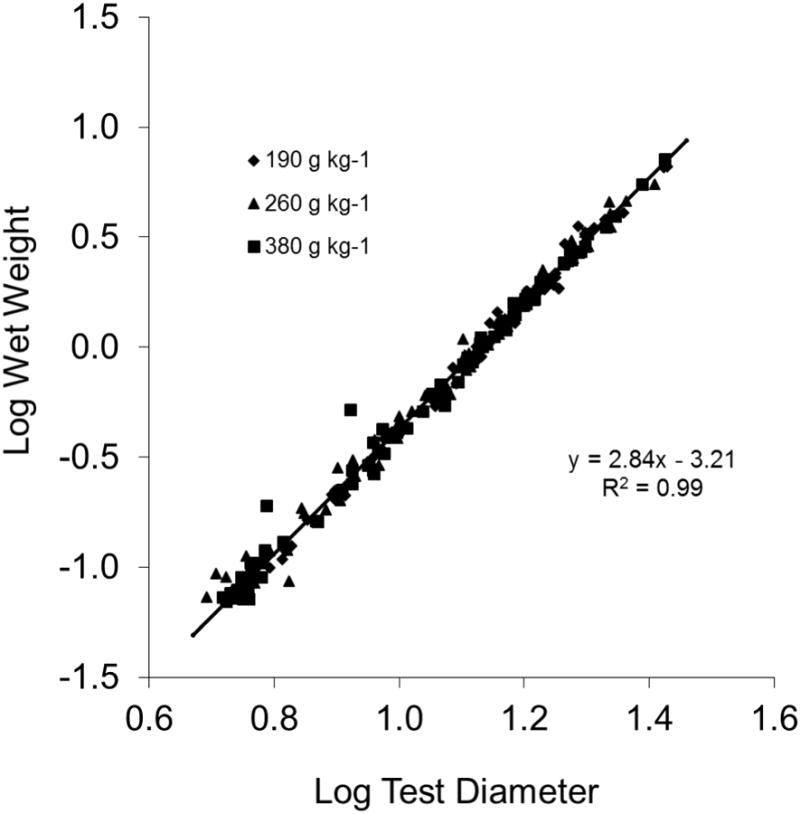
Linear regression analysis of test diameters and wet weights (log transformation) of juvenile sea urchins, Lytechinus variegatus, fed one of three semi-purified, formulated diets (190, 260, or 380 g kg-1 carbohydrate and 310 g kg-1 protein). Values represent means ± SE (n = 16 sea urchins treatment-1).
Organ Analyses
Test wet and dry weight did not vary with treatment (p = 0.686 and p = 0.864, respectively; Table 2). Lantern wet and dry weights were not significantly different among treatments when co-varied with test diameter (p = 0.08 and p = 0.87, respectively; Table 2). Gut wet or dry weights did not vary significantly among treatments (p = 0.93 and p = 0.41, respectively; Table 3). Although significant differences among the wet or dry gonad weight of sea urchins were not observed at p < 0.05, a trend was observed in that wet weight of the gonads in those fed the 190 or 260 g kg-1 carbohydrate diets was higher than those fed 380 g kg-1 carbohydrate (p = 0.08; Table 3).
Table 2.
Test (g) and lantern (mg) wet and dry weights and moisture content (g kg-1) of juvenile sea urchins, Lytechinus variegatus, fed one of three semi-purified, formulated diets (190, 260, or 380 g kg-1 carbohydrate and 310 g kg-1 protein). Values represent means ± SEM (n = 16 sea urchins treatment-1). Means with similar superscripts are not significantly different (p > 0.05).
| Diets | |||
|---|---|---|---|
|
| |||
| 190 g kg-1 | 260 g kg-1 | 380 g kg-1 | |
| Test Wet Weight (g) | 1.33 ± 0.14 A | 1.16 ± 0.14 A | 1.05 ± 0.15 A |
| Test Dry Weight (g) | 0.58 ± 0.06 A | 0.50 ± 0.06 A | 0.48 ± 0.06 A |
| Test Moisture Content (g kg-1) | 56.5 A | 55.9 A | 53.3 A |
| Lantern Wet Weight (mg) | 81.2 ± 10.9 A | 66.7 ± 9.22 A | 72.7 ± 10.0 A |
| Lantern Dry Weight (mg) | 43.4 ± 5.32 A | 38.6 ± 5.13 A | 40.8 ± 5.56 A |
| Lantern Moisture Content (g kg-1) | 45.5 A | 41.1 A | 42.6 A |
Table 3.
Gut and gonad wet and dry weights (mg, ±SEM) and moisture (g kg-1, ± SEM) content of juvenile sea urchins, Lytechinus variegatus, fed one of three semi-purified, formulated diets (190, 260, or 380 g kg-1 carbohydrate and 310 g kg-1 protein. Values represent means ± SE (n = 16 sea urchins treatment-1). Means with similar superscripts are not significantly different (p > 0.05).
| Diets | |||
|---|---|---|---|
|
| |||
| 190 g kg-1 | 260 g kg-1 | 380 g kg-1 | |
| Gut Wet Weight (mg) | 101 ± 11.4 A | 89.2 ± 11.0 A | 79.9 ± 13.6 A |
| Gut Dry Weight (mg) | 21.9 ± 2.44 A | 19.0 ± 2.36 A | 16.7 ± 2.85 A |
| Gut Moisture Content (g kg-1) | 78.3 A | 78.7 A | 78.9 A |
| Gonad Wet Weight (mg) | 55.1 ± 25.4 A | 43.4 ± 20.3 A | 25.9 ± 11.5 A |
| Gonad Dry Weight (mg) | 13.0 ± 6.96 A | 11.0 ± 5.85 A | 5.37 ± 2.47 A |
| Gonad Moisture Content (g kg-1) | 80.9 A | 78.1 A | 79.5 A |
Measurable gonads were first apparent in sea urchins of ca. 13 mm diameter or 0.9 g wet weight (Fig. 6). However, gonad mass in all treatments showed appreciable increases after sea urchins reached a size of ca. 18.5 mm diameter or 2.7 g wet weight (Fig. 6).
Figure 6.
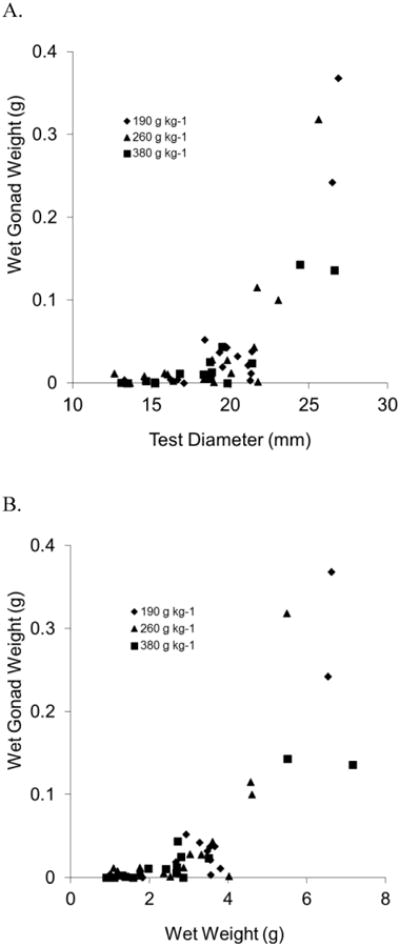
Relation of wet gonad weight to (A) diameter and (B) wet weight of individual juvenile sea urchins, Lytechinus variegatus, fed one three semi-purified, formulated diets (190, 260, or 380 g kg-1 carbohydrate and 310 g kg-1 protein).
Experiment 2: Diet Intake and Digestibility
Although significant differences in diet or protein intake rates (expressed as a percentage of body weight per day) were not observed among treatments, total daily diet intake (mg day-1) and total daily protein intake (mg day-1) were highest in those fed 190 g kg-1 carbohydrate (Table 4). Total daily carbohydrate intake of sea urchins fed 380 g kg-1 carbohydrate was significantly higher than in those fed 190 or 260 g kg-1 carbohydrate (Table 4). Apparent dry matter digestibility was significantly higher in individuals fed the 380 g kg-1 carbohydrate diet (Table 4). No significant differences in apparent dry matter digestibility were detected between the 190 and 260 g kg-1 carbohydrate diets. Although digestible energy did not vary with treatment, the amount of energy consumed varied directly with dietary carbohydrate and was highest in those fed 380 g kg-1, although differences were not significant among dietary treatments (Table 4). Individuals fed 380 g kg-1 carbohydrate consumed 84 kcal of digestible energy kg-1 body weight day-1, whereas individuals fed 190 and 260 g kg-1 carbohydrate consumed 65 and 62 kcal of digestible energy kg-1 body weight day-1, respectively, an average of ca. 70 kcal/kg-1 for all diets (Table 4). Weight gain was 420, 360 or 320 g kg-1 of the initial weight for those fed the 190, 260 or 380 g kg-1 carbohydrate diets, respectively, for the 12 day period; weight gain was inversely related to the carbohydrate content of the diet (p=0.49; Table 4). This would suggest, most likely, that sufficient energy was provided by both 190 and 260 g kg-1 carbohydrate to produce similar outcomes in several metrics.
Table 4.
Diet intake parameters of individual juvenile sea urchins, Lytechinus variegatus, fed one of three semi-purified, formulated diets (190, 260, or 380 g kg-1 carbohydrate and 310 g kg-1 protein; n = 10 individuals treatment-1). Means with similar superscripts are not significantly different (p > 0.05).
| Diets | |||
|---|---|---|---|
|
| |||
| 190 g kg-1 | 260 g kg-1 | 380 g kg-1 | |
| Total diet intake (mg day-1) | 162.2 ± 10.1 A | 120.0 ± 8.43 B | 141.0 ± 12.0 AB |
| Total protein intake (mg day-1) | 51.1 ± 2.61 A | 38.0 ± 3.27 B | 45.0 ± 3.73 AB |
| Total carbohydrate intake (mg day-1) | 30.8± 1.92 A | 31.2 ± 2.19 A | 53.6 ± 4.25 B |
| Digestible energy consumed (cal day-1) | 405.6 ± 20.4 AB | 328.0 ± 20.8 B | 447.0 ± 35.7 A |
| Kcal consumed kg-1 body weight day-1 | 64.7±7.2 A | 61.8±7.8 A | 84.4±14.7 A |
| ADMD (g kg-1) | 743 ± 15.6 B | 733 ± 24.8 B | 812 ± 13.1 A |
| Weight gain (g kg-1) | 417± 0.5 A | 351± 0.5 AB | 316± 0.6 B |
| Digestible energy (g kg-1) | 82.2 ± 1.07 A | 83.4 ± 0.84 A | 85.2 ± 1.23 A |
| Diet intake rate (g kg-1 body weight consumed day-1, as fed) | 3.35 ± 0.33 A | 2.87 ± 0.34 A | 3.37 ± 0.44 A |
Discussion
Experiment 1: Survival and Growth
Formulated diets used in this study promoted 100% survival, indicating juvenile and small L. variegatus are capable of utilizing nutrients in formulated diets. Survival rates were equal or higher than those of individuals of similar size fed a live, mixed-taxa algal biofilm (Taylor et al. 2009). Previous studies demonstrate that formulated diets can support survival and growth in small juvenile L. variegatus (reviewed by Watts et al. 2013).
At this size class, juvenile L. variegatus are rapidly growing, indicated by weight gain and their corresponding high specific growth rates. Similar rates of growth have been observed in this size class of L. variegatus in related studies (Gibbs et al. 2015; Heflin et al. 2013). In the current study, carbohydrate levels were adjusted by co-varying the levels of acid-washed diatomaceous earth. At these levels of diatomaceous earth, no effects on growth have been found (unpublished data). Fat levels were held constant in all diets. Thus, the results of this study are attributed to changes in dietary carbohydrate or protein intake and assimilation. Under the conditions of this study, carbohydrate level in the diet was inversely correlated with weight gain. Data from this and another investigation (Heflin, 2015) indicate that dietary carbohydrate levels of ca. 190 g kg-1 support high weight gain. Lower dietary carbohydrate levels (≤ 120 g kg-1) appear to be inadequate for growth (Heflin, 2015). However, the rate of weight gain was not directly related to caloric energy of the diet. Results of this and other studies (Hammer et al. 2006; 2012; Heflin et al. 2012; Heflin 2015) suggest that, while adequate energy must be consumed, the caloric value of the diet is not the only determinant of sea urchin growth. Instead, we suggest the difference in weight gain is attributed to the amount of protein consumed relative to the amount of energy provided (protein: energy ratio).
Previous studies show strong correlation between protein consumption and somatic weight gain among sea urchins (Cook et al. 1998; Fernandez & Bourdouresque 1998; Fernandez & Pergent 1998; Meidel & Scheibling 1999; Agatsuma 2000; Akiyama 2001; Hammer et al. 2004; Hammer et al. 2006; Taylor 2006; Hammer et al. 2012; Heflin et al. 2012). Hammer et al. (2012) found that adult L. variegatus fed diets with high levels of protein (310 g kg-1 dry weight) had significantly more wet weight gain, larger test diameters, and higher specific growth rates than sea urchins fed low levels of protein (170 g kg-1 dry weight). Likewise, Heflin et al. (2012) observed increased growth among adult L. variegatus with increased dietary protein level and in a 9-week study recorded a 0.5 g increase in wet weight for every 10 g kg-1 increase dietary protein consumed. Exact dietary protein requirements for juvenile L. variegatus are not yet known but intake ranges have been identified: Both juvenile Strongylocentrotus droebachiensis (Pearce 2002; Eddy et al. 2012) and L. variegatus (Hammer et al. 2004) appear to have a minimum requirement for ca. 200 g kg-1 dietary protein to support optimal growth. It will be important that a formulated diet provide juvenile sea urchins with adequate dietary protein to promote satisfactory growth. However, consumption of excess protein should be avoided, as nitrogenous waste contributes greatly to water fouling (Basuyaux & Mathieu 1999) and excess dietary protein may contribute a bitter taste to the developing gonad (Pearce et al. 2002; Woods et al. 2008). In addition, production (assimilation) efficiency is greatly reduced when protein levels are high (Heflin 2015).
The protein: energy ratio of the feeds used in this study ranged from 82 to 104 mg protein kcal-1. The formulated feed that supported the highest weight gain, 190 g kg-1 carbohydrate, had the highest protein: energy ratio of 104 mg protein kcal-1. The feed with the highest carbohydrate content and, consequently, the lowest protein: energy ratio, resulted in the lowest growth rate. Hammer et al. (2012) found that adult sea urchins fed four different formulated diets exhibited the highest weight gain in individuals fed the diet having the highest protein: energy ratio (81 mg protein kcal-1). Heflin et al. (2012) identified a direct correlation between protein: energy ratio and growth in adult L. variegatus. We suggest that, in addition to adequate dietary protein, high protein: energy ratios are needed to promote high rates of growth of juvenile sea urchins. Comparatively, Cuzon & Guillaume (1997) suggested that feeds with protein: energy ratios ranging from 90-160 mg protein kcal-1 are optimal for many commercial shrimp species.
Interactions between dietary nutrients often result in undesirable outcomes (Simpson & Raubenheimer 2012), many of which are related to growth and/or nutrient utilization. Among sea urchins, dietary nutrient ratios affect feed utilization efficiency (Hammer et al. 2012; Heflin et al. 2012). In particular, interactions between high protein and mid to high carbohydrate levels are demonstrated to result in reduced protein efficiency ratio among adult L. variegatus (Heflin et al. 2012) and reduced protein efficiency and production energy efficiency (mg dry tissue produced relative to caloric intake) among juvenile L. variegatus (Heflin 2015). Interactions between dietary protein and carbohydrate are not evident among outcomes in the current study; however, carbohydrate appears to show a hormetic effect. That is, low to medium dietary content of soluble carbohydrate promotes efficient growth outcomes, but a high dietary content of soluble carbohydrates reduces growth and growth efficiencies. Similar results were reported by Heflin et al. (2012), as diets containing high protein and carbohydrate decreased gut size and protein retention in small L. variegatus. The initial SGR of sea urchins fed the formulated diets in this study were higher (83 to 91 g kg-1 body weight gain day-1) than the reported SGR of L. variegatus observed in the field (Moore et al. 1963; Beddingfield & McClintock 2000). These initial values decreased as individuals increased in size. These values were also higher that the SGR for juvenile L. variegatus (55 g kg-1 body weight gain day-1) in the study reported by Taylor et al. (2009) in which juvenile sea urchins were fed a commercial diet formulated for sea urchins (320 g kg-1 protein, 370 g kg-1 carbohydrate, as fed). Wallace (2001) reported SGRs of approximately 50 g kg-1 body weight gain day-1 in small L. variegatus (14.6 ± 0.15 mm, 1.1 ± 0.35 g) fed formulated diets. These data suggest that the quality of the formulated diets used in this study exceeded the quality of the diets used in these previous studies.
Organ Analyses
Organ growth (test, lantern, and gut) appeared to be isometrically proportioned, with these body parts growing at the same rate. A contrast of the size of the gonad observed in relation to body size shows that there is a threshold at which gonads can be visually seen along the body wall (ca. 0.9 g or 12 mm diameter) and a threshold at which time gonads will begin to increase rapidly in size (ca. 2 g wet weight or 18 mm diameter). Some of these gonads were observed to be in advanced stages of gametogenesis, because mature sperm and eggs obtained from gonad exudates were collected and successful fertilization was observed. In contrast, wild, L. variegatus develop mature gonads (containing significant numbers of gametes) only after reaching a size of ca. 35-40 mm diameter (Moore et al. 1963). Precocious gonad development is not unusual among juvenile sea urchins fed formulated feeds (Kelly et al. 1998; Akiyama et al. 2001; Olave 2001; Hammer et al. 2004). Findings from this and previous investigations suggest diets high in nutrient content induce sexual maturity at a much smaller size than reported in natural conditions (Watts et al. 2013). The consequences of conflict between precocious sexual development and somatic growth are not yet quantified but will be of importance for consideration in commercial sea urchin aquaculture (Lawrence 2000). Early gonad development will be unfavorable if it restricts energy allocation to somatic growth (Lawrence 2000).
Additional studies are needed to determine factors affecting the onset of gonad development in juvenile sea urchins. A development timeline representing cellular proliferation and growth (nutritive phagocytes vs gametes) would provide information concerning the nutritional requirements and control of nutrient storage and gametogenesis. We hypothesize that once gonads have begun to develop, the metabolism and nutrient requirements of the sea urchin will change. This could alter animal husbandry techniques, and reflect a need for changes in the nutrient content of required diets.
Experiment 2: Feed Intake and Digestibility
Under the conditions of this study, weight gain and test diameter in experiment 1 were inversely related to the carbohydrate content and caloric value of the diet. Growth parameters were directly related to protein: energy ratio. Since sea urchins fed the 190 g kg-1 carbohydrate diet (higher protein: energy ratio) consumed significantly more dry diet, they also consumed more protein than the 260 g kg-1 carbohydrate diet (lower protein: energy ratio) even though the amount of energy consumed was similar. However, a simple explanation for observed feed and nutrient intake in relation to growth observed in experiment 1 is most likely not possible.
Across animal taxa, feed intake and nutrient utilization are often regulated by nutrient balance within the available food (Simpson & Raubenheimer 2012). The sea urchins in this study were fed a single diet during the experimental time period, and thus were limited to the nutrients available in that single diet. Other studies have shown that many organisms will consume to a nutrient intake target, or an energy intake target (reviewed by Simpson & Raubenheimer 2012; Heflin 2015). The sea urchins in this study appeared to consume to a similar carbohydrate (energy) target when fed 190 or 260 g kg-1 carbohydrate, but may have overconsumed carbohydrate to meet a protein intake requirement at 380 g kg-1 carbohydrate. Over consumed carbohydrate would most likely be used inefficiently, perhaps to the detriment of the urchin health. Protein intake targets can be inferred from previous studies, as Fernandez & Boudouresque (1998) and Daggett et al. (2005) suggested that sea urchins consume high quantities of low nitrogen diets. In addition, adult L. variegatus fed diets with 310 g kg-1 protein exhibited decreased total feed and energy intake (Hammer et al. 2012). Hammer et al. (2012) and Heflin (2015) further suggesting that protein intake can regulate diet intake in L. variegatus. We hypothesize that both protein content and protein: energy regulate diet intake and, consequently, weight gain in L. variegatus.
The ADMD values were inversely proportional to the amount of ash in the diets. In this study, ash was substituted with purified starch to produce various carbohydrate levels. Consequently, the diets with less carbohydrate (190 and 260 g kg-1 carbohydrate) had a higher ash content, which could account for the overall differences detected among ADMD values in the various treatments. These ADMD values are similar to those reported by Hammer (2006).
The diet intake rate (g kg-1 body weight consumed day-1) of juvenile sea urchins in this study was comparable with feeding rates of adult Strongylocentrotus intermedius fed various algae, but lower than sub-adult S. intermedius (average test diameter = 24.6) fed Laminaria japonica (Fuji 1967). Diet intake rates were also similar to S. droebachiensis fed formulated diets during the summer months (Daggett 2005), and slightly higher than the diet intake rates reported by Barker et al. (1998) for small Evechinus chloroticus fed formulated diets.
In summary, although weight gain among sea urchins in this study was inversely proportional to the carbohydrate level in the diet, these differences can, most likely, be attributed to differences in protein intake and/or in protein: energy ratios in the proffered diets. If we interpret the results of experiment 1 with regard to insights gained from experiment 2, sea urchins that had higher feed intake also consumed higher levels of protein leading to highest weight gain. Consequently, protein content and/or protein: energy ratio may be important in determining nutrient utilization and growth. Carbohydrate levels used in this study were all adequate to support high rates of weight gain in small juvenile L. variegatus; however, high dietary carbohydrate (or a high carbohydrate to protein ratio) can be inhibitory to weight gain. Finally, the average digestible energy intake was approximately 70 kcal kg-1 body weight day-1, suggesting daily caloric intake of juvenile L. variegatus is lower than in shrimp and fish.
Acknowledgments
The authors would like to thank Victoria Gibbs, Warren Jones, Hugh Hammer, Randy Watts, Adele Cunningham, Natalie Warren and Dorothy Moseley for assistance and support. We would especially like to thank Dr. Anthony Siccardi for his contribution to the statistical evaluation of the data. This publication was supported by the National Sea Grant College Program of the U.S. Department of Commerce's National Oceanic and Atmospheric Administration under NOAA Grant #'s NA16RG2258 and NA06OAR4170078, the Mississippi-Alabama Sea Grant Consortium project #'s R/SP-9 and R/SP-15, respectively, and the University of Alabama at Birmingham. Animal resources were supported in part by NIH P30DK056336, Aquatic Animal Research Core (NORC). The views expressed herein do not necessarily reflect the views of any of those organizations.
Contributor Information
Anna M. Taylor, University of Alabama at Birmingham, Department of Biology, 1300 University Blvd., CH 374A, Birmingham, AL 35294
Laura E. Heflin, University of Alabama at Birmingham, Department of Biology, 1300 University Blvd., CH 374A, Birmingham, AL 35294
Mickie L. Powell, University of Alabama at Birmingham, Department of Biology, 1300 University Blvd., CH 374A, Birmingham, AL 35294
Addison L. Lawrence, Texas A&M University, Texas AgriLIFE Mariculture Research Laboratory, 1300 Port Street, Port Aransas, Texas 78373
Stephen A. Watts, University of Alabama at Birmingham, Department of Biology, 1300 University Blvd., CH 375, Birmingham, AL 35294
References
- Agatsuma Y. Food consumption and growth of the juvenile sea urchin Strongylocentrotus intermedius. Fish Sci. 2000;66(3):467–472. [Google Scholar]
- Akiyama T, Unuma T, Yamamoto T. Optimal protein level in a purified diet for young red sea urchins Pseudocentrotus depressus. Fish Sci. 2001;67(2):361–363. [Google Scholar]
- Andrews NL, Agatsuma Y, Ballesteros E, Bazhin AG, Creaser EP, Barnes DKA, Botsford LW, Bradbury A, Campbell A, Dixon JD, Einarsson S, Gerring PK, Hebert K, Hunter M, Hur SB, Johnson CR, Juinio-Menez MA, Kalviss P, Miller RJ, Moreno CA, Palleiro JS, Rivas D, Robinson SML, Schroeter SC, Steneck RS, Vadas RL, Woodby DA, Xiaoqi Z. Status and management of world sea urchin fisheries. Oceanogr Mar Biol: an Annual Review. 2002;40:343–425. [Google Scholar]
- AOAC. Association of Official Analytical Chemists. 15th. Vol. 1. Arlington, VA, USA: 1990. Official Methods of Analysis. [Google Scholar]
- Barker MF, Keogh JA, Lawrence JM, Lawrence AL. Feeding rate, absorption efficiencies, growth, and enhancement of gonad production in the New Zealand sea urchin Evechinus chloroticus Valenciennes (Echinoidea: Echinodermetridae) fed prepared and natural diets. J Shellfish Res. 1998;17(5):1583–1590. [Google Scholar]
- Basuyaux O, Mathieu M. Inorganic nitrogen and its effect on growth of the abalone Haliotis tuberculata Linnaeus and the sea urchin Paracentrodus lividus Lamarck. Aquaculture. 1999;174:95–107. [Google Scholar]
- Beddingfield SD, McClintock JB. Demographic characteristics of Lytechinus variegatus Echinoidea: Echinodermata from three habitats in a North Florida Bay, Gulf of Mexico. Mar Ecol. 2000;21:17–40. [Google Scholar]
- Cook E, Kelly M. Enhanced production of the sea urchin Paracentrotus lividus in integrated open-water cultivation with Atlantic salmon Salmo salar. Aquaculture. 2007;273(4):573–585. [Google Scholar]
- Cook E, Kelly M, McKenzie J. Somatic and gonadal growth of the sea urchin Psammechinus miliaris (Gmelin) fed artificial salmon feed compared with a macroalgal diet. J Shellfish Res. 1998;17(5):1549–1555. [Google Scholar]
- Cuzon G, Guillaume J. Energy and Protein: Energy Ratio. In: D'Abramo L, Conklin D, Akiyama D, editors. Crustacean Nutrition. Vol. 6. World Aquaculture Society; 1997. pp. 51–70. [Google Scholar]
- Daggett TL, Pearce CM, Tingley M, Robinson SMC, Chopin T. Effect of prepared and macroalgal diets and seed stock source on somatic growth of juvenile green sea urchins (Strongylocentrotus droebachiensis) Aquaculture. 2005;244:263–281. [Google Scholar]
- Eddy SD, Brown NP, Kling AL, Watts SA, Lawrence AL. Growth of juvenile green sea urchins, Strongylocentrotus droebachiensis, fed formulated feeds with varying protein levels compared with a macroalgal diet and a commercial abalone feed. J World Aquac Soc. 2012;43(2):159–173. [Google Scholar]
- Fernandez C, Boudouresque CF. Evaluating artificial diets for small Paracentrotus lividus (Echinodermata: Echinoidea) In: Mooi R, Telford M, editors. Echinoderms: San Francisco. Balkema; Rotterdam, Amsterdam: 1998. pp. 651–656. [Google Scholar]
- Fernandez C, Pergent G. Effect of different formulated diets and rearing conditions on growth parameters in the sea urchin Paracentrotus lividus. J Shellfish Res. 1998;17:1571–1582. [Google Scholar]
- Fuji A. Memoirs of the Faculty of Fisheries. Vol. 15. Hokkaido University; 1967. Ecological studies on the growth and food consumption of the Japanese common littoral sea urchin, Strongylocentrotus intermedius (A. Agassiz) p. 2. [Google Scholar]
- Gibbs VK, Heflin LE, Jones WT, Powell ML, Lawrence AL, Makowsky R, Watts SA. Optimizing dietary levels of menhaden and soybean oil and soybean lecithin for pre-gonadal somatic growth in juveniles of the sea urchin Lytechinus variegatus. Aquaculture. 2015 doi: 10.1016/j.aquaculture.2015.05.013. doi.org/10.1016/j.aquaculture.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer BW, Hammer HS, Watts SA, Desmond RA, Lawrence JM, Lawrence AL. The effects of dietary protein concentration on feeding and growth of small Lytechinus variegatus (Echinodermata: Echinoidea) Mar Biol. 2004;145:1143–1157. [Google Scholar]
- Hammer HS. Ph D Dissertation. University of Alabama at Birmingham; Birmingham, Alabama, USA: 2006. Determination of dietary protein, carbohydrate, and lipid requirements for the sea urchin, Lytechinus variegatus, fed semi-purified feeds. [Google Scholar]
- Hammer HS, Powell ML, Jones WT, Gibbs VK, Lawrence AL, Lawrence JM, Watts SA. Effect of feed protein and carbohydrate levels on feed intake, growth, and gonad production of the sea urchin, Lytechinus variegatus. Journal of the World Aquaculture Society. 2012;43(2):145–158. [Google Scholar]
- Hammer H, Watts SA, Lawrence AL, Lawrence JM, Desmond RA. The effect of dietary protein on consumption, survival, growth and production of the sea urchin Lytechinus variegatus. Aquaculture. 2006;254(1):483–495. [Google Scholar]
- Heflin LE, Gibbs VK, Powell ML, Makowsky R, Lawrence JM, Lawrence AL, Watts SA. Effect of dietary protein and carbohydrate levels on weight gain and gonad production in the sea urchin Lytechinus variegatus. Aquaculture. 2012;358:253–61. doi: 10.1016/j.aquaculture.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heflin LE. Ph D Dissertation. University of Alabama at Birmingham; Birmingham, Alabama, USA: 2015. Nutrient intake, nutrient targets and feed application to promote optimal production and feed efficiency in the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea) [Google Scholar]
- Heflin LE, Gibbs VK, Jones WT, Makowsky R, Lawrence AL, Watts SA. Growth rates are related to production efficiencies in juveniles of the sea urchin Lytechinus variegatus. J Mar Biol Assoc UK. 2013;93(06):1673–1683. doi: 10.1017/S0025315412001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinegardner RT. Growth and development of the laboratory cultured sea urchin. Biol Bull. 1969;137(3):465–475. doi: 10.2307/1540168. [DOI] [PubMed] [Google Scholar]
- Humphries M, Hughes A, Cook E. U S Aquac Soc. Las Vegas, NV: 2012. Aquacultural study of sea urchin Paracentrotus lividus diet trials, lipid metabolism and the effect of broodstock diet on larval growth and survivorship. [Google Scholar]
- Keesing JK, Hall KC. Urchin aquaculture: molecules to market- Review of harvests and status of world sea urchin fisheries points to opportunities for aquaculture. J Shellfish Res. 1998;17(5):1597–1608. [Google Scholar]
- Kelly MS, Brodie CC, McKenzie JD. Somatic and gonadal growth of the sea urchin Psammechinus miliaris (Gmelin) maintained in polyculture with the Atlantic salmon. J Shellfish Res. 17:1557–1562. [Google Scholar]
- Kelly M. Echinodermata. Springer; Berlin, Heidelberg: 2005. Echinoderms: their culture and bioactive compounds; pp. 139–165. [PubMed] [Google Scholar]
- Lawrence AL, Lawrence JM. Importance status and future research needs for formulated feeds for sea urchins aquaculture. In: Lawrence JM, Guzman O, editors. Sea Urchins: Fisheries and Ecology. DEStech Publications, Inc.; Lancaster, PA, USA: 2004. pp. 275–283. [Google Scholar]
- Lawrence JM. Conflict between somatic and gonadal growth in sea urchins: a review. Workshop on coordination of green sea urchin research in Atlantic Canada, Moncton. 2000 http://crdpm.umcs.ca/oursin/
- Lawrence JM, Lawrence AL, Watts SA. Feeding, digestion and digestibility of sea urchins. In: Lawrence JM, editor. Sea Urchins: Biology and Ecology. 3rd. Elsevier Science, BV; Amsterdam: 2013. pp. 135–154. [Google Scholar]
- Lesser P, Walker CW. Introduction to the special section on sea urchin aquaculture. J Shellfish Res. 1998;17:1505–1506. [Google Scholar]
- Marsh AG, Powell ML, Watts SA. Energy metabolism and gonad development. In: Lawrence JM, editor. Sea Urchins: Biology and Ecology. 3rd. Elsevier Science, B.V.; Amsterdam: 2013. pp. 27–42. [Google Scholar]
- Meidel SK, Scheibling RE. Effects of food type and ration on reproductive maturation and growth of the sea urchin Strongylocentrotus droebachiensis. Mar Biol. 1999;134:155–166. [Google Scholar]
- Moore H, Jutary T, Bauer J, Jones J. The biology of Lytechinus variegatus. Bull Mar Sci Gulf Caribb. 1963;13:23–53. [Google Scholar]
- Morris JG. Nutrition. In: Prosser CL, editor. Environmental and metabolic animal physiology. Wiley-Liss; New York, USA: 1991. pp. 231–276. [Google Scholar]
- Olave S, Bustos E, Lawrence JM, Carcamo P. The effect of size and diet on gonad production by the Chilean sea urchin Loxechinus albus. J World Aquac Soc. 2001;32(2):210–214. [Google Scholar]
- Pearce CM, Daggett TL, Robinson SMC. Effect of protein source ratio and protein concentration in prepared diets on gonad yield and quality of the green sea urchin Strongylocentrotus droebachiensis. Aquaculture. 2002;214:307–322. [Google Scholar]
- Pearse JS, Cameron RA. Echinoderm: Echinoidea. In: Pearse JS, Pearse VB, editors. Reproduction of marine invertebrates: Echinoderms and lophorates. Vol. 6. Boxwood Press; Pacific Grove, CA, USA: 1991. pp. 513–662. [Google Scholar]
- Rahman MA, Arshad A, Yusoff FM. International Conference on Agricultural, Ecological and Medical Sciences (AEMS) London, United Kingdom: 2014. Sea Urchins (Echinodermata: Echinoidea): Their Biology, Culture and Bioactive Compounds. [Google Scholar]
- Robinson SMC. Sea urchins: fisheries and ecology. DEStech Publications, Inc.; Lancaster, PA, USA: 2004. The evolving role of aquaculture in the global production of sea urchins; pp. 343–57. [Google Scholar]
- Simpson SJ, Raubenheimer D. The nature of nutrition: a unifying framework from animal adaptation to human obesity. Princeton University Press; Princeton, NJ, USA: 2012. [Google Scholar]
- Sloan N. Echinoderm fisheries of the world: a review. AA Balkema; Rotterdam, Amsterdam: 1985. pp. 109–24. [Google Scholar]
- Taylor AM. Master's Thesis. University of Alabama at Birmingham; Birmingham, Alabama, USA: 2006. The effects of dietary carbohydrate on weight gain and gonad production in juvenvile sea urchins, Lytechinus variegatus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Powell ML, Watts SA, Lawrence AL. Formulated feed supports weight gain and survivorship in juvenile sea urchins Lytechinus variegatus. J World Aquac Soc. 2009;40(6):780–787. [Google Scholar]
- Unuma T. Gonadal growth and its relationship to aquaculture in sea urchins. In: Yokota, Matranga, Smolenicka, editors. The sea urchin: from basic biology to aquaculture. Swets and Zeitlinger; Lisse, The Netherlands: 2002. pp. 115–127. [Google Scholar]
- Wallace BD. Masters Thesis. University of Alabama Birmingham; Birmingham, Alabama, USA: 2001. The effects of dietary protein concentration on feeding and growth of small Lytechinus variegatus (Echinodermata: Echinoidea) [Google Scholar]
- Watts SA, Lawrence AL, Lawrence JM. Nutrition. In: Lawrence JM, editor. Sea Urchins: Biology and Ecology. 3rd. Elsevier Science, B.V; Amsterdam: 2013. pp. 155–166. [Google Scholar]
- Woods CM, James PJ, Moss GA, Wright J, Siikavuopio S. A comparison of the effect of urchin size and diet on gonad yield and quality in the sea urchin Evechinus chloroticus Valenciennes. Aquacult Int. 2008;16(1):49–68. [Google Scholar]


