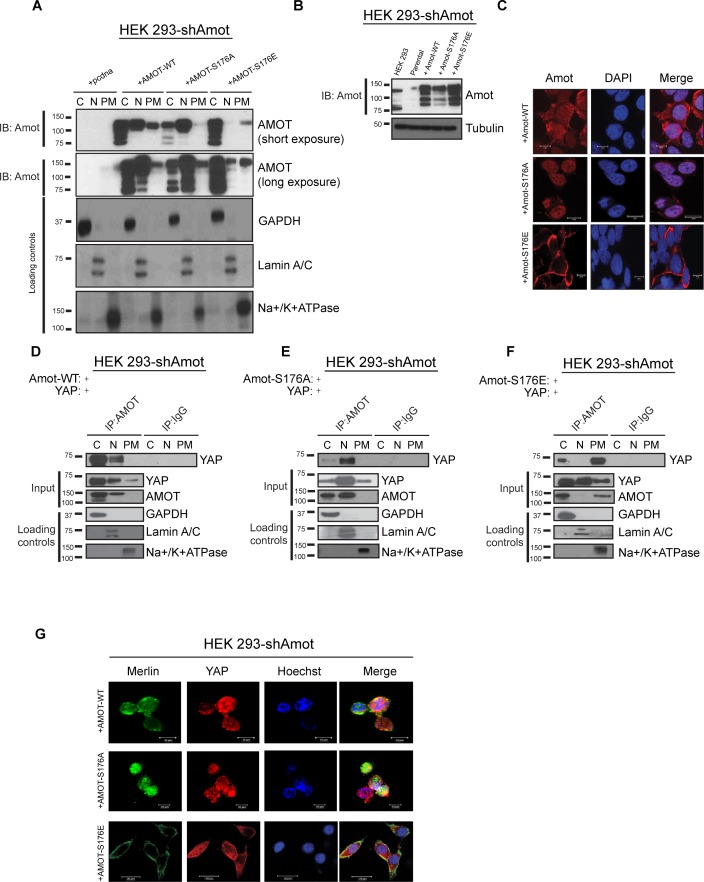
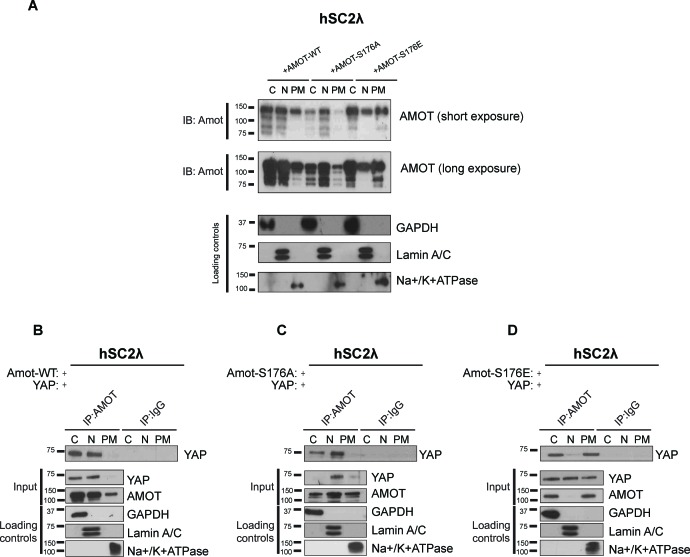
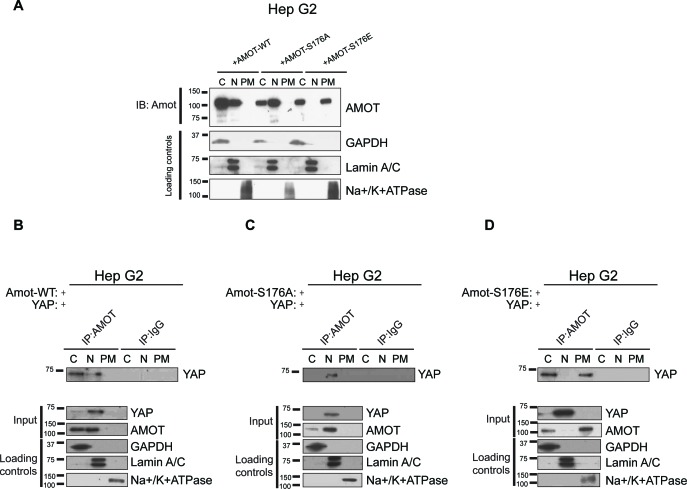
Figure 4. Amot-p130S176 shifts localization of the YAP/Merlin complex.
(A) Phosphorylation of Amot-p130 shifts its localization at the plasma membrane. HEK293-shAmot cells were transfected with Amot-WT, Amot-p130S176A or Amot-p130S176E expression plasmids and fractionated into cytoplasm (C), nuclear (N) and plasma membrane (PM) fractions. Immunoblot analysis was conducted using an anti-Amot antibody. GAPDH, Lamin A/C, and Na+/K+ATPase were using as controls for the cytoplasmic, nuclear, and plasma membrane fractions, respectively. (B) IB analysis was used to verify the transfection efficiency of the indicated constructs in total lysates of HEK293-shAmot cells. Tubulin was used as loading control. Blots shown are representative of three independent biological experiments (n = 3). (C) HEK293-shAmot cells were transfected with Amot-WT, Amot-p130S176A or Amot-p130S176E and subjected to immunofluorescence staining using an antibody against Amot. DAPI was used for nuclei fluorescence staining. Pictures show fields at 63x magnification and are representative of three independent biological replicates, in each of which 20 independent fields were examined. Scale bar = 10 μm. (D–F) Phosphorylation state of Amot-p130 mediates YAP localization. HEK293-shAmot cells were co-transfected with YAP and (D) Amot-WT, (E) Amot-p130S176A or (F) Amot-p130S176E and subjected to subcellular fractionation as in (A). Cell lysates (input) and Amot IPs of each one of the subcellular fractions were subjected to immunoblot analysis with anti-YAP antibodies as indicated. Loading controls were as in (A). All western blots shown are representative of three independent biological replicates (n = 3). (G) Double-immunofluorescence staining with anti-YAP and anti-Merlin antibodies on HEK293-shAmot cells transfected with expression vectors for Amot-WT, Amot-p130S176A or Amot-p130S176E. Hoechst was used for staining of nuclei. Pictures show fields at 63x magnification and are representative of three independent biological replicates, in each of which 20 independent fields were examined. Scale bar = 10 μm.