Abstract
Viruses are a common cause of central nervous system (CNS) infections with many host, agent, and environmental factors influencing the expression of viral diseases. Viruses can be responsible for CNS disease through a variety of mechanisms including direct infection and replication within the CNS resulting in encephalitis, infection limited to the meninges, or immune-related processes such as acute disseminated encephalomyelitis. Common pathogens including herpes simplex virus, varicella zoster, and enterovirus are responsible for the greatest number of cases in immunocompetent hosts. Other herpes viruses (eg, cytomegalovirus, John Cunningham virus) are more common in immunocompromised hosts. Arboviruses such as Japanese encephalitis virus and Zika virus are important pathogens globally, but the prevalence varies significantly by geographic region and often season. Early diagnosis from radiographic evidence and molecular (eg, rapid) diagnostics is important for targeted therapy. Antivirals may be used effectively against some pathogens, although several viruses have no effective treatment. This article provides a review of epidemiology, diagnostics, and management of common viral pathogens in CNS disease.
Keywords: Central nervous system, antivirals, encephalitis, meningoencephalitis
Introduction
Viruses are a common cause of central nervous system (CNS) infections with many factors influencing the expression of viral diseases. These include complex interactions between the host, agent, and environment that ultimately result in the specific CNS infection. Viruses can be responsible for CNS disease through a variety of mechanisms including direct infection and replication within the CNS resulting in encephalitis, infection limited to the meninges, or immune-related processes such as acute disseminated encephalomyelitis.1 Pathogenicity, virulence, and immunogenicity strongly determine the expression of CNS infection. Host risk factors such as immunosuppression due to disease or medications often increase the risk of infection.2
Epidemiology and Clinical Presentation
Epidemiology
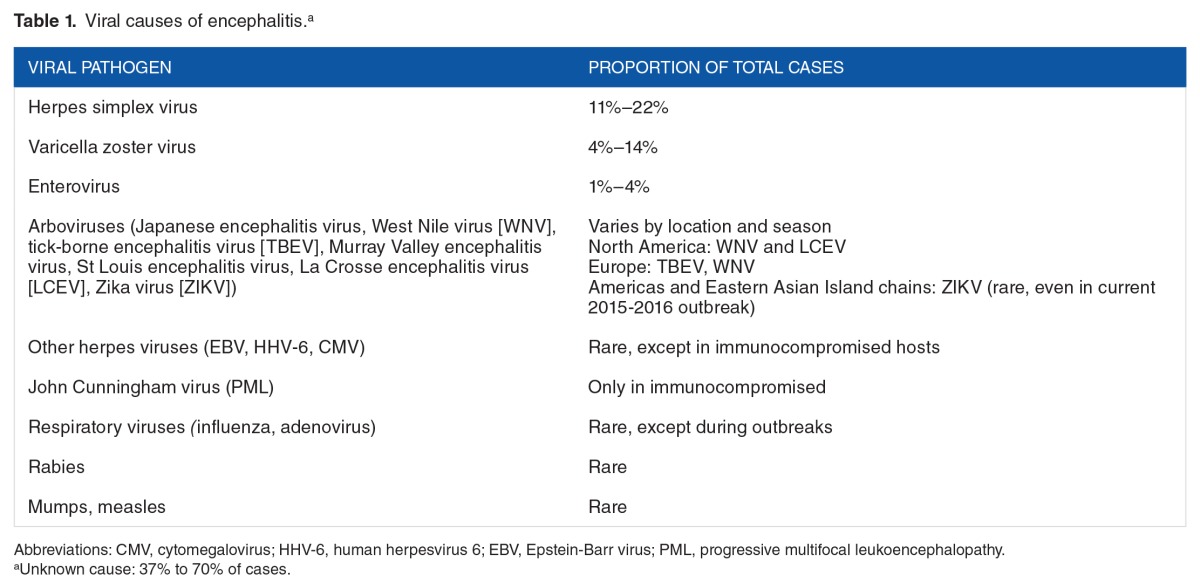
Precise epidemiology of CNS infections can be difficult to determine due to the variation in the constellation of symptoms throughout the disease process. There are a number of viruses that cause CNS infections such as meningitis, meningoencephalitis, or encephalitis, which is the most common. Disease patterns of CNS viral infections can be sporadic, endemic, epidemic, or pandemic. Viruses can cause CNS infections in any of these patterns. They can often change over time, such as epidemic to endemic. Table 1 displays the most common causes of CNS encephalitis.
Table 1.
Viral causes of encephalitis.a
A detailed discussion on the viruses that cause these CNS infections is beyond the scope of this review; however, some viruses have unique clinical presentations that we will highlight. The clinical presentation of viral infections, especially encephalitis is often nonspecific and requires the clinician to consider a range of differential diagnoses.3 Meningitis, which affects the lining of the CNS, produces characteristic symptoms of fever, neck stiffness, photophobia, and/or phonophobia. Photophobia and nuchal rigidity are reflective of the presence of meningeal irritation. Patients with encephalitis have the manifestations of infection within the brain itself and have signs and symptoms of altered brain function. These include altered mental status, personality change, abnormal behavior or speech, and movement disorders. Focal neurologic signs such as hemiparesis, flaccid paralysis, or paresthesias may also be present. Seizures can occur with viral meningitis and encephalitis. Patients may be critically ill with respiratory failure or unable to maintain their airway which prompts intubation and intensive care unit admission.
Relative to bacterial CNS infections, patients with viral CNS infections are more likely to present with altered mental status, coma or stupor, or new onset seizure activity that may indicate encephalitis. Assessment of these patients should include consideration of past medical history for other differential diagnoses including stroke risk, use of CNS depressants, and risk factors for viral infection. Many patients may report a viral prodrome with fevers or mild CNS changes prior to presentation. Additional findings or patient history may be suggestive for specific viral causes. The presence of vesicles with dermatomal distribution can indicate varicella zoster virus (VZV). However, the virus may be present without dermal involvement. Mumps, a paramyxovirus, is accompanied by par-otitis. Although vaccination has dramatically reduced the incidence, recent changes in vaccination practices should be considered. Up to 50% of the patients with West Nile virus (WNV) have a maculopapular rash. In addition, flaccid paralysis, which can be mistaken for other neurologic abnormalities such as Guillain-Barré, is highly indicative of WNV infection. St Louis encephalitis produces eyelid, tongue, lip, and extremity tremors in up to two-thirds of the patients. Physical examination findings of cranial nerve abnormalities are likely to be present. Rabies produces characteristic findings of hydrophobia, aerophobia, pharyngeal spasm, and hyperactivity.
Clinical presentation—CNS disease
Herpes simplex virus (HSV) encephalitis is one of the most devastating, common causes of fatal encephalitis worldwide, and this virus can also cause meningitis and meningoencephalitis. In the United States, it accounts for nearly 20% of all cases of encephalitis with peak occurrences in patients aged 5 to 30 years and those greater than 50 years.4,5 The estimated incidence is 2.3 cases per million in the population per year, with HSV type 1 accounting for approximately 95% of all cases.5,6
Varicella zoster virus can cause a wide range of different CNS manifestations including encephalitis, meningitis, cerebellitis, myelitis, and Ramsay Hunt syndrome. Cerebellar involvement, with cerebellar ataxia, is the most common manifestation and usually resolves after illness.7 Varicella zoster virus is the second most common cause of encephalitis after HSV and the second most common cause of viral meningitis after enterovirus (EV) in developed countries.7–10 Despite this, VZV encephalitis is a rare complication with an incidence of 1.8 cases per 10 000 cases of varicella zoster infection.11 Immunocompromised patients are at greater risk of disseminated disease with an incidence of up to 36%.
Cytomegalovirus (CMV) is a ubiquitous virus with an estimated 50% to 80% of adults infected by age 40.12 Variations in seropositivity exist among subgroups including increased rates in women of childbearing age and 1% of infants with congenital infection.13 Despite these high rates of seropositivity, CMV is a rare cause of CNS infection with one study reporting only a single positive polymerase chain reaction (PCR) result among 354 patients with suspected viral CNS disease.14 Patients who are immunocompromised including patients with human immunodeficiency virus (HIV) with CD4 counts below 100 cells/µL or transplant recipients are more susceptible to invasive disease. Retinitis is the most common presentation in patients with HIV infection, accounting for 85% of CMV disease. Over half of solid organ transplant patients have evidence of disease with a smaller percentage (10%-50%) manifesting symptoms. Hematopoietic allogeneic stem cell transplant (HSCT) patients are at greater risk for both primary infection and reactivation of latent disease.15 Primary infection is common in seronegative recipients, occurring in 30% of HSCT patients. Reactivation of latent disease is common and occurs in 80% of seropositive recipients. Early-onset CMV disease (<100 days after SCT) is typically associated with pneumonitis and enterocolitis, whereas late-onset CMV disease (>100 days after SCT) also includes retinitis and encephalitis as rare complications. Nearly 18% of patients in one study developed late-onset CMV disease approximately 169 days posttransplant with a mortality rate approaching 50%. Specific data on CNS-associated CMV disease are unavailable.
Epstein-Barr virus (EBV) is commonly known as the causative pathogen of infectious mononucleosis. Although most infections are mild, it can cause meningitis, encephalitis, and other CNS disease. The incidence of CNS involvement ranges from 0.7% to 5% in immunocompetent patients, with an increased incidence in immunocompromised individuals.16
Enteroviruses are RNA viruses of the Picornaviridae family with more than 100 identified serotypes. Although 1 virulent subtype, EV 71, has longed been linked with encephalitis, many other serotypes including coxsackievirus strains have been associated with acute CNS disease.17 Enterovirus 71 is commonly associated with hand, foot, and mouth disease in children. Echoviruses 13, 18, and 30 have been linked to viral meningitis outbreaks in the United States. Most recently, in 2014, a nationwide outbreak of EV infection (serotype 68) occurred in the United States, infecting nearly 1200 people, primarily infants, and young children.18,19 During this outbreak, approximately 100 patients were also diagnosed with an acute flaccid myelitis. This neurologic involvement has not been conclusively linked; evidence suggests and experts agree that this is a unique presentation of EV-D68. Many of these patients have remained symptomatic and only a small percentage of patients have fully recovered.20
Arboviruses represent a broad group of viruses with arthropod vectors, most commonly mosquitos and ticks.20 Japanese encephalitis virus (JEV) is the most common definitive cause of viral encephalitis with approximately 10 000 cases annually in East Asia.21 West Nile virus was first identified in 1937 before being recognized in the United States at the turn of the century.22 Throughout history, these viruses have caused sporadic epidemics but rarely cause significant burden of disease in the United States. Globalization with enhanced international travel over the past 3 to 4 decades has increased the spread and emergence. Dengue fever is the most common arbovirus infection, behind only malaria for infection-related sequelae in the tropics, but is less commonly associated with CNS disease. The rate of infections worldwide has increased 30-fold in the past 50 years.23
Recently, the Zika virus (ZIKV), a flavivirus also primarily transmitted by mosquito and first isolated in 1947 from a rhesus macaque in the Zika Forest of Uganda, has emerged as a public health emergency for much of the Americas and parts of island chains of Eastern Asia.24 General viral syndromes are common with about 45% of patients with ZIKV infection reporting headache and an additional 39% reporting retro-orbital pain.24 There appears to be a relationship between ZIKV outbreaks and incidence of Guillain-Barré syndrome (odds ratio [OR]: >34) supported by findings from electrophysiological studies.25 Meningoencephalitis and acute myelitis have also been reported. At least 1 case report has demonstrated the potential for ZIKV neurotropism in a patient with profound neurodeficits present for several weeks. Profound effects on the unborn child are most concerning.26 Maternal ZIKV infection has led to high rates of microcephaly among infants, supported by evidence of ZIKV RNA in amniotic fluid and brain tissue of newborns with microcephaly. Zika virus infection is the first major infectious diseases linked to serious birth defects to be discovered in more than 50 years.24
The rabies virus is an RNA virus transmitted through saliva of an infected animal. In the United States, bats, raccoons, skunks, and foxes are the primary sources of infection correlated with region. Each year in the United States, approximately 6000 animals are infected with rabies, with 92% of infections occurring in nondomestic animals. By contrast, human infections in the United States are rare with 2 to 3 cases per year; nearly 75 000 cases occur annually worldwide.27
Diagnosis of CNS Viral Infections
Brain imaging is the first step in the workup of a potential CNS infection. The main purpose of brain imaging is to rule out space-occupying lesions such as abscess, tumor, edema, or hydrocephalus that can lead to brain herniation during lumbar puncture (LP). Brain imaging must be performed before diagnostic LP. Computed tomographic scan is a screening tool to rule out intracranial space-occupying lesions, but the utility is limited. Magnetic resonance imaging (MRI) is now widely accepted as the preferred test to detect early changes in the CNS if any viral infections are suspected.28
Magnetic resonance imaging
In herpes simplex encephalitis (HSE), T2-weighted (T2W), and fluid attenuation inversion recovery (FLAIR) sequences of MRI show abnormal hyperintensities in limbic and hypothalamic areas, whereas the same areas appear hypointense on T1 weighted (T1W). If the lesion is complicated by hemorrhage, T1W can also show areas of hyperintensity within the hypointense lesion. Usually, changes on T2W and FLAIR sequence become visible in the first 48 hours.29 The same area appears hyperintense on diffusion-weighted imaging (DWI) and hypointense on apparent diffusion coefficient (ADC) sequence. Diffusion-weighted imaging is more sensitive than T2-weighted imaging (T2WI) or FLAIR imaging in early detection of edema changes.30 In the late acute and early subacute stages, the DWI starts to become less hyperintense and ADC starts to become less hypointense.31 T1-weighted gadolinium–contrasted sequence will not show any enhancement in early stage but will show contrast enhancement in late acute to subacute stage.32
John Cunningham (JC) virus causes progressive multifocal leukoencephalopathy (PML). These lesions are typically multifocal and asymmetric in the subcortical location. Periventricular white matter is usually spared. Magnetic resonance imaging findings in PML include hypointense lesions on T1W and hyperintense lesions on T2W sequence.33 Diffusion-weighted imaging sequence shows patchy peripheral hyperintensities.34 Typically, the lesions do not enhance with gadolinium contrast.35
Varicella zoster virus can cause CNS vasculitis which subsequently results in ischemic stroke.36 Magnetic resonance imaging shows abnormal signal intensity and swelling in cerebral cortex, gray-white matter junction, subcortical white matter, basal ganglia, or cerebellum.28 The lesions may occasionally be enhanced by contrast.37
In CMV encephalitis, there is usually nonspecific periventricular white matter hyperintensity on T2W and FLAIR imaging. If CMV is associated with ventriculitis, then MRI may also show contrast enhancement of the ependymal and subependymal surfaces along with hydrocephalus. Cytomegalovirus encephalitis is often associated with concurrent atrophy. Usually there is no mass effect but in rare cases, CMV may manifest as a ring-enhancing or space-occupying lesion.38
Japanese encephalitis virus classically affects bilateral thalami. Magnetic resonance imaging shows hypointense lesions on T1W and hyperintense lesions on T2W and FLAIR sequence in the bilateral thalami. Diffusion-weighted imaging will show variable amount of restricted diffusion. If hemorrhagic component is present, hypointensity can be seen within the lesion on gradient echo sequence.31,39–41
West Nile virus encephalitis gives abnormal MRI findings in more than 33% of the cases.42 The findings are generally nonspecific. Magnetic resonance imaging may show leptomeningeal thickening and contrast enhancement.43 The T2W and FLAIR sequences show patchy white matter hyperintensities.44–46 These lesions typically do not enhance with contrast.
In acute disseminated encephalomyelitis, MRI typically shows bilateral lesions, but they may be asymmetric with ill-defined margins.47 The lesions are mostly in the periventricular and subcortical white matter including corpus callosum. They also involve gray matter in the cortex as well as thalamus and basal ganglia.47–49 On T2W and FLAIR sequences, the lesions are hyperintense, whereas on T1W sequences, these lesions appear hypointense.50,51 Infratentorial lesions are also commonly seen.48,52 These lesions variably enhance with gadolinium contrast.52,53 The lesions appear hyperintense on DWI and hypointense on ADC in an acute phase.49,52
Magnetic resonance spectroscopy
Advanced magnetic resonance (MR) techniques such as MR spectroscopy are frequently used in the hospital setting for the evaluation of CNS lesions. It is a noninvasive technique that is helpful for the assessment of metabolic profiles of normal and pathologic brain tissue. It is mainly helpful in the workup for malignancy, but sometimes plays an important role in the infectious workup.
In acute HSE, MR spectroscopy shows decreased concentrations of N-acetylaspartate (NAA) and increased concentrations of choline (Cho) with a resultant decrease in the NAA/Cho and NAA/Cr (creatine) ratios. Lactate values are usually elevated, but the peak is variable.54 However, this pattern is not very specific as it can be seen in multiple viruses. By comparison in HIV encephalopathy, MR spectroscopy reveals decreased NAA and increased Cho and myoinositol.55,56
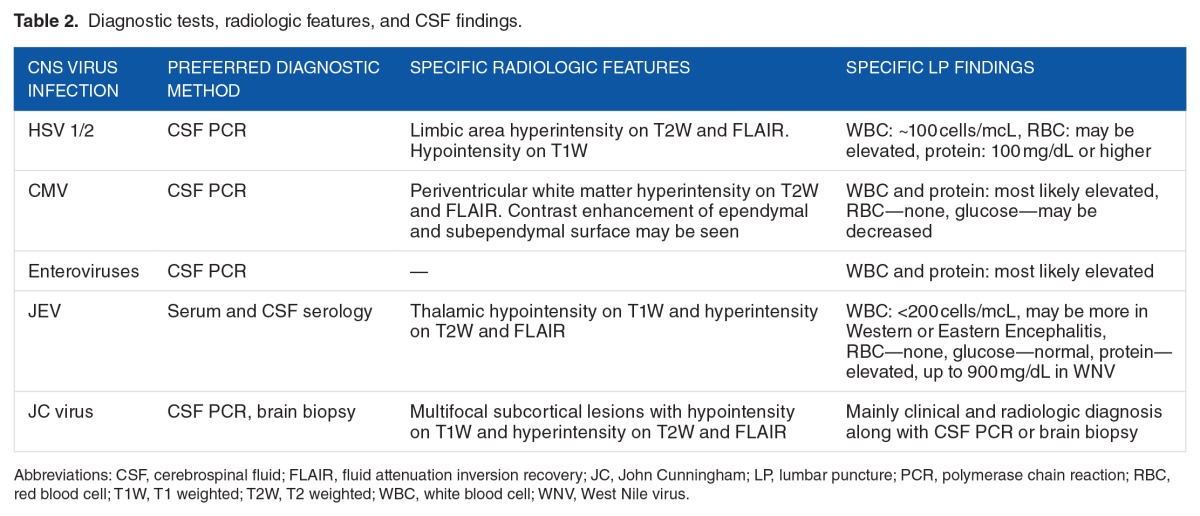
In JEV infections, the data on MR spectroscopic finding are limited. Based on one study, PML lesions show reduced NAA and lactate but increased Cho.57 Characteristic radio-logic findings in some of the CNS viral infections are described in Table 2.
Table 2.
Diagnostic tests, radiologic features, and CSF findings.
Lumbar puncture
Once the space-occupying lesion is ruled out, the next step is the diagnostic LP. The typical findings in viral encephalitis include normal or mildly elevated opening pressure, normal glucose concentration, and mildly elevated protein. Cerebrospinal fluid (CSF) shows increased number of nucleated cells but usually <100 cells/mm3. The cells are predominantly lymphocytic, but PMNs may predominate early in the course. In HSV, increased number of red blood cells (RBCs) may be seen even in a nontraumatic tap. Characteristic CSF findings in some CNS viral infections are described in Table 2, but the absence of those findings does not rule out a particular infection if clinical suspicion is high.
Other—ultrasound
Zika virus infection in adults is atypically associated with CNS disease. In unborn children, ultrasound, while not highly sensitive, may detect several things in addition to microcephaly including an absent corpus callosum, cerebral calcifications, ventricular dilatation, brain atrophy, and other abnormalities. These may be detected as early as 18 to 20 weeks, although may not occur until later in pregnancy.24,26,58
Molecular diagnostics
Diagnosis of viral infections of the CNS has been revolutionized by the advent of new molecular diagnostic technologies to amplify viral nucleic acid from CSF, most notably PCR. Detection of viral PCR in CSF has emerged as the preferred method of diagnosis of most viral meningitis cases. High sensitivity and specificity of such testing, especially for HSV CNS infections, and rapid availability of results are driving forces in making CSF viral PCR testing the preferred method. The HSV CSF PCR test has become the gold standard for the diagnosis of HSV meningitis and encephalitis with specificity around 98% and sensitivity around 94% to 100%.59 The HSV CSF PCR test is usually positive early in the course of the infection (within first 24 hours) and remains positive during the first week of treatment.60 Regardless of the high specificity and sensitivity of HSV CSF PCR, results of the test should be correlated clinically. In patients with high clinical suspicion of HSV CNS infection, CSF PCR should be repeated if negative and performed very early in the course of illness.61 Although antiviral therapy lasting less than 1 week has no effect on detection of HSV DNA in CSF, therapy lasting more than 1 week can significantly affect the result, with only 47% of specimens from patients who received 8 to 14 days of antiviral therapy remained positive for HSV PCR.62
Although EVs (coxsackie A and B, echoviruses, poliovi-ruses) rarely cause encephalitis, unlike HSV and arboviruses, they predominate as a cause of viral meningitis, with increasing frequency during summer and fall. Enterovirus PCR has sensitivity and specificity estimated at >95%.63 Although most of the EV infections are self-limiting, early detection of EV CSF PCR has a significant positive impact on hospital costs, leading to a reduction in unnecessary use of antimicrobials and imaging.64,65 Although less readily available, detecting CSF PCR is also an extremely useful test for the diagnosis of other herpes viruses, including CMV, HHV-6, VZV, and EBV. The CMV CSF PCR test has a sensitivity and specificity of >90% and it is a specific indicator of CNS disease, including retinitis.61 Epstein-Barr virus is associated with primary CNS lymphoma and AIDS-related non-Hodgkin lymphoma with CNS involvement in patients with AIDS. The sensitivity and specificity of positive EBV CSF PCR in patients with those malignancies are 100% and 98.5%, respectively.66 The CSF PCR test of JC virus, the etiologic pathogen in PML, has sensitivity of 50% to 75% and specificity of 100% for the diagnosis of PML in HIV-infected patients presenting with neurologic signs and symptoms.67 Unlike all viruses mentioned thus far, WNV CSF PCR is not a particularly sensitive test to rule out CNS infection because sensitivity is <60% in immunocompetent patients with CNS disease.68 Diagnosis of WNV CNS disease is mainly based on serology, including detection of IgM antibodies in CSF. Cerebrospinal fluid viral PCR testing is much less readily available for arboviruses other than WNV. Currently, the primary diagnostic methodology for ZIKV is serum PCR and detection of IgM antibodies. PCR testing has been most successful within 1 week of clinical illness, whereas viral RNA can be detected for weeks to months after infection in pregnant woman.24 Serology is also the choice method for adenovirus, measles, and BK virus–related CNS infections diagnosis. Usefulness of such testing for these viruses is mostly unproven.
Overview of Antiviral Agents
Acyclovir and valacyclovir
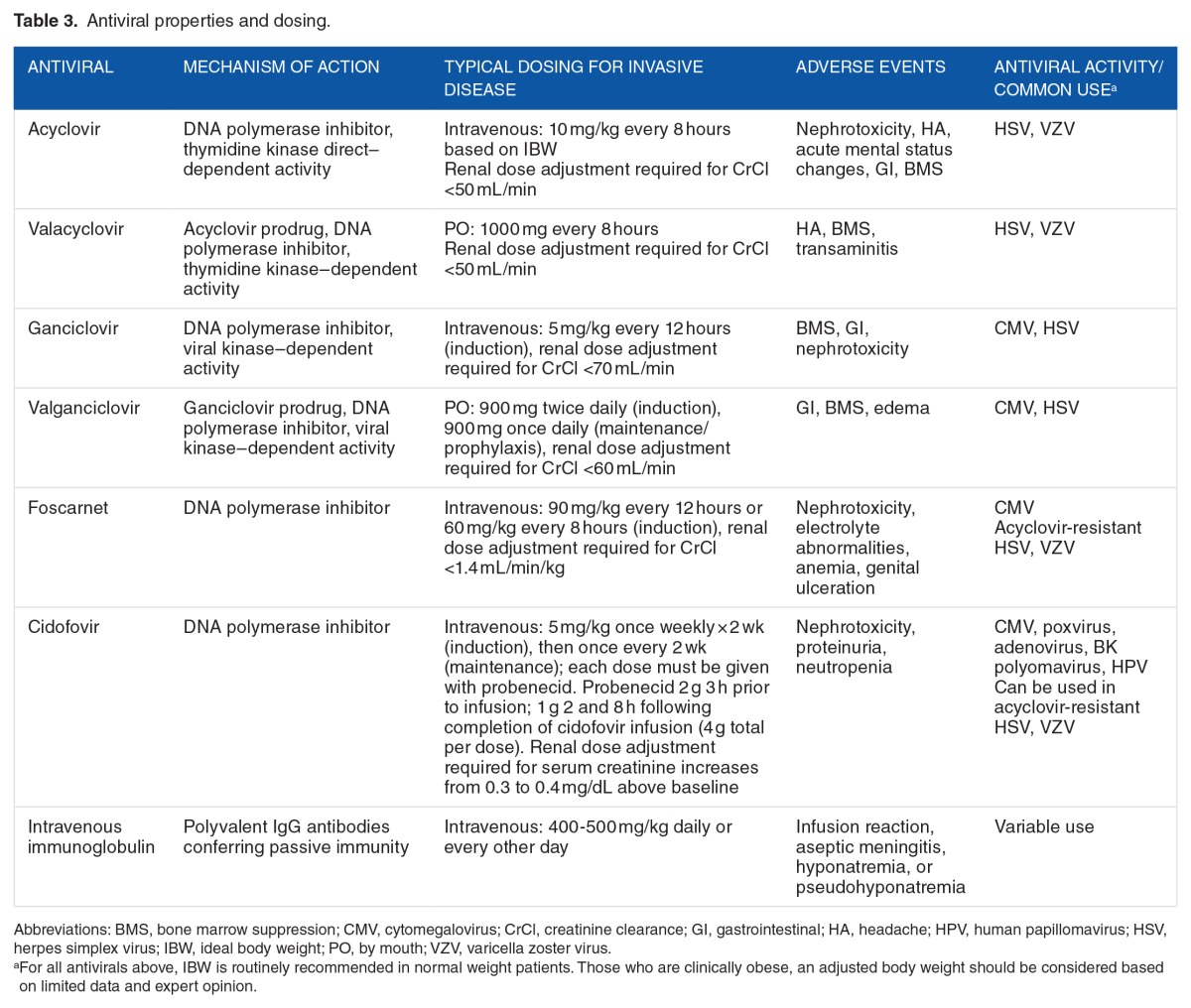
The antiviral acyclovir can be used in the treatment of HSV and VZV and is activated by the viral enzyme, thymidine kinase, present in essentially all strains of HSV. Acyclovir is typically dosed at 10 to 15 mg/kg intravenous every 8 hours based on ideal body weight (IBW), although this recommendation is based on very limited data.69 Adjustment due to renal insufficiency is required. Table 3 discusses antiviral properties and dosing. Commonly associated toxicities include nephrotoxicity, and less frequently, headache, gastrointestinal effects, altered mental status, or bone marrow suppression. The mechanism of nephrotoxicity is thought to be obstructive secondary to crystallization of acyclovir in the renal tubules exceeding maximum solubility. Intravenous hydration is an important mechanism of prevention and should be initiated in critically ill patients. Rapid administration may also contribute to the development of acute kidney injury.70
Table 3.
Antiviral properties and dosing.
Oral valacyclovir, the prodrug l-valyl ester of acyclovir, possesses excellent bioavailability and at doses of 1000 mg every 8 hours has demonstrated sustained CSF concentrations above target over a 20-day treatment period.71,72 Although treatment data are limited, in cases where intravenous access or intravenous acyclovir is unavailable, this may be a potential option. Rarely, HSV exhibits tolerance or resistance to acyclovir, primarily due to deficiency or mutations in thymidine kinase. In these cases, alternative antiviral therapies including foscarnet or less commonly cidofovir (discussed below) should be considered.
Ganciclovir and valganciclovir
Ganciclovir requires triphosphorylation to a substrate that competitively inhibits viral DNA synthesis by inhibiting the binding of deoxyguanosine triphosphate to DNA polymerase. The first phosphorylation is known as the rate-limiting step and is induced by enzymes produced by CMV. Acyclovir, in contrast, is inactivated by CMV. Although relatively uncommon, ganciclovir resistance can be incurred through mutations around in protein UL97 responsible for phosphorylation. Ganciclovir should not be used in suspected acyclovir resistance or clinical failures.73 Recommended ganciclovir dosing is 5 mg/kg intravenous every 12 hours. The oral prodrug of ganciclovir, valganciclovir, is rapidly converted to ganciclovir and is approximately 60% bioavailable. Cerebrospinal fluid concentrations (calculated as ratio of AUCs in CSF to plasma) of ganciclovir in nonhuman primates were approximately 15.5% of plasma.74 In limited case reports, CSF penetration has been demonstrated following both intravenous ganciclovir and oral valganciclovir.75–77 In a single case study, brain extracellular fluid concentrations were approximately 50% of serum following a single 900 mg oral valganciclovir dose.78 Both agents require renal dose adjustment and close monitoring of renal function. Ganciclovir causes numerous adverse effects with hematologic effects including neutropenia, thrombocytopenia, and anemia being most prominent. It may also cause fever, rash, diarrhea, and less commonly, neurological impairment. Adverse events are dose dependent and may be mitigated with adjunctive therapies. Drug-drug interactions, while uncommon, may be significant especially with concomitant use of zidovudine, tenofovir, or tacrolimus. Although the optimal body weight dosing for ganciclovir is unknown, it is suggested that an IBW be used in most individuals or consider an adjusted body weight in obese individuals.79 Significant inter-patient variability in serum concentrations has been noted, especially in solid organ transplant patients, primarily driven by changes in creatinine clearance.80
Foscarnet
Foscarnet is a pyrophosphate analogue that acts as a noncompetitive inhibitor of many viral RNA and DNA polymerases as well as HIV reverse transcriptase. Typical dosing strategies employed include 90 mg/kg intravenous twice daily or 60 mg/kg intravenous 3 times daily. Ideal body weight should be used for most individuals.81 The appropriate dosing weight for obese patients is unknown; however, IBW or an adjusted body weight is recommended.79 Foscarnet is a highly toxic agent causing considerable nephrotoxicity and electrolyte disturbances (hypomagnesemia, hypokalemia, hypocalcemia). Aggressive hydration is used to decrease renal toxicity, and appropriate dose adjustments must be made in patients with renal dysfunction. Other documented adverse events are genital ulcers, dysuria, nausea, and paresthesia. An infusion pump, at a rate not to exceed 1 mg/kg/min, is necessary for administration.81 Foscarnet does not require phosphorylation to be active and therefore can treat ganciclovir-resistant isolates.82
Cidofovir
Cidofovir acts through inhibition of viral DNA synthesis by incorporation of cidofovir into replicating viral DNA. Infusion times should be greater than 1 hour with 1 L of intravenous 0.9% normal saline administered prior to cidofovir infusion.83 A second liter may be administered over a 1- to 3-hour period immediately following infusion, if tolerated. Serum creatinine must be monitored for dose adjustments and contraindications to cidofovir include SCr values >1.5 mg/dL, creatinine clearance <55 mL/min, history of clinically severe hypersensitivity to probenecid, or other sulfa-containing medications and use of nephrotoxic agents within 7 days. Dosing is 5 mg/kg once weekly × 2 weeks (induction), then once every 2 weeks (maintenance) with coadministration of probenecid. Although renal toxicity is the primary adverse effect of cidofovir administration, gastrointestinal, hematologic (black boxed warning for neutropenia), and CNS effects have been reported.83,84 Routine therapeutic drug monitoring is unavailable for all of these antiviral agents.
Management of CNS Viral Disease
Herpes viruses
Herpes simplex virus
Acyclovir is the only US Food and Drug Administration (FDA)–approved therapy for the treatment of HSE.85 Empiric use of acyclovir at a dose of 10 mg/kg intravenous every 8 hours is recommended in all presumed cases of HSE. Because acyclovir is only effective in halting viral replication, therapy should be initiated as quickly as possible after HSE is suspected. Whitley and colleagues demonstrated that acyclovir was the treatment of choice for HSE when they compared it with vidarabine, a purine nucleoside, in 208 patients who underwent brain biopsy for presumptive HSE. Vidarabine was dosed at 15 mg/kg/d, and acyclovir was dosed at 30 mg/kg/d. Both therapies were given for a total of 10 days. Overall, 69 patients had proven disease. Thirty-seven (54%) of the proven disease patients received vidarabine and 32 (46%) of the proven disease patients received acyclovir. Mortality was significantly reduced in the acyclovir-treated patients (28% vs 54%, P = .008), and a higher percentage of patients who received acyclovir were also functioning normally at 6 months as compared with those patients who received vidarabine (38% vs 14%, P = .021).86 Even though this study and others only treated HSE for 10 days, longer durations of therapy (usually 14–21 days) are recommended given the potential for relapses with shorter durations of treatment.87
Valacyclovir has been studied as an alternative oral first-line treatment for patients with HSE. Pouplin and colleagues reported the use of valacyclovir 1 g 3 times daily for a total of 21 days. Four patients with a positive PCR for HSV-1 received the full 21-day course of valacyclovir. All patients had a negative PCR at day 10.72 Because 60% of HSE survivors have neuropsychological sequelae in a year, valacyclovir has also been studied as extended antiviral therapy to improve neuropsychological outcomes. Gnann and colleagues studied the use of valacyclovir (2 g 3 times daily) after the completion of a standard course of intravenous acyclovir in 87 patients with HSE. Of the 87 patients, 40 patients received valacyclovir, whereas 47 patients received placebo. At 12 months, there was no significant difference in survival with no or mild neuropsychological impairment, the primary endpoint, as measured by the Mattis Dementia Rating Scale (85.7% vs 90.2% for valacyclovir and placebo, respectively, P = .72).71 Further studies with larger patient populations are needed before valacyclovir can be routinely recommended as first-line treatment or additive therapy.
Adjunct therapy with corticosteroids has been investigated by Kamei and colleagues in a nonrandomized, retrospective study of 45 patients treated with acyclovir. Twenty-two patients received corticosteroids. Eighteen (82%) of these patients received dexamethasone, whereas 4 of these patients (18%) received prednisolone. Corticosteroid therapy was started at the same time as the initiation of acyclovir treatment. The average dose was 64.6 mg/d of prednisolone equivalent, and the average duration of corticosteroid treatment was 13.6 days (range of 2 days-6 weeks). Corticosteroid administration was associated with a higher odds of a good outcome (defined as normal and mild sequelae) (OR: 8.96, 95% confidence interval: 1.13–70.99, P = .038). Corticosteroid therapy administration is not routinely recommended; however, more prospective, randomized studies are required to confirm benefit.3 A large, multi-center, randomized trial comparing acyclovir alone and acyclovir plus dexamethasone initiated on patient admission is currently ongoing and may provide more clarity on the use of corticosteroids for HSE.88
Cytomegalovirus
The antivirals intravenous ganciclovir, intravenous foscarnet, or oral valganciclovir are all potential options for management of CMV disease. Use of valganciclovir in encephalitis has not been studied specifically, but administration in patients with glioblastoma multiforme has shown improved survival.88,89 The management of CMV encephalitis is based primarily on published data from the pre-highly active antiretroviral therapy (HAART) era in HIV-infected patients.90 Thirty-one patients with either CMV encephalitis (n = 17) or CMV myelitis (n = 14) received induction therapy with foscarnet 90 mg/kg plus ganciclovir 5 mg/kg twice daily and were then transitioned to maintenance therapy. Twenty-three patients (74%) demonstrated stabilization or clinical improvement following a median of 41 days of induction therapy. Of the 23 patients who received maintenance therapy, 10 had CMV disease progression with a median time of relapse of 126 days. Maintenance therapy was given as combination therapy in 13 patients and primarily monotherapy in 10 patients for a median duration of 74 days. Safety and tolerability are concerns with the use of ganciclovir and foscarnet, especially in combination. Approximately, one-third of the patients discontinued at least 1 drug during the induction phase in the study.90 Other case reports and series have demonstrated mixed results with dual therapy and monotherapy.
Based on the available data, most experts recommend combination therapy (foscarnet plus ganciclovir) in CMV CNS disease especially during the induction phase of therapy.3 Continuation of dual therapy beyond the induction phase should be individualized based on clinical response, tolerability, and feasibility. Oral valganciclovir may be considered in specific situations.
Varicella zoster virus
Recommended management of invasive VZV disease is intravenous acyclovir 10 to 15 mg/kg every 8 hours (Table 3).3,91 Compared with HSV, increased doses of acyclovir may be required as higher concentrations against VZV are needed for some strains. There are limited controlled trials supporting this recommendation; however, reduction in disease severity and recovery time has been demonstrated. Therapy should not be delayed due to lack of confirmed diagnostics from CSF if suspicion of VZV is high. Many patients will have concomitant immunocompromising states (eg, malignancy and HIV) that will require management of these conditions. Toxicities associated with acyclovir, which are often dose dependent, remain significant and may be exacerbated in immunocompromised patients with underlying renal dysfunction. The duration of therapy should be 14 days; however, 21 days should be strongly considered in patients with underlying immunocompromise.36,92,93 Use of oral agents such as valacyclovir has not been studied and cannot be recommended at this time. Because of the accompanying vasculitis, adjunct corticosteroids are recommended by many experts. A prednisone equivalent dose of 1 mg/kg daily should be considered. Limited use in varicella pneumonia demonstrated some beneficial effects. No definite duration for prednisone has been established, although some have recommended 3 to 5 days of therapy.3,94,95 Short course therapy may still be associated with significant ADEs and should be evaluated and managed as required. In patients with VZV optic disease, specifically progressive ocular retinal necrosis, acyclovir monotherapy has produced suboptimal results. Ganciclovir or foscarnet, or in combination, should be considered first-line therapy. Dosage is consistent with other invasive viral diseases (Table 3). Because of the immunocompromised state of many of these patients, specialist management including infectious diseases consultation is recommended. Although many patients recover from the VZV infection itself, full recovery is highly dependent on manifestations of disease (eg, stroke) and the immune status of the patient.96
Epstein-Barr virus
Management of EBV-infected patients is targeted at restoration of T-cell (and B-cell) immune function and supportive care. Antiviral therapy, including acyclovir, exhibits activity in vitro; however, clinical trials have failed to show morbidity or mortality benefit.97,98 This may be due to lack of phosphorylation, and thus activation, of the antivirals by viral enzymes. In addition, failure to concentrate in circulating infected B lymphocytes and inability to target the virus in the latent state may also be contributing factors.97 Corticosteroids are used in the acute phase of these infections by some experts, although clinical data to support outcomes are lacking.3
Enterovirus
There is no conclusive evidence of effectiveness of pharmacologic interventions for enteroviral CNS diseases, aside from symptomatic care.17 Through limited investigations, antivirals have shown some benefits.17,99 Ribavirin specifically has shown promise in animal models against EV 71 with no published data in patients with CNS disease.100,101 The compound pleconaril, which was rejected by the FDA in 2011, has been shown to alter the course of enteroviral disease. Although it does cross the blood-CNS barrier, it has limited study in CNS disease and is currently unavailable.102,103 Intravenous immunoglobulin (IVIG) has been used in some outbreaks, although correlation with successful outcomes is unknown.104 Mortality rates vary depending on vulnerability of the infected host and viral serotype but may be as high as 10%. Other therapeutic interventions such as convalescent serum have been tried, but there is no conclusive evidence to support benefit at this time.
Arboviruses
Symptomatic care is the hallmark of treatment for arbovirus infections with no active antiviral therapies available. In some instances, IVIG has been used on a limited basis with mixed results. In a pediatric population in Nepal with JEV, IVIG at 400 mg/kg daily for 5 days resulted in higher antibodies and interleukin (IL)-4 and IL-6 concentrations in treated patients compared with those receiving standard of care.105 Clinical outcomes, however, remained the same in both the groups. Sporadic case reports demonstrate mixed results on the effectiveness of IVIG in WNV encephalitis.106 Immunoglobulin lots obtained from endemic areas are likely to have higher viral titers specific to many of these viral infections and potentially enhanced effects. In viral encephalitis, some experts hypothesize using intrathecal or intraventricular administration to enhance antibody exposure across the blood-CSF barrier. Although IVIG may be considered in many of the flavivirus infections with progression despite aggressive symptomatic care, caution with untoward effects is prudent. The optimal dosing and route of administration in suspected CNS infection are unknown.
Dengue virus
Management of severe dengue fever is primarily symptom care and focused on appropriate fluid balance. Repeated boluses may be needed in severe plasma leak; however, maintenance fluids should be carefully balanced and adjusted per patient requirement.23,107 Further management of resultant hypotension beyond intravenous hydration (IVF) may be required in rare situations. Electrolyte shifts are also common. Antibiotics are not indicated unless a secondary bacterial infection occurs. The mortality rate is <1%. Prevention is a primary focus with the lack of antiviral therapies.
Zika virus
Symptomatic management of patients with ZIKV infection is currently the only therapeutic option available.24 Patients often have generalized viral syndromes with headache, fatigue, and lethargy, which can be managed with appropriate fluid balance, antipyretics, and nonsteroidal anti-inflammatory agents. Managing neonates with microcephaly, or what is now referred to as the congenital ZIKV syndrome, is complex and beyond the scope of this review. Interim guidance is offered by the World Health Organization.108
Other zoonotic infections
Rabies virus
Early presentation following a bite but prior to onset of symptoms will trigger a proactive response to determine necessary prophylaxis. Animal testing can be done quickly using direct fluorescent antibody testing on the brain tissue. If it is determined that a high-risk exposure has occurred, the previously unvaccinated patient will receive a single dose of human rabies immunoglobulin (HRIG) infiltrated into the wound and surrounding areas.27,109 Patients will also receive a 4-dose series of rabies vaccine with the first dose beginning the same day with subsequent doses on days 3, 7, and 14. The vaccine should be administered intramuscular in the deltoid area at a site distant from the HRIG. If the entire volume of the HRIG cannot be administered local to the bite, the remaining volume can be administered intramuscular at a site distant from the vaccine. This is to reduce the potential inactivation of the rabies vaccine. Patients who were previously vaccinated should receive 2 doses of the vaccine, but HRIG is not indicated.27
Among the 3 survivors known to date who did not receive postexposure prophylaxis, a 15-year-old girl who survived was placed in a therapeutic coma with intravenous midazolam and supplemental phenobarbital for a burst suppression pattern on electroencephalogram. In addition, she was maintained on continuous infusion ketamine and provided antiviral therapy with ribavirin and amantadine. This protocol, based on very limited evidence, has been labeled the “Milwaukee protocol.”27,109,110 Despite success in this patient, at least 20 failures have been documented using the similar approach since its publication.109 Although these agents are under investigation, there is no evidence currently to suggest this pharmacologic approach promotes clearance of rabies virus or resolution of symptoms.
CNS viral disease drug pipeline
There are multiple drugs or vaccines in all stages of development to prevent a variety of viral infections. Development focuses heavily on vaccines rather than acute treatments. The focus here is on antivirals indicated for treatment of active infection in phase 2 or 3 of US development as of July 2016 that could potentially be used for CNS infections given the ability to penetrate the blood-brain or -CSF barrier or be injected via the intrathecal route. Each is identified on the sponsor’s Web site.
Brincidofovir (CMX001) is a phase 3, orally administered lipid conjugate of cidofovir, administered twice weekly for 12 weeks, being developed by Chimerix for the treatment of adenovirus infection in immunocompromised, pediatric, and adult patients. Due to its lipophilic nature, it is believed to be able to cross cell membranes by passive diffusion.111 There is also a phase 2 investigator-sponsored study, although not yet recruiting, that aims to identify whether brincidofovir can be used to treat infants with neonatal HSV involving the CNS. Brincidofovir has received fast-track designation from the FDA for CMV, adenovirus, and smallpox.112
Contravir is developing FV-100, a once-daily (400 mg) oral therapy for the treatment of VZV. The duration of therapy is 7 days. Potential advantages of FV-100 over currently marketed shingles therapies are its once daily dosing, higher specificity for VZV, and no renal dose adjustment needed.113
Globavir is in phase 2 development of GBV006 for the treatment of Ebola. It has also shown in vivo activity against Dengue virus.114
Epiphany Biosciences is developing valomaciclovir (EPB-348), an oral drug with broad-spectrum antiviral activity against HSV, EBV, and VZV.115
An interrogation of FDA-approved compounds and their potential anti-ZIKV activity resulted approximately 20 compounds of 774 tested, some with no previously known antiviral activity, that demonstrated inhibitory effects in cervical, placental, and neural stem cell lines. This may provide an avenue for future clinical studies.116 Candidate vaccines are currently in development, with at least 1 entering early clinical trials as of this publication.117
Although there are a number of agents available for the acute treatment of CNS viral infections, there are still significant needs for antiviral therapy specifically related to arboviruses for which there is no currently available active antiviral agent.
Acknowledgments
The authors acknowledge Jason Lockhart, PharmD Candidate, and Chelsea Campbell, PharmD Candidate, for their assistance in referencing and editing. The views expressed are those of the authors and do not necessarily represent the views of the FDA or the Federal Government.
Footnotes
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 720 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
All authors had shared responsibilities for searching the literature, drafting the manuscript and editing for final submission.
REFERENCES
- 1.Young NP, Weinshenker BG, Lucchinetti CF. Acute disseminated encephalomyelitis: current understanding and controversies. Semin Neurol. 2008;28:84–94. doi: 10.1055/s-2007-1019130. [DOI] [PubMed] [Google Scholar]
- 2.Sejvar J. Neuroepidemiology and the epidemiology of viral infections of the nervous system. Handb Clin Neurol. 2014;123:67–87. doi: 10.1016/B978-0-444-53488-0.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–327. doi: 10.1086/589747. [DOI] [PubMed] [Google Scholar]
- 4.Olson LC, Buescher EL, Artenstein MS, Parkman PD. Herpesvirus infections of the human central nervous system. N Engl J Med. 1967;277:1271–1277. doi: 10.1056/NEJM196712142772401. [DOI] [PubMed] [Google Scholar]
- 5.Whitley RJ, Lakeman F. Herpes simplex virus infections of the central nervous system: therapeutic and diagnostic considerations. Clin Infect Dis. 1995;20:414–420. doi: 10.1093/clinids/20.2.414. [DOI] [PubMed] [Google Scholar]
- 6.Moon SM, Kim T, Lee EM, Kang JK, Lee SA, Choi SH. Comparison of clinical manifestations, outcomes and cerebrospinal fluid findings between herpes simplex type 1 and type 2 central nervous system infections in adults. J Med Virol. 2014;86:1766–1771. doi: 10.1002/jmv.23999. [DOI] [PubMed] [Google Scholar]
- 7.Kaewpoowat Q, Salazar L, Aguilera E, Wootton SH, Hasbun R. Herpes simplex and varicella zoster CNS infections: clinical presentations, treatments and outcomes. Infection. 2016;44:337–345. doi: 10.1007/s15010-015-0867-6. [DOI] [PubMed] [Google Scholar]
- 8.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 9.de Ory F, Avellon A, Echevarria JE, et al. Viral infections of the central nervous system in Spain: a prospective study. J Med Virol. 2013;85:554–562. doi: 10.1002/jmv.23470. [DOI] [PubMed] [Google Scholar]
- 10.Mailles A, Stahl JP, Steering Committee and Investigators Group Infectious encephalitis in France in 2007: a national prospective study. Clin Infect Dis. 2009;49:1838–1847. doi: 10.1086/648419. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention . Varicella. In: Hamborsky J, Kroger A, Wolfe S, editors. Epidemiology and Prevention of Vaccine-Preventable Diseases. 13th ed. Washington, DC: Public Health Foundation; 2015. [Google Scholar]
- 12.Centers for Disease Control and Prevention Cytomegalovirus (CMV) and Congenital CMV Infection. 2016. http://www.cdc.gov/cmv/overview.html.
- 13.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 14.Parisi SG, Basso M, Del Vecchio C, et al. Viral infections of the central nervous system in elderly patients: a retrospective study. Int J Infect Dis. 2016;44:8–10. doi: 10.1016/j.ijid.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am. 2011;25:151–169. doi: 10.1016/j.hoc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duniewicz M, Lasovska J, Kouba K, et al. Central nervous system involvement in infectious mononucleosis with studies for Epstein-Barr virus. Infection. 1976;4:55–57. doi: 10.1007/BF01638352. [DOI] [PubMed] [Google Scholar]
- 17.Jain S, Patel B, Bhatt GC. Enteroviral encephalitis in children: clinical features, pathophysiology, and treatment advances. Pathog Glob Health. 2014;108:216–222. doi: 10.1179/2047773214Y.0000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention Enterovirus D68. 2016. https://www.cdc.gov/non-polio-enterovirus/hcp.html.
- 19.Greninger AL, Naccache SN, Messacar K, et al. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012–14) a retrospective cohort study. Lancet Infect Dis. 2015;15:671–682. doi: 10.1016/S1473-3099(15)70093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan F. Enterovirus D68. Emerg Med Clin N Am. 2015;33:e19–e32. doi: 10.1016/j.emc.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Japanese Encephalitis Virus. 2015. http://www.cdc.gov/japaneseencephalitis/
- 22.Centers for Disease Control and Prevention West Nile Virus. 2015. http://www.cdc.gov/westnile/index.html.
- 23.Halstead SB, Cohen SN. Dengue hemorrhagic fever at 60 years: early evolution of concepts of causation and treatment. Microbiol Mol Biol Rev. 2015;79:281–291. doi: 10.1128/MMBR.00009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 25.Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicastri E, Castilletti C, Balestra P, Galgani S, Ippolito G. Zika virus infection in the central nervous system and female genital tract. Emerg Infect Dis. 2016;22:2228–2230. doi: 10.3201/eid2212.161280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Rabies. 2016. http://www.cdc.gov/rabies/index.html.
- 28.Kawamura N, Kizawa M, Ueda A, Niimi Y, Mutoh T. An update on diagnostic imaging studies for viral encephalitis. Future Virol. 2012;7:901–909. [Google Scholar]
- 29.Maschke M, Kastrup O, Forsting M, Diener HC. Update on neuroimaging in infectious central nervous system disease. Curr Opin Neurol. 2004;17:475–480. doi: 10.1097/01.wco.0000137540.29857.bf. [DOI] [PubMed] [Google Scholar]
- 30.Teixeira J, Zimmerman RA, Haselgrove JC, Bilaniuk LT, Hunter JV. Diffusion imaging in pediatric central nervous system infections. Neuroradiology. 2001;43:1031–1039. doi: 10.1007/s002340100625. [DOI] [PubMed] [Google Scholar]
- 31.Prakash M, Kumar S, Gupta RK. Diffusion-weighted MR imaging in Japanese encephalitis. J Comput Assist Tomogr. 2004;28:756–761. doi: 10.1097/00004728-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Bulakbasi N, Kocaoglu M. Central nervous system infections of herpesvirus family. Neuroimaging Clin N Am. 2008;18:53–84. viii. doi: 10.1016/j.nic.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Shah R, Bag AK, Chapman PR, Cure JK. Imaging manifestations of progressive multifocal leukoencephalopathy. Clin Radiol. 2010;65:431–439. doi: 10.1016/j.crad.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Buckle C, Castillo M. Use of diffusion-weighted imaging to evaluate the initial response of progressive multifocal leukoencephalopathy to highly active antiretroviral therapy: early experience. AJNR Am J Neuroradiol. 2010;31:1031–1035. doi: 10.3174/ajnr.A2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garrels K, Kucharczyk W, Wortzman G, Shandling M. Progressive multifocal leukoencephalopathy: clinical and MR response to treatment. AJNR Am J Neuroradiol. 1996;17:597–600. [PMC free article] [PubMed] [Google Scholar]
- 36.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vascu-lopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8:731–740. doi: 10.1016/S1474-4422(09)70134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilden DH. Brain imaging abnormalities in CNS virus infections. Neurology. 2008;70:84. doi: 10.1212/01.wnl.0000286937.09760.e4. [DOI] [PubMed] [Google Scholar]
- 38.Smith AB, Smirniotopoulos JG, Rushing EJ. From the archives of the AFIP: central nervous system infections associated with human immunodeficiency virus infection: radiologic-pathologic correlation. Radiographics. 2008;28:2033–2058. doi: 10.1148/rg.287085135. [DOI] [PubMed] [Google Scholar]
- 39.Kumar S, Misra UK, Kalita J, Salwani V, Gupta RK, Gujral R. MRI in Japanese encephalitis. Neuroradiology. 1997;39:180–184. doi: 10.1007/s002340050388. [DOI] [PubMed] [Google Scholar]
- 40.Handique SK, Barkataky N. MR imaging in biphasic Japanese encephalitis. AJNR Am J Neuroradiol. 2008;29:E3. doi: 10.3174/ajnr.A0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehra S. Role of imaging in herpes and Japanese encephalitis—two cases and review of literature. J Indian Acad Clin Med. 2012;13:338–343. [Google Scholar]
- 42.Zak IT, Altinok D, Merline JR, Chander S, Kish KK. West Nile virus infection. AJR Am J Roentgenol. 2005;184:957–961. doi: 10.2214/ajr.184.3.01840957. [DOI] [PubMed] [Google Scholar]
- 43.Olsan AD, Milburn JM, Baumgarten KL, Durham HL. Leptomeningeal enhancement in a patient with proven West Nile virus infection. AJR Am J Roentgenol. 2003;181:591–592. doi: 10.2214/ajr.181.2.1810591. [DOI] [PubMed] [Google Scholar]
- 44.Jeha LE, Sila CA, Lederman RJ, Prayson RA, Isada CM, Gordon SM. West Nile virus infection: a new acute paralytic illness. Neurology. 2003;61:55–59. doi: 10.1212/01.wnl.0000073617.08185.0a. [DOI] [PubMed] [Google Scholar]
- 45.Ohry A, Karpin H, Yoeli D, Lazari A, Lerman Y. West Nile virus myelitis. Spinal Cord. 2001;39:662–663. doi: 10.1038/sj.sc.3101228. [DOI] [PubMed] [Google Scholar]
- 46.Vidwan G, Bryant KK, Puri V, Stover BH, Rabalais GP. West Nile virus encephalitis in a child with left-side weakness. Clin Infect Dis. 2003;37:e91–e94. doi: 10.1086/377263. [DOI] [PubMed] [Google Scholar]
- 47.Ketelslegers IA, Visser IE, Neuteboom RF, Boon M, Catsman-Berrevoets CE, Hintzen RQ. Disease course and outcome of acute disseminated encephalomyelitis is more severe in adults than in children. Mult Scler. 2011;17:441–448. doi: 10.1177/1352458510390068. [DOI] [PubMed] [Google Scholar]
- 48.Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B. Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology. 2001;56:1313–1318. doi: 10.1212/wnl.56.10.1313. [DOI] [PubMed] [Google Scholar]
- 49.Balasubramanya KS, Kovoor JM, Jayakumar PN, et al. Diffusion-weighted imaging and proton MR spectroscopy in the characterization of acute disseminated encephalomyelitis. Neuroradiology. 2007;49:177–183. doi: 10.1007/s00234-006-0164-2. [DOI] [PubMed] [Google Scholar]
- 50.Atlas SW, Grossman RI, Goldberg HI, Hackney DB, Bilaniuk LT, Zimmerman RA. MR diagnosis of acute disseminated encephalomyelitis. J Comput Assist Tomogr. 1986;10:798–801. doi: 10.1097/00004728-198609000-00017. [DOI] [PubMed] [Google Scholar]
- 51.Singh S, Alexander M, Korah IP. Acute disseminated encephalomyelitis: MR imaging features. AJR Am J Roentgenol. 1999;173:1101–1107. doi: 10.2214/ajr.173.4.10511187. [DOI] [PubMed] [Google Scholar]
- 52.van der Knaap MS, Valk J. Acute disseminated encephalomyelitis and acute hemorrhagic encephalomyelitis. In: Marjo S, van der Knaap MS, Valk J, editors. Magnetic Resonance of Myelination and Myelin Disorders. 3rd ed. New York, NY: Springer; 2005. pp. 604–615. [Google Scholar]
- 53.Honkaniemi J, Dastidar P, Kahara V, Haapasalo H. Delayed MR imaging changes in acute disseminated encephalomyelitis. AJNR Am J Neuroradiol. 2001;22:1117–1124. [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta RK, Lufkin RB, editors. MR Imaging and Spectroscopy of Central Nervous System Infection. 1st ed. New York, NY: Springer; 2001. Viral infections; pp. 147–175. [Google Scholar]
- 55.Laubenberger J, Haussinger D, Bayer S, et al. HIV-related metabolic abnormalities in the brain: depiction with proton MR spectroscopy with short echo times. Radiology. 1996;199:805–810. doi: 10.1148/radiology.199.3.8638009. [DOI] [PubMed] [Google Scholar]
- 56.Patel SH, Inglese M, Glosser G, Kolson DL, Grossman RI, Gonen O. Whole-brain N-acetylaspartate level and cognitive performance in HIV infection. AJNR Am J Neuroradiol. 2003;24:1587–1591. [PMC free article] [PubMed] [Google Scholar]
- 57.Iranzo A, Moreno A, Pujol J, et al. Proton magnetic resonance spectroscopy pattern of progressive multifocal leukoencephalopathy in AIDS. J Neurol Neurosurg Psychiatry. 1999;66:520–523. doi: 10.1136/jnnp.66.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jouannic J-M, Friszer S, Leparc-Goffart I, Garel C, Eyrolle-Guignot D. Zika virus infection in French Polynesia. Lancet. 2016;387:1051–1052. doi: 10.1016/S0140-6736(16)00625-5. [DOI] [PubMed] [Google Scholar]
- 59.Tebas P, Nease RF, Storch GA. Use of the polymerase chain reaction in the diagnosis of herpes simplex encephalitis: a decision analysis model. Am J Med. 1998;105:287–295. doi: 10.1016/s0002-9343(98)00259-9. [DOI] [PubMed] [Google Scholar]
- 60.Wildemann B, Ehrhart K, Storch-Hagenlocher B, et al. Quantitation of herpes simplex virus type 1 DNA in cells of cerebrospinal fluid of patients with herpes simplex virus encephalitis. Neurology. 1997;48:1341–1346. doi: 10.1212/wnl.48.5.1341. [DOI] [PubMed] [Google Scholar]
- 61.Weil AA, Glaser CA, Amad Z, Forghani B. Patients with suspected herpes simplex encephalitis: rethinking an initial negative polymerase chain reaction result. Clin Infect Dis. 2002;34:1154–1157. doi: 10.1086/339550. [DOI] [PubMed] [Google Scholar]
- 62.Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis. 1995;171:857–863. doi: 10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- 63.Verstrepen WA, Kuhn S, Kockx MM, Van De Vyvere ME, Mertens AH. Rapid detection of enterovirus RNA in cerebrospinal fluid specimens with a novel single-tube real-time reverse transcription-PCR assay. J Clin Microbiol. 2001;39:4093–4096. doi: 10.1128/JCM.39.11.4093-4096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramers C, Billman G, Hartin M, Ho S, Sawyer MH. Impact of a diagnostic cerebrospinal fluid enterovirus polymerase chain reaction test on patient management. JAMA. 2000;283:2680–2685. doi: 10.1001/jama.283.20.2680. [DOI] [PubMed] [Google Scholar]
- 65.Marshall GS, Hauck MA, Buck G, Rabalais GP. Potential cost savings through rapid diagnosis of enteroviral meningitis. Pediatr Infect Dis J. 1997;16:1086–1087. doi: 10.1097/00006454-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 66.Tachikawa N, Goto M, Hoshino Y, et al. Detection of Toxoplasma gondii, Epstein-Barr virus, and JC virus DNAs in the cerebrospinal fluid in acquired immunodeficiency syndrome patients with focal central nervous system complications. Intern Med. 1999;38:556–562. doi: 10.2169/internalmedicine.38.556. [DOI] [PubMed] [Google Scholar]
- 67.Hirsch HH, Meylan PR, Zimmerli W, Iten A, Battegay M, Erb P. HIV-1-infected patients with focal neurologic signs: diagnostic role of PCR for Toxoplasma gondii, Epstein-Barr virus, and JC virus. Clin Microbiol Infect. 1998;4:577–584. doi: 10.1111/j.1469-0691.1998.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 68.Petersen LR, Marfin AA. West Nile virus: a primer for the clinician. Ann Intern Med. 2002;137:173–179. doi: 10.7326/0003-4819-137-3-200208060-00009. [DOI] [PubMed] [Google Scholar]
- 69.Davis RL, Quenzer RW, Weller S, et al. Acyclovir pharmacokinetics in morbid obesity; Interscience Conference on Antimicrobial Agents and Chemotherapy; 29 September-2 October, 1991; Chicago, IL. [Google Scholar]
- 70.Hernandez JO, Norstrom J, Wysock G. Acyclovir-induced renal failure in an obese patient. Am J Health Syst Pharm. 2009;66:1288–1291. doi: 10.2146/ajhp080307. [DOI] [PubMed] [Google Scholar]
- 71.Gnann JW, Jr, Skoldenberg B, Hart J, et al. Herpes simplex encephalitis: lack of clinical benefit of long-term valacyclovir therapy. Clin Infect Dis. 2015;61:683–691. doi: 10.1093/cid/civ369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pouplin T, Pouplin JN, Van Toi P, et al. Valacyclovir for herpes simplex encephalitis. Antimicrob Agents Chemother. 2011;55:3624–3626. doi: 10.1128/AAC.01023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.James SH, Prichard MN. Current and future therapies for herpes simplex virus infections: mechanism of action and drug resistance. Curr Opin Virol. 2014;8:54–61. doi: 10.1016/j.coviro.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Serabe BM, Murry DJ, Dauser R, et al. Plasma and CSF pharmacokinetics of ganciclovir in nonhuman primates. Cancer Chemother Pharmacol. 1999;43:415–418. doi: 10.1007/s002800050916. [DOI] [PubMed] [Google Scholar]
- 75.Sakamoto H, Hirano M, Nose K, et al. A case of severe ganciclovir-induced encephalopathy. Case Rep Neurol. 2013;5:183–186. doi: 10.1159/000355638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peyriere H, Jeziorsky E, Jalabert A, et al. Neurotoxicity related to valganciclovir in a child with impaired renal function: usefulness of therapeutic drug monitoring. Ann Pharmacother. 2006;40:143–146. doi: 10.1345/aph.1G214. [DOI] [PubMed] [Google Scholar]
- 77.Natale F, Bizzarri B, Cardi V, et al. Ganciclovir penetrates into the cerebrospinal fluid of an infant with congenital cytomegalovirus infection. Ital J Pediatr. 2015;41:26. doi: 10.1186/s13052-015-0132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peredo I, Hellden A, Wolmer-Solberg N, et al. Ganciclovir concentrations in the cerebral extracellular space after valganciclovir treatment; a case study. BMJ Case Rep. 2015 doi: 10.1136/bcr-2014-207694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Polso AK, Lassiter JL, Nagel JL. Impact of hospital guideline for weight-based antimicrobial dosing in morbidly obese adults and comprehensive literature review. J Clin Pharm Ther. 2014;39:584–608. doi: 10.1111/jcpt.12200. [DOI] [PubMed] [Google Scholar]
- 80.Caldes A, Colom H, Armendariz Y, et al. Population pharmacokinetics of ganciclovir after intravenous ganciclovir and oral valganciclovir administration in solid organ transplant patients infected with cytomegalovirus. Antimicrob Agents Chemother. 2009;53:4816–4824. doi: 10.1128/AAC.00085-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wagstaff AJ, Bryson HM. Foscarnet. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs. 1994;48:199–226. doi: 10.2165/00003495-199448020-00007. [DOI] [PubMed] [Google Scholar]
- 82.Foscavir. 2006. http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/020068s017lbl.pdf.
- 83.Cidofovir approved. PI Perspect. 1996;(19):14–15. [PubMed] [Google Scholar]
- 84.Lea AP, Bryson HM. Cidofovir. Drugs. 1996;52:225–230. doi: 10.2165/00003495-199652020-00006. discussion 31. [DOI] [PubMed] [Google Scholar]
- 85.Zovirax. 2016. http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/18603slr027_zovirax_lbl.pdf.
- 86.Whitley RJ, Alford CA, Hirsch MS, et al. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med. 1986;314:144–149. doi: 10.1056/NEJM198601163140303. [DOI] [PubMed] [Google Scholar]
- 87.VanLandingham KE, Marsteller HB, Ross GW, Hayden FG. Relapse of herpes simplex encephalitis after conventional acyclovir therapy. JAMA. 1988;259:1051–1053. [PubMed] [Google Scholar]
- 88.Martinez-Torres F, Menon S, Pritsch M, et al. Protocol for German trial of acyclovir and corticosteroids in herpes-simplex-virus-encephalitis (GACHE): a multi-center, multinational, randomized, double-blind, placebo-controlled German, Austrian and Dutch trial [ISRCTN45122933] BMC Neurol. 2008;8:40. doi: 10.1186/1471-2377-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soderberg-Naucler C, Rahbar A, Stragliotto G. Survival in patients with glioblastoma receiving valganciclovir. N Engl J Med. 2013;369:985–986. doi: 10.1056/NEJMc1302145. [DOI] [PubMed] [Google Scholar]
- 90.Anduze-Faris BM, Fillet AM, Gozlan J, et al. Induction and maintenance therapy of cytomegalovirus central nervous system infection in HIV-infected patients. AIDS. 2000;14:517–524. doi: 10.1097/00002030-200003310-00007. [DOI] [PubMed] [Google Scholar]
- 91.Poscher ME. Successful treatment of varicella zoster virus meningoencephalitis in patients with AIDS: report of four cases and review. AIDS. 1994;8:1115–1117. [PubMed] [Google Scholar]
- 92.Hagiya H, Kimura M, Miyamoto T, Otsuka F. Systemic varicella-zoster virus infection in two critically ill patients in an intensive care unit. Virol J. 2013;10:225. doi: 10.1186/1743-422X-10-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gilden D. Varicella zoster virus and central nervous system syndromes. Herpes. 2004;11:89A–94A. [PubMed] [Google Scholar]
- 94.Wood MJ, Johnson RW, McKendrick MW, Taylor J, Mandal BK, Crooks J. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. 1994;330:896–900. doi: 10.1056/NEJM199403313301304. [DOI] [PubMed] [Google Scholar]
- 95.Whitley RJ, Weiss H, Gnann JW, Jr, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376–383. doi: 10.7326/0003-4819-125-5-199609010-00004. [DOI] [PubMed] [Google Scholar]
- 96.Nagel MA, Gilden D. Neurological complications of varicella zoster virus reactivation. Curr Opin Neurol. 2014;27:356–360. doi: 10.1097/WCO.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gershburg E, Pagano JS. Epstein-Barr virus infections: prospects for treatment. J Antimicrob Chemother. 2005;56:277–281. doi: 10.1093/jac/dki240. [DOI] [PubMed] [Google Scholar]
- 98.Rafailidis PI, Mavros MN, Kapaskelis A, Falagas ME. Antiviral treatment for severe EBV infections in apparently immunocompetent patients. J Clin Virol. 2010;49:151–157. doi: 10.1016/j.jcv.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 99.Smee DF, Evans WJ, Nicolaou KC, Tarbet EB, Day CW. Susceptibilities of enterovirus D68, enterovirus 71, and rhinovirus 87 strains to various antiviral compounds. Antiviral Res. 2016;131:61–65. doi: 10.1016/j.antiviral.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li ZH, Li CM, Ling P, et al. Ribavirin reduces mortality in enterovirus 71-infected mice by decreasing viral replication. J Infect Dis. 2008;197:854–857. doi: 10.1086/527326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gross AE, Bryson ML. Oral ribavirin for the treatment of noninfluenza respiratory viral infections: a systematic review. Ann Pharmacother. 2015;49:1125–1135. doi: 10.1177/1060028015597449. [DOI] [PubMed] [Google Scholar]
- 102.Webster AD. Pleconaril—an advance in the treatment of enteroviral infection in immuno-compromised patients. J Clin Virol. 2005;32:1–6. doi: 10.1016/j.jcv.2004.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rotbart HA, Webster AD, Pleconaril Treatment Registry Group Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin Infect Dis. 2001;32:228–235. doi: 10.1086/318452. [DOI] [PubMed] [Google Scholar]
- 104.Wang JN, Yao CT, Yeh CN, et al. Critical management in patients with severe enterovirus 71 infection. Pediatr Int. 2006;48:250–256. doi: 10.1111/j.1442-200X.2006.02198.x. [DOI] [PubMed] [Google Scholar]
- 105.Rayamajhi A, Nightingale S, Bhatta NK, et al. A preliminary randomized double blind placebo-controlled trial of intravenous immunoglobulin for Japanese encephalitis in Nepal. PLoS ONE. 2015;10:e0122608. doi: 10.1371/journal.pone.0122608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kramer AH. Viral encephalitis in the ICU. Crit Care Clin. 2013;29:621–649. doi: 10.1016/j.ccc.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 107.Ranjit S, Kissoon N. Dengue hemorrhagic fever and shock syndromes. Pediatr Crit Care Med. 2011;12:90–100. doi: 10.1097/PCC.0b013e3181e911a7. [DOI] [PubMed] [Google Scholar]
- 108.Russell K, Oliver SE, Lewis L, et al. Update: interim guidance for the evaluation and management of infants with possible congenital Zika virus infection—United States, August 2016. MMWR Morb Mortal Wkly Rep. 2016;65:870–878. doi: 10.15585/mmwr.mm6533e2. [DOI] [PubMed] [Google Scholar]
- 109.Jackson AC. Current and future approaches to the therapy of human rabies. Antiviral Res. 2013;99:61–67. doi: 10.1016/j.antiviral.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 110.Caicedo Y, Paez A, Kuzmin I, et al. Virology, immunology and pathology of human rabies during treatment. Pediatr Infect Dis J. 2015;34:520–528. doi: 10.1097/INF.0000000000000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Research & Development. 2015. http://www.chimerix.com/pipeline-technologies/pipeline/
- 112.Chimerix Chimerix completes targeted enrollment for brincidofovir phase 3 AdVise trial for adenovirus infection. 2015. http://ir.chimerix.com/releasede-tail.cfm?releaseid=925188.
- 113.Valnivudine™ (FV-100) 2016. http://contravir.com/fv-100/
- 114.Globavir leverages its data driven platform technology to develop 505(b)(2) candidates for the treatment of cancer and infectious disease. Read more about Globavir’s Drug Discovery Platform (GDDP) 2016. http://www.globavir.com/pipeline.php.
- 115.Advancing therapies for infectious disease. 2009. http://www.epiphanybio.com/pipeline/pipe.html.
- 116.Barrows NJ, Campos RK, Powell ST, et al. A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe. 2016;20:259–270. doi: 10.1016/j.chom.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dawes BE, Smalley CA, Tiner BL, et al. Research and development of Zika virus vaccines. npj Vaccines. 2016;1:16007. doi: 10.1038/npjvaccines.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]