Abstract
BACKGROUND:
Although antibiotics are widely used during pregnancy, evidence regarding their fetal safety remains limited. Our aim was to quantify the association between antibiotic exposure during pregnancy and risk of spontaneous abortion.
METHODS:
We conducted a nested case–control study within the Quebec Pregnancy Cohort (1998–2009). We excluded planned abortions and pregnancies exposed to fetotoxic drugs. Spontaneous abortion was defined as having a diagnosis or procedure related to spontaneous abortion before the 20th week of pregnancy. The index date was defined as the calendar date of the spontaneous abortion. Ten controls per case were randomly selected and matched by gestational age and year of pregnancy. Use of antibiotics was defined by filled prescriptions between the first day of gestation and the index date and was compared with (a) non-exposure and (b) exposure to penicillins or cephalosporins. We studied type of antibiotics separately using the same comparator groups.
RESULTS:
After adjustment for potential confounders, use of azithromycin (adjusted odds ratio [OR] 1.65, 95% confidence interval [CI] 1.34–2.02; 110 exposed cases), clarithromycin (adjusted OR 2.35, 95% CI 1.90–2.91; 111 exposed cases), metronidazole (adjusted OR 1.70, 95% CI 1.27–2.26; 53 exposed cases), sulfonamides (adjusted OR 2.01, 95% CI 1.36–2.97; 30 exposed cases), tetracyclines (adjusted OR 2.59, 95% CI 1.97–3.41; 67 exposed cases) and quinolones (adjusted OR 2.72, 95% CI 2.27–3.27; 160 exposed cases) was associated with an increased risk of spontaneous abortion. Similar results were found when we used penicillins or cephalosporins as the comparator group.
INTERPRETATION:
After adjustment for potential confounders, use of macro-lides (excluding erythromycin), quinolones, tetracyclines, sulfonamides and metronidazole during early pregnancy was associated with an increased risk of spontaneous abortion. Our findings may be of use to policy-makers to update guidelines for the treatment of infections during pregnancy.
Although antibiotics are widely used during pregnancy, the fetal safety of macrolides, quinolones, tetracyclines, metronidazole and nitrofurantoin remains a concern. Existing literature on the association between gestational antibiotic use and the risk of spontaneous abortion provides conflicting results. Four studies using data from teratology information services did not find an association between the use of macrolides and spontaneous abortion.1–4 In contrast, a Danish study based on data from a prescription database found an increased risk of spontaneous abortion associated with the use of clarithromycin.5 Three studies using data from teratology information services failed to show a link between the use of quinolones and the risk of spontaneous abortion,6–8 and an early Medicaid study showed an association with the use of metronidazole.9 However, the studies were all limited by either small samples, or recall or indication bias.1–9 Studies of the risk of spontaneous abortion associated with tetracyclines and nitrofurantoin are lacking.
We aimed to quantify the association between exposure to antibiotics during pregnancy and the risk of spontaneous abortion, taking into account methodologic limitations of previous studies.
Methods
Study design
We conducted a nested case–control study within the Quebec Pregnancy Cohort.
Data sources
We used data from the Quebec Pregnancy Cohort, which is an ongoing population-based cohort with prospective data collection on all pregnancies of women covered by the Quebec Public Prescription Drug Insurance Plan, from January 1998 to December 2009. Information for each pregnancy is obtained from province-wide databases and linked using unique personal identifiers.
The Quebec Pregnancy Cohort was constructed by identifying all pregnancies in the Régie de l’assurance maladie du Québec (RAMQ) and the provincial hospital discharge database (MED-ÉCHO). We determined the day of the last menstrual period (defined as the first day of gestation) using data on gestational age, which was validated against data in patient charts and ultrasound measures.10
Prospective follow-up data were available from 1 year before the first day of gestation, during pregnancy and until December 2009. The data sources for this study included RAMQ (diagnoses, medical procedures, socioeconomic status of women and prescribers), the province’s Public Prescription Drug Insurance Plan database (drug name, start date, dosage and duration), MED-ÉCHO (in-hospital diagnoses and procedures, and gestational age) and the Institut de la statistique du Quebec (patient sociodemographic data and birth weight). The data sources used, as well as the Quebec Pregnancy Cohort, have been described previously.11
Study population
We included women who were 15–45 years of age on the first day of gestation and were continuously insured by the province’s drug plan for at least 12 months before and during their pregnancy. We excluded pregnancies with exposure to known teratogenic drugs12,13 and those terminated by planned abortion. We included all pregnancies meeting our inclusion criteria for women who had more than 1 pregnancy during the study period.
Cases
For cases, we identified pregnancies with a diagnosis or procedure related to spontaneous abortion before the 20th week of gestation using diagnostic codes of the International Classification of Diseases (ICD-9 codes 630–634 and ICD-10 codes O01–O03). We considered only women with clinically detected sponaneous abortion. We defined the index date as the calendar date of the spontaneous abortion.
For each case, we determined the first day of gestation by subtracting the gestational age (based on ultrasound) from the calendar date of the spontaneous abortion. If the information was missing in the database, we used an algorithm to estimate the gestational age (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.161020/-/DC1).
Controls
We selected 10 controls for each case at the index date and matched them by gestational age (within 3 d) and year of pregnancy. We chose this number of controls because the control–case ratio needs to increase to 10 or more to increase statistical power when the prevalence of exposure to the study drug is less than 15% among controls (as in our study).14 Suissa and colleagues have used such numbers in many studies in similar situations.15,16 On the basis of a relative power table for nested case–control studies, if we assumed a prevalence of exposure of 5% among controls, a number of case–control sets fixed to 100, a control–case ratio of 10, a relative power of 80% and a type 1 error of 5%, we planned to detect an odds ratio (OR) of 1.5.17
Exposure
We defined antibiotic exposure as having filled at least 1 prescription for any type of antibiotic either between the first day of gestation and the index date, or before pregnancy but with a duration that overlapped the first day of gestation.
We considered the following classes of antibiotics, defined according to the American Hospital Formulary Service (AHFS) categories: cephalosporins (AHFS 8:12.06), macrolides (AHFS 8:12.12), penicillins (AHFS 8:12.16), quinolones (AHFS 8:12.18) sulfonamides (AHFS 8:12.20), tetracyclines (AHFS 8:12.24), other antibacterials (AHFS 8:12.28), antiprotozoals (AHFS 8:30.92) and urinary anti-infectives (AHFS 8:36).
We considered the following individual antibiotics: amoxicillin, amoxicillin–potassium clavulanate, phenoxymethylpenicillin, cephalexin, azithromycin, clarithromycin, ciprofloxacin, norfloxacin, levofloxacin, clindamycin, doxycycline, minocycline, erythromycin, metronidazole and nitrofurantoin.
To take into account the baseline risk of spontaneous abortion in the general population, we defined a non-exposure category as pregnancy with no antibiotic exposure between the first day of gestation and the index date.
To take into account the underlying indication, we used pregnancies with exposure to penicillins as an active comparator group because penicillin is the most commonly prescribed antimicrobial class during pregnancy and has the most robust safety data during this period.18 Further, we also used cephalosporin exposure as an active comparator group because it is an alternative to penicillin for patients allergic or intolerant to penicillins and it has a long history of documented use in pregnancy.18
Covariates
We measured covariates for 3 periods: (a) as of the first day of gestation (maternal age, area of residence [urban v. rural], receipt of social assistance during pregnancy, education level in years [≤ 12 v. > 12] and marital status [living alone v. cohabiting]; (b) in the year before and during pregnancy until the index date (physician-based diagnoses or filled prescriptions of related medications for chronic comorbidities [depression, asthma, diabetes mellitus, chronic hypertension, thyroid disorder, epilepsy, and rheumatoid polyarthritis and systemic lupus erythematosus]; physician-based diagnoses of endometriosis, uterine malformations and maternal infections [urinary tract infection, respiratory tract infection, bacterial vaginosis and sexually transmitted infections]; and use of other anti-infective agents); and (c) in the year before pregnancy (use of health services, and history of planned and spontaneous abortions).
Diagnostic and medication codes used to identify covariates are listed in Appendix 2. To illustrate the structure of confounding with maternal infections, we used a Directed Acyclic Graph19 (Appendix 3). (The appendices are available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.161020/-/DC1.)
Statistical analysis
We calculated descriptive analyses to summarize the characteristics of the study population using t tests and χ2 tests for continuous and categorical variables, respectively.
We calculated crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) using conditional generalized estimation equations.
We performed several sensitivity analyses. First, we defined the time window for antibiotic exposure as the period from the first day of gestation to 15 days before the index date, to ensure that exposure to the study medication preceded the study outcome. Second, to address indication bias, we restricted our analysis to pregnancies with urinary or respiratory tract infections. Third, we considered only spontaneous abortions that had gestational age determined on the basis of ultrasound. Fourth, given that many early pregnancy losses are not recognized clinically, we excluded spontaneous abortions that occurred before 6 completed weeks of gestation. Fifth, given that we performed 27 hypothesis tests in our study, we adjusted for multiple testing using the Bonferroni correction.20 We considered an association between antibiotic class or type and spontaneous abortion to be statistically significant if the p value was less than 0.002 (i.e., 0.05/27).
We considered all analyses significant at a p value of less than 0.05 (2-tailed). When results were not significant, we performed a post hoc power analysis. We conducted all analyses using SAS version 9.3 software (SAS Institute Inc.).
Ethics approval
This study was approved by the research ethics board of the Centre hospitalier universitaire Sainte-Justine. The linkage between administrative databases was authorized by the Commission d’accès à l’information du Québec.
Results
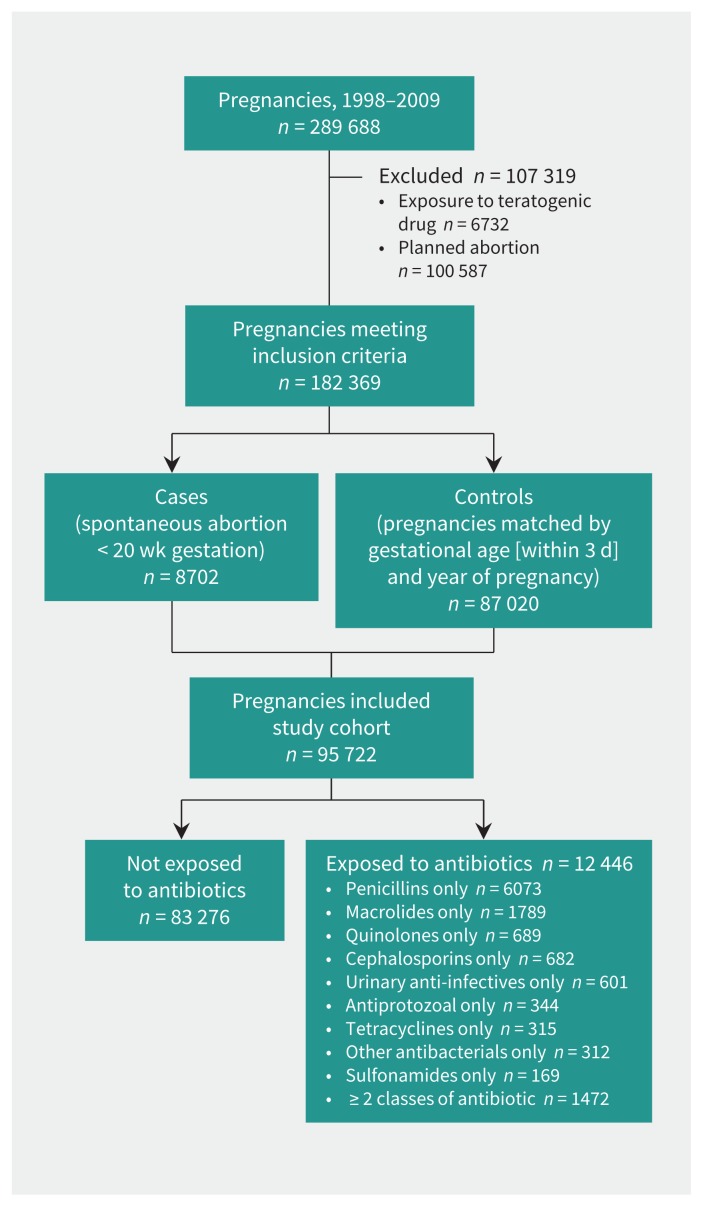
We identified 182 369 pregnancies that met the inclusion criteria, 8702 (4.7%) of which ended with a clinically detected spontaneous abortion (Figure 1). The mean gestational age was 14.1 (standard deviation 3.1) weeks (median 14 wk). For 1068 (12.3%) of the cases, the gestational age was based on ultrasound. Compared with the 87 020 matched controls, women with spontaneous abortion were more likely to be older, to be living alone, and to have comorbidities and infections (Table 1).
Figure 1:
Selection of pregnancies for analysis.
Table 1.
(part 1 of 2): Characteristics of study population
| Characteristic | No. (%) of pregnancies* | p value† | |
|---|---|---|---|
| Cases n = 8702 |
Controls n = 87 020 |
||
| Gestational age at index date,‡ wk | 14.1 ± 3.1 | 14.1 ± 3.1 | 0.9 |
| As of first day of gestation | |||
| Age, yr, mean ± SD | 28.7 ± 6.3 | 27.8 ± 5.5 | < 0.001 |
| Age group, yr | |||
| 18–34 | 6732 (77.3) | 73 947 (84.9) | < 0.001 |
| < 18 | 145 (1.6) | 1410 (1.6) | 0.7 |
| > 34 | 1825 (20.9) | 11 663 (13.4) | < 0.001 |
| Urban residence | 7237 (83.1) | 71 754(82.4) | 0.09 |
| Recipient of social assistance | 2337 (26.8) | 22 390 (25.7) | 0.02 |
| Education (≤ 12 yr) | 3773 (43.3) | 37 309 (42.8) | 0.4 |
| Living alone | 1554 (17.8) | 13 557 (15.5) | < 0.001 |
| In year before and during pregnancy until index date‡ | |||
| Chronic comorbidity or maternal infection | |||
| Depression§ | 1806 (20.7) | 12 508 (14.3) | < 0.001 |
| Asthma§ | 1418(16.3) | 11 319 (13.0) | < 0.001 |
| Diabetes mellitus§ | 176 (2.0) | 1625 (1.8) | 0.3 |
| Chronic hypertension§ | 228 (2.6) | 1946 (2.2) | 0.02 |
| Thyroid disorder§ | 348 (4.0) | 3216 (3.7) | 0.2 |
| Epilepsy§ | 204 (2.3) | 1130 (1.3) | < 0.001 |
| Endometriosis¶ | 106 (1.2) | 558 (0.6) | < 0.001 |
| Uterine malformations¶ | 6 (< 0.1) | 25 (< 0.1) | 0.06 |
| Rheumatoid polyarthritis and systemic lupus erythematosus§ | 2 (< 0.1) | 43 (< 0.1) | 0.3 |
| Urinary tract infection¶ | 1158 (13.3) | 9702 (11.1) | < 0.001 |
| Respiratory tract infection¶ | 2847 (32.7) | 24 857 (28.5) | < 0.001 |
| Bacterial vaginosis¶ | 884(10.1) | 7311 (8.4) | < 0.001 |
| Sexually transmitted infection¶ | 357(4.1) | 3289 (3.7) | 0.1 |
| Medication use | |||
| Other anti-infectives** | 860 (9.8) | 7056 (8.1) | < 0.001 |
| In year before pregnancy | |||
| Health care utilization | |||
| Hospital admission or emergency department visit | 3285 (37.7) | 29 030 (33.3) | < 0.001 |
| No. of physician visits | |||
| 0 | 2289 (26.3) | 25 956 (29.8) | < 0.001 |
| 1–2 | 1728 (19.8) | 18 936 (21.7) | < 0.001 |
| ≥3 | 4685 (53.8) | 42 128 (48.4) | < 0.001 |
| Medication use | |||
| Antibiotics | 3560 (40.9) | 31 576 (36.2) | < 0.001 |
| Drugs other than anti-infectives | |||
| 0 | 2216 (25.4) | 25 730 (29.5) | < 0.001 |
| 1–2 | 1720 (19.7) | 18 107 (20.8) | 0.02 |
| ≥ 3 | 4766 (54.7) | 43 183 (49.6) | < 0.001 |
| History of obstetric complications | |||
| Prior spontaneous abortion | 135 (1.5) | 856 (0.9) | < 0.001 |
| Prior abortion | 562 (6.4) | 3645 (4.1) | < 0.001 |
| Antibiotic use during pregnancy | 1428 (16.4) | 11 018 (12.6) | < 0.001 |
| Cephalosporins†† | 60 (0.69) | 622 (0.71) | |
| Penicillins†† | 500 (5.75) | 5573 (6.40) | |
| Macrolides†† | 264 (3.03) | 1525 (1.75) | |
| Quinolones†† | 160 (1.84) | 529 (0.61) | |
| Sulfonamides†† | 30 (0.34) | 139 (0.16) | |
| Tetracyclines†† | 67 (0.77) | 248 (0.28) | |
| Other antibacterials†† | 34 (0.39) | 278 (0.32) | |
| Antiprotozoals†† | 53 (0.61) | 291 (0.33) | |
| Urinary anti-infectives†† | 39 (0.45) | 562 (0.65) | |
| Use of ≥ 2 classes of antibiotics‡‡ | 221 (2.54) | 1251 (1.44) | < 0.001 |
Note: SD = standard deviation.
Unless stated otherwise.
Pearson χ2 test for categorical variables and t test for continuous variables.
Index date = date of spontaneous abortion for cases and corresponding date for matched controls.
Based on ICD-9 or ICD-10 codes or prescriptions filled for chronic diseases (depression, asthma, diabetes mellitus, chronic hypertension, thyroid disorder, epilepsy, and rheumatoid polyarthritis and systemic lupus erythematosus).
Based on ICD-9 or ICD-10 codes for urinary tract infections, respiratory tract infections, bacterial vaginosis, endometriosis, sexually transmitted infection and uterine malformations.
Includes prescriptions for anti-infectives other than antibiotics (e.g., antituberculosis drugs, antifungals, antivirals, antimalarial, highly active antiretroviral therapy, anthelmintics, amebicides).
Includes prescriptions for at least one dose of only this class of antibiotic between first day of gestation and index date.
Includes prescriptions for at least one dose of ≥ 2 different classes of antibiotic between first day of gestation and index date.
Classes and types of antibiotics
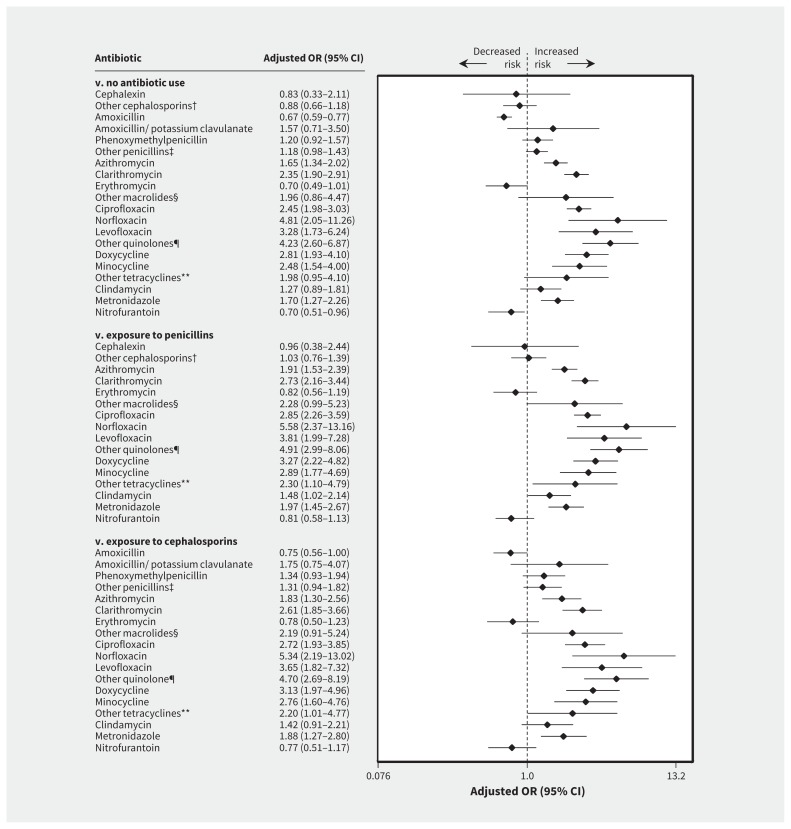
After adjustment for potential confounders, use of azithromycin (adjusted OR 1.65, 95% CI 1.34–2.02; 110 exposed cases), clarithromycin (adjusted OR 2.35, 95% CI 1.90–2.91; 111 exposed cases), tetracyclines (adjusted OR 2.59, 95% CI 1.97–3.41; 67 exposed cases), doxycycline (adjusted OR 2.81, 95% CI 1.93–4.10; 36 exposed cases), minocycline (adjusted OR 2.48, 95% CI 1.54–4.00; 21 exposed cases), quinolones (adjusted OR 2.72, 95% CI 2.27–3.27; 160 exposed cases), ciprofloxacin (adjusted OR 2.45, 95% CI 1.98–3.03; 114 exposed cases), norfloxacin (adjusted OR 4.81, 95% CI 2.05–11.26; 8 exposed cases), levofloxacin (adjusted OR 3.28, 95% CI 1.73–6.24; 14 exposed cases), sulfonamides (adjusted OR 2.01, 95% CI 1.36–2.97; 30 exposed cases) and metronidazole (adjusted OR 1.70, 95% CI 1.27–2.26; 53 exposed cases) was associated with an increased risk of spontaneous abortion. An increased risk of spontaneous abortion was not associated with use of nitrofurantoin (adjusted OR 0.70, 95% CI 0.51–0.96; 39 exposed cases) or erythromycin (adjusted OR 0.70, 95% CI 0.49–1.01; 29 exposed cases) (Table 2; Figure 2; Appendices 4 and 5, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.161020/-/DC1). Similar results were found when we used penicillins or cephalosporins as the comparator group.
Table 2:
Risk of spontaneous abortion associated with use of antibiotics during pregnancy, by drug class and type
| Drug exposure; class/type | No. (%) of spontaneous abortions n = 8702 |
Crude OR (95% CI) | Adjusted OR* (95% CI) |
|---|---|---|---|
| No antibiotic use (ref) | 7274 (83.6) | 1.00 | 1.00 |
| Cephalosporins | 60 (0.7) | 1.01 (0.78–1.31) | 0.90 (0.69–1.18) |
| Cephalexin | 9 (0.1) | 1.13 (0.60–2.10) | 0.83 (0.33–2.11) |
| Other cephalosporins | 48 (0.6) | 0.97 (0.72–1.29) | 0.88 (0.66–1.18) |
| ≥ 2 drugs in class | 3 (0.03) | 1.93 (0.58–6.45) | 1.87 (0.57–6.19) |
| Macrolides | 264 (3.0) | 1.78 (1.56–2.03) | 1.61 (1.41–1.85) |
| Azithromycin | 110 (1.3) | 1.76 (1.44–2.14) | 1.65 (1.34–2.02) |
| Clarithromycin | 111 (1.3) | 2.66 (2.16–3.27) | 2.35 (1.90–2.91) |
| Erythromycin | 29 (0.3) | 0.76 (0.53–1.10) | 0.70 (0.49–1.01) |
| Other macrolides | 7 (0.1) | 2.30 (1.02–5.14) | 1.96 (0.86–4.47) |
| ≥ 2 drugs in class | 7 (0.1) | 2.30 (1.03–5.15) | 1.89 (0.85–4.23) |
| Penicillins | 500 (5.7) | 0.94 (0.86–1.03) | 0.86 (0.78–0.95) |
| Amoxicillin | 257 (3.0) | 0.75 (0.66–0.85) | 0.67 (0.59–0.77) |
| Amoxicillin–potassium clavulanate | 7 (0.1) | 1.99 (0.90–4.43) | 1.57 (0.71–3.50) |
| Phenoxymethylpenicillin (penicillin V) | 74 (0.9) | 1.33 (1.05–1.67) | 1.20 (0.92–1.57) |
| Other penicillins | 117 (1.3) | 1.22 (1.01–1.47) | 1.18 (0.98–1.43) |
| ≥ 2 drugs in class | 45 (0.5) | 1.44 (1.06–1.95) | 1.33 (0.98–1.81) |
| Quinolones | 160 (1.8) | 3.07 (2.57–3.65) | 2.72 (2.27–3.27) |
| Ciprofloxacin | 114 (1.3) | 2.74 (2.23–3.37) | 2.45 (1.98–3.03) |
| Norfloxacin | 8 (0.1) | 5.32 (2.30–12.27) | 4.81 (2.05–11.26) |
| Levofloxacin | 14 (0.2) | 3.90 (2.12–7.16) | 3.28 (1.73–6.24) |
| Other quinolone | 23 (0.3) | 4.88 (2.99–7.96) | 4.23 (2.60–6.87) |
| ≥ 2 drugs in class | 1 (0.01) | 1.29 (0.17–9.92) | 0.92 (0.11–7.90) |
| Sulfonamides† | 30 (0.3) | 2.20 (1.49–3.24) | 2.01 (1.36–2.97) |
| Tetracyclines | 67 (0.8) | 2.75 (2.11–3.60) | 2.59 (1.97–3.41) |
| Doxycycline | 36 (0.4) | 3.12 (2.16–4.51) | 2.81 (1.93–4.10) |
| Minocycline | 21 (0.2) | 2.47 (1.54–3.94) | 2.48 (1.54–4.00) |
| Other tetracyclines | 9 (0.1) | 2.10 (1.04–4.24) | 1.98 (0.95–4.10) |
| ≥ 2 drugs in class | 1 (0.01) | NA | NA |
| Other antibacterials | 34 (0.4) | 1.26 (0.89–1.65) | 1.25 (0.88–1.79) |
| Clindamycin | 34 (0.4) | 1.29 (0.90–1.83) | 1.27 ( 0.89–1.81) |
| Antiprotozoals | |||
| Metronidazole | 53 (0.6) | 1.86 (1.40–2.48) | 1.70 (1.27–2.26) |
| Urinary anti-infectives | 39 (0.4) | 0.73 (0.53–1.00) | 0.69 (0.50–0.95) |
| Nitrofurantoin | 39 (0.4) | 0.73 (0.53–1.00) | 0.70 (0.51–0.96) |
| ≥ 2 classes of antibiotics | 221 (2.5) | 1.82 (1.58–2.09) | 1.54 (1.33–1.78) |
Note: CI = confidence interval, NA = not applicable, OR = odds ratio, ref = reference category.
Adjusted for variables (a) on first day of gestation (maternal age, area of residence [urban v. rural], receipt of social assistance during pregnancy, education level in years [≤ 12 v. > 12] and marital status [living alone v. cohabiting]); (b) in the year before and during pregnancy until the index date (physician-based diagnoses or filled prescriptions of related medications for maternal chronic comorbidities [depression, asthma, diabetes mellitus, chronic hypertension, thyroid disorder, epilepsy, endometriosis, uterine malformation, and rheumatoid polyarthritis and systemic lupus erythematosus]; physician-based diagnoses of maternal infections [urinary tract infection, respiratory tract infection, bacterial vaginosis and sexually transmitted infections]; and use of other anti-infective agents); and (c) and in the year before pregnancy (use of health services, and history of planned and spontaneous abortions).
Analysis considered only sulfonamides class, not individual types of sulfonamides.
Figure 2:
Risk of spontaneous abortion associated with use of antibiotics during pregnancy (3 comparator groups: no antibiotic use, exosure to penicillins and exposure to cephalosporins). Values greater than 1.0 indicate an increased risk of spontaneous abortion. CI = confidence interval, OR = odds ratio. *Odds ratios were adjusted for covariates listed in Methods. †Includes cefixime, cefuroxime, cefaclor and cefprozil. ‡Includes pivampicillin, ampicillin and cloxacillin sodium. §Includes spiramycin and telithromycin. ¶Includes moxifloxacin and ofloxacin. **Includes tetracycline and demethylchlortetracycline.
Sensitivity analyses
When we looked at the risk of spontaneous abortion in pregnancies exposed to antibiotics between the first day of gestation until 15 days before the index date, our results were similar to those in the main analysis (Appendices 6 and 7, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.161020/-/DC1). In a subgroup analysis of pregnancies with urinary tract infection, quinolone exposure was associated with an increased risk of spontaneous abortion when compared with penicillin use (adjusted OR 8.73, 95% CI 3.08–24.77; 17 exposed cases) (Appendix 8, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.161020/-/DC1).
When we restricted the analysis to pregnancies with respiratory tract infections, we found that use of macrolides had an increased OR for spontaneous abortion compared with penicillin, but the association was not statistically significant (adjusted OR 1.89, 95% CI 0.97–3.69; 17 exposed cases; post hoc power analysis = 52%) (Appendix 9, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.161020/-/DC1).
In the analysis restricted to spontaneous abortions with gestational age determined by ultrasound, our results were consistent with those in the main analysis except for quinolones (adjusted OR 0.17, 95% CI 0.01–2.10; 4 exposed cases; post hoc power analysis = 54%; Appendix 10, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.161020/-/DC1). When we excluded spontaneous abortions occurring before 6 weeks’ gestation, our results were also similar to those in the main analysis (Appendix 11, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.161020/-/DC1).
After adjustment for multiple testing, our results were similar to those in the main analysis (Appendices 12 and 13, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.161020/-/DC1).
Interpretation
After adjustment for potential confounders, use of macrolides (excluding erythromycin), quinolones, tetracyclines, sulfonamides and metronidazole during early pregnancy was associated with an increased risk of spontaneous abortion. Nitrofurantoin exposure was not associated with an increased risk of spontaneous abortion; this finding supports its use as an alternative to trimethoprim–sulfamethoxazole for the treatment of urinary tract infection during pregnancy.21
The increased risk of spontaneous abortion associated with the use of azithromycin and clarithromycin (65% and twofold increased risk, respectively) was consistent with findings in 2 previous studies (56% and 2-fold increased risk of spontaneous abortion with clarithromycin exposure).4,5 Conversely, our findings on azithromycin are different from those in a teratology information services study;3 the difference may be explained by the study’s small sample.
A class effect was reported for the tetracyclines and quinolone classes. This finding supports current guidelines used in obstetrics that do not recommend use of these drugs in early pregnancy.22 In contrast, previous studies failed to show an association. These discrepancies may be explained by a lack of statistical power and a recall bias.6–8
Metronidazole exposure was associated with a 70% increased risk of spontaneous abortion, which is consistent with a Medicaid cohort study that showed a 67% increased risk of spontaneous abortion.9
Strengths and limitations
Our study included a large sample, valid information on filled prescriptions, and prospectively and routinely collected data on physician-based diagnoses or procedures related to spontaneous abortion, which limited the potential for detection bias. Other strengths include the use of 2 active comparator groups, adjustment for maternal infections and sensitivity analyses.
The use of a nested case–control design minimized selection bias, and adjustment for multiple comparisons ruled out a chance finding.
Confounding by infection severity is a potential limitation. Penicillins or cephalosporins have overlapping indications with other antibiotics (quinolones and macrolides) in Quebec. For example, β-lactams are among the first-line treatments of noncomplicated urinary tract infections and the treatment of community-acquired pneumonia in patients with comorbidities.23,24 Therefore, women receiving penicillins or cephalosporins would not be very different from those receiving other antibiotics. Nevertheless, we acknowledge that the main reason for confounding by indication in our study may be differences in the severity of infections. However, we adjusted for several documented proxies for infection severity, such as prior exposure to antibiotics, comorbidities, hospital-based diagnosis of maternal infections and prior hospital admissions.25–27 We feel confident that confounding by infection severity cannot fully explain our findings, although residual confounding cannot be completely rule out.
Many classes of antibiotics were associated with an increased risk of spontaneous abortion. The presence of unmeasured confounders could be a possible explanation for these results. Some potential confounders are not available in the Quebec Pregnancy Cohort, including smoking status, folic acid use, alcohol intake, body mass index and other dietary factors. However, we used 2 active comparator groups to attenuate potential differences related to these variables between treatment groups.28 Therefore, unmeasured confounding, if present, would not fully explain this finding.
Biological plausibility of the association between the use of those antibiotic classes and the risk of spontaneous abortion has been reported in the literature. A possible mechanism explaining this effect for clarithromycin involves its proarrhythmic properties (through inhibition of a specific cardiac potassium channel) that play a role in cardiac rhythm regulation in early embryonic development.29 Quinolone may act as a DNA gyrase inhibitor as well as a mitotic inhibitor.30 Tetracycline inhibits the production of proinflammatory cytokines, and of matrix metalloproteinases known to play a role in tissue remodelling, trophoblast invasion and endometrial decidualization.31,32 Metronidazole may increase the production of reactive intermediates, which may result in DNA damage.33
Data on filled prescriptions in the Quebec Pregnancy Cohort may not reflect actual drug use. However, they have been validated against maternal reports and showed a high positive and negative predictive value for antibiotic exposure (86.7% and 92.3%, respectively).34
Although we performed subgroup analyses, some of them were underpowered given the small number of exposed cases.
We included only clinically detected spontaneous abortions. If there is an association between antibiotic exposure and the risk of nonclinically detected spontaneous abortion, our findings are conservative and thus underestimate the true risk. However, if there is no such association, a nondifferential misclassification is likely, and again our estimates are conservative.
The gestational age at the time of spontaneous abortion was not systematically determined on the basis of ultrasound. Nevertheless, when we restricted our analysis to spontaneous abortions with gestational age based on ultrasound, our findings were consistent with those in our main analysis except for quinolones; however, the analysis was underpowered.
We included only pregnant women who were insured by the province’s Prescription Drug Insurance program. Therefore, our results may not be generalizable to those with private drug insurance.35
Conclusion
Use of macrolides (excluding erythromycin), quinolones, tetracyclines, sulfonamides and metronidazole during early pregnancy was associated with an increased risk of spontaneous abortion. However, residual confounding by severity of infection cannot be ruled out. Our findings may be of use to policy-makers to update guidelines for the treatment of infections during pregnancy.
Acknowledgements
Flory Muanda is supported by a scholarship from the CHU Sainte-Justine Foundation and the Foundation of Stars, and a scholarship from the Canadian Network for Advanced Interdisciplinary Methods for Comparative Effectiveness Research. Anick Bérard is a recipient of a career award from the FRSQ and is on the endowment Research Chair of the Famille Louis-Boivin on Medications, Pregnancy and Lactation at the Faculty of Pharmacy, Université de Montréal.
Footnotes
Competing interests: Anick Bérard is a consultant for plaintiffs in litigations involving antidepressants and birth defects. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Flory Muanda and Anick Bérard conceived of and designed the study and drafted the manuscript. Anick Bérard acquired the data and supervised the study; she had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Flory Muanda, Odile Sheehy and Anick Bérard analyzed and interpreted the data. Odile Sheehy and Anick Bérard provided administrative, technical and material support. Flory Muanda performed the statistical analyses. All of the authors critically revised the manuscript for important intellectual content, approved the final version to be published and agreed to act as guarantors of the work.
Funding: This study was supported by the Fonds de la recherche en santé du Québec (FRSQ) and the Réseau Québécois de recherche sur l’usage des médicaments.
References
- 1.Bar-Oz B, Diav-Citrin O, Shechtman S, et al. Pregnancy outcome after gestational exposure to the new macrolides: a prospective multi-center observational study. Eur J Obstet Gynecol Reprod Biol 2008;141:31–4. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Oz B, Weber-Schoendorfer C, Berlin M, et al. The outcomes of pregnancy in women exposed to the new macrolides in the first trimester: a prospective, multicentre, observational study. Drug Saf 2012;35:589–98. [DOI] [PubMed] [Google Scholar]
- 3.Sarkar M, Woodland C, Koren G, et al. Pregnancy outcome following gestational exposure to azithromycin. BMC Pregnancy Childbirth 2006;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Einarson A, Phillips E, Mawji F, et al. A prospective controlled multicentre study of clarithromycin in pregnancy. Am J Perinatol 1998;15:523–5. [DOI] [PubMed] [Google Scholar]
- 5.Andersen JT, Petersen M, Jimenez-Solem E, et al. Clarithromycin in early pregnancy and the risk of miscarriage and malformation: a register based nationwide cohort study. PLoS One 2013;8:e53327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loebstein R, Addis A, Ho E, et al. Pregnancy outcome following gestational exposure to fluoroquinolones: a multicenter prospective controlled study. Antimicrob Agents Chemother 1998;42:1336–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padberg S, Wacker E, Meister R, et al. Observational cohort study of pregnancy outcome after first-trimester exposure to fluoroquinolones. Antimicrob Agents Chemother 2014;58:4392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer C, Amoura-Elefant E, Vial T, et al. Pregnancy outcome after prenatal quinolone exposure. Evaluation of a case registry of the European Network of Teratology Information Services (ENTIS). Eur J Obstet Gynecol Reprod Biol 1996;69:83–9. [DOI] [PubMed] [Google Scholar]
- 9.Rosa FW, Baum C, Shaw M. Pregnancy outcomes after first-trimester vaginitis drug therapy. Obstet Gynecol 1987;69:751–5. [PubMed] [Google Scholar]
- 10.Vilain A, Otis S, Forget A, et al. Agreement between administrative databases and medical charts for pregnancy-related variables among asthmatic women. Pharmacoepidemiol Drug Saf 2008;17:345–53. [DOI] [PubMed] [Google Scholar]
- 11.Bérard A, Sheehy O. The Quebec Pregnancy Cohort — prevalence of medication use during gestation and pregnancy outcomes. PLoS One 2014;9:e93870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulaga S, Zargarzadeh AH, Bérard A. Prescriptions filled during pregnancy for drugs with the potential of fetal harm. BJOG 2009;116:1788–95. [DOI] [PubMed] [Google Scholar]
- 13.Koren G, Pastuszak A, Ito S. Drugs in pregnancy. N Engl J Med 1998;338:1128–37. [DOI] [PubMed] [Google Scholar]
- 14.Hennessy S, Bilker WB, Berlin JA, et al. Factors influencing the optimal control-to-case ratio in matched case-control studies. Am J Epidemiol 1999;149:195–7. [DOI] [PubMed] [Google Scholar]
- 15.Spitzer WO, Suissa S, Ernst P, et al. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med 1992;326:501–6. [DOI] [PubMed] [Google Scholar]
- 16.Suissa S, Hemmelgarn B, Spitzer WO, et al. The Saskatchewan oral contraceptive cohort study of oral contraceptive use and cardiovascular risks. Pharmacoepidemiol Drug Saf 1993;2:33–49. [Google Scholar]
- 17.Pang D. A relative power table for nested matched case–control studies. Occup Environ Med 1999;56:67–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamont HF, Blogg HJ, Lamont RF. Safety of antimicrobial treatment during pregnancy: a current review of resistance, immunomodulation and teratogenicity. Expert Opin Drug Saf 2014;13:1569–81. [DOI] [PubMed] [Google Scholar]
- 19.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48. [PubMed] [Google Scholar]
- 20.Gordi T, Khamis H. Simple solution to a common statistical problem: interpreting multiple tests. Clin Ther 2004;26:780–6. [DOI] [PubMed] [Google Scholar]
- 21.Macejko AM, Schaeffer AJ. Asymptomatic bacteriuria and symptomatic urinary tract infections during pregnancy. Urol Clin North Am 2007;34:35–42. [DOI] [PubMed] [Google Scholar]
- 22.Lynch CM, Sinnott JT, IV, Holt DA, et al. Use of antibiotics during pregnancy. Am Fam Physician 1991;43:1365–8. [PubMed] [Google Scholar]
- 23.Pneumonie acquise en communauté chez l’adulte. Quebec City: Conseil du Médicament; 2009. Available: https://www.inesss.qc.ca/fileadmin/doc/CDM/UsageOptimal/Guides-serieI/CdM-Antibio1-Pneumonie-Adulte-fr.pdf (accessed 2016 Dec. 1). [Google Scholar]
- 24.Infections urinaires chez l’adulte. Quebec City: Conseil du médicament; 2009. Available: https://www.inesss.qc.ca/fileadmin/doc/CDM/UsageOptimal/Guides-serieI/CdM-Antibio1-InfectionsUrinaires-Adultes-fr.pdf (accessed 2016 Dec. 1). [Google Scholar]
- 25.Hillier S, Roberts Z, Dunstan F, et al. Prior antibiotics and risk of antibiotic-resistant community-acquired urinary tract infection: a case–control study. J Antimicrob Chemother 2007;60:92–9. [DOI] [PubMed] [Google Scholar]
- 26.Mazzulli T. Diagnosis and management of simple and complicated urinary tract infections (UTIs). Can J Urol 2012;19(Suppl 1):42–8. [PubMed] [Google Scholar]
- 27.Hertz FB, Schønning K, Rasmussen SC, et al. Epidemiological factors associated with ESBL- and non ESBL-producing E. coli causing urinary tract infection in general practice. Infect Dis (Lond) 2016;48:241–5. [DOI] [PubMed] [Google Scholar]
- 28.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf 2006;15:291–303. [DOI] [PubMed] [Google Scholar]
- 29.Källén BA, Otterblad Olausson P, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol 2005;20:209–14. [DOI] [PubMed] [Google Scholar]
- 30.Aboubakr M, Elbadawy M, Soliman A, et al. Embryotoxic and teratogenic effects of norfloxacin in pregnant female albino rats. Adv Pharmacol Sci 2014;2014:924706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shlopov BV, Stuart JM, Gumanovskaya ML, et al. Regulation of cartilage collagenase by doxycycline. J Rheumatol 2001;28:835–42. [PubMed] [Google Scholar]
- 32.Moutier R, Tchang F, Caucheteux SM, et al. Placental anomalies and fetal loss in mice, after administration of doxycycline in food for tet-system activation. Transgenic Res 2003;12:369–73. [DOI] [PubMed] [Google Scholar]
- 33.Mudry MD, Martinez-Flores I, Palermo AM, et al. Embryolethality induced by metro-nidazole (MTZ) in Rattus norvegicus. Teratog Carcinog Mutagen 2001;21:197–205. [DOI] [PubMed] [Google Scholar]
- 34.Jobin-Gervais K, Sheehy O, Bérard A. Can we rely on pharmacy claims databases to ascertain maternal use of medications during pregnancy? Pharmacoepidemiol Drug Saf 2013;22:155. [DOI] [PubMed] [Google Scholar]
- 35.Bérard A, Lacasse A. Validity of perinatal pharmacoepidemiologic studies using data from the RAMQ administrative database. Can J Clin Pharmacol 2009;16:e360–9. [PubMed] [Google Scholar]