Abstract
Allogeneic hematopoietic cell transplantation (allo HCT) remains a valuable alternative for relapsed/refractory (R/R) Hodgkin lymphoma (HL). Data on allo HCT outcomes in the era of new HL therapies are needed. We evaluated 72 R/R HL patients who received reduced intensity conditioning (RIC) allo HCT and compared the time periods 2009-2013 (n=20) to 2000-2008 (n=52). Grafts included HLA-matched sibling (35%), unrelated donor (8%) and umbilical cord blood (UCB, 56%). In recent period, patients more often received brentuximab vedotin (BV, 60% vs 2%), had fewer comorbidities (Sorror index 0: 60% vs 12%) and were in complete remission (50% vs 23%). Median follow-up was 4.4 years. Three-year progression-free survival (PFS) improved for patients treated between 2009-2013 (49%, 95% CI 26-68%) as compared to the earlier era (23%, 95% CI 13-35%, p=0.02). Overall survival (OS) at 3-years was 84% (95% CI 57-94%) vs 50% (95% CI 36-62%, p=0.01), reflecting lower non-relapse mortality and relapse rates. In multivariate analysis mortality was higher among those with chemoresistance (HR 3.83, 95% CI 1.38-10.57), while treatment during the recent era was associated with better OS (HR for period 2009-2013: 0.24, 95% CI 0.07-0.79) and PFS (HR 0.46, 95% CI 0.23-0.92). Allo HCT in patients with R/R HL is now a more effective treatment.
Keywords: Allogeneic transplantation, Hodgkin lymphoma, Brentuximab vedotin
Introduction
Hodgkin lymphoma (HL) is curable in the majority of patients however upwards of 25% will fail standard front-line chemotherapy.1, 2 For these patients, high-dose chemotherapy followed by autologous hematopoietic cell transplantation (auto HCT) can be curative but half will eventually relapse with poor prognosis.3-5 Median survival of patients who relapse after auto HCT has historically been approximately 2 years.6-8 Allogeneic hematopoietic transplantation (allo HCT) has been applied for patients with advanced HL who relapse after auto HCT, are poorly responsive to chemotherapy, or fail to collect an adequate autologous graft. Reduced intensity conditioning (RIC) regimens decreased non-relapse mortality (NRM) after allo HCT as compared to myeloablative conditioning and are now widely applied for HL.9-12 Brentuximab vedotin (BV), an anti-CD30 targeting antibody-drug conjugate has been studied for relapsed/refractory (R/R) HL since 200913 and demonstrated an overall response rate of 75% in patients relapsing after autoHCT.14 Given this improvement in disease control and wider use of RIC and alternative donors, more patients with advanced HL can now consider a potentially curative alloHCT.15-17 To guide treatment decisions in the current era and to counsel patients in whom allograft is considered, we present updated data on survival after allo HCT from a single institution.
Patients and Methods
Using prospectively collected data from University of Minnesota Blood and Marrow Transplantation Database, we identified 72 consecutive patients with HL who underwent RIC allo HCT between 2000-2013. The review of all available medical records were supplemented by chart review for the use of BV therapy. To evaluate transplant outcomes over time, the 13-year period was divided into a historical cohort from 2000-2008 and more recent cohort during the BV era from 2009-2013. The University of Minnesota Institutional Review Board approved the study. Eligibility criteria included relapse after prior auto HCT or not an auto HCT candidate because of insufficient stem cell collection or chemorefractory disease. RIC conditioning consisted of 200 cGy of total body irradiation (TBI) plus fludarabine (Flu) 40 mg/m2/day intravenously (IV) for days -6 through -2 and either cyclophosphamide (CY) 50 mg/kg/day IV day -6 (n=60) or busulfan (BU) 1 mg/kg/day orally every 6 hours days -8 and -7 (n=12). Five patients received anti-thymocyte globulin (ATG). HLA-matched sibling donor, HLA-matched unrelated donor, and umbilical cord blood (UCB) donor sources were included. Choice of donor was based on availability and institutional preference and experience with cord blood transplantation.
For graft versus host disease (GVHD) prophylaxis, 69 patients received cyclosporine IV or orally targeting a therapeutic trough range of 200-400 mg/mL from day -3 for a minimum for 100 days, followed by a taper thorough day +180. Mycophenolate mofetil (1 gram IV or orally twice a day for 30 days) was increased to 1 gram three times a day in 200618. All patients received filgrastim (5 μg/kg/day IV) from day +1 until absolute neutrophil count (ANC) ≥2500/μL for 2 days. Comorbidities were scored according to the Sorror hematopoietic cell transplantation index (HCT-CI).19 Acute and chronic GVHD were graded as extensive or limited prior to 200520 and by the NIH consensus criteria after 2005.21 Disease evaluation using computed tomography, bone marrow biopsies, and variable number of tandem repeats (VNTR) engraftment analysis occurred on day 30 day, day 100, and years 1 and 2 after transplantation. Positron emission tomography (PET) was routinely used to measure disease status prior to transplant after 2004 and all patients underwent PET scan at day 100. Disease response was scored using standard International Working Group criteria.22
Patients and disease characteristics were summarized using descriptive statistics. Statistical comparisons of variables between 2 groups were completed by nonparametric Wilcoxon test for continuous factors and Pearson chi-square test for categorical factors. The endpoints were progression free survival (PFS), overall survival (OS), cumulative incidence of relapse (REL), and non-relapse mortality (NRM). The Kaplan-Meier method23 was used to estimate the probabilities of PFS and OS, and the log-rank test was used for univariate comparisons between groups. Cumulative incidence estimator was used to calculate the probabilities of relapse reflecting nonevent deaths as a competing risk. The cumulative incidence of NRM was also calculated reflecting the relapse as a competing risk.24 Fine and Gray regression analyses were used to compare the differences between cumulative incidence curves for the endpoints of relapse and NRM.25 The Cox proportional hazard regression model was used to estimate adjusted cumulative incidence curves. Prognostic factor models for all endpoints were created using backward selection method considering a P value of <0.20. The cut-off significance level for all P values was 0.05. All statistical analyses were performed with Statistical Analysis System statistical software version 9.3 (SAS Institute, Inc., Cary, NC) and R Statistical Software (Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org).
Results
Patient and donor characteristics
We evaluated 72 patients with R/R HL who received RIC allo HCT and compared the outcomes of 52 patients treated between 2000-2008 to 20 patients treated between 2009-2013. Patient, disease, and treatment characteristics were similar between the groups (Table 1). For all patients, median age was 31 years (range 6-59), 58% were males, and 10% had Karnofsky performance status≤80%. All patients had advanced HL; primary induction failure was non-significantly less common in recent era (5% vs 23%) possibly due to early intensification of chemotherapy based on interim PET. Most patients (82%) had failed prior auto HCT. Median time from diagnosis to allo HCT was 3 years in both groups. Patients received a median of 4 lines of prior therapy, and there was no significant difference between the two time periods. Graft source included HLA-matched sibling (35%) or UCB (56%) grafts, of which majority received double umbilical cord blood units (90%).
Table 1. Patient, disease, and transplant characteristics.
| Characteristics | All Groups (n=72) | 2000-2008 (n=52) | 2009-2013 (n=20) | p-value | |
|---|---|---|---|---|---|
| Recipient age at allo HCT, median (range), yr | 31 (6-58) | 33 (6-58) | 26 (17-54) | 0.12 | |
| Donor age, median (range), yr | 36 (14-57) | 39 (14-57) | 27 (15-39) | 0.03 | |
| Recipient gender, male, n (%) | 42 (58%) | 29 (56%) | 13 (65%) | 0.48 | |
| Donor gender, male, n (%) | 17 (24%) | 11 (52%) | 6 (60%) | 0.69 | |
| Karnofsky Score >90, n (%) | 7 (10%) | 6 (12%) | 1 (5%) | 0.35 | |
| HCT-CI, n (%) | <0.01 | ||||
| 0 | 18 (25%) | 6 (12%) | 12 (60%) | ||
| 1-2 | 17 (24%) | 14 (27%) | 3 (15%) | ||
| >=3 | 33 (46%) | 28 (54%) | 5 (25%) | ||
| Stage at Diagnosis, n (%) | 0.32 | ||||
| I | 2 (3%) | 2 (5%) | 0 | ||
| II | 23 (36%) | 13 (30%) | 10 (50%) | ||
| III | 23 (36%) | 18 (41%) | 5 (25%) | ||
| IV | 16 (25%) | 11 (25%) | 5 (25%) | ||
| Primary refractory to induction, n (%) | 13 (18%) | 12 (23%) | 1 (5%) | 0.64 | |
| No. previous regimens, median (range) | 4 (2-7) | 4 (2-7) | 4 (2-7) | 0.52 | |
| Received BV, n (%) | 13 (18%) | 1 (2%) | 12 (60%) | <0.01 | |
| No. cycles of BV, median (range) | 4 (1-16) | 1(1-1) | 4.5 (2-16) | 0.11 | |
| Prior auto HCT, n (%) | 56 (82%) | 38 (79%) | 18 (90%) | 0.29 | |
| Post auto-HCT relapse, n (%) | 0.40 | ||||
| <1 year | 20 (28%) | 12 (23%) | 8 (40%) | ||
| >1 year | 5 (7%) | 4 (8%) | 1 (5%) | ||
| Absence of prior radiotherapy, n (%) | 15 (21%) | 6 (12%) | 9 (45%) | 0.03 | |
| CR status at allo HCT, n (%) | 22 (31%) | 12 (23%) | 10 (50%) | 0.03 | |
| Disease status at allo HCT, n (%) | 0.07 | ||||
| CR | 22 (32%) | 12 (24%) | 10 (53%) | ||
| Chemoresistant | 7 (10%) | 6 (12%) | 1 (5%) | ||
| Chemosensitive non-CR | 40 (58%) | 32 (64%) | 8 (42%) | ||
| Time from diagnosis to transplant, median (range), yr | 3 (1-19) | 3 (1-19) | 3 (1-9) | 0.99 | |
| Preparative regimen, n (%) | 0.22 | ||||
| BU/CLAD/TBI | 4 (6%) | 4 (8%) | 0 | ||
| BU/FLU/TBI | 8 (11%) | 8 (15%) | 0 | ||
| CY/FLU/TBI | 55 (76%) | 37 (71%) | 18 (90%) | ||
| CY/FLU/TBI/ATG | 5 (7%) | 3 (6%) | 2 (10%) | ||
| Received ATG, n (%) | 5 (7%) | 3 (6%) | 2 (10%) | 0.53 | |
| Donor type, n (%) | 0.53 | ||||
| SIB | 26 (36%) | 17 (33%) | 9 (45%) | ||
| MUD | 6 (8%) | 4 (8%) | 2 (10%) | ||
| UCB | 40 (56%) | 31 (60%) | 9 (45%) | ||
| Stem-cell source, n (%) | 0.56 | ||||
| BM | 7 (10%) | 5 (10%) | 2 (10%) | ||
| PB | 24 (33%) | 15 (29%) | 9 (45%) | ||
| UCB | 40 (56%) | 31 (60%) | 9 (45%) | ||
| GVHD Prophylaxis, n (%) | 0.02 | ||||
| CSA/MMF | 69 (96%) | 52 (100%) | 17 (85%) | ||
| Siro/MMF | 1 (1%) | 0 | 1 (5%) | ||
| Tacro/Siro/MTX | 2 (3%) | 0 | 2 (10%) | ||
| HLA match, n (%) | 0.11 | ||||
| 4/6 | UCB, n (%) | 20 (28%) | 18 (35%) | 2 (10%) | |
| 5/6 | 19 (26%) | 12 (23%) | 7 (35%) | ||
| SIB, n (%) | 1 (1%) | ||||
| UCB, n (%) | 18 (25%) | ||||
| 6/6, 8/8 | 33 (46%) | 22 (42%) | 11 (55%) | ||
| SIB, n (%) | 25 (35%) | ||||
| MUD, n (%) | 6 (8%) | ||||
| UCB, n (%) | 2 (3%) | ||||
| CD34+ cell count, median (range), cells ×10ˆ6 | 0.7 (0.1-13.7) | 0.7 (0.1-13.7) | 2.3 (0.1-12.8) | 0.20 | |
| SIB | 6.2 (3.0-13.7) | 6.0 (3.0-13.7) | 6.2 (3.3-12.8) | 1.00 | |
| MUD | 1.5 (0.8-5.3) | 1.3 (0.8-1.8) | 3.3 (1.2-5.3) | 0.72 | |
| UCB | 0.5 (0.1-1.2) | 0.5 (0.1-1.2) | 0.5 (0.1-0.7) | 0.81 | |
| Follow-up of survivors, median (range), mo | 53 (12-146) | 66 (26-146) | 32 (12-72) | ||
Abbreviations: allo HCT = allogeneic hematopoeitic cell transplantation; CMV = cytomegalovirus; auto HCT = autologous hematopoetic cell transplantation; HCT-CI = hematopoeitic cormobidity index; SIB=sibling; MUD = matched unrelated donor; UCB = umbilical cord blood; BM = bone marrow; PB = peripheral blood; CR = complete remission; BU = busulfan; CLAD = cladarabine; FLU = fludarabine; TBI = total body irradiation; ATG = anti-thymocyte globulin; GVHD = graft-versus-host disease,; CSA = cyclosporine; MMF = mycophenolate; Siro = sirolimus; Tacro = tacrlimus; MTX = methotrexate; BV = brentuximab vedotin.
BV was used primarily after 2009 (before 2009 2% vs after 2009 60%) at a median of 4.5 cycles. Four patients received BV as bridge immediately prior to allo HCT, while nine other patients received it at some point in therapy prior to allo HCT. Only one patient had received BV as maintenance therapy after auto HCT. Patients treated more recently (2009-2013) had significantly fewer comorbidities (HCT-CI 0: 60% vs 12%, p<0.01), and attained higher rates of complete response (CR) pre-HCT (50% vs 23%, p=0.03) than patients treated from 2000-2008. More than half of patients treated with BV pre-transplant experienced CR (54%) compared to 35% CR rate without BV (p=0.04).
Transplantation outcomes
PFS, OS, NRM and relapse after allo HCT are shown in Figure 1. PFS at 3-years was remarkably improved for patients treated from 2009-2013 (49%, 95% CI 26-68%) as compared to 23% (95% CI 13-35%, p=0.02) for patients treated prior to 2009. Three-year OS was 84% (95% CI 57-94%) vs 50% (95% CI 36-62%, p=0.01), reflecting lower 1-yearNRM (6%, 95% CI 0-16% vs 16%, 95% CI 6-26%; p=0.15) and 3-year relapse rates (40%, 95% CI 17-63% vs 50%, 95% CI 34-65%; p=0.11) during the more recent treatment era. In univariate analysis, OS with UCB and HLA-matched sibling transplants were 70%, 95% CI 53-82% and 52%, 95% CI 29-70%, respectively; p=0.02. Median follow-up of surviving patients was 4.4 years (range 1-12.2 years). In multivariate analysis (MVA), treatment in 2009-2013 and chemosensitive disease was associated with improved PFS and OS (Table 2). Adjusted OS was comparable between HLA-matched sibling and UCB transplantation (HR 0.65; 95% CI 0.28-1.48; p=0.30). Factors significant in UVA and MVA are summarized in Table 2. HCT-CI ≥1 was associated with increased NRM at 1 year, however this trend was not significant (HCT-CI ≥1 15% vs 0% in patients with HCT-CI=0; p-0.1). Graft source, KPS, ATG use and CMV status did not impact on NRM, relapse rate, PFS, or OS. For the entire cohort, median PFS was 26 months (range 1-146) and median OS was 53 months (range 12-146). In MVA, chemoresistant disease increased mortality (3.83, 95% CI 1.38-10.57), while treatment from 2009-2013 was associated with better OS (HR 0.24, 95% CI 0.07-0.79) and PFS (HR 0.46, 95% CI 0.23-0.92; Table 2).
Figure 1. Transplantation Outcomes by Transplant Period.
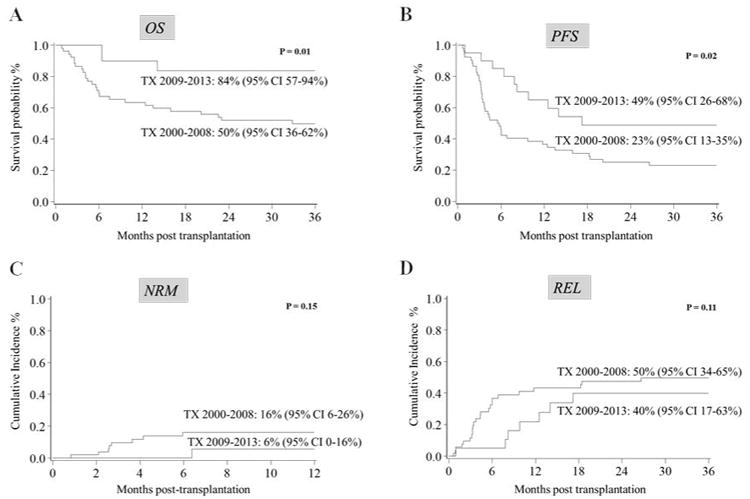
Progression-free survival (PFS), overall survival (OS), non-relapse mortality (NRM), and relapse (REL) comparing two transplant periods (TX): 2000-2008 (n=52) versus 2009-2013 (n=20). Median survival is 32.8 months and not reached, respectively. (A) Kaplan-Meier survival probabilities for 3-year OS. (B) Kaplan-Meier survival probabilities for 3-year PFS. (C) Cumulative incidence of NRM at 1-year. (D) Cumulative incidence of 3-year relapse for each time period. NRM were calculated as competing risks.
Table 2. Univariate and Multivariate Analyses of Prognostic Factors Post-Transplant.
| Factor | Value | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Estimate | 95% CI | p-value | HR | 95% CI | p-value | ||
| OS (3-year) | |||||||
| Disease status | <0.01 | <0.01 | |||||
| Chemosensitive | 62% | 49-73% | 1.00 | ||||
| Chemoresistant | 29% | 4-61% | 3.83 | 1.38-10.57 | |||
| Donor type | 0.02 | <0.01 | |||||
| SIB | 52% | 29-70% | 1.00 | ||||
| MUD | 14% | 1-46% | 3.25 | 1.13-9.32 | 0.03 | ||
| UCB | 70% | 53-82% | 0.65 | 0.28-1.48 | 0.30 | ||
| Transplant year | 0.01 | 0.02 | |||||
| 2000-2008 | 50% | 36-62% | 1.00 | ||||
| 2009-2013 | 84% | 57-94% | 0.24 | 0.07-0.79 | |||
| Acute GVHD II-IV | <0.01 | ||||||
| No | 81% | 64-91% | |||||
| Yes | 30% | 15-46% | |||||
| PFS (3-year) | |||||||
| Disease status | 0.01 | 0.02 | |||||
| Chemosensitive | 33% | 22-45% | 1.00 | ||||
| Chemoresistant | 0% | 2.68 | 1.17-6.10 | ||||
| Transplant year | 0.02 | 0.03 | |||||
| 2000-2008 | 23% | 13-35% | 1.00 | ||||
| 2009-2013 | 49% | 26-68% | 0.46 | 0.23-0.92 | |||
| Chronic GVHD | 0.01 | ||||||
| No | 25% | 15-37% | |||||
| Yes | 46% | 21-69% | |||||
| NRM (1-year) | |||||||
| Acute GVHD II-IV | 0.03 | ||||||
| No | 25% | 9-41% | |||||
| Yes | 5% | 0-12% | |||||
| Relapse (3-year) | |||||||
| Chronic GVHD | 0.02 | 0.03 | |||||
| No | 53% | 38-69% | 1.00 | ||||
| Yes | 27% | 5-49% | 0.34 | 0.13-0.89 | |||
Abbreviations: HR=hazard ratio; OS=overall survival; PFS=progression-free survival; NRM=non-relapse mortality; CI=confidence interval; SIB=sibling; MUD = matched unrelated donor; UCB = umbilical cord blood; GVHD=graft-versus-host disease.
Toxicity and GVHD
Cumulative incidence of grade 2 to 4 acute GVHD was 44% and declined in the recent time period (25% vs 52%, p=0.03). The cumulative incidence of chronic GVHD was 33% and also decreased in the recent time period (21% vs 39%). Our group previously reported that GVHD improvements are partially due to increased MMF dosing (up to 3 grams per day) in UCB transplants.18 Patients with evidence of chronic GVHD diagnosed before relapse experienced lower cumulative incidence of relapse at 3 years (27%, 95% CI 5-49%) than patients without chronic GVHD (53%, 95% CI 38-69%; p=0.02) leading to better 3-year PFS of 46% (95% CI 21-69%) versus 25% (95%CI 15-37%; p=0.01) suggesting a graft-versus-lymphoma effect. Grade III-IV acute GVHD was associated with worse 3-year OS (22%, 95% CI 7-43% vs 71%, 95% CI 56-81%; p<0.01).
Discussion
Allo HCT represents the only potentially curative option for patients who have R/R HL failing prior auto HCT. Here, we report that survival in patients with advanced R/R HL undergoing allo HCT improved from 2000-2008 to 2009-2013. The survival rate for patients treated after 2009 was above 80%, while NRM has been steadily declined to below 10%. These findings suggest that donor transplantation remains a viable modality for suitable patients with advanced HL.
We defined two time periods for allo HCT for comparison (before and after 2009) based upon availability of BV. The differences in the two groups reflect the efficacy of BV. We showed improved health in potential candidates undergoing allo HCT. Patients in the recent era undergo allo HCT with less frequent use of prior radiation therapy, lower HCT-CI and better disease control. Both improvement in CR rates which occurred in recent era and the reduced morbidity may be attributable to the use of less toxic BV as a safer and more effective salvage alternative. Additionally, better selection of appropriate candidates for transplantation is possible. Our data suggest that improved survival of patients with HL undergoing allo HCT overtime resulted from both reduction in NRM and decreased early relapse. Decreased morbidity and mortality after allo HCT in lymphoma has been attributed to RIC conditioning regimen, advances in supportive care, and improved strategies to prevent GVHD. Improvements in supportive measures include better infection prophylaxis and treatment, more comprehensive physical therapy and rehabilitation, and early nutritional evaluation and intervention. We showed that GVHD, both acute and chronic, has declined overtime. The decrease in acute GVHD may be due to more effective GVHD prophylaxis, such as increased MMF dosing in UCB transplants at our institution.18 Using better HLA-matched UCB units (less 4/6- matched), younger donors, and higher proportion receiving ATG in recent era may contribute to decreased chronic GHVD. Also, the potential role of BV in GVHD treatment has been suggested as CD30 appears increased on effector and memory CD8+ T cells and serum soluble CD30 levels are elevated in acute GVHD.26 Further investigation is needed on whether BV is playing a beneficial role in GVHD control.
The efficacy of allo HCT for HL has been controversial. In a few series, two critical prognostic factors for allo HCT that are predictive of outcomes are disease status at transplant and patient comorbidity/performance status.15, 27 Lack of effective therapies to control advanced HL prior to transplant and comorbidities in heavily treated patients historically limited the benefit of allo HCT. Previous reports on the feasibility of RIC allo HCT for R/R HL in the era prior to 2009 showed 2-year PFS of 23-29% (Fred Hutchinson), 32% (MD Anderson), and 3-year PFS of 22% (Dana Farber); similar to our 3-year PFS of 23% for time period 2000-2008.28-30 Relapse rates were as high as 50%.11, 30-36 Our data also support a recent study by Chen et al. from 2014 who reported 2-year OS of 71% and 2-year PFS of 59%, which is comparable to 3-year OS of 84% and 3-year PFS of 49% from our 2009-2013 period.37 PFS and OS for allo HCT for R/R HL have more than doubled in the recent BV era.
Regarding donor selection, the majority of patients received UCB transplants in this study with excellent long-term outcomes. Although overall numbers were small, we speculate that cord transplants have better graft versus tumor effect than matched sibling. This supports smaller recent studies on UCB HCT in HL, which suggest promising survival in patients lacking a suitable adult donor38, but caution about higher risk of lymphoproliferative disorders (PTLD) with use of anti-thymocyte globulin.39 At our center, we developed a strategy of prospective EBV titer monitoring and treat EBV viremia pre-emptively with rituximab with marked reduction of PTLD.40 Our data concur with recent registry data on alternative donor use in patients with lymphoma41 and a review by Messer et al. which indicates that unrelated donor and alternative donor sources versus sibling donor allo SCT result in similar outcomes in advanced HL.42
A limitation of this study is that it is a retrospective and single institution study, yet the design is reflective of actual practice as it has transformed over the last decade. Only one patient had received BV as maintenance therapy after auto HCT given only recent approval of this indication per AETHERA trial.43 We report on one of the largest series of HL patients treated with RIC allo HCT after BV therapy.
Use of the salvage treatment BV yields improved responses over conventional multi-agent chemotherapy with less toxicity, thereby providing better candidates for allo HCT. The improved disease control prior to transplant most significantly impacts survival, resulting is a doubling of OS.6-8 Additionally, the impressive activity of novel regimens, such as PD1 inhibitors, has potential to further enhance responses and survival in HL; however the timing with respect to allo HCT needs to be judiciously studies.44 Allo HCT for R/R HL is now a more effective treatment.
Acknowledgments
The authors would like to thank Michael Franklin for editorial support. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114 (VB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported in part by NIH P30 CA77598 utilizing the Biostatistics and informatics core, Masonic Cancer Center, University of Minnesota shared resource.
Footnotes
Authorship and Conflict of Interest Statement: VB is a member of Seattle Genetics Advisory Board. Other authors declare no competing financial interests. L.H. and V.B. contributed to the conception and design of the study and drafted the manuscript. L.H., V.B., Q.C., and A.L. contributed to data analysis and interpretation. L.H., Q.C., A.L., B.L.M., D.J.W., C.G.B., and V.B. reviewed and approved the final version.
References
- 1.Ansell SM. Hodgkin lymphoma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(7):771–779. doi: 10.1002/ajh.23750. [DOI] [PubMed] [Google Scholar]
- 2.Canellos GP, Niedzwiecki D. Long-term follow-up of Hodgkin's disease trial. N Engl J Med. 2002;346(18):1417–1418. doi: 10.1056/NEJM200205023461821. [DOI] [PubMed] [Google Scholar]
- 3.Majhail NS, Weisdorf DJ, Defor TE, Miller JS, McGlave PB, Slungaard A, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12(10):1065–1072. doi: 10.1016/j.bbmt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359(9323):2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 5.Gopal AK, Metcalfe TL, Gooley TA, Pagel JM, Petersdorf SH, Bensinger WI, et al. High-dose therapy and autologous stem cell transplantation for chemoresistant Hodgkin lymphoma: the Seattle experience. Cancer. 2008;113(6):1344–1350. doi: 10.1002/cncr.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moskowitz AJ, Perales MA, Kewalramani T, Yahalom J, Castro-Malaspina H, Zhang Z, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009;146(2):158–163. doi: 10.1111/j.1365-2141.2009.07727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arai S, Fanale M, DeVos S, Engert A, Illidge T, Borchmann P, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54(11):2531–2533. doi: 10.3109/10428194.2013.798868. [DOI] [PubMed] [Google Scholar]
- 8.Sureda A, Constans M, Iriondo A, Arranz R, Caballero MD, Vidal MJ, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol. 2005;16(4):625–633. doi: 10.1093/annonc/mdi119. [DOI] [PubMed] [Google Scholar]
- 9.Champlin R, Khouri I, Komblau S, Molidrem J, Giralt S. Reinventing bone marrow transplantation. Non myeloablative preparative regimens and induction of graft-vs-malignancy effect. Oncology (Williston Park) 1999;13(5):621–628. discussion 631, 635-628, 641. [PubMed] [Google Scholar]
- 10.Martino M, Festuccia M, Fedele R, Console G, Cimminiello M, Gavarotti P, et al. Salvage treatment for relapsed/refractory hodgkin lymphoma: role of allografting, brentuximab vedotin and newer agents. Expert Opin Biol Ther. 2015 doi: 10.1517/14712598.2015.1130821. [DOI] [PubMed] [Google Scholar]
- 11.Gajewski JL, Phillips GL, Sobocinski KA, Armitage JO, Gale RP, Champlin RE, et al. Bone marrow transplants from HLA-identical siblings in advanced Hodgkin's disease. J Clin Oncol. 1996;14(2):572–578. doi: 10.1200/JCO.1996.14.2.572. [DOI] [PubMed] [Google Scholar]
- 12.Peggs KS, Sureda A, Qian W, Caballero D, Hunter A, Urbano-Ispizua A, et al. Reduced-intensity conditioning for allogeneic haematopoietic stem cell transplantation in relapsed and refractory Hodgkin lymphoma: impact of alemtuzumab and donor lymphocyte infusions on long-term outcomes. Br J Haematol. 2007;139(1):70–80. doi: 10.1111/j.1365-2141.2007.06759.x. [DOI] [PubMed] [Google Scholar]
- 13.de Claro RA, McGinn K, Kwitkowski V, Bullock J, Khandelwal A, Habtemariam B, et al. U.S. Food and Drug Administration approval summary: brentuximab vedotin for the treatment of relapsed Hodgkin lymphoma or relapsed systemic anaplastic large-cell lymphoma. Clin Cancer Res. 2012;18(21):5845–5849. doi: 10.1158/1078-0432.CCR-12-1803. [DOI] [PubMed] [Google Scholar]
- 14.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30(18):2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson SP, Sureda A, Canals C, Russell N, Caballero D, Bacigalupo A, et al. Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin's lymphoma: identification of prognostic factors predicting outcome. Haematologica. 2009;94(2):230–238. doi: 10.3324/haematol.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sureda A, Robinson S, Canals C, Carella AM, Boogaerts MA, Caballero D, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26(3):455–462. doi: 10.1200/JCO.2007.13.2415. [DOI] [PubMed] [Google Scholar]
- 17.Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Ritz J, et al. A prognostic score for patients with acute leukemia or myelodysplastic syndromes undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14(1):28–35. doi: 10.1016/j.bbmt.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bejanyan N, Rogosheske J, DeFor T, Lazaryan A, Esbaum K, Holtan S, et al. Higher Dose of Mycophenolate Mofetil Reduces Acute Graft-versus-Host Disease in Reduced-Intensity Conditioning Double Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21(5):926–933. doi: 10.1016/j.bbmt.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006;12(1):31–47. doi: 10.1016/j.bbmt.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric-Estimation from incomplete Observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 24.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 25.Fine JP, G RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 26.Martino M, Festuccia M, Fedele R, Console G, Cimminiello M, Gavarotti P, et al. Salvage treatment for relapsed/refractory Hodgkin lymphoma: role of allografting, brentuximab vedotin and newer agents. Expert Opin Biol Ther. 2016;16(3):347–364. doi: 10.1517/14712598.2015.1130821. [DOI] [PubMed] [Google Scholar]
- 27.Sarina B, Castagna L, Farina L, Patriarca F, Benedetti F, Carella AM, et al. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: a retrospective study based on the time of HLA typing and donor availability. Blood. 2010;115(18):3671–3677. doi: 10.1182/blood-2009-12-253856. [DOI] [PubMed] [Google Scholar]
- 28.Burroughs LM, O'Donnell PV, Sandmaier BM, Storer BE, Luznik L, Symons HJ, et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following non myeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14(11):1279–1287. doi: 10.1016/j.bbmt.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armand P, Kim HT, Ho VT, Cutler CS, Koreth J, Antin JH, et al. Allogeneic transplantation with reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma: importance of histology for outcome. Biol Blood Marrow Transplant. 2008;14(4):418–425. doi: 10.1016/j.bbmt.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderlini P, Saliba R, Acholonu S, Giralt SA, Andersson B, Ueno NT, et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin's lymphoma: the updated M.D. Anderson Cancer Center experience. Haematologica. 2008;93(2):257–264. doi: 10.3324/haematol.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez I, Sureda A, Caballero MD, Urbano-Ispizua A, Ribera JM, Canales M, et al. Non myeloablative stem cell transplantation is an effective therapy for refractory or relapsed hodgkin lymphoma: results of a spanish prospective cooperative protocol. Biol Blood Marrow Transplant. 2006;12(2):172–183. doi: 10.1016/j.bbmt.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Anderlini P, Saliba R, Acholonu S, Okoroji GJ, Donato M, Giralt S, et al. Reduced-intensity allogeneic stem cell transplantation in relapsed and refractory Hodgkin's disease: low transplant-related mortality and impact of intensity of conditioning regimen. Bone Marrow Transplant. 2005;35(10):943–951. doi: 10.1038/sj.bmt.1704942. [DOI] [PubMed] [Google Scholar]
- 33.Corradini P, Dodero A, Farina L, Fanin R, Patriarca F, Miceli R, et al. Allogeneic stem cell transplantation following reduced-intensity conditioning can induce durable clinical and molecular remissions in relapsed lymphomas: pre-transplant disease status and histotype heavily influence outcome. Leukemia. 2007;21(11):2316–2323. doi: 10.1038/sj.leu.2404822. [DOI] [PubMed] [Google Scholar]
- 34.Devetten MP, Hari PN, Carreras J, Logan BR, van Besien K, Bredeson CN, et al. Unrelated donor reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2009;15(1):109–117. doi: 10.1016/j.bbmt.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majhail NS, Weisdorf DJ, Wagner JE, Defor TE, Brunstein CG, Burns LJ. Comparable results of umbilical cord blood and HLA-matched sibling donor hematopoietic stem cell transplantation after reduced-intensity preparative regimen for advanced Hodgkin lymphoma. Blood. 2006;107(9):3804–3807. doi: 10.1182/blood-2005-09-3827. [DOI] [PubMed] [Google Scholar]
- 36.Peggs KS, Hunter A, Chopra R, Parker A, Mahendra P, Milligan D, et al. Clinical evidence of a graft-versus-Hodgkin's-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet. 2005;365(9475):1934–1941. doi: 10.1016/S0140-6736(05)66659-7. [DOI] [PubMed] [Google Scholar]
- 37.Chen R, Palmer JM, Tsai NC, Thomas SH, Siddiqi T, Popplewell L, et al. Brentuximab vedotin is associated with improved progression-free survival after allogeneic transplantation for Hodgkin lymphoma. Biol Blood Marrow Transplant. 2014;20(11):1864–1868. doi: 10.1016/j.bbmt.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson PA, Perera T, Marin D, Oran B, Popat U, Qazilbash M, et al. Double umbilical cord blood transplant is effective therapy for relapsed or refractory Hodgkin lymphoma. Leuk Lymphoma. 2016;57(7):1607–1615. doi: 10.3109/10428194.2015.1105370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinana JL, Sanz J, Esquirol A, Martino R, Picardi A, Barba P, et al. Umbilical cord blood transplantation in adults with advanced hodgkin's disease: high incidence of post-transplant lymphoproliferative disease. Eur J Haematol. 2016;96(2):128–135. doi: 10.1111/ejh.12557. [DOI] [PubMed] [Google Scholar]
- 40.Blaes AH, Cao Q, Wagner JE, Young JA, Weisdorf DJ, Brunstein CG. Monitoring and preemptive rituximab therapy for Epstein-Barr virus reactivation after antithymocyte globulin containing non myeloablative conditioning for umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2010;16(2):287–291. doi: 10.1016/j.bbmt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bachanova V, Burns LJ, Wang T, Carreras J, Gale RP, Wiernik PH, et al. Alternative donors extend transplantation for patients with lymphoma who lack an HLA matched donor. Bone Marrow Transplant. 2015;50(2):197–203. doi: 10.1038/bmt.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messer M, Steinzen A, Vervolgyi E, Lerch C, Richter B, Dreger P, et al. Unrelated and alternative donor allogeneic stem cell transplant in patients with relapsed or refractory Hodgkin lymphoma: a systematic review. Leuk Lymphoma. 2014;55(2):296–306. doi: 10.3109/10428194.2013.802780. [DOI] [PubMed] [Google Scholar]
- 43.Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–1862. doi: 10.1016/S0140-6736(15)60165-9. [DOI] [PubMed] [Google Scholar]
- 44.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]


