Abstract
Estrogens are potent and efficacious neuroprotectants both in vitro and in vivo in a variety of models of neurotoxicity. We determined the structural requirements for neuroprotection in an in vitro assay using a panel of more than 70 novel estratrienes, synthesized to reduce or eliminate estrogen receptor (ER) binding. We observed that neuroprotection could be enhanced by as much as 200-fold through modifications that positioned a large bulky group at the C2 or C4 position of the phenolic A ring of the estratriene. Further, substitutions on the B, C or D rings either reduced or did not markedly change neuroprotection. Collectively, there was a negative correlation between binding to ERs and neuroprotection with the more potent compounds showing no ER binding. In an in vivo model for neuroprotection, transient cerebral ischemia, efficacious compounds were active in protection of brain tissue from this pro-oxidant insult. We demonstrated that these non-feminizing estrogens engage in a redox cycle with glutathione, using the hexose monophosphate shunt to apply cytosolic reducing potential to cellular membranes. Together, these results demonstrate that non-feminizing estrogens are neuroprotective and protect brain from the induction of ischemic- and Alzheimer’s disease (AD)-like neuropathology in an animal model. These features of non-feminizing estrogens make them attractive compounds for assessment of efficacy in AD and stroke, as they are not expected to show the side effects of chronic estrogen therapy that are mediated by ER actions in the liver, uterus and breast.
Key Works: Estrogens, estradiol, non-feminizing estrogens, structure-activity relationships, redox cycling
1. Introduction
1.1 Need for non-feminizing estrogens
Menopause, in which a human female transitions to reproductive senescence, occurs during the fifth decade of life among women (Timiras et al., 1995). This transition is characterized by depleted ovarian follicles, declines in naturally circulating levels of sex hormones, such as estrogens and progesterone, and a dysregulation of gonadotrophin feedback loops marked by increasing levels of follicular stimulating hormone and lutenizing hormone (Rannevik et al., 1995). Further, menopause is associated with hot flashes, urogenital atrophy, cognitive decline (specifically learning and memory), and other symptoms that reduce quality of life (Freedman, 2002; Sherwin & Henry, 2008). To alleviate these symptoms, estrogen-containing hormone therapy (HT) is given. Premarin® (conjugated equine estrogens), a purified pregnant mare urine compound first developed by Wyeth, is the most widely used estrogen-based menopausal HT in North America (Hersh et al., 2004) although a plethora of other estrogen-containing treatment options exist as well (Sood et al., 2014). Despite these treatments showing attenuation of undesirable menopausal symptoms (Sood et al., 2014) and possible protection against brain aging and injury (Engler-Chiurazzi et al., 2016a), recent comprehensive studies have demonstrated that in older women, chronic exposure to feminizing estrogens alone or in combination with a synthetic progestin leads to an increase in pro-thrombotic and pro-mitotic side effects (Manson et al., 2003; Wassertheil-Smoller et al., 2003; Anderson et al., 2004). These chronic toxicities of feminizing estrogens are mediated by the effects of persistent estrogen exposure in estrogen responsive tissues, like the liver, uterus, and breast. This, combined with the controversial findings of the Women’s Health Initiative regarding the potential increased risk for adverse outcomes among reproductively senescent women taking HT has spurred an intense debate as to whether estrogen-containing HTs should continue to be administered for treatment of menopausal symptoms and brain aging. Given that approximately half of the aging adult population is female, there is an important medical need to develop novel treatments for the menopausal transition and aging processes with a more acceptable risk-to-benefit ratio.
1.2. Neuroprotective effects of estrogens
We were among the first research groups to document the beneficial actions of estrogens on the central nervous system (reviewed in Engler-Chiurazzi et al., 2016b). We first demonstrated potent neuroprotective activity of the feminizing estrogen, 17β-estradiol (17β-E2), in 1994 (Bishop & Simpkins, 1994). Since then, the neuroprotective effects of feminizing estrogens have been confirmed using neuronal cultures and primary cells against a variety of toxicities including serum deprivation (Green et al., 1997a; Green et al., 1997b), β-amyloid toxicity (Green et al., 1998; Pike, 1999), and oxidative stress (Behl et al., 1995; Goodman et al., 1996; Sawada et al., 1998; Sawada et al., 2000)), among others, in hippocampal, amygdala, cortical and mesencephalic neurons (for review, please see Green & Simpkins, 2000; Garcia-Segura et al., 2001; Lee & McEwen, 2001). Similarly, in vivo feminizing estrogens have been shown to enhance cognitive outcomes (Engler-Chiurazzi et al., 2016a). As well, in in vivo animal models of brain injury, feminizing estrogens impart protection in models of cerebral ischemia (Simpkins et al., 1997; Dubal et al., 1998; Yang et al., 2000), following kainic acid treatment (Azcoitia et al., 1998), and in contusion injury models (Nakamizo et al., 2000; Gatson et al., 2012). Indeed, in stroke, the protective effects of estrogens are seen in a variety of models including transient and permanent middle cerebral artery occlusion models (Simpkins et al., 1997; Alkayed et al., 1998; Dubal et al., 1998; Perez et al., 2005b), global forebrain ischemia models (Sudo et al., 1997), photothrombotic focal ischemia models (Fukuda et al., 2000), and glutamate-induced focal cerebral ischemia models (Mendelowitsch et al., 2001). The protection afforded by estrogens is seen in rats, mice, and gerbils (Simpkins et al., 1997; Culmsee et al., 1999; Chen et al., 2001) and in adult and middle-aged female rats, as well as in reproductively senescent female rats (Wise et al., 2001). This protection is seen even in the presence of diabetes and hypertension (Carswell et al., 2000; Toung et al., 2000). Similarly, the neuroprotective effects of estrogens are observed against subarachnoid hemorrhage (Yang et al., 2001). Finally, the neuroprotective actions of estrogen are also seen in males (Hawk et al., 1998; Toung et al., 1998). Collectively, potent estrogen protection in these model systems suggest that this steroid hormone may play an important role in preserving neurons in the face of a variety of insults and represents an important therapeutic target for alleviating brain aging and disease. However, given the potential for undesirable peripheral activity of feminizing estrogens, the development of novel estrogen analogues that act in brain but not on reproductive organs represents a promising future therapeutic option for the treatment of brain aging.
1.3. Discovery of neuroprotection by non-feminizing estrogens
In the process of conducting controled studies for the neuroprotective effects of 17β-E2, we discovered that 17α-estradiol (17α-E2) was as potent as 17β-E2 in protection of neurons from toxicity (Green et al., 1997a). 17α-E2 is a weak diastereomer of 17β-E2 and despite the fact that 17β-E2 binds avidly to estrogen receptors (ERs) α and β and activates tissues in a hormonally-responsive manner, 17α-E2 is biologically weak at both receptors. We went on to show that the enantiomer of 17β-E2 (ent-17β-E2), which has identical physiochemical properties as 17β-E2 except for interactions with other stereospecific molecules such as ERs is potently neuroprotective (Green et al., 2001). ent-17β-E2 is reported to interact only weakly with ERs (Chernayaev et al., 1975; Payne & Katzenellenbogen, 1979) and lacks estrogenic effects on reproductive tissues in rodents (Terenius, 1968; 1971). Importantly, although ent-17β-E2 exerts only slight anti-uterotrophic activity and can antagonize the uterotrophic effects of 17β-E2 (Edgren & Jones, 1969; Terenius, 1971), ent-17β-E2 is still a potent neuroprotectant (Green et al., 2001). These collective findings suggest that neuroprotective effects of estrogens do not necessarily require action at the ER.
2. Structure-activity relationship among estrogens
In view of the observation that many of chronic estrogen treatment side-effects are likely due to peripheral effects of orally administered estrogen preparations acting via known ERs (Dubey et al., 2005; Maki, 2006; Salpeter et al., 2006; Coker et al., 2009; Resnick et al., 2009), we sought to determine if non-feminizing estrogens could have the beneficial effects of estrogens on brain protection, without the negative peripheral side effects of traditional feminizing preparations. We undertook a series of studies to define the structure-activity relationship among estrogen-like compounds for both neuroprotection and ER binding, based on our and other’s observations of a disparity between ER binding and neuroprotection (Behl et al., 1997; Green et al., 1997b; Moosmann & Behl, 1999; Green et al., 2001). We initially assessed over 70 synthetic estrogens to determine the structure activity relationship among the compounds.
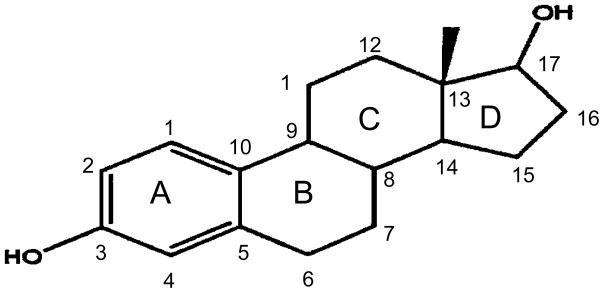
We defined that the minimal structural requirement for neuroprotection by estrogens is the steroid A ring. Estrogens are the only class of steroids that have a phenolic A ring (Figure 1). Any modifications that eliminated the phenolic nature of the A ring completely eliminated neuroprotective activity. These modifications included saturation of the A ring or creation of a covalent bond with substituents through the 3 carbon oxygen.
Figure 1.
Structure of 17 β-E2. Letters denote the 4 rings of the molecule and numbers indicate the carbons positions.
Modifications of the D ring had little effect on the neuroprotective activity, including changing the orientation of the 17 carbon hydroxyl group (as is the case with 17α-E2), eliminating the 17 carbon hydroxyl group (estratriene-3-ol), or opening the D ring (Perez et al., 2005a). The addition of polar groups in the B and C rings tended to reduce neuroprotective activity of estrogens indicating that the center of the molecular requires sufficient lipophilicity for neuroprotective activity (Perez et al., 2005a).
Finally, we observed a marked enhancement in neuroprotection with the addition of non-polar group to the 2 and/or 4 carbons of the A ring (Perez et al., 2005a). One particularly potent non-feminizing estrogen is ZYC-26, which has a large adamantly group on the 2 carbon and a methyl group on the 4 carbon (Figure 2).
Figure 2.
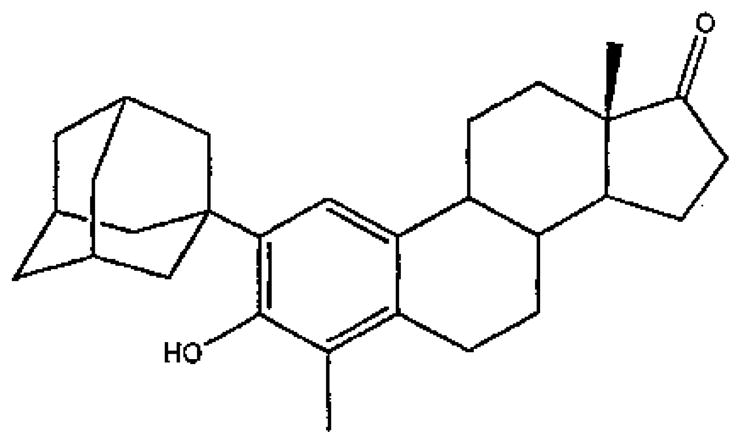
Structure of 2-(1-Adamantyl)-4 methyl-3-hydroxyestra-1, 3, 5(10)-trien-17-one (ZYC-26).
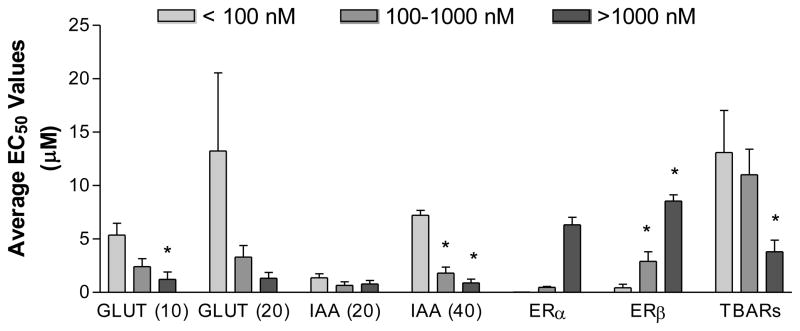
When compounds were categorized by their binding affinity to ERα, we observed the expected positive correlations with ERβ binding (Figure 3) but a negative correlation with neuroprotective activity and lipid peroxidation (Figure 3).
Figure 3.
Relationship among ER binding, neuroprotective activity and lipid peroxidation among estrogens. All activities were categorized by ERα binding affinity and we observed a marked negative relationship between ERα binding and neuroprotection in 4 assays of neurotoxicity. GLUT indicated glutamate toxicity at 10 and 20 mM concentrations of glutamate. IAA indicated indolacetic acid at 20 and 40 mM concentrations (IAA). TBARs indicated thiobarbituric acid reactive substances (TBARs). Reproduced from Perez et al., 2006, with permission.
Collectively these structure-activity relationships argue for a neuroprotective mechanism(s) that do not require action at the classical ERs. As such, we began a program of research to determine the mechanism(s) by which non-feminizing estrogens are potently neuroprotective.
3. Mechanism of non-feminizing estrogen neuroprotection
Two observations suggested a potential mechanism by which non-feminizing estrogens could be potently neuroprotective. First, the need for the estrogen molecule to avoid polar groups on the B and C rings suggested that their interaction with lipid membranes was a critical component of their neuroprotective activity. Given the high lipophilicity of estrogens, most estrogens are associated with lipid membranes. Indeed, we have shown that estrogens insert into lipid bilayers and that the presence of an adamantyl group at the C-2 position affects the position of the A ring 3-hydroxyl group such that it is possible to detect an orientation that would bring it into close proximity to the double bonds of membrane lipids; a position that is optimal for terminating lipid peroxidation (Cegelski et al., 2006).
Second, we demonstrated that 17β-E2, 17α-E2, and estratriene-3-ol all synergizes with the aqueous soluble antioxidant, glutathione to result in a 400-fold enhancement of the neuroprotective activity of both feminizing and non-feminizing estrogens (Green et al., 1998). This suggests that lipid resident estrogens can interact with soluble cytosolic antioxidants to halt an oxidative/inflammatory cellular cascade and achieve neuroprotection.
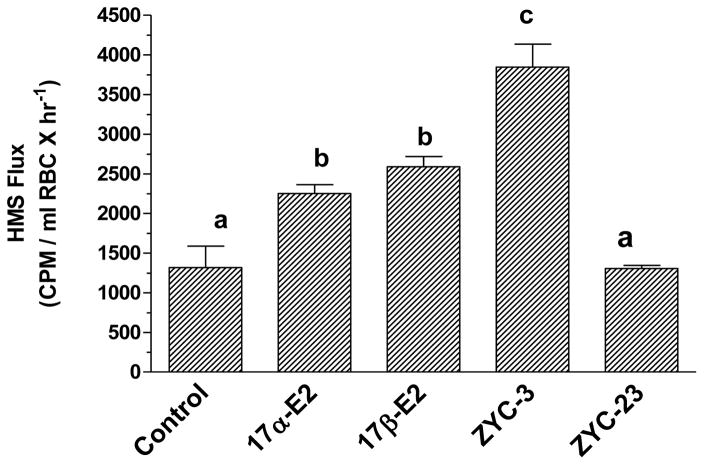
We then assessed the possible redox cycling of estrogens with glutathione using human erythrocytes (Dykens et al., 2004). Human erythrocytes (RBCs) lack mitochondria and nuclei, making them a useful model to assess estrogens for assessing the redox potential of non-feminizing estrogens. In this model, the hexose monophosphate shunt (HMS) generates NADPH, which serves to reduce glutathione (GSH) from oxidized glutathione (GSSG), allowing us to test the extent to which estrogens enhance the activity of this redox cycle. With H2O2 exposure, the HMS is activated. In the presence of either 17β-E2 or 17α-E2, an approximate doubling in HMS activity was observed (Figure 4). The potent neuroprotectant, ZYC-3 increased HMS activity about 3-fold, but the inactive compound, ZYC-23 did not affect HMS activity (Figure 4). These results indicate that estrogens are able to tap the large reducing potential of the HMS, and through NADPH-induced reduction of GSSH to GSH, and able to apply this reducing potential to lipid membranes using estrogens as a mediator.
Figure 4.
Carbon flux through the hexose monophosphate shunt in normal human erythrocytes (RBCs). In the absence of an oxidative insult, the compounds have no effect on HMS flux. However, exposure to 10 mM H2O2 significantly increases HMS flux in untreated control RBCs. Treatment with the indicated compounds at 1 mM for 10 min prior to addition of 0.3 mCi 14C-U-glucose results in varying increases in HMS flux, with 17β-E2 and 17α-E2, showing comparable responses. ZYC-3 significantly increased HMS flux over the estrogens, and ZYC-23, a non-neuroprotective negative control, yields HMS flux rates indistinguishable from untreated controls. Means not significantly different at P < 0.05 (Bonferroni), share superscripts; ANOVA F = 22.18, * P < 0.0001. Adapted and reprinted with permission from Dykens et al., 2004
4. Summary and Conclusions
The present series of studies provide evidence that synthetic estrogens that do not interact with ERs are potent neuroprotectants, likely working through a redox cycle that involves glutathione and the HMS. The neuroprotection depends on a phenolic A ring and potency is enhanced through aliphatic substituents on the 2 and/or 4 carbons of the estrogen molecule. These compounds avoid ERα and ERβ, and as such are candidates for chronic therapy aimed at preserving the brain from insults sustained by diseases, like AD, or more acute traumas, like stroke. However, of important clinical significance is that, despite the strong supportive evidence for their neuroprotective actions, because these non-feminizing estrogens impart these effects independent of the classical ERs, the known peripheral benefits of estrogen-containing HTs, including on urogenital tract, bone, and cardiovascular tissues when administered near to the time of menopause initiation (Freedman, 2002; Gambacciani & Levancini, 2014; Hale & Shufelt, 2015), may not be observed. Thus, in conclusion, non-feminizing estrogens represent a novel and effective therapeutic intervention approach for the prevention of injury-induced neuropathology.
Highlights.
Estrogen neuroprotection can be independent of estrogen receptors
The phenolic A ring of estrogen molecule is essential to its neuroprotective activity
Allophalic substitutions at the 2 and 4 carbon of the A ring enhance neuroprotective potency
Non-feminizing estrogens represent a novel target for post-menopausal brain aging
Yet, with these agents, peripheral estrogenic benefits will not be observed
Acknowledgments
Funding: This work was supported by the National Institutes of Health grants P20 GM109098, P01 AG022550, P01 AG027956, U54 GM1049492 and by WVU College of Medicine intramural funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke; a journal of cerebral circulation. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S Women’s Health Initiative Steering C. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. Jama. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Estradiol prevents kainic acid-induced neuronal loss in the rat dentate gyrus. Neuroreport. 1998;9:3075–3079. doi: 10.1097/00001756-199809140-00029. [DOI] [PubMed] [Google Scholar]
- Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton CJ, Holsboer F. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Molecular pharmacology. 1997;51:535–541. [PubMed] [Google Scholar]
- Behl C, Widmann M, Trapp T, Holsboer F. 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochemical and biophysical research communications. 1995;216:473–482. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- Bishop J, Simpkins JW. Estradiol treatment increases viability of glioma and neuroblastoma cells in vitro. Molecular and cellular neurosciences. 1994;5:303–308. doi: 10.1006/mcne.1994.1036. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Dominiczak AF, Macrae IM. Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. American journal of physiology. Heart and circulatory physiology. 2000;278:H290–294. doi: 10.1152/ajpheart.2000.278.1.H290. [DOI] [PubMed] [Google Scholar]
- Cegelski L, Rice CV, O’Connor RD, Caruano AL, Tochtrop GP, Cai ZY, Covey DF, Schaefer J. Mapping the locations of estradiol and potent neuroprotective analogues in phospholipis bilayers by REDOR. Drug development research. 2006;66:93–102. [Google Scholar]
- Chen J, Xu W, Jiang H. 17 beta-estradiol protects neurons from ischemic damage and attenuates accumulation of extracellular excitatory amino acids. Anesthesia and analgesia. 2001;92:1520–1523. doi: 10.1097/00000539-200106000-00033. [DOI] [PubMed] [Google Scholar]
- Chernayaev GA, Barkova TI, Egorova VV, Sorokina IB, Ananchenko SN, Mataradze GD, Sokolova NA, Rozen VB. A series of optical structural and isomeric analogs of estradiol: a comparative study of the biological activity and affinity to cytosol receptor of rabbit uterus. Journal of steroid biochemistry. 1975;6:1483–1488. doi: 10.1016/0022-4731(75)90201-0. [DOI] [PubMed] [Google Scholar]
- Coker LH, Hogan PE, Bryan NR, Kuller LH, Margolis KL, Bettermann K, Wallace RB, Lao Z, Freeman R, Stefanick ML, Shumaker SA. Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI Study. Neurology. 2009;72:125–134. doi: 10.1212/01.wnl.0000339036.88842.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Vedder H, Ravati A, Junker V, Otto D, Ahlemeyer B, Krieg JC, Krieglstein J. Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: evidence for a receptor-independent antioxidative mechanism. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1999;19:1263–1269. doi: 10.1097/00004647-199911000-00011. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Imthurn B, Barton M, Jackson EK. Vascular consequences of menopause and hormone therapy: importance of timing of treatment and type of estrogen. Cardiovascular research. 2005;66:295–306. doi: 10.1016/j.cardiores.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Dykens JA, Carroll AK, Wiley S, Covey DF, Cai ZY, Zhao L, Wen R. Photoreceptor preservation in the S334ter model of retinitis pigmentosa by a novel estradiol analog. Biochemical pharmacology. 2004;68:1971–1984. doi: 10.1016/j.bcp.2004.06.042. [DOI] [PubMed] [Google Scholar]
- Edgren RA, Jones RC. An anti-estradiol effect of ent-estradiol-17beta (1-estradiol) Steroids. 1969;14:335–341. doi: 10.1016/0039-128x(69)90021-x. [DOI] [PubMed] [Google Scholar]
- Engler-Chiurazzi EB, Brown CM, Povroznik JM, Simpkins JW. Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury. Progress in neurobiology. 2016a doi: 10.1016/j.pneurobio.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi EB, Singh M, Simpkins JW. From the 90’s to now: A brief historical perspective on more than two decades of estrogen neuroprotection. Brain research. 2016b;1633:96–100. doi: 10.1016/j.brainres.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman MA. Quality of Life and Menopause: The Role of Estrogen. Journal of Women’s Health. 2002;11:703–718. doi: 10.1089/15409990260363661. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Yao H, Ibayashi S, Nakahara T, Uchimura H, Fujishima M, Hall ED. Ovariectomy exacerbates and estrogen replacement attenuates photothrombotic focal ischemic brain injury in rats. Stroke; a journal of cerebral circulation. 2000;31:155–160. doi: 10.1161/01.str.31.1.155. [DOI] [PubMed] [Google Scholar]
- Gambacciani M, Levancini M. Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Przeglad menopauzalny = Menopause review. 2014;13:213–220. doi: 10.5114/pm.2014.44996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Progress in neurobiology. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Gatson JW, Liu MM, Abdelfattah K, Wigginton JG, Smith S, Wolf S, Simpkins JW, Minei JP. Estrone is neuroprotective in rats after traumatic brain injury. Journal of neurotrauma. 2012;29:2209–2219. doi: 10.1089/neu.2011.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. Journal of neurochemistry. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- Green PS, Bishop J, Simpkins JW. 17 alpha-estradiol exerts neuroprotective effects on SK-N-SH cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997a;17:511–515. doi: 10.1523/JNEUROSCI.17-02-00511.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PS, Gordon K, Simpkins JW. Phenolic A ring requirement for the neuroprotective effects of steroids. The Journal of steroid biochemistry and molecular biology. 1997b;63:229–235. doi: 10.1016/s0960-0760(97)00124-6. [DOI] [PubMed] [Google Scholar]
- Green PS, Gridley KE, Simpkins JW. Nuclear estrogen receptor-independent neuroprotection by estratrienes: a novel interaction with glutathione. Neuroscience. 1998;84:7–10. doi: 10.1016/s0306-4522(97)00595-2. [DOI] [PubMed] [Google Scholar]
- Green PS, Simpkins JW. Neuroprotective effects of estrogens: potential mechanisms of action. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2000;18:347–358. doi: 10.1016/s0736-5748(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Green PS, Yang SH, Nilsson KR, Kumar AS, Covey DF, Simpkins JW. The nonfeminizing enantiomer of 17beta-estradiol exerts protective effects in neuronal cultures and a rat model of cerebral ischemia. Endocrinology. 2001;142:400–406. doi: 10.1210/endo.142.1.7888. [DOI] [PubMed] [Google Scholar]
- Hale GE, Shufelt CL. Hormone therapy in menopause: An update on cardiovascular disease considerations. Trends in cardiovascular medicine. 2015;25:540–549. doi: 10.1016/j.tcm.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain research. 1998;796:296–298. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. Jama. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McEwen BS. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annual review of pharmacology and toxicology. 2001;41:569–591. doi: 10.1146/annurev.pharmtox.41.1.569. [DOI] [PubMed] [Google Scholar]
- Maki PM. Potential importance of early initiation of hormone therapy for cognitive benefit. Menopause. 2006;13:6–7. doi: 10.1097/01.gme.0000194822.76774.30. [DOI] [PubMed] [Google Scholar]
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M Women’s Health Initiative I. Estrogen plus progestin and the risk of coronary heart disease. The New England journal of medicine. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- Mendelowitsch A, Ritz MF, Ros J, Langemann H, Gratzl O. 17beta-Estradiol reduces cortical lesion size in the glutamate excitotoxicity model by enhancing extracellular lactate: a new neuroprotective pathway. Brain research. 2001;901:230–236. doi: 10.1016/s0006-8993(01)02359-9. [DOI] [PubMed] [Google Scholar]
- Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamizo T, Urushitani M, Inoue R, Shinohara A, Sawada H, Honda K, Kihara T, Akaike A, Shimohama S. Protection of cultured spinal motor neurons by estradiol. Neuroreport. 2000;11:3493–3497. doi: 10.1097/00001756-200011090-00019. [DOI] [PubMed] [Google Scholar]
- Payne DW, Katzenellenbogen JA. Binding specificity of rat alpha-fetoprotein for a series of estrogen derivatives: studies using equilibrium and nonequilibrium binding techniques. Endocrinology. 1979;105:745–753. doi: 10.1210/endo-105-3-743. [DOI] [PubMed] [Google Scholar]
- Perez E, Cai ZY, Covey DF, Simpkins JW. Neuroprotective effects of estratriene analogs: structure-activity relationships and molecular optimization. Drug Development Research. 2005a;66:78–92. [Google Scholar]
- Perez E, Liu R, Yang SH, Cai ZY, Covey DF, Simpkins JW. Neuroprotective effects of an estratriene analog are estrogen receptor independent in vitro and in vivo. Brain research. 2005b;1038:216–222. doi: 10.1016/j.brainres.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer’s disease. Journal of neurochemistry. 1999;72:1552–1563. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;21:103–113. doi: 10.1016/0378-5122(94)00869-9. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Espeland MA, Jaramillo SA, Hirsch C, Stefanick ML, Murray AM, Ockene J, Davatzikos C. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009;72:135–142. doi: 10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter SR, Walsh JM, Greyber E, Salpeter EE. Brief report: Coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. Journal of general internal medicine. 2006;21:363–366. doi: 10.1111/j.1525-1497.2006.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H, Ibi M, Kihara T, Urushitani M, Akaike A, Shimohama S. Estradiol protects mesencephalic dopaminergic neurons from oxidative stress-induced neuronal death. Journal of neuroscience research. 1998;54:707–719. doi: 10.1002/(SICI)1097-4547(19981201)54:5<707::AID-JNR16>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Sawada H, Ibi M, Kihara T, Urushitani M, Honda K, Nakanishi M, Akaike A, Shimohama S. Mechanisms of antiapoptotic effects of estrogens in nigral dopaminergic neurons. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000;14:1202–1214. doi: 10.1096/fasebj.14.9.1202. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Frontiers in neuroendocrinology. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. Journal of neurosurgery. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Sood R, Faubion SS, Kuhle CL, Thielen JM, Shuster LT. Prescribing menopausal hormone therapy: an evidence-based approach. International journal of women’s health. 2014;6:47–57. doi: 10.2147/IJWH.S38342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo S, Wen TC, Desaki J, Matsuda S, Tanaka J, Arai T, Maeda N, Sakanaka M. Beta-estradiol protects hippocampal CA1 neurons against transient forebrain ischemia in gerbil. Neuroscience research. 1997;29:345–354. doi: 10.1016/s0168-0102(97)00106-5. [DOI] [PubMed] [Google Scholar]
- Terenius L. Differential inhibition in vitro of 17 beta-estradiol binding in the mouse uterus and vagina by optical antipodes of estrogens. Molecular pharmacology. 1968;4:301–310. [PubMed] [Google Scholar]
- Terenius L. The Allen-Doisy test for estrogens reinvestigated. Steroids. 1971;17:653–661. doi: 10.1016/0039-128x(71)90081-x. [DOI] [PubMed] [Google Scholar]
- Timiras P, Quay W, Vernadakis A, editors. Hormones and aging. CRC Press; New York: 1995. [Google Scholar]
- Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke; a journal of cerebral circulation. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- Toung TK, Hurn PD, Traystman RJ, Sieber FE. Estrogen decreases infarct size after temporary focal ischemia in a genetic model of type 1 diabetes mellitus. Stroke; a journal of cerebral circulation. 2000;31:2701–2706. doi: 10.1161/01.str.31.11.2701. [DOI] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ Investigators WHI. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. Jama. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Bottner M, Rosewell KL. Estradiol is a protective factor in the adult and aging brain: understanding of mechanisms derived from in vivo and in vitro studies. Brain research Brain research reviews. 2001;37:313–319. doi: 10.1016/s0165-0173(01)00136-9. [DOI] [PubMed] [Google Scholar]
- Yang SH, He Z, Wu SS, He YJ, Cutright J, Millard WJ, Day AL, Simpkins JW. 17-beta estradiol can reduce secondary ischemic damage and mortality of subarachnoid hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21:174–181. doi: 10.1097/00004647-200102000-00009. [DOI] [PubMed] [Google Scholar]
- Yang SH, Shi J, Day AL, Simpkins JW. Estradiol exerts neuroprotective effects when administered after ischemic insult. Stroke; a journal of cerebral circulation. 2000;31:745–749. doi: 10.1161/01.str.31.3.745. discussion 749–750. [DOI] [PubMed] [Google Scholar]