Abstract
In chronic lymphocytic leukemia (CLL), STAT3 is constitutively phosphorylated on serine 727 and plays a role in the pathobiology of CLL. However, what induces constitutive phosphorylation of STAT3 is currently unknown. Mass spectrometry was used to identify casein kinase 2 (CK2), a serine/threonine kinase that co-immunoprecipitated with serine phosphorylated STAT3 (pSTAT3). Furthermore, activated CK2 incubated with recombinant STAT3 induced phosphorylation of STAT3 on serine 727. Although STAT3 and CK2 are present in normal B- and T-cells, STAT3 is not constitutively phosphorylated in these cells. Further study found that CD5 and BLNK co-expressed in CLL, but not in normal B- or T-cells, are required for STAT3 phosphorylation. To elucidate the relationship of CD5 and BLNK to CK2 and STAT3, STAT3 was immunoprecipitated from CLL cells and CK2, CD5, and BLNK were detected in the immunoprecipitate. Conversely, STAT3, CD5, and BLNK were in the immunoprecipitate of CLL cells immunoprecipitated with CK2 antibodies. Furthermore, siRNA knockdown of CD5 or BLNK, or treatment with CD5-neutralizing antibodies significantly reduced the levels of serine pSTAT3 in CLL cells. Finally, confocal microscopy determined that CD5 is cell membrane bound and fractionation studies revealed that the CK2/CD5/BLNK/STAT3 complex remains in the cytoplasm, whereas serine pSTAT3 is shuttled to the nucleus.
Implications
These data show that the cellular proteins CK2, CD5, and BLNK are required for constitutive phosphorylation of STAT3 in CLL. Whether this protein complex phosphorylates other proteins or inhibiting its activity would have clinical benefit in patients has yet to be determined.
Keywords: Casein Kinase 2 (CK2), Signal Transducer and Activator of Transcription 3 (STAT3), Phosphorylation, Chronic lymphocytic Leukemia (CLL)
Introduction
Chronic lymphocytic leukemia (CLL), the most common leukemia in the Western Hemisphere, is characterized by a gradual accumulation of mature-appearing lymphocytes co-expressing typical B-cell surface antigens (1) and CD5. CD5 is usually expressed on T-cells (2), although only on a rare B-cell subtype, but not on most B-cells (3). CLL cells are also characterized by constitutive activation of the signal transducer and activator of transcription 3 (STAT3) (4, 5).
Typically, STAT3 is activated by extracellular molecules such as cytokines and growth factors that bind to their corresponding receptors and induce the phosphorylation of STAT3 on tyrosine 705 residues. This phosphorylated (p) STAT3 forms dimers, translocates to the nucleus, binds to DNA, and activates STAT3-regulated genes (6). STAT3 plays a key role in cell growth, suppression of apoptosis, cell motility (7), tumorigenesis, and malignant transformation (8).
Unlike in normal B-cells, in CLL cells STAT3 is constitutively phosphorylated on serine 727 residues rather than tyrosine residues (4, 5). Serine pSTAT3 has biologic activities similar to those of tyrosine pSTAT3: Serine pSTAT3 is shuttled to the nucleus, binds to DNA, activates genes known to be activated by tyrosine pSTAT3, and provides CLL cells with a survival advantage (5, 9–12) and proliferation capacity (12, 13).
STAT3 is ubiquitously expressed in various cell types (14), and its phosphorylation and biologic activation are usually induced by tyrosine kinases such as Janus kinase 2 (15). What induces the phosphorylation of STAT3 on serine residues is currently unknown. Unexpectedly, although STAT3 is constitutively phosphorylated exclusively on serine residues in CLL cells, (14), several large-scale whole-exome sequencing analysis did not identify a recurrent activating mutation in a serine kinase nor inactivating mutation in a phosphatase (16, 17). Therefore, we hypothesized that a combination of several proteins uniquely assembled in CLL cells induces the phosphorylation of STAT3 on serine 727 residues.
Materials and methods
Patients’ characteristics
After Institutional Review Board approval and written informed consent were obtained, peripheral blood (PB) samples were obtained from 28 patients with CLL who were treated in the Leukemia Department at The University of Texas MD Anderson Cancer Center from 2011 to 2016. The clinical characteristics of all the patients are summarized in Supplementary Table 1.
CLL cell fractionation
To isolate low-density cells, PB cells were fractionated using Histopaque-1077 (Sigma-Aldrich). More than 95% of the fractionated PB lymphocytes obtained from these patients were CD19+/CD5+, as assessed by flow cytometry.
Western immunoblotting
Western immunoblotting was performed as described previously (5). The following primary antibodies were used: monoclonal mouse anti-human STAT3, mouse anti-human CD5, and mouse anti-human α-catalytic subunit of casein kinase 2 (CK2) (BD Biosciences) and rabbit anti-human serine pSTAT3 and rabbit anti–B-cell linker protein (BLNK) (Cell Signaling Technology). Densitometry analysis was performed using an Epson Expression 1680 scanner (Epson America, Inc.). Densitometry values were normalized by dividing the numerical value of each sample signal by the numerical value of the corresponding β-actin signal, used as a loading control.
Mass spectrometry analysis
Mass spectrometry was performed as previously described (18). Briefly, the silver-stained bands from the pull-down assay were de-stained and subjected to in-gel digestion. The resulting peptides were analyzed by nano-liquid chromatography-coupled ion trap mass spectrophotometry. Electrospray ion trap mass spectrometry was performed on an LTQ linear ion trap mass spectrometer (Thermo Fisher Scientific). The resulting proteins were then identified by a database search for the fragment spectra using the National Center for Biotechnology Information database of non-redundant proteins. Resulting peptide matches were manually curated.
Incubation of CLL cells with neutralizing antibodies
A total of 1 × 106 cells/ml were placed in flasks in minimum essential medium alpha (Invitrogen) supplemented with 10% bovine calf serum (HyClone Laboratories). CD5-neutralizing antibodies (Abcam) were added at a concentration of 1 to 2500 per ml. Cells were incubated at 37°C for 16 hours and harvested for further studies.
Immunoprecipitation studies
Immunoprecipitation studies were done as previously described (19). Briefly, CLL cell lysates were incubated with anti–serine pSTAT3, anti-CK2, anti-CD5, or anti-BLNK antibodies for 16 hours at 4°C. Protein A agarose beads (EMD Millipore) were added for 2 hours at 4°C. For negative controls, the cytoplasmic lysates were incubated either with rabbit serum plus protein A agarose beads or with protein A agarose beads alone. After three washes with radioimmunoprecipitation assay buffer, the beads were suspended in sodium dodecyl sulfate (SDS) sample buffer, boiled for 5 minutes, and removed by centrifugation; and the supernatant proteins were separated by SDS–polyacrylamide gel electrophoresis (PAGE). Human Embryonic Kidney 293 (HEK293) cells, HeLa cells, Jurkat T lymphoblastic leukemia cells, and cells from the myeloid-derived lines K-562 and RAMOS were used as controls in the immunoprecipitation studies.
Isolation of nuclear and cytoplasmic extracts
Non-denatured nuclear and cytoplasmic extracts of CLL cells were prepared using an NE-PER extraction kit (Thermo Fisher Scientific) and confirmed by Western immunoblotting–based detection of the nuclear protein lamin B and the cytoplasmic ribosomal protein S6 (5).
In vitro kinase assay
Recombinant STAT3 from Novus Biologicals and active CK2 from New England Biolabs were used according to the manufacturers’ instructions. Briefly, 360 ng STAT3 was incubated with 100 ng CK2 in adenosine 5′-triphosphate–supplemented (200 μM) kinase buffer (20 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 5 mM dithiothreitol) for 30 minutes at 37°C. Subsequently, the reaction was stopped with 2× Laemmli buffer, and phosphorylation of STAT3 was determined using anti–serine pSTAT3 antibodies in Western immunoblotting.
RNA extraction
After thawing in warm water, cells were washed twice with Roswell Park Memorial Institute 1640 medium (Gibco), and TRIzol (Thermo Fisher Scientific) was added. The RNA was purified using an RNeasy purification procedure (QIAGEN). RNA quality and concentration were analyzed with a spectrophotometer (ND-1000; NanoDrop Technologies).
Quantitative reverse-transcription polymerase chain reaction analysis (qRT-PCR)
We used 500 ng of total RNA in one-step qRT-PCR (Applied Biosystems) with an ABI PRISM 7700 sequence detection system (Applied Biosystems) using a TaqMan gene expression assay for CD5 and BLNK according to the manufacturer’s instructions. Samples were run in triplicate, and relative quantification was performed by using the comparative CT method.
Transfection of CLL cells with CK2, CD5 or BLNK small interfering RNA (siRNA)
Five microliters of siPORT NeoFX agent and 50 pmol of CD5 siRNA, BLNK-siRNA, or FAM-labeled siRNA targeting human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or scrambled control (Applied Biosystems) were each diluted in 50 μl of OPTI-MEM I and then mixed together and incubated at room temperature for 10 minutes. A total of 5 × 106 cells suspended in 0.2 ml of OPTI-MEM I medium containing the siRNA and transfection agents were incubated at room temperature. After 1 hour of incubation, transfections were performed by electroporation (Bio-Rad Laboratories), and then the cells were cultured in complete Roswell Park Memorial Institute 1640 medium. Transfection efficiency was calculated on the basis of the green fluorescent protein (GFP)–conjugated siRNA measured by flow cytometry (Becton, Dickinson and Company). The apoptosis rate of the evaluated transfected cells was ≤ 30% as assessed by Annexin V/PI using flow cytometry analysis.
Confocal microscopy
CLL cells were incubated in microtubes with phosphate-buffered saline (PBS) supplemented with 2% bovine serum (HyClone) and with anti–S6 ribosomal protein antibodies (Cell Signaling Technology) and anti-CD5 antibodies (Becton, Dickinson and Company) for 1 hour. After being washed three times with phosphate-buffered saline, the cells were suspended in 5 mg/ml solution of 4′,6-diamidino-2-phenylindole (DAPI) dye (Invitrogen) for 5 minutes and then washed in phosphate-buffered saline to remove the unbound dye. The cells were then placed into μ-slide VI0.4 chamber slides (ibidi, LLC) for microscopic analysis. The slides were viewed using an Olympus FluoView 500 confocal laser scanning microscope (Olympus America), and images were analyzed using the FluoView software (Olympus America).
Results
CK2 binds to STAT3 and phosphorylates STAT3 on serine 727 residues
To identify a serine kinase that induces phosphorylation of STAT3 on serine residues in CLL cells, we immunoprecipitated protein extract of CLL cells obtained from three patients with anti–serine pSTAT3 antibodies and analyzed the immunoprecipitated protein using mass spectrometry. One of the 635 proteins that were pulled down with anti–serine pSTAT3 antibodies was the β-regulatory subunit of the serine/threonine kinase CK2. Therefore we sought to determine whether CK2 is the kinase that induces constitutive phosphorylation of STAT3 on serine 727 residues in CLL cells.
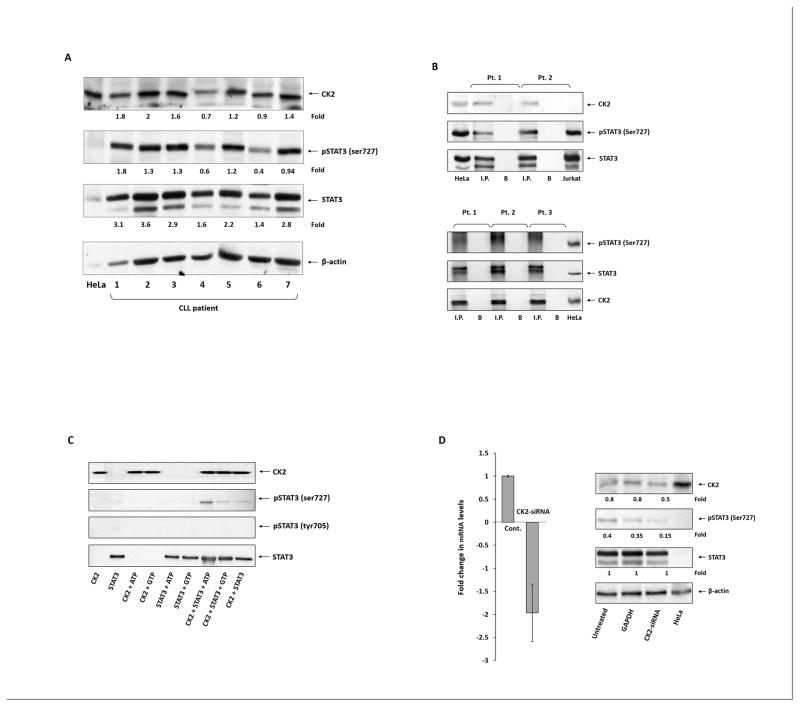
Although CK2 is ubiquitously expressed in mammalian cells (20), we first sought to determine whether the catalytically active subunit of CK2 is present in CLL cells. We obtained low-density PB cells from seven patients with CLL and, using Western immunoblotting, found that the α-catalytic subunit of CK2 as well as serine pSTAT3 were readily detected in all samples (Figure 1A).
Figure 1. CK2 phosphorylates STAT3 on serine in CLL cells.
(A) CK2 and serine pSTAT3 are expressed in CLL cells. CLL cells from the PB of seven patients were lysed and subjected to Western immunoblotting using anti-CK2, anti-STAT3, and anti–serine pSTAT3 antibodies. β-actin served as a loading control, and HeLa cells were used as a positive control. (B) STAT3 and CK2 co-immunoprecipitate. CLL cell lysates were immunoprecipitated either with anti-STAT3 (upper panel) or with anti-CK2 (lower panel) antibodies. The immune complex was separated using SDS-PAGE. STAT3, serine pSTAT3, and CK2 were detected in CLL cell lysates that were immunoprecipitated with anti-STAT3 antibodies (upper panel). Similarly, CK2, STAT3, and serine pSTAT3 were detected in CLL cell lysates that were immunoprecipitated with anti-CK2 antibodies (lower panel) using Western immunoblotting. HeLa and Jurkat cells were used as controls. I.P., immunoprecipitate; B, beads. (C) CK2 phosphorylates STAT3 on serine 727 residues. Recombinant human STAT3 was incubated with (treated) or without (control) active CK2 in adenosine 5′-triphosphate (ATP)– or guanosine 5′-trisphosphate (GTP)-supplemented buffer for 30 minutes and analyzed by western immunoblotting. As shown, maximal phosphorylation of STAT3 occurred in the presence of CK2 and ATP. (D) CK2-siRNA reduces the phosphoserine STAT3 levels in CLL cells. CLL PB cells from two patients were transfected with CK2-siRNA using electroporation. After 48 hours, the cells were harvested and processed. Transfection efficiency was 30%, as assessed by flow cytometry detecting cells with intracellular GFP-conjugated siRNA. Left panel: CK2-siRNA significantly reduced CK2 mRNA levels. qRT-PCR was used to detect CK2 transcripts. The δ-δ cycle threshold method was used to determine the relative fold change in CK2 transcripts after transfection with CD5-siRNA. Right panel: Western immunoblotting of CLL cells from two patients transfected with CK2-siRNA or GAPDH were analyzed using Western immunoblotting. As shown, CK2-siRNA, but not GAPDH, significantly reduced the protein levels of CK2 and serine pSTAT3 compared with levels in untreated CLL cells. HeLa cells were used as positive controls.
Then, to validate the mass spectrometry results, we immunoprecipitated CLL cell extracts obtained from two CLL patients using anti-STAT3 antibodies. As expected, we found the α-catalytic subunit of CK2 along with phosphoserine STAT3 co-immunoprecipitated with STAT3 (Figure 1B, upper panel). To confirm these findings, we immunoprecipitated CLL cell extracts from three patients with anti-CK2 antibodies. We found that the α-catalytic subunit of CK2 co-immunoprecipitated with STAT3 and phosphoserine STAT3 (Figure 1B, lower panel), suggesting that CK2 binds to STAT3 and phosphoserine STAT3.
Because we found that the serine/threonine kinase CK2 binds to STAT3 in CLL cells, we sought to determine whether CK2 indeed phosphorylates STAT3 on serine residues. To test this hypothesis, we incubated active CK2 with recombinant human (rh) STAT3 for 30 minutes and, as shown in Figure 1C, found that rhSTAT3 became phosphorylated on serine 727 residues when rhSTAT3 was incubated in the presence, but not in the absence, of active CK2. Then, to confirm these observations, we transfected CLL cells with a CK2-siRNA construct and found that when the levels of CK2 transcripts were significantly downregulated, protein levels of CK2 and serine pSTAT3 were markedly reduced (Figure 1D), suggesting that CK2 induces the induction of STAT3 phosphorylation of serine 727 residues.
CD5 is required for the induction of STAT3 serine phosphorylation in CLL cells
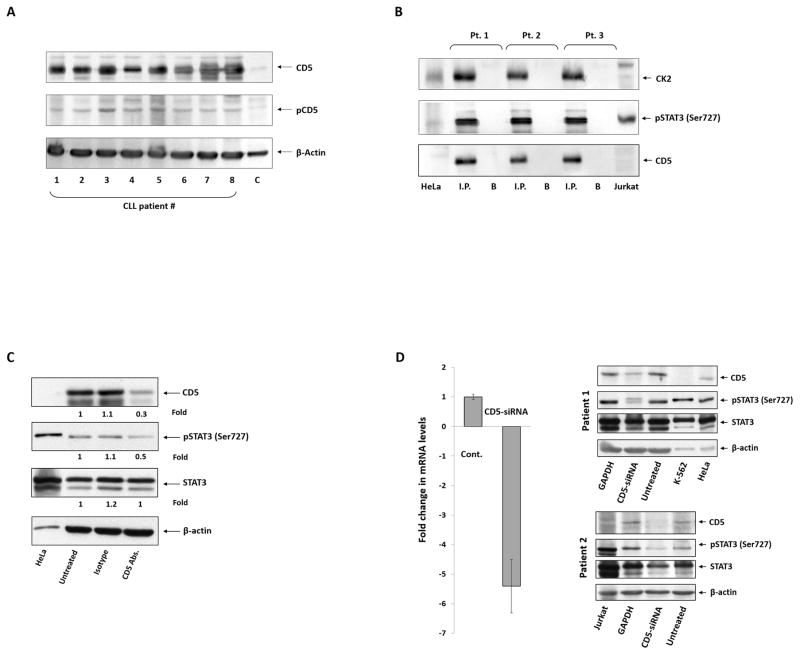
CK2 and STAT3 are ubiquitously expressed in mammalian cells (14, 20). However, in most cells, unphosphorylated STAT3, but not serine phosphorylated STAT3, is commonly detected, likely because CK2 has to be activated to exert its enzymatic activity. CD5 is a transmembrane glycoprotein present on the surface of CLL cells. Only a rare population of non-malignant B-cells express CD5 (3). Because CD5 is thought to be biologically active upon its phosphorylation, we obtained CLL cells from eight CLL patients and found that CD5 is constitutively phosphorylated in CLL cells (Figure 2A). Because the interaction between CK2 and CD5 induces activation of CK2 (21–24), we sought to determine whether CD5 binds CK2 in CLL cells. Therefore, we immunoprecipitated CLL cell lysates with anti-CK2 antibodies and, using Western immunoblotting, found that CD5, as well as serine pSTAT3, co-immunoprecipitated with the α-catalytic subunit of CK2 (Figure 2B).
Figure 2. CD5 is required for CK2-induced phosphorylation of STAT3 in CLL cells.
(A) CD5 is constitutively phosphorylated in CLL cells. Western immunoblotting detected CD5 and tyrosine pCD5 in CLL cells from the PB of eight randomly selected CLL patients. C, control (Jurkat cells). (B) CD5, CK2, and serine pSTAT3 co-immunoprecipitate. CLL cell lysates from three patients were immunoprecipitated with anti-CD5 antibodies. The immune complex was separated using SDS-PAGE, and serine pSTAT3, CK2, and CD5 were detected in the immunoprecipitate by Western immunoblotting. HeLa and Jurkat cells were used as controls. I.P., immunoprecipitate; B, beads. (C) CD5-neutralizing antibodies reduce serine pSTAT3 levels in CLL cells. CLL cells were incubated with CD5-neutralizing antibodies, isotype antibodies, or culture media. After 2 hours, the cells were harvested and subjected to Western immunoblotting. As shown, compared with untreated cells, the levels of CD5 and serine pSTAT3 were lower in cells that were incubated with CD5-neutralizing antibodies, but not in cells incubated with the isotype control antibodies, whereas the levels of STAT3 were unchanged by any treatment. Densitometry analysis was used to quantify protein levels. Abs.: antibodies. (D) CD5-siRNA reduces the phosphoserine STAT3 levels in CLL cells. CLL PB cells from two patients were transfected with CD5-siRNA using electroporation. After 48 hours, the cells were harvested and processed. Transfection efficiency was 35%, as assessed by flow cytometry detecting cells with intracellular GFP-conjugated siRNA. Left panel: CD5-siRNA significantly reduced CD5 mRNA levels. qRT-PCR was used to detect CD5 transcripts. The δ-δ cycle threshold method was used to determine the relative fold change in CD5 transcripts after transfection with CD5-siRNA. Right panel: Western immunoblotting of CLL cells from two patients transfected with CD5-siRNA or GAPDH were analyzed using Western immunoblotting. As shown, CD5-siRNA, but not GAPDH, significantly reduced the protein levels of CD5 and serine pSTAT3 compared with levels in untreated CLL cells. K-562, HeLa, and Jurkat cells were used as positive controls.
Anti-CD5 antibodies have been found to block the homophilic interactions of B-cells and to inhibit B-cell activation, suggesting that CD5 binds to and induces CD5 (25). To determine whether CK2 requires CD5 to induce STAT3 phosphorylation in CLL cells, we first incubated CLL cells with CD5-neutralizing antibodies, and we found a significant reduction in levels of both CD5 and serine pSTAT3 (Figure 2C). Then, to confirm this observation, we transfected CLL cells with a CD5-siRNA construct and found that when the levels of CD5 transcripts were significantly downregulated, protein levels of CD5 and serine pSTAT3 were markedly reduced (Figure 2D), suggesting that CD5 is indeed required for the induction of STAT3 phosphorylation of serine 727 residues.
BLNK contributes to serine phosphorylation of STAT3 in CLL cells
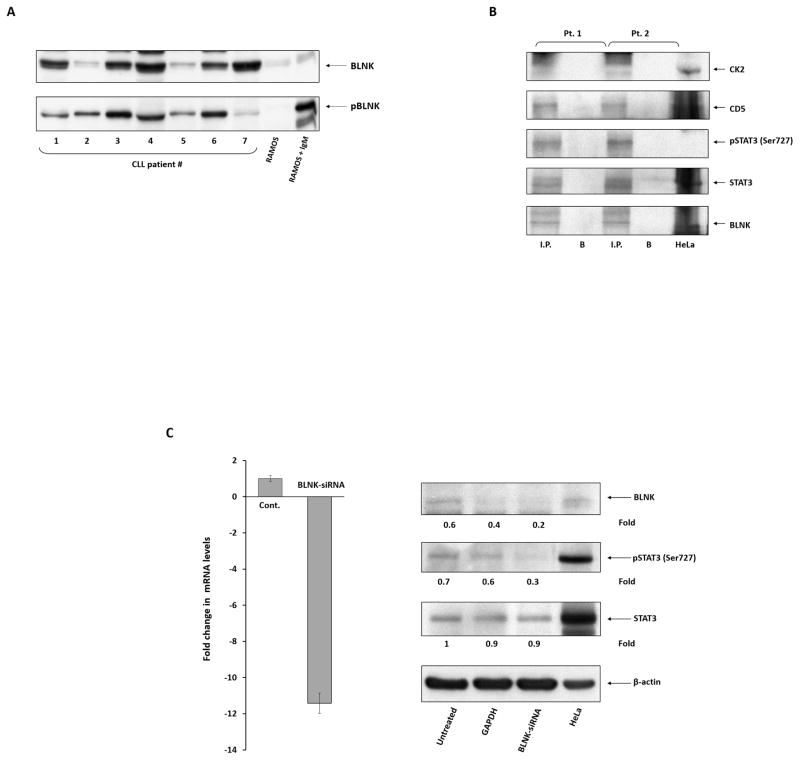
We found that CK2 and CD5 conjoin in phosphorylating STAT3 on serine 727 residues in CLL cells. However, unlike in CLL cells, STAT3 is not constitutively phosphorylated in T-lymphocytes, although both CK2 and CD5 are commonly detected there (26). Therefore, we wondered whether an additional factor not present in T-cells is required for the induction of STAT3 phosphorylation on serine residues in CLL cells. BLNK, also known as SLP-65, is an adaptor protein that is expressed in B-cells (27) but not in T-cells and was found to be required for lipopolysaccharide-induced STAT3 phosphorylation (28). To determine whether BLNK is present and activated in CLL cells, we performed a Western immunoblot analysis, and we detected BLNK and tyrosine pBLNK in CLL cells in seven out of seven patients (Figure 3A). Furthermore, when we immunoprecipitated CLL cell extracts with anti-BLNK antibodies, we found that BLNK co-immunoprecipitated CK2, CD5, STAT3, and serine pSTAT3 (Figure 3B). We then transfected CLL cells with BLNK siRNA and found that when BLNK transcript levels were significantly downregulated, protein levels of BLNK decreased and that while the levels of STAT3 did not change, the levels of serine pSTAT3 were markedly reduced, suggesting that BLNK is required for serine phosphorylation of STAT3 in CLL cells (Figure 3C).
Figure 3. BLNK is required for CK2-induced phosphorylation of STAT3 in CLL cells.
(A) BLNK is constitutively phosphorylated in CLL cells. We obtained PB CLL cells from seven randomly selected CLL patients and, using Western immunoblotting, detected tyrosine pBLNK in all samples. Equal loading was confirmed by Ponceau staining (not shown). Untreated and IgM-treated RAMOS cells were used as controls. IgM: immunoglobulin M. (B) CK2, CD5, STAT3, and serine pSTAT3 co-immunoprecipitated with BLNK. CLL cell lysates were immunoprecipitated with anti-BLNK antibodies. The immune complex was separated using SDS-PAGE, and STAT3, serine pSTAT3, CK2, and CD5 were detected in the immunoprecipitate by Western immunoblotting. I.P., immunoprecipitate; B, beads. (C) BLNK-siRNA inhibits the phosphorylation of STAT3 on serine residues. CLL cells from three different patients were transfected by electroporation with BLNK-siRNA or GAPDH or were left untreated (controls). Left panel: BLNK-siRNA significantly reduced BLNK mRNA levels. qRT-PCR was used to detect BLNK transcripts. The δ-δ cycle threshold method was used to determine the relative fold change in BLNK transcripts after treatment with BLNK-siRNA. Right panel: CLL cells transfected with BLNK-siRNA or GAPDH were analyzed using Western immunoblotting. As shown, BLNK-siRNA, but not GAPDH, significantly reduced the protein levels of BLNK and serine pSTAT3, whereas the levels of STAT3 remained unchanged by either treatment. HeLa cells were used as positive controls.
A protein complex comprising CK2, CD5, and BLNK phosphorylates STAT3 in CLL cells
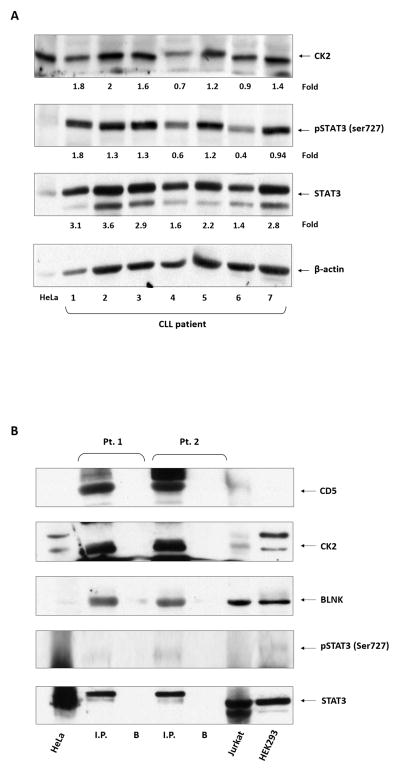
Our data suggest that both CD5 and BLNK, uniquely present in CLL cells, bind CK2 and are required for the induction of STAT3 phosphorylation. Therefore, we hypothesized that CD5, CK2, BLNK, and STAT3 form a complex that enables the phosphorylation of STAT3 by CK2. To test this hypothesis, we immunoprecipitated CLL cell protein extracts with anti-STAT3 antibodies. Then, using Western immunoblotting, we found that STAT3 co-immunoprecipitated with CD5, CK2, BLNK, and serine pSTAT3 (Figure 4A). To confirm these data, we immunoprecipitated CLL cell protein extracts with anti-CD5 antibodies and, using Western immunoblotting, found that CD5 co-immunoprecipitated with STAT3, CK2, BLNK, and serine pSTAT3 (Figure 4B). Taken together, our data suggest that a protein complex consisting of CD5, CK2, and BLNK binds STAT3 and that the entire protein complex is required for the phosphorylation of STAT3 on serine 727 residues.
Figure 4. CK2, CD5, and BLNK form a phosphorylation complex that facilitates the phosphorylation of STAT3 on serine residues.
(A) CLL cell lysates from two randomly selected CLL patients were immunoprecipitated with anti-STAT3 antibodies. The immune complex was separated using SDS-PAGE, and STAT3, serine pSTAT3, CK2, BLNK, and CD5 were detected in the immunoprecipitate by Western immunoblotting. HeLa and Jurkat cells were used as controls. I.P., immunoprecipitate; B, beads. (B) Similarly, CLL cell lysates were immunoprecipitated with anti-CD5 antibodies. The immune complex was separated using SDS-PAGE, and STAT3, serine pSTAT3, BLNK, CD5, and CK2 were detected in the immunoprecipitate by Western immunoblotting. HeLa, Jurkat, and HEK293 cells were used as controls.
Serine pSTAT3 detaches from the phosphorylation complex and translocates to the nucleus
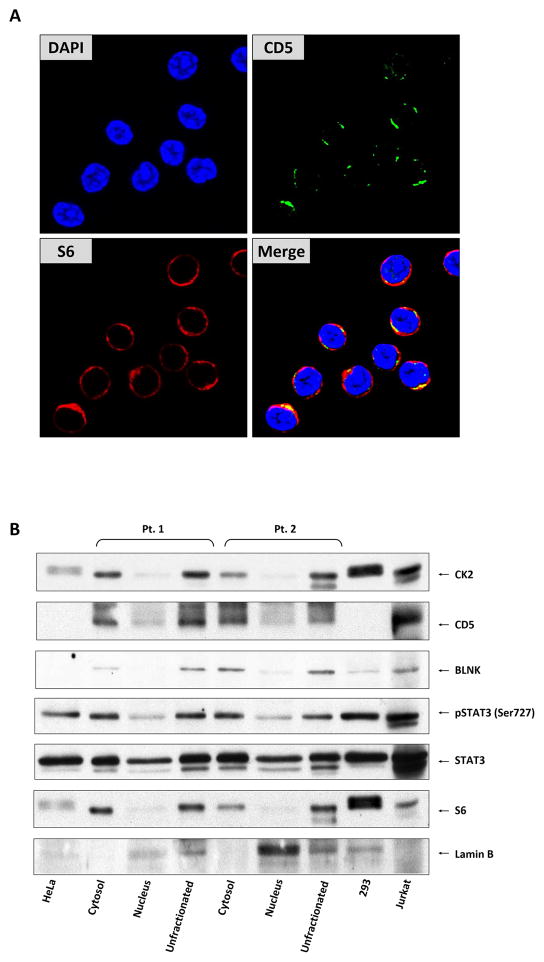
Because CD5 is a surface protein, we wondered whether the STAT3 phosphorylation complex is cell surface bound or whether a CD5 intracytoplasmic isoform participates in the phosphorylation complex. Using confocal microscopy of CLL cells, we found that CD5 remains membrane bound (Figure 5A), suggesting that CD5 anchors the STAT3 phosphorylation protein complex to the cell membrane.
Figure 5. STAT3 phosphorylation complex is anchored to membranal CD5 and de-assembles after STAT3 phosphorylation.
(A) CD5, a crucial member of the STAT3 phosphorylation complex, is expressed on the cell membrane but not in the cytosol or nucleus of CLL cells. Confocal microscopy images (400×) of freshly isolated CLL cells were stained with anti-CD5 antibodies for 1 hour. CD5 was detected on the cell surface but not the cytoplasm or nucleus of CLL cells. DAPI was used to detect the nucleus and S6 the cytoplasm of CLL cells. (B) The BLNK/CD5/CK2/STAT3 phosphorylation complex is de-assembled following the phosphorylation of STAT3. Cytosolic and nuclear fractions from PB CLL cells of two patients were analyzed using Western immunoblotting. As shown, the proteins participating in the formation of the STAT3 phosphorylation complex—BLNK, CD5, and CK2—were detected only in the cytosolic fractions whereas STAT3 and serine pSTAT3 were detected in both the cytosol and the nucleus of CLL cells. Lamin B was used as the nuclear fraction control and S6 as the cytoplasmic fraction control. HeLa, HEK293, and Jurkat cells were used as controls.
In a previous study, we demonstrated that after STAT3 phosphorylation, phosphoserine STAT3 forms dimers and translocates to the nucleus, where it binds to DNA (5). To determine whether any of the components of the STAT3 phosphorylation protein complex are co-shuttled with serine pSTAT3 to the nucleus, we obtained CLL cell cytoplasmic and nuclear fractions and performed a Western immunoblotting analysis. While proteins from the entire complex—CD5, CK2, BLNK, STAT3, and serine pSTAT3—were detected in the cytosolic fractions, only serine pSTAT3 was detected in the nuclear extracts (Figure 5B). Taken together, these data suggest that the STAT3 phosphorylation protein complex is anchored by CD5 to the cell membrane and that upon phosphorylation, pSTAT3 detaches from the complex and is shuttled to the nucleus.
Discussion
We and others have previously shown that in circulating CLL cells, STAT3 is constitutively phosphorylated on serine 727 residues (4, 5); However, what induces this post-translational modification of STAT3 was largely unknown.
Here, we show that a protein complex consisting of CK2, CD5, and BLNK induces phosphorylation of STAT3 on serine 727 residues. Whereas CK2 is expressed in all hematopoietic cells, BLNK is expressed exclusively in B-cells (27); and CD5, primarily present on the surface of T-cells (29), is usually detected on the cellular membrane of CLL cells. Unlike normal B-cells, CLL cells co-express CD5 and BLNK (3), and these two proteins participate in the formation of a protein complex that is likely unique to CLL cells. Because pSTAT3 activates numerous pathways that provide CLL cells with a survival advantage (9, 10, 19, 30, 31), the cellular mechanism that induces STAT3 phosphorylation is a potential therapeutic target to inhibit in CLL.
The threonine/serine kinase CK2 is ubiquitously expressed in eukaryotic cells (20). However, although STAT3 is one of approximately 300 well-established CK2 substrates (32), STAT3 is rarely phosphorylated on serine residues in unstimulated B-cells, suggesting that in order to induce STAT3 phosphorylation, CK2 has to be activated and attach to STAT3. CD5 is known to associate with and activate CK2 (21). Our data show that in CLL cells, CD5 contributes to STAT3 phosphorylation, as transfection of CLL cells with CD5-siRNA significantly downregulated CD5 mRNA and protein levels and markedly reduced the levels of serine pSTAT3. In addition, CD5-neutralizing antibodies, known to inhibit the activation of CD5, significantly reduced CD5 and serine pSTAT3 protein levels in CLL cells, likely because CD5 and STAT3 form a feed-forward loop (33).
Both CLL cells and T-cells express CD5. Yet, while in T-cells CD5 activates CK2 (34), STAT3 is typically not constitutively phosphorylated, suggesting that another component, not shared by CLL cells and T-cells is required for the induction of STAT3 phosphorylation on serine residues. Like their normal B-cell counterparts, CLL cells express BLNK. BLNK is a cytoplasmic B-cell–specific protein that plays a critical role in B-cell development (35) and participates in the induction of STAT3 phosphorylation in CLL cells (36). We found that in CLL cells, BLNK is a crucial component of the STAT3 phosphorylation complex and is required for maximal STAT3 serine phosphorylation. Remarkably, BLNK is also a part of a protein complex that includes phospholipase Cγ2 and Bruton tyrosine kinase phosphorylation in CLL cells (37).
CK2 is a stable tetrameric enzyme consisting of two α-catalytic and two β-regulatory subunits (38). Recent studies suggested that some of the CK2 β-subunit functions are independent of the CK2 tetramer (39). In a search for a serine kinase that phosphorylates STAT3 in CLL cells we used a proteomics approach and found that the β-regulatory subunit of CK2 co-immunoprecipitated with STAT3. Then, to determine whether CK2 phosphorylates STAT3 we obtained recombinant human tetrameric CK2 and found that it phosphorylated rhSTAT3 on serine 727 residues. Because in all other experiments we used anti-CK2 antibodies directed against the α-catalytic subunit or CK2-α-siRNA, we were unable to determine whether the CK2 β-subunit plays a role in the induction of STAT3 phosphorylation.
Several investigators reported that CK2 is overexpressed and activated in a variety of hematological malignancies such as multiple myeloma (40, 41), mantle cell lymphoma (40, 41), follicular lymphoma (42), diffuse large B-cell lymphoma (42), acute B-lymphoblastic leukemia (43–45), and CLL (46–49). These observations led to the development of CK2 inhibitors. One such inhibitor CX-4945, whose activity is being investigated in clinical trials, was found to possess anti-neoplastic activity in CLL (50). Ex vivo studies showed that a combination of fludarabine and CX-4945 significantly reduced CLL cell viability (47) and that CX-4945 synergistically interacted with the Bruton tyrosine kinase inhibitor ibrutinib (47) in eliminating CLL cells. Because CK2 is present in all mammalian cells, the specificity of CK2 inhibitors might be a crucial limiting factor in the development of CK2 inhibitors as anti-neoplastic agents. In contrast, targeting the STAT3 phosphorylation complex might prove to be a specific and effective approach.
Supplementary Material
Acknowledgments
We thank Sarah J. Bronson for editing our manuscript. This study was supported by grants from the CLL Global Research Foundation and the Cancer Center Support Grant from the NIH/NCI, P30 CA016672.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Authorship
Conceived: ZE
Designed: UR, ZE
Analysis and bioinformatics: UR
Provision of study materials or patients: AF, JB, SO, PB, PT, NJ, WW, MK, ZE
Performed the Western blot and the IP experiments: ZL, DMH
Performed the RNA studies: DMH, PL,
Performed the confocal, mass spectrometry and in-vitro kinase assays: DMH, IV
Wrote the manuscript: UR, ZE
Final approval of manuscript: All authors.
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Raman C. CD5, an important regulator of lymphocyte selection and immune tolerance. Immunol Res. 2002;26:255–63. doi: 10.1385/IR:26:1-3:255. [DOI] [PubMed] [Google Scholar]
- 3.Caligaris-Cappio F, Gobbi M, Bofill M, Janossy G. Infrequent normal B lymphocytes express features of B-chronic lymphocytic leukemia. J Exp Med. 1982;155:623–8. doi: 10.1084/jem.155.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank DA, Mahajan S, Ritz J. B lymphocytes from patients with chronic lymphocytic leukemia contain signal transducer and activator of transcription (STAT) 1 and STAT3 constitutively phosphorylated on serine residues. J Clin Invest. 1997;100:3140–8. doi: 10.1172/JCI119869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazan-Halevy I, Harris D, Liu Z, Liu J, Li P, Chen X, et al. STAT3 is constitutively phosphorylated on serine 727 residues, binds DNA, and activates transcription in CLL cells. Blood. 2010;115:2852–63. doi: 10.1182/blood-2009-10-230060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–5. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 7.Akira S. Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells. 1999;17:138–46. doi: 10.1002/stem.170138. [DOI] [PubMed] [Google Scholar]
- 8.Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell research. 2008;18:254–67. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li P, Harris D, Liu Z, Liu J, Keating M, Estrov Z. Stat3 activates the receptor tyrosine kinase like orphan receptor-1 gene in chronic lymphocytic leukemia cells. PLoS One. 2010;5:e11859. doi: 10.1371/journal.pone.0011859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Hazan-Halevy I, Harris DM, Li P, Ferrajoli A, Faderl S, et al. STAT-3 activates NF-kappaB in chronic lymphocytic leukemia cells. Mol Cancer Res. 2011;9:507–15. doi: 10.1158/1541-7786.MCR-10-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P, Grgurevic S, Liu Z, Harris D, Rozovski U, Calin GA, et al. Signal transducer and activator of transcription-3 induces MicroRNA-155 expression in chronic lymphocytic leukemia. PLoS One. 2013;8:e64678. doi: 10.1371/journal.pone.0064678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozovski U, Grgurevic S, Bueso-Ramos C, Harris DM, Li P, Liu Z, et al. Aberrant LPL Expression, Driven by STAT3, Mediates Free Fatty Acid Metabolism in CLL Cells. Mol Cancer Res. 2015;13:944–53. doi: 10.1158/1541-7786.MCR-14-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozovski U, Keating MJ, Estrov Z. Targeting inflammatory pathways in chronic lymphocytic leukemia. Critical reviews in oncology/hematology. 2013;88:655–66. doi: 10.1016/j.critrevonc.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arinobu Y, Sugimoto R, Akaiwa M, Arima K, Otsuka T, Hamasaki N, et al. Augmentation of signal transducer and activation of transcription (STAT)6 and STAT3 expression in stimulated B and T cells. Biochem Biophys Res Commun. 2000;277:317–24. doi: 10.1006/bbrc.2000.3674. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nature reviews Cancer. 2014;14:736–46. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Lawrence MS, Wan Y, Stojanov P, Sougnez C, Stevenson K, et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011;365:2497–506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quesada V, Conde L, Villamor N, Ordonez GR, Jares P, Bassaganyas L, et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet. 2012;44:47–52. doi: 10.1038/ng.1032. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Y, Yang W, Xia Y, Hawke D, Liu DX, Lu Z. Ras-induced and extracellular signal-regulated kinase 1 and 2 phosphorylation-dependent isomerization of protein tyrosine phosphatase (PTP)-PEST by PIN1 promotes FAK dephosphorylation by PTP-PEST. Molecular and cellular biology. 2011;31:4258–69. doi: 10.1128/MCB.05547-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D, Huang Y, Zeng J, Chen B, Huang N, Guo N, et al. Down-regulation of JAK1 by RNA interference inhibits growth of the lung cancer cell line A549 and interferes with the PI3K/mTOR pathway. Journal of cancer research and clinical oncology. 2011;137:1629–40. doi: 10.1007/s00432-011-1037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turowec JP, Duncan JS, French AC, Gyenis L, St Denis NA, Vilk G, et al. Protein kinase CK2 is a constitutively active enzyme that promotes cell survival: strategies to identify CK2 substrates and manipulate its activity in mammalian cells. Methods in enzymology. 2010;484:471–93. doi: 10.1016/B978-0-12-381298-8.00023-X. [DOI] [PubMed] [Google Scholar]
- 21.Raman C, Kimberly RP. Differential CD5-dependent regulation of CD5-associated CK2 activity in mature and immature T cells: implication on TCR/CD3-mediated activation. J Immunol. 1998;161:5817–20. [PubMed] [Google Scholar]
- 22.Calvo J, Vilda JM, Places L, Simarro M, Padilla O, Andreu D, et al. Human CD5 signaling and constitutive phosphorylation of C-terminal serine residues by casein kinase II. J Immunol. 1998;161:6022–9. [PubMed] [Google Scholar]
- 23.Axtell RC, Xu L, Barnum SR, Raman C. CD5-CK2 binding/activation-deficient mice are resistant to experimental autoimmune encephalomyelitis: protection is associated with diminished populations of IL-17-expressing T cells in the central nervous system. J Immunol. 2006;177:8542–9. doi: 10.4049/jimmunol.177.12.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Axtell RC, Webb MS, Barnum SR, Raman C. Cutting edge: critical role for CD5 in experimental autoimmune encephalomyelitis: inhibition of engagement reverses disease in mice. J Immunol. 2004;173:2928–32. doi: 10.4049/jimmunol.173.5.2928. [DOI] [PubMed] [Google Scholar]
- 25.Brown MH, Lacey E. A ligand for CD5 is CD5. J Immunol. 2010;185:6068–74. doi: 10.4049/jimmunol.0903823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng J, Cantrell D. STAT3 is a serine kinase target in T lymphocytes. Interleukin 2 and T cell antigen receptor signals converge upon serine 727. J Biol Chem. 1997;272:24542–9. doi: 10.1074/jbc.272.39.24542. [DOI] [PubMed] [Google Scholar]
- 27.Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 28.Meng A, Zhang X, Shi Y. Role of p38 MAPK and STAT3 in lipopolysaccharide-stimulated mouse alveolar macrophages. Experimental and therapeutic medicine. 2014;8:1772–6. doi: 10.3892/etm.2014.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 30.Rozovski U, Calin GA, Setoyama T, D’Abundo L, Harris DM, Li P, et al. Signal transducer and activator of transcription (STAT)-3 regulates microRNA gene expression in chronic lymphocytic leukemia cells. Molecular cancer. 2013;12:50. doi: 10.1186/1476-4598-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozovski U, Grgurevic S, Bueso-Ramos C, Harris DM, Li P, Liu Z, et al. Aberrant LPL Expression, Driven by STAT3, Mediates Free Fatty Acid Metabolism in CLL Cells. Mol Cancer Res. 2015 doi: 10.1158/1541-7786.MCR-14-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–68. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 33.Zhang C, Xin H, Zhang W, Yazaki PJ, Zhang Z, Le K, et al. CD5 Binds to Interleukin-6 and Induces a Feed-Forward Loop with the Transcription Factor STAT3 in B Cells to Promote Cancer. Immunity. 2016;44:913–23. doi: 10.1016/j.immuni.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sestero CM, McGuire DJ, De Sarno P, Brantley EC, Soldevila G, Axtell RC, et al. CD5-dependent CK2 activation pathway regulates threshold for T cell anergy. J Immunol. 2012;189:2918–30. doi: 10.4049/jimmunol.1200065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Y, Ballicora MA, Leykam JF, Preiss J. Mechanism of reductive activation of potato tuber ADP-glucose pyrophosphorylase. J Biol Chem. 1998;273:25045–52. doi: 10.1074/jbc.273.39.25045. [DOI] [PubMed] [Google Scholar]
- 36.Jin G, Hamaguchi Y, Matsushita T, Hasegawa M, Le Huu D, Ishiura N, et al. B-cell linker protein expression contributes to controlling allergic and autoimmune diseases by mediating IL-10 production in regulatory B cells. J Allergy Clin Immunol. 2013;131:1674–82. doi: 10.1016/j.jaci.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 37.Kim YJ, Sekiya F, Poulin B, Bae YS, Rhee SG. Mechanism of B-cell receptor-induced phosphorylation and activation of phospholipase C-gamma2. Mol Cell Biol. 2004;24:9986–99. doi: 10.1128/MCB.24.22.9986-9999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allende JE, Allende CC. Protein kinases. 4. Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995;9:313–23. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- 39.Bibby AC, Litchfield DW. The multiple personalities of the regulatory subunit of protein kinase CK2: CK2 dependent and CK2 independent roles reveal a secret identity for CK2beta. Int J Biol Sci. 2005;1:67–79. doi: 10.7150/ijbs.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manni S, Brancalion A, Mandato E, Tubi LQ, Colpo A, Pizzi M, et al. Protein kinase CK2 inhibition down modulates the NF-kappaB and STAT3 survival pathways, enhances the cellular proteotoxic stress and synergistically boosts the cytotoxic effect of bortezomib on multiple myeloma and mantle cell lymphoma cells. PLoS One. 2013;8:e75280. doi: 10.1371/journal.pone.0075280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piazza FA, Ruzzene M, Gurrieri C, Montini B, Bonanni L, Chioetto G, et al. Multiple myeloma cell survival relies on high activity of protein kinase CK2. Blood. 2006;108:1698–707. doi: 10.1182/blood-2005-11-013672. [DOI] [PubMed] [Google Scholar]
- 42.Pizzi M, Piazza F, Agostinelli C, Fuligni F, Benvenuti P, Mandato E, et al. Protein kinase CK2 is widely expressed in follicular, Burkitt and diffuse large B-cell lymphomas and propels malignant B-cell growth. Oncotarget. 2015;6:6544–52. doi: 10.18632/oncotarget.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mishra S, Pertz V, Zhang B, Kaur P, Shimada H, Groffen J, et al. Treatment of P190 Bcr/Abl lymphoblastic leukemia cells with inhibitors of the serine/threonine kinase CK2. Leukemia. 2007;21:178–80. doi: 10.1038/sj.leu.2404460. [DOI] [PubMed] [Google Scholar]
- 44.Mishra S, Reichert A, Cunnick J, Senadheera D, Hemmeryckx B, Heisterkamp N, et al. Protein kinase CKIIalpha interacts with the Bcr moiety of Bcr/Abl and mediates proliferation of Bcr/Abl-expressing cells. Oncogene. 2003;22:8255–62. doi: 10.1038/sj.onc.1207156. [DOI] [PubMed] [Google Scholar]
- 45.Song C, Gowda C, Pan X, Ding Y, Tong Y, Tan BH, et al. Targeting casein kinase II restores Ikaros tumor suppressor activity and demonstrates therapeutic efficacy in high-risk leukemia. Blood. 2015;126:1813–22. doi: 10.1182/blood-2015-06-651505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shehata M, Schnabl S, Demirtas D, Hilgarth M, Hubmann R, Ponath E, et al. Reconstitution of PTEN activity by CK2 inhibitors and interference with the PI3-K/Akt cascade counteract the antiapoptotic effect of human stromal cells in chronic lymphocytic leukemia. Blood. 2010;116:2513–21. doi: 10.1182/blood-2009-10-248054. [DOI] [PubMed] [Google Scholar]
- 47.Prins RC, Burke RT, Tyner JW, Druker BJ, Loriaux MM, Spurgeon SE. CX-4945, a selective inhibitor of casein kinase-2 (CK2), exhibits anti-tumor activity in hematologic malignancies including enhanced activity in chronic lymphocytic leukemia when combined with fludarabine and inhibitors of the B-cell receptor pathway. Leukemia. 2013;27:2094–6. doi: 10.1038/leu.2013.228. [DOI] [PubMed] [Google Scholar]
- 48.Martins LR, Lucio P, Silva MC, Anderes KL, Gameiro P, Silva MG, et al. Targeting CK2 overexpression and hyperactivation as a novel therapeutic tool in chronic lymphocytic leukemia. Blood. 2010;116:2724–31. doi: 10.1182/blood-2010-04-277947. [DOI] [PubMed] [Google Scholar]
- 49.Martins LR, Lucio P, Melao A, Antunes I, Cardoso BA, Stansfield R, et al. Activity of the clinical-stage CK2-specific inhibitor CX-4945 against chronic lymphocytic leukemia. Leukemia. 2014;28:179–82. doi: 10.1038/leu.2013.232. [DOI] [PubMed] [Google Scholar]
- 50.Chon HJ, Bae KJ, Lee Y, Kim J. The casein kinase 2 inhibitor, CX-4945, as an anti-cancer drug in treatment of human hematological malignancies. Front Pharmacol. 2015;6:70. doi: 10.3389/fphar.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.