Abstract
Age is an important consideration for recovery and repair after spinal cord injury. Spinal cord injury is increasingly affecting the middle-aged and aging populations. Despite rapid progress in research to promote axonal regeneration and repair, our understanding of how age can modulate this repair is rather limited. In this review, we discuss the literature supporting the notion of an age-dependent decline in axonal growth after central nervous system (CNS) injury. While both neuron-intrinsic and extrinsic factors are involved in the control of axon growth after injury, here we focus on possible intrinsic mechanisms for this age-dependent decline.
Keywords: Spinal cord injury, Aging, Axon growth, Axon regeneration, Neuron-intrinsic, CNS injury
Introduction
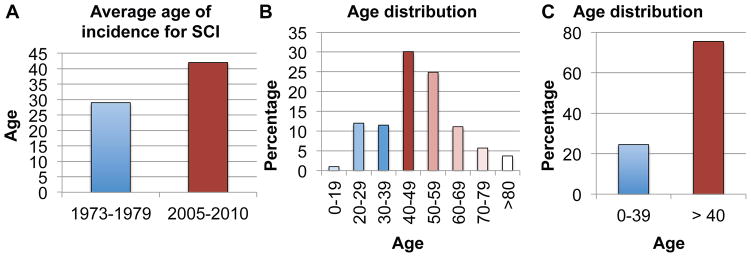
Age is an important factor for spinal cord injury (SCI) and repair. SCI is increasingly inflicted in the middle aged and aging populations [21, 90]. The average age of incidence for SCI has risen substantially in recent years, from ~29 in the 1970s to ~42 since 2010 in the United States (National Spinal Cord Injury Statistical Center), partly due to an increasingly active older population. In a census study initiated by the Christopher and Dana Reeve Foundation, the average age of people in the United States who reported being paralyzed due to a SCI is now at ~48, with the peak age group of 40–49 followed closely by the 50–59 age group (Fig. 1). Together, the 40 and above age groups represent about 75% of all people with a paralyzing SCI. Thus, whereas SCI used to preferentially affect young individuals, today this condition most widely impacts older individuals and especially the middle-aged group. These changing demographics call for a critical need to better understand how age and aging impact recovery and repair after SCI.
Figure 1. Spinal Cord Injury and Age.
A: Average Age of Incidence for SCI increased from ~29 in the 1970s to ~42 since 2010 in the US (from National Spinal Cord Injury Statistical Center); B–C: Age distribution for people who live with a paralyzing spinal cord injury in the US. (Adapted From One Degree of Separation, 2009, Christopher and Dana Reeve Foundation).
The field of SCI has certainly recognized the importance of age in both the basic and clinical arenas [24, 30, 38–40, 101]. However, our understanding of how age and aging impact repair and recovery after SCI is still rather limited. In particular, despite the critical importance of axon regeneration in central nervous system (CNS) repair and the rapid progress in understanding its molecular regulation [6, 9, 11, 61, 64, 73, 74, 80, 84, 89, 91, 93], a major gap exists in our knowledge of how age impacts CNS axon regeneration. This is in large part due to the fact that CNS axons even in young adult mammals have a very limited natural ability to regenerate after injury. Meanwhile, most of the studies in the field use young animals as the model system, corresponding at best to teenagers/young adults in humans. It is understandable that studying how aging impacts spinal cord repair can be intimidating: it is extremely time and resource consuming, and experimental manipulations may be less likely to have a detectable effect relative to experiments performed in young animals.
As this dichotomy in age between human spinal cord injury populations and experimental animal models will inevitably impede translational efforts for restorative therapies, it is of special importance to better understand the impact age has on spinal cord repair. A parallel can be drawn in the field of stroke research, where age has been recognized as an important variable in translating basic research findings into clinical practice [31]. In this review, we will discuss the evidence for an age-dependent decline in axon growth after CNS injury. Although both neuron-intrinsic and -extrinsic factors are likely to play significant roles in this age-dependent decline, here we focus on potential neuron-intrinsic mechanisms as the first step to start a discourse on this important topic.
Age-dependent decline in axon growth after injury in diverse systems
In model organisms, axon regeneration has been reported to decline with age. In aging zebrafish, axon regeneration occurs at a reduced speed with an increased latency, both of which were tentatively attributed to factors intrinsic to the neurons [37]. Similarly, in C. elegans, efficiency of axon regeneration declines with age, and intra-neuronal mechanisms seem to be at play [13]. An important question in the relationship between aging and axon regeneration is whether molecular pathways involved in lifespan and organismal aging also play a significant role in aging-associated alterations in regeneration. Indeed, worms deficient in the insulin/IGF1 (insulin-like growth factor 1) receptor DAF-2, which have an increased lifespan, exhibit enhanced regeneration in aged but not young adults [13]. These effects require the activity of the downstream forkhead transcription factor DAF-16/FOXO. However, DAF-16 appears to regulate axon regeneration independently of its role in lifespan as it is required in different cell/tissue types for these two functions. On the other hand, DAF-18/PTEN inhibits regeneration in both young and old worms via the TOR pathway independently of age. Unlike in organismal aging, the DAF-2/DAF-16 pathway does not appear to cross-talk with DAF-18/TOR in regulating age-dependent regeneration. These complex relationships, which remain to be fully elucidated, indicate that the molecular pathways involved in organismal aging can regulate axon regeneration in aging adults, but the same molecular machinery can regulate lifespan and regeneration independently.
In the mammalian peripheral nervous system (PNS), where axons regenerate robustly compared to in the CNS, an age-dependent decline in regeneration has been known for over 30 years [83, 99, 100]. There has been a debate on whether this age-dependent decline is mediated by neuron-intrinsic or extrinsic mechanisms [32, 53, 57]. Recent evidence from in vivo imaging and reciprocal nerve graft experiments between young and old animals implicates a neuron-extrinsic mechanism in which Schwann cells have a reduced ability to clear up axon and myelin debris in aging adults, thus impeding regeneration [51, 79]. However, the molecular underpinnings for the proposed extrinsic mechanism have not been identified and it remains possible that manipulating neuron-intrinsic factors may alleviate this age-dependent decline in PNS regeneration.
In experimental models of mammalian spinal cord injury, relatively few studies have assessed the relationship between age and various outcome measures. Aging reduces locomotor recovery after SCI and is linked to changes in inflammation and myelination [33, 40, 55, 88]. Even fewer studies have examined the effect of age or aging on axon growth after injury. Obviously, since CNS axons have a very limited natural ability to regenerate, it would be difficult, if not impossible, to detect a further reduction in regeneration at an increased age. One study reported that aging impacts axon growth in a tract-specific manner, reducing sprouting of the corticospinal tract (CST), serotonergic (5-HT), raphespinal and catecholaminergic (TH) coerulospinal tracts rostral to the injury site, while the regenerative growth of 5-HT, TH and calcitonin gene-related peptide positive (CGRP+) sensory axons into the lesion site is not impaired by aging [47]. However, this study did not examine true axon regeneration beyond a lesion site.
The recent identification of neuron-intrinsic factors whose manipulation reproducibly promotes axon regeneration in the CNS [64] makes it now possible to address the impact that age has on CNS axon regeneration. One of the most effective targets to promote regeneration to date is the PTEN/mTOR pathway. PTEN (phosphatase and tensin homolog) is a negative regulator of the mTOR (mammalian target of rapamycin) signaling pathway [66]. mTOR activity undergoes developmental decline and is further down-regulated after axonal injury in CST neurons [61]. PTEN deletion in young animals prevents axotomy-induced reduction of mTOR activity and promotes the regeneration of retinal ganglion and CST axons after injury [35, 61, 80].
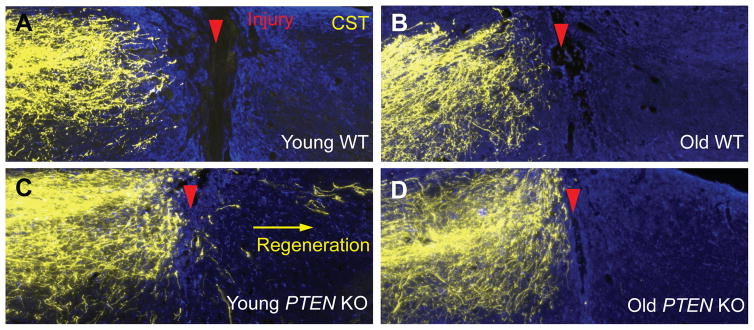
In collaboration with Dr. Wolfram Tetzlaff’s lab at the University of British Columbia, we have examined the effect of age on CST and rubrospinal tract (RST) axon regeneration induced by PTEN deletion [34]. Somewhat surprisingly, as in young mice, PTEN deletion in older mice (12–18 month old for the CST study and 7–8 month old for the RST study) remains effective in preventing axotomy-induced decline in neuron-intrinsic growth state, as assessed by phospho-S6 (p-S6) immunoreactivity (an indicator of mTOR activity, but see below), neuronal soma size and axonal growth proximal to a spinal cord injury. However, axonal regeneration distal to the injury is greatly diminished in both the CST (Fig. 2) and the RST. This decrease in regeneration is accompanied by increased expression of astroglial and inflammatory markers at the injury site such as GFAP and CD68, suggesting neuron-extrinsic mechanisms underlying the age-dependent decline. In essence, PTEN deletion unmasked an age-dependent decline in axon regeneration in the adult mammalian CNS. Two research teams, each working on a different experimental system (the CST in the Zheng lab and the RST in the Tetzlaff lab), reached the same conclusion, supporting the general applicability of this finding [34]. Thus, just as in model organisms and the mammalian PNS, the mammalian CNS also exhibits an age-dependent decline in axon regeneration after injury.
Figure 2. Age-dependent decline in axon regeneration after spinal cord injury.
Age at the time of PTEN deletion influences axon regeneration of the CST 6 weeks after T8 dorsal hemisection. In 12-month old WT animals (B) few CST axons are found at close proximity of the injury, similar to young WT animals (A). After PTEN deletion (C–D), a high number of CST axons grow rostral to the lesion all the way to the injury border, independently of the age PTEN deletion occurs. However, only animals with PTEN deleted at a young age (C) present strong regeneration caudal to the lesion site (Adapted from Geoffroy et al. 2016).
While our study [34] implicated the importance of neuron-extrinsic mechanisms for the age-dependent decline in CNS regeneration, the molecular pathways involved are not known. More importantly, the involvement of neuron-intrinsic factors cannot be excluded for several reasons: 1) An enhanced sensitivity to extrinsic inhibitory cues can manifest itself as increased extrinsic inhibition, e.g. by changes in receptor compositions and/or concentrations at the neuronal or axonal surface, which are neuron intrinsic in nature; 2) There are likely changes in other aspects of neuron-intrinsic growth state in aging neurons that we did not examine in our published study; 3) Even if extrinsic influence were the dominant force in the age-dependent decline in CNS regeneration, manipulating other neuron-intrinsic pathways in combination with PTEN deletion may still reverse at least some of the age-dependent decline in regeneration.
In a study examining CST regeneration in a chronic injury, Du et al. compared PTEN deletion at 1 month and 12 months after thoracic crush injury in 8-week old young mice [25]. The authors found that while deleting PTEN one month after injury (gene deletion at ~12 weeks of age) promoted CST regeneration as assessed 4 months later, very little regeneration was observed at the same time point after gene deletion when PTEN deletion was performed 12 months after injury (i.e., at the age of ~14 months). Interestingly, however, regeneration was observed in the chronic injury model if they assessed regeneration 7 months after PTEN deletion. This result indicates that CST regeneration in older animals is still possible after PTEN deletion but either the speed of regeneration is reduced or the latency between axotomy and the initiation of regeneration is increased, both of which are consistent with diminished regeneration. However, in this study it is not clear whether the diminished regeneration was due to the greater amount of time between injury and PTEN deletion or simply the advanced age. Indeed, this study illustrates the complexity associated with age when studying the effects of chronic injury in axonal repair after CNS injury with rodent models. Future studies need to take into consideration this complexity in order to tease out the effects of chronic injury versus age.
Together, the studies discussed above indicate that an age-dependent decline in axon regeneration exists in C. elegans, and in both the mammalian PNS and CNS. While either neuron-intrinsic or extrinsic mechanisms have been emphasized in different experimental systems, neither could be excluded. Without a careful exploration of both intrinsic and extrinsic mechanisms, we may miss important opportunities to counteract this age-dependent decline. Below we focus on one aspect, the intrinsic aspect, and will consider extrinsic influences elsewhere.
Candidate neuron-intrinsic signaling pathways in the age-dependent decline
As discussed above, PTEN deletion has recently been used to create an enhanced axonal growth state in order to demonstrate an age-dependent decline in CNS regeneration after SCI [34]. This reduced regeneration in older mice may be due to a slower speed of regeneration or a prolonged latency between axonal injury and regeneration [25], although this remains to be firmly established. Regardless, regeneration is diminished in older mice with PTEN deletion. While changes in neuron-extrinsic factors likely contribute to this phenomenon, manipulating neuron-intrinsic pathways may also counteract this age-dependent decline. Here we discuss relevant literature and speculate on signaling pathways that may be involved in such mechanisms, focusing on neuron-intrinsic regulators (Fig. 3 and Fig. 4).
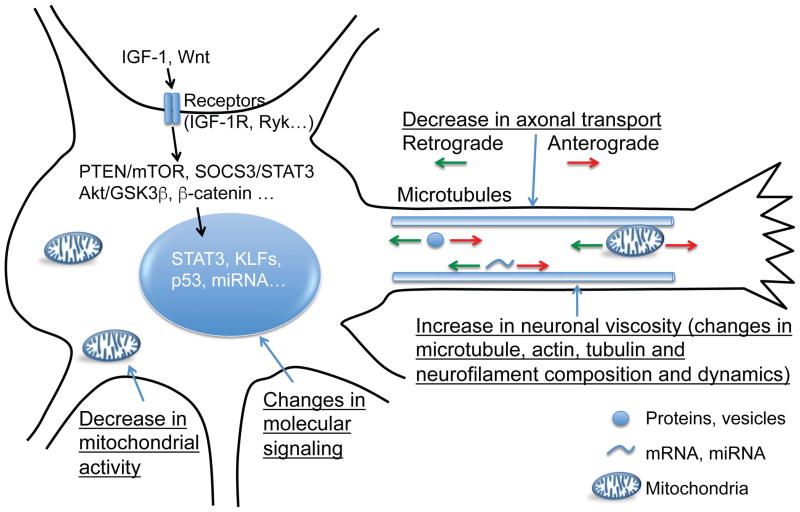
Figure 3. Neuronal intrinsic changes occurring with age.
Diagram of known or speculated intrinsic changes occurring with aging in neurons that may contribute to the age-dependent decline in axon growth. These changes include, but are not limited to, differences in signaling pathways, cytoskeleton composition, axonal transport and mitochondria activity.
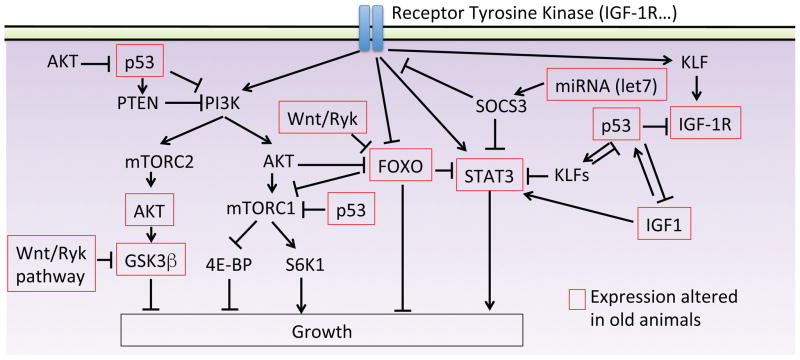
Figure 4. Schematic representation of possible molecular pathways involved in the age-dependent regeneration decline.
Simplified diagram of molecular pathways known or speculated to alter with aging, and potential interactions between them.
1. Components of the PTEN/mTOR signaling pathway
We previously demonstrated that PTEN deletion in older animals remains effective in elevating p-S6 levels and neuronal soma size [34]. However, other aspects of PTEN-mTOR signaling may be compromised in older animals that could still contribute to the age-dependent decline in regeneration, a hypothesis that has not yet been tested. After PTEN deletion, mammalian target of rapamycin complex 1 (mTORC1) is activated via Akt, leading to both the activation of ribosomal protein S6 kinase 1 (S6K1) and the inhibition of the eukaryotic translation initiation factor 4E-binding protein (4E-BP). Although PTEN deletion in aged animals may activate S6K1 to a level similar to that in young animals [34], a recent study indicates that at least in the optic nerve, inhibition of 4E-BP is also required for PTEN deletion-induced axon regeneration [107]. Therefore, it remains possible that 4E-BP is still active in old mice with PTEN deletion, thereby reducing the effect of S6K1. It would be interesting to assess the expression pattern and activity of 4E-BP in old animals after PTEN deletion and determine its role in the age-dependent decline in regeneration.
A second downstream effector of PTEN is mTORC2. Only recently has its role in regeneration been studied [70]. Miao et al. elegantly demonstrated the complexity of the PTEN-mTORC1-mTORC2 balance in controlling axon regeneration in the optic nerve, with Akt as the central point. They found that mTORC2 inhibits regeneration by inhibiting the inactivating phosphorylation of Glycogen Synthase Kinase 3 beta (GSK3β). GSK3β phosphorylation has been suggested to promote peripheral axons regeneration [87, 109]. Additionally, inactivation of the GSK3β-CRMP-2 pathway in dorsal root ganglion (DRG) neurons enhances axon regeneration via increasing microtubule dynamics in the growth cone [63]. Intriguingly, a decrease in p-Akt and p-GSK3β in the hippocampus has been reported in aged mice [78]. One interesting theory to test would be that in aged animals, a decrease of p-GSK3β results in regeneration decline, counterbalancing the regenerative effect of S6K1 activation following PTEN deletion.
2. IGF signaling
Among the intrinsic pathways, insulin/IGF signaling is unusual because of evidence for its apparently contradictory roles in axon growth in the developing versus aging nervous system. In the developing CNS, IGF-1 promotes CST axon growth, although IGF-1 delivery in adult rodents does not promote CST regeneration, likely because of the lack of IGF-receptor expression in axons [2, 44]. Indeed, re-expression and activation of the IGF-1 receptor is required for axon regeneration in adult retinal ganglion cells in vitro [26]. As discussed above, IGF-1-recepor DAF2 in C. elegans has been implicated in an age-dependent decline in axon regeneration, via daf-16/FOXO [13]. This mechanism is independent of PTEN and mTOR. If this finding can be extrapolated to the mammalian CNS, IGF signaling would be expected to inhibit axon regeneration in aged mice. In mammals, Akt directly inhibits activity of FOXO and FOXO can also inhibit mTORC1 [17, 42]. It is noteworthy that IGF-1/IGF-1-R also acts, through Akt, on mTOR and GSK3-β activity [85]. Interestingly, IGF-1/IGF-1-R signaling is altered in the aging mammalian CNS, and has been linked to aging-related dysfunction such as memory impairment, cognitive dysfunction and synaptic dysfunction [20, 92]. Altogether, these data suggest a role of IGF-1/IGF-1-R in axon regeneration at an advanced age and it remains to be seen whether this signaling pathway promotes or inhibits axon regeneration in old mammals.
3. SOCS3/STAT3
SOCS3 has recently been identified as another neuron-intrinsic inhibitor of axon growth in the mammalian CNS [91]. Deleting SOCS3 increases axon regeneration and sprouting via promoting STAT3 activity. Interestingly, PTEN and SOCS3 co-deletion synergistically increase optic nerve regeneration and CST sprouting, at least in young animals [49, 93]. In the PNS, STAT3 deletion decreases regeneration and STAT3 over-expression promotes axon growth [5]. Intriguingly, STAT3 expression level is specifically decreased in mature and aging brains [19] and STAT3 phosphorylation is decreased in hippocampal neurons of mice modeling Alzheimer’s disease and patients with the disease [18]. Moreover, STAT3 is activated by IGF-1 signaling [111] and, in addition, PTEN/SOCS3 co-deletion increases IGF-1 expression [93]. Finally, STAT3 is inhibited by FOXO [76]. Altogether, these data support SOCS3/STAT3 signaling as an attractive target to dissect the relationship between axon growth and aging in SCI paradigms.
4. KLF
The Krüppel-like factors have been identified in retinal ganglion cells (RGCs) as mediators of a developmental stage-dependent decline in axonal growth [36, 73]. KLF-4 was identified via an in vitro screen as a repressor of axon growth and KLF-7 as an activator, which in an artificially activated form promotes CST regeneration and sprouting [9, 73]. In that same screen, another KLF member, KLF-1, was also found to inhibit axon growth at a level similar to KLF-4 [73]. This inhibitory effect of KLF-1 was recently confirmed in C. elegans [75]. Intriguingly, KLF-1 was recently implicated in lifespan and longevity in C. elegans [15]. Indeed, knockdown of KLF-1 suppresses the extended lifespan induced by dietary restriction whereas its overexpression in the intestine is sufficient to extend the lifespan of wild-type animals. It is not known if these findings apply to mammals. Finally, in the cancer field, KLFs have been associated with p53 and IGF-1 signaling [7, 108]. It will be interesting to address the relation between KLFs, axon growth and aging in the CNS.
5. Wnt signaling
Wnt/Ryk is another attractive target to promote axon growth after SCI. Indeed, Wnt has been shown to inhibit axon regeneration and its blockade to promote CST growth and plasticity [45, 62]. Although an extrinsic molecule, its intracellular signaling pathways may be implicated in the effect of age on axon regeneration. Indeed, one downstream effector of Wnt signaling is GSK3-β (discussed above as part of the PTEN signaling pathway)[104]. This is interesting, as Wnt signaling has previously been linked to aging. Indeed, Wnt signaling is increased during aging, and plays a central role in tissue-specific stem cell aging and age-related increases in tissue fibrosis [10]. In an accelerated mouse model of aging, increased Wnt activity induces senescence [60]. Interestingly, Ryk has been shown to repress activity of the longevity-promoting factor FOXO in C. elegans, which is involved in axon regeneration through the IGF-1 signaling pathway [13, 28, 96]. Altogether, these data would suggest that inhibition of the Wnt/Ryk pathway may be beneficial in promoting axon growth in aging mice. However, contradictory results are found in the literature. Indeed, some studies reported a decrease in Wnt/β-catenin signaling in hippocampal cells of aged rats, which may be involved in a decline of neurogenesis with age [77, 78]. Additionally, Wnt deficiency is associated with another age-associated disease, Alzheimer disease [14]. Therefore, Wnts may have divergent, yet unexplored, roles in axonal responses to injury in the aging CNS [67].
6. p53
Another strong candidate in mediating the age-dependent regeneration decline is the tumor suppressor p53, as it has been implicated in both axon regeneration and the aging process. Indeed, p53 is required for neurite outgrowth and axonal regeneration in the CNS [22, 23]. Additionally, the role of p53 in aging is well established and documented [86]. Interestingly, in cancer and aging, p53 is linked to IGF-1 and mTOR pathways [29, 58]. Not only does p53 down-regulate IGF-1, but IGF-1 can also mediate p53 activation [97]. Recently, Di Giovanni’s group identified IGF-1-R as a key player in mediating the regenerative effect of p53, as its activity and expression was found to be required for axon growth [50]. Altogether, these studies suggest a complex relationship between p53, IGF-1 and mTOR in controlling the decline of axon regeneration in aged animals that remains to be explored.
7. Effects of miRNA
Literature on the roles of miRNA is quickly expanding and evolving in many fields, including neuroscience [27, 52, 54]. In neurons, several miRNAs have been associated with axon growth and regeneration [41, 46, 59]. miRNAs have been associated with axon regeneration via controlling the PTEN pathway [110], GSK3β inhibition [48] and even Wnt signaling [103]. The roles of miRNAs can be complex as some have been implicated in modulating astrogliosis [8] and others in angiogenesis [98]. Intriguingly, it has been suggested that miRNAs are involved in the developmental decline in neuronal regeneration in C. elegans [112]. The authors demonstrated that the balance between the miRNA let-7 and its target lin-41 contributes to the decrease in axon regeneration in older neurons. Interestingly, in pancreatic cancer cells, let-7 can enhance SOCS3 expression, leading to a blockage of STAT3 activation [81]. It will be interesting to determine whether miRNAs play a part in regulating axon growth in aging.
Other neuron-intrinsic considerations
In addition to the specific neuron-intrinsic molecular species and pathways listed above, other neuron-intrinsic properties may influence axon regeneration specifically in aging adults (Fig. 3).
1. Neuronal viscosity
In aging zebrafish, not only do axons regenerate slower but there is also an increase in the latency to regenerate [37]. In the aging mammalian PNS slower regeneration is also observed and has been linked to an increased viscosity (stiffness) of aged axons, which are 3 times intrinsically stiffer than neonatal axons [56]. Generally speaking, axon stiffness correlates with increased neurofilament expression and decreased tubulin expression. The converse of this state, i.e. decreased neurofilament expression and increased tubulin expression, is associated with growth during development or regeneration [43]. It is possible that the increase of stiffness in aged axons results from an increased stability of neurofilaments or reduced microtubule dynamics. Indeed, levels of neurofilaments and tubulins are altered in aging pyramidal neurons, with microtubule density and assembly decreasing with aging [16, 102]. Changes associated with aging in the relative composition of these proteins and in their assembly/disassembly may explain, at least in part, the slower regeneration observed in different models of axon injury and even after genetic manipulation of PTEN after SCI in mammals. This also highlights that, no matter how much the regenerative system is boosted by the intrinsic manipulations proposed above, the bottleneck may still be the physical limitation of aged neurons for growing. It would be interesting to test whether drugs modifying microtubule dynamics result in a modification in axon growth in aged animals.
2. Changes in axonal transport
Axonal transport is essential for the regeneration process, as it brings supplies necessary for growth to the axon tips, including organelles, cytoskeletal proteins and other necessary components. Speed of regeneration has been shown to depend on the rate of the slow component of anterograde transport in DRG neurons [105]. More recently, pre-conditioning lesion in the PNS, known to induce axon regeneration, has been linked to an increase in axon transport [68]. Perhaps not surprisingly, axonal transport has been reported to decline with age. In the optic nerve and sciatic nerve, the rate of slow anterograde transport declines with age [69, 95]. Similarly, fast axonal trafficking declines with age in both the PNS and CNS [71]. Nonetheless, PNS axon regeneration in aged mice can occur, albeit slower than in young animals, and has been linked to changes in axonal transport rate [71]. Fawcett and colleagues recently showed that axonal transport of growth-promoting, transmembrane integrins is both age and neuron-type dependent [1]. Although this study focused on the comparison between the postnatal and adult stages rather than different ages in adulthood, it provides an interesting example that differential transport of specific proteins can be involved in the age-dependent decline in regeneration. In addition to protein and vesicular transport, mitochondrial trafficking is also altered with aging. This is interesting because an increase in anterograde transport of mitochondria accompanies axon regeneration after PNS injury [72] and there is an age-related decline in mitochondrial transport in both the PNS and the CNS [71, 94]. Thus, one interesting hypothesis for future studies is that an age-dependent decline in axonal transport is responsible, at least in part, for the decline in axon regeneration.
3. Free radical theory and mitochondria activity
Free radical formation (including reactive oxygen species, or ROS, and reactive nitrogen species, or RNS) and oxidative damage after CNS injury have been extensively described and have been proposed to be key mediators of the pathophysiology in acute and secondary injury response [3]. Several antioxidant therapies are currently in pre-clinical and clinical trials after SCI [3]. In the aging field, the free radical theory is one of the main theories advanced to explain the biological process of aging [4]. In this model, mitochondria, a primary target of free radicals, exhibit a decrease in activity with aging [82]. Interestingly, after SCI, mitochondrial dysfunction is worsened by oxidative damage, which activates proteases and the degradation of cytoskeletal proteins [106]. Therefore, the combination of aging and injury could exacerbate mitochondrial malfunction, leading to a further reduction of regeneration in aging individuals. Indeed, an increase in oxidative-related mechanisms has been reported after SCI in aged rats [33]. Additionally, mitochondria dysfunction results in a decrease of ATP production. A likely connection could be that this decrease in energy production in aged cells directly impacts axon growth. Recently, it has been shown that enhancing mitochondrial activity via STAT3 activation increases axon growth in the CNS [65]. How free radicals, mitochondrial activity and aging are related in modulating the age-dependent decline in axon growth remains to be better understood. Finally, it is noteworthy that other mechanisms in addition to the mitochondrial free radical theory of aging may play a role in the decrease of mitochondrial activity. Indeed, mTOR, IGF-1 and p53 signaling have recently been linked to changes in mitochondrial metabolism and aging [12, 82].
Conclusion
It is well accepted in several model organisms and the mammalian PNS that age negatively impacts axon regeneration after injury. This observation has now been extended to the mammalian CNS. Our knowledge of the age-dependent decline in axon regeneration in the context of spinal cord injury is rather limited, although testable hypotheses exist. As the average age of incidence of SCI and the average age of people living with a paralyzing SCI are increasing, there is a strong need to better understand the molecular and cellular players involved in the age-dependent hurdle for axonal repair. The spinal cord injury field can learn from the experience of the stroke field, where a mismatch in age between human patients and animal models has been recognized as an important barrier to the successful clinical translation of research findings. The relationship between age/aging and axon growth is complicated and multifactorial. Indeed, differences in a multitude of signaling pathways, cytoskeleton composition, axonal transport, mitochondrial activity and oxidative stress are likely at play. Furthermore, while this review focuses on neuron-intrinsic factors, it is noteworthy that extrinsic cues are also at play, including but not limited to, changes in the glial and fibrotic scar, inflammation, growth factor expression and extra-cellular matrix components.
Acknowledgments
This work was supported by grants from NIH/NINDS (R01NS054734, R01NS093055), Craig H. Neilsen Foundation (190181 and 384971) and Wings for Life Foundation (WFL-US-014/13).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrews MR, Soleman S, Cheah M, Tumbarello DA, Mason MR, Moloney E, Verhaagen J, Bensadoun JC, Schneider B, Aebischer P, Fawcett JW. Axonal Localization of Integrins in the CNS Is Neuronal Type and Age Dependent. eNeuro. 2016;3 doi: 10.1523/ENEURO.0029-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 3.Bains M, Hall ED. Antioxidant therapies in traumatic brain and spinal cord injury. Biochimica et biophysica acta. 2012;1822:675–684. doi: 10.1016/j.bbadis.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Bareyre FM, Garzorz N, Lang C, Misgeld T, Buning H, Kerschensteiner M. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6282–6287. doi: 10.1073/pnas.1015239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belin S, Nawabi H, Wang C, Tang S, Latremoliere A, Warren P, Schorle H, Uncu C, Woolf CJ, He Z, Steen JA. Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics. Neuron. 2015;86:1000–1014. doi: 10.1016/j.neuron.2015.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bentov I, Narla G, Schayek H, Akita K, Plymate SR, LeRoith D, Friedman SL, Werner H. Insulin-like growth factor-i regulates Kruppel-like factor-6 gene expression in a p53-dependent manner. Endocrinology. 2008;149:1890–1897. doi: 10.1210/en.2007-0844. [DOI] [PubMed] [Google Scholar]
- 8.Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, Kessler JA. microRNA-21 regulates astrocytic response following spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:17935–17947. doi: 10.1523/JNEUROSCI.3860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackmore MG, Wang Z, Lerch JK, Motti D, Zhang YP, Shields CB, Lee JK, Goldberg JL, Lemmon VP, Bixby JL. Kruppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7517–7522. doi: 10.1073/pnas.1120684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 11.Bradke F, Marin O. Editorial overview: development and regeneration: nervous system development and regeneration. Current opinion in neurobiology. 2014;27:iv–vi. doi: 10.1016/j.conb.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Bratic A, Larsson NG. The role of mitochondria in aging. The Journal of clinical investigation. 2013;123:951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrne AB, Walradt T, Gardner KE, Hubbert A, Reinke V, Hammarlund M. Insulin/IGF1 signaling inhibits age-dependent axon regeneration. Neuron. 2014;81:561–573. doi: 10.1016/j.neuron.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, Storto M, Gaviraghi G, Terstappen GC, Nicoletti F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrano AC, Dillin A, Hunter T. A Kruppel-like factor downstream of the E3 ligase WWP-1 mediates dietary-restriction-induced longevity in Caenorhabditis elegans. Nat Commun. 2014;5:3772. doi: 10.1038/ncomms4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cash AD, Aliev G, Siedlak SL, Nunomura A, Fujioka H, Zhu X, Raina AK, Vinters HV, Tabaton M, Johnson AB, Paula-Barbosa M, Avila J, Jones PK, Castellani RJ, Smith MA, Perry G. Microtubule reduction in Alzheimer’s disease and aging is independent of tau filament formation. The American journal of pathology. 2003;162:1623–1627. doi: 10.1016/s0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, Park Y, Hay N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiba T, Yamada M, Sasabe J, Terashita K, Shimoda M, Matsuoka M, Aiso S. Amyloid-beta causes memory impairment by disturbing the JAK2/STAT3 axis in hippocampal neurons. Molecular psychiatry. 2009;14:206–222. doi: 10.1038/mp.2008.105. [DOI] [PubMed] [Google Scholar]
- 19.De-Fraja C, Conti L, Govoni S, Battaini F, Cattaneo E. STAT signalling in the mature and aging brain. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2000;18:439–446. doi: 10.1016/s0736-5748(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 20.Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1, The journals of gerontology. Series A. Biological sciences and medical sciences. 2012;67:611–625. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeVivo MJ, Chen Y. Trends in new injuries, prevalent cases, and aging with spinal cord injury. Archives of physical medicine and rehabilitation. 2011;92:332–338. doi: 10.1016/j.apmr.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 22.Di Giovanni S, Knights CD, Rao M, Yakovlev A, Beers J, Catania J, Avantaggiati ML, Faden AI. The tumor suppressor protein p53 is required for neurite outgrowth and axon regeneration. The EMBO journal. 2006;25:4084–4096. doi: 10.1038/sj.emboj.7601292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Giovanni S, Rathore K. p53-Dependent pathways in neurite outgrowth and axonal regeneration. Cell and tissue research. 2012;349:87–95. doi: 10.1007/s00441-011-1292-5. [DOI] [PubMed] [Google Scholar]
- 24.Dolbow JD, Dolbow DR, Gorgey AS, Adler RA, Gater DR. The effects of aging and electrical stimulation exercise on bone after spinal cord injury. Aging Dis. 2013;4:141–153. [PMC free article] [PubMed] [Google Scholar]
- 25.Du K, Zheng S, Zhang Q, Li S, Gao X, Wang J, Jiang L, Liu K. Pten Deletion Promotes Regrowth of Corticospinal Tract Axons 1 Year after Spinal Cord Injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:9754–9763. doi: 10.1523/JNEUROSCI.3637-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupraz S, Grassi D, Karnas D, Nieto Guil AF, Hicks D, Quiroga S. The insulin-like growth factor 1 receptor is essential for axonal regeneration in adult central nervous system neurons. PloS one. 2013;8:e54462. doi: 10.1371/journal.pone.0054462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eacker SM, Dawson TM, Dawson VL. The interplay of microRNA and neuronal activity in health and disease. Frontiers in cellular neuroscience. 2013;7:136. doi: 10.3389/fncel.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 29.Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, Levine AJ. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer research. 2007;67:3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 30.Fenn AM, Hall JC, Gensel JC, Popovich PG, Godbout JP. IL-4 signaling drives a unique arginase+/IL-1beta+ microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Ralpha after traumatic spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:8904–8917. doi: 10.1523/JNEUROSCI.1146-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke; a journal of cerebral circulation. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gavazzi I, Cowen T. Axonal regeneration from transplanted sympathetic ganglia is not impaired by age. Exp Neurol. 1993;122:57–64. doi: 10.1006/exnr.1993.1107. [DOI] [PubMed] [Google Scholar]
- 33.Genovese T, Mazzon E, Di Paola R, Crisafulli C, Muia C, Bramanti P, Cuzzocrea S. Increased oxidative-related mechanisms in the spinal cord injury in old rats. Neuroscience letters. 2006;393:141–146. doi: 10.1016/j.neulet.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 34.Geoffroy CG, Hilton BJ, Tetzlaff W, Zheng B. Evidence for an Age-Dependent Decline in Axon Regeneration in the Adult Mammalian Central Nervous System. Cell reports. 2016;15:238–246. doi: 10.1016/j.celrep.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geoffroy CG, Lorenzana AO, Kwan JP, Lin K, Ghassemi O, Ma A, Xu N, Creger D, Liu K, He Z, Zheng B. Effects of PTEN and Nogo Codeletion on Corticospinal Axon Sprouting and Regeneration in Mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:6413–6428. doi: 10.1523/JNEUROSCI.4013-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- 37.Graciarena M, Dambly-Chaudiere C, Ghysen A. Dynamics of axonal regeneration in adult and aging zebrafish reveal the promoting effect of a first lesion. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1610–1615. doi: 10.1073/pnas.1319405111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groah SL, Stiens SA, Gittler MS, Kirshblum SC, McKinley WO. Spinal cord injury medicine. 5. Preserving wellness and independence of the aging patient with spinal cord injury: a primary care approach for the rehabilitation medicine specialist. Arch Phys Med Rehabil. 2002;83:S82–89. S90–88. doi: 10.1053/apmr.2002.32182. [DOI] [PubMed] [Google Scholar]
- 39.Gwak YS, Hains BC, Johnson KM, Hulsebosch CE. Effect of age at time of spinal cord injury on behavioral outcomes in rat. J Neurotrauma. 2004;21:983–993. doi: 10.1089/0897715041650999. [DOI] [PubMed] [Google Scholar]
- 40.Gwak YS, Hains BC, Johnson KM, Hulsebosch CE. Locomotor recovery and mechanical hyperalgesia following spinal cord injury depend on age at time of injury in rat. Neuroscience letters. 2004;362:232–235. doi: 10.1016/j.neulet.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Hancock ML, Preitner N, Quan J, Flanagan JG. MicroRNA-132 is enriched in developing axons, locally regulates Rasa1 mRNA, and promotes axon extension. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:66–78. doi: 10.1523/JNEUROSCI.3371-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hay N. Interplay between FOXO, TOR, and Akt. Biochimica et biophysica acta. 2011;1813:1965–1970. doi: 10.1016/j.bbamcr.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffman PN, Cleveland DW. Neurofilament and tubulin expression recapitulates the developmental program during axonal regeneration: induction of a specific beta-tubulin isotype. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:4530–4533. doi: 10.1073/pnas.85.12.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollis ER, 2nd, Lu P, Blesch A, Tuszynski MH. IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury. Exp Neurol. 2009;215:53–59. doi: 10.1016/j.expneurol.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollis ER, 2nd, Zou Y. Expression of the Wnt signaling system in central nervous system axon guidance and regeneration. Frontiers in molecular neuroscience. 2012;5:5. doi: 10.3389/fnmol.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu YW, Jiang JJ, Yan G, Wang RY, Tu GJ. MicroRNA-210 promotes sensory axon regeneration of adult mice in vivo and in vitro. Neuroscience letters. 2016;622:61–66. doi: 10.1016/j.neulet.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 47.Jaerve A, Schiwy N, Schmitz C, Mueller HW. Differential effect of aging on axon sprouting and regenerative growth in spinal cord injury. Exp Neurol. 2011;231:284–294. doi: 10.1016/j.expneurol.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Jiang JJ, Liu CM, Zhang BY, Wang XW, Zhang M, Saijilafu, Zhang SR, Hall P, Hu YW, Zhou FQ. MicroRNA-26a supports mammalian axon regeneration in vivo by suppressing GSK3beta expression. Cell death & disease. 2015;6:e1865. doi: 10.1038/cddis.2015.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin D, Liu Y, Sun F, Wang X, Liu X, He Z. Restoration of skilled locomotion by sprouting corticospinal axons induced by co-deletion of PTEN and SOCS3. Nat Commun. 2015;6:8074. doi: 10.1038/ncomms9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joshi Y, Soria MG, Quadrato G, Inak G, Zhou L, Hervera A, Rathore KI, Elnaggar M, Cucchiarini M, Marine JC, Puttagunta R, Di Giovanni S. The MDM4/MDM2-p53-IGF1 axis controls axonal regeneration, sprouting and functional recovery after CNS injury. Brain : a journal of neurology. 2015;138:1843–1862. doi: 10.1093/brain/awv125. [DOI] [PubMed] [Google Scholar]
- 51.Kang H, Lichtman JW. Motor axon regeneration and muscle reinnervation in young adult and aged animals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:19480–19491. doi: 10.1523/JNEUROSCI.4067-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaplan BB, Kar AN, Gioio AE, Aschrafi A. MicroRNAs in the axon and presynaptic nerve terminal. Frontiers in cellular neuroscience. 2013;7:126. doi: 10.3389/fncel.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawabuchi M, Tan H, Wang S. Age affects reciprocal cellular interactions in neuromuscular synapses following peripheral nerve injury. Ageing Res Rev. 2011;10:43–53. doi: 10.1016/j.arr.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Kosik KS. The neuronal microRNA system. Nature reviews. Neuroscience. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 55.Kumamaru H, Saiwai H, Ohkawa Y, Yamada H, Iwamoto Y, Okada S. Age-related differences in cellular and molecular profiles of inflammatory responses after spinal cord injury. Journal of cellular physiology. 2012;227:1335–1346. doi: 10.1002/jcp.22845. [DOI] [PubMed] [Google Scholar]
- 56.Lamoureux PL, O’Toole MR, Heidemann SR, Miller KE. Slowing of axonal regeneration is correlated with increased axonal viscosity during aging. BMC neuroscience. 2010;11:140. doi: 10.1186/1471-2202-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le TB, Aszmann O, Chen YG, Royall RM, Brushart TM. Effects of pathway and neuronal aging on the specificity of motor axon regeneration. Exp Neurol. 2001;167:126–132. doi: 10.1006/exnr.2000.7538. [DOI] [PubMed] [Google Scholar]
- 58.Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes & development. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 59.Liu CM, Wang RY, Saijilafu, Jiao ZX, Zhang BY, Zhou FQ. MicroRNA-138 and SIRT1 form a mutual negative feedback loop to regulate mammalian axon regeneration. Genes & development. 2013;27:1473–1483. doi: 10.1101/gad.209619.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 61.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nature neuroscience. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, Wang X, Lu CC, Kerman R, Steward O, Xu XM, Zou Y. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8376–8382. doi: 10.1523/JNEUROSCI.1939-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liz MA, Mar FM, Santos TE, Pimentel HI, Marques AM, Morgado MM, Vieira S, Sousa VF, Pemble H, Wittmann T, Sutherland C, Woodgett JR, Sousa MM. Neuronal deletion of GSK3beta increases microtubule speed in the growth cone and enhances axon regeneration via CRMP-2 and independently of MAP1B and CLASP2. BMC biology. 2014;12:47. doi: 10.1186/1741-7007-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu Y, Belin S, He Z. Signaling regulations of neuronal regenerative ability. Current opinion in neurobiology. 2014;27:135–142. doi: 10.1016/j.conb.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo X, Ribeiro M, Bray ER, Lee DH, Yungher BJ, Mehta ST, Thakor KA, Diaz F, Lee JK, Moraes CT, Bixby JL, Lemmon VP, Park KK. Enhanced Transcriptional Activity and Mitochondrial Localization of STAT3 Co-induce Axon Regrowth in the Adult Central Nervous System. Cell reports. 2016;15:398–410. doi: 10.1016/j.celrep.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nature reviews. Molecular cell biology. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 67.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: aging gracefully as a protectionist? Pharmacology & therapeutics. 2008;118:58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mar FM, Simoes AR, Leite S, Morgado MM, Santos TE, Rodrigo IS, Teixeira CA, Misgeld T, Sousa MM. CNS axons globally increase axonal transport after peripheral conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:5965–5970. doi: 10.1523/JNEUROSCI.4680-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McQuarrie IG, Brady ST, Lasek RJ. Retardation in the slow axonal transport of cytoskeletal elements during maturation and aging. Neurobiology of aging. 1989;10:359–365. doi: 10.1016/0197-4580(89)90049-3. [DOI] [PubMed] [Google Scholar]
- 70.Miao L, Yang L, Huang H, Liang F, Ling C, Hu Y. mTORC1 is necessary but mTORC2 and GSK3beta are inhibitory for AKT3-induced axon regeneration in the central nervous system. eLife. 2016;5 doi: 10.7554/eLife.14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milde S, Adalbert R, Elaman MH, Coleman MP. Axonal transport declines with age in two distinct phases separated by a period of relative stability. Neurobiology of aging. 2015;36:971–981. doi: 10.1016/j.neurobiolaging.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Misgeld T, Kerschensteiner M, Bareyre FM, Burgess RW, Lichtman JW. Imaging axonal transport of mitochondria in vivo. Nature methods. 2007;4:559–561. doi: 10.1038/nmeth1055. [DOI] [PubMed] [Google Scholar]
- 73.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nawabi H, Belin S, Cartoni R, Williams PR, Wang C, Latremoliere A, Wang X, Zhu J, Taub DG, Fu X, Yu B, Gu X, Woolf CJ, Liu JS, Gabel CV, Steen JA, He Z. Doublecortin-Like Kinases Promote Neuronal Survival and Induce Growth Cone Reformation via Distinct Mechanisms. Neuron. 2015;88:704–719. doi: 10.1016/j.neuron.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nix P, Hammarlund M, Hauth L, Lachnit M, Jorgensen EM, Bastiani M. Axon regeneration genes identified by RNAi screening in C. elegans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:629–645. doi: 10.1523/JNEUROSCI.3859-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oh HM, Yu CR, Dambuza I, Marrero B, Egwuagu CE. STAT3 protein interacts with Class O Forkhead transcription factors in the cytoplasm and regulates nuclear/cytoplasmic localization of FoxO1 and FoxO3a proteins in CD4(+) T cells. The Journal of biological chemistry. 2012;287:30436–30443. doi: 10.1074/jbc.M112.359661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okamoto M, Inoue K, Iwamura H, Terashima K, Soya H, Asashima M, Kuwabara T. Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:3570–3582. doi: 10.1096/fj.11-184697. [DOI] [PubMed] [Google Scholar]
- 78.Orellana AM, Vasconcelos AR, Leite JA, de Sa Lima L, Andreotti DZ, Munhoz CD, Kawamoto EM, Scavone C. Age-related neuroinflammation and changes in AKT-GSK-3beta and WNT/ beta-CATENIN signaling in rat hippocampus. Aging. 2015;7:1094–1111. doi: 10.18632/aging.100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Painter MW, Brosius Lutz A, Cheng YC, Latremoliere A, Duong K, Miller CM, Posada S, Cobos EJ, Zhang AX, Wagers AJ, Havton LA, Barres B, Omura T, Woolf CJ. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron. 2014;83:331–343. doi: 10.1016/j.neuron.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel K, Kollory A, Takashima A, Sarkar S, Faller DV, Ghosh SK. MicroRNA let-7 downregulates STAT3 phosphorylation in pancreatic cancer cells by increasing SOCS3 expression. Cancer letters. 2014;347:54–64. doi: 10.1016/j.canlet.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Payne BA, Chinnery PF. Mitochondrial dysfunction in aging: Much progress but many unresolved questions. Biochimica et biophysica acta. 2015;1847:1347–1353. doi: 10.1016/j.bbabio.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pestronk A, Drachman DB, Griffin JW. Effects of aging on nerve sprouting and regeneration. Exp Neurol. 1980;70:65–82. doi: 10.1016/0014-4886(80)90006-0. [DOI] [PubMed] [Google Scholar]
- 84.Qin S, Zou Y, Zhang CL. Cross-talk between KLF4 and STAT3 regulates axon regeneration. Nat Commun. 2013;4:2633. doi: 10.1038/ncomms3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nature cell biology. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 86.Rufini A, Tucci P, Celardo I, Melino G. Senescence and aging: the critical roles of p53. Oncogene. 2013;32:5129–5143. doi: 10.1038/onc.2012.640. [DOI] [PubMed] [Google Scholar]
- 87.Saijilafu, Hur EM, Liu CM, Jiao Z, Xu WL, Zhou FQ. PI3K-GSK3 signalling regulates mammalian axon regeneration by inducing the expression of Smad1. Nat Commun. 2013;4:2690. doi: 10.1038/ncomms3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siegenthaler MM, Ammon DL, Keirstead HS. Myelin pathogenesis and functional deficits following SCI are age-associated. Exp Neurol. 2008;213:363–371. doi: 10.1016/j.expneurol.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Silver J, Schwab ME, Popovich PG. Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harbor perspectives in biology. 2015;7:a020602. doi: 10.1101/cshperspect.a020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clinical epidemiology. 2014;6:309–331. doi: 10.2147/CLEP.S68889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Frontiers in aging neuroscience. 2013;5:27. doi: 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, He Z. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–375. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takihara Y, Inatani M, Eto K, Inoue T, Kreymerman A, Miyake S, Ueno S, Nagaya M, Nakanishi A, Iwao K, Takamura Y, Sakamoto H, Satoh K, Kondo M, Sakamoto T, Goldberg JL, Nabekura J, Tanihara H. In vivo imaging of axonal transport of mitochondria in the diseased and aged mammalian CNS. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:10515–10520. doi: 10.1073/pnas.1509879112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tashiro T, Komiya Y. Impairment of cytoskeletal protein transport due to aging or beta,beta′-iminodipropionitrile intoxication in the rat sciatic nerve. Gerontology. 1994;40(Suppl 2):36–45. doi: 10.1159/000213626. [DOI] [PubMed] [Google Scholar]
- 96.Tourette C, Farina F, Vazquez-Manrique RP, Orfila AM, Voisin J, Hernandez S, Offner N, Parker JA, Menet S, Kim J, Lyu J, Choi SH, Cormier K, Edgerly CK, Bordiuk OL, Smith K, Louise A, Halford M, Stacker S, Vert JP, Ferrante RJ, Lu W, Neri C. The Wnt receptor Ryk reduces neuronal and cell survival capacity by repressing FOXO activity during the early phases of mutant huntingtin pathogenicity. PLoS biology. 2014;12:e1001895. doi: 10.1371/journal.pbio.1001895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tran D, Bergholz J, Zhang H, He H, Wang Y, Zhang Y, Li Q, Kirkland JL, Xiao ZX. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging cell. 2014;13:669–678. doi: 10.1111/acel.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ujigo S, Kamei N, Hadoush H, Fujioka Y, Miyaki S, Nakasa T, Tanaka N, Nakanishi K, Eguchi A, Sunagawa T, Ochi M. Administration of microRNA-210 promotes spinal cord regeneration in mice. Spine. 2014;39:1099–1107. doi: 10.1097/BRS.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 99.Verdu E, Buti M, Navarro X. The effect of aging on efferent nerve fibers regeneration in mice. Brain research. 1995;696:76–82. doi: 10.1016/0006-8993(95)00762-f. [DOI] [PubMed] [Google Scholar]
- 100.Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. Journal of the peripheral nervous system : JPNS. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 101.Wecht JM, Weir JP, DeMeersman RE, Schilero GJ, Handrakis JP, LaFountaine MF, Cirnigliaro CM, Kirshblum SC, Bauman WA. Cold face test in persons with spinal cord injury: age versus inactivity. Clin Auton Res. 2009;19:221–229. doi: 10.1007/s10286-009-0009-2. [DOI] [PubMed] [Google Scholar]
- 102.Willcox BJ, Scott JN. Growth-associated proteins and regeneration-induced gene expression in the aging neuron. Mechanisms of ageing and development. 2004;125:513–516. doi: 10.1016/j.mad.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 103.Wu D, Murashov AK. MicroRNA-431 regulates axon regeneration in mature sensory neurons by targeting the Wnt antagonist Kremen1. Frontiers in molecular neuroscience. 2013;6:35. doi: 10.3389/fnmol.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends in biochemical sciences. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wujek JR, Lasek RJ. Correlation of axonal regeneration and slow component B in two branches of a single axon. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1983;3:243–251. doi: 10.1523/JNEUROSCI.03-02-00243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiong Y, Rabchevsky AG, Hall ED. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. Journal of neurochemistry. 2007;100:639–649. doi: 10.1111/j.1471-4159.2006.04312.x. [DOI] [PubMed] [Google Scholar]
- 107.Yang L, Miao L, Liang F, Huang H, Teng X, Li S, Nuriddinov J, Selzer ME, Hu Y. The mTORC1 effectors S6K1 and 4E-BP play different roles in CNS axon regeneration. Nat Commun. 2014;5:5416. doi: 10.1038/ncomms6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoon HS, Chen X, Yang VW. Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. The Journal of biological chemistry. 2003;278:2101–2105. doi: 10.1074/jbc.M211027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang BY, Saijilafu, Liu CM, Wang RY, Zhu Q, Jiao Z, Zhou FQ. Akt-independent GSK3 inactivation downstream of PI3K signaling regulates mammalian axon regeneration. Biochemical and biophysical research communications. 2014;443:743–748. doi: 10.1016/j.bbrc.2013.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou S, Shen D, Wang Y, Gong L, Tang X, Yu B, Gu X, Ding F. microRNA-222 targeting PTEN promotes neurite outgrowth from adult dorsal root ganglion neurons following sciatic nerve transection. PloS one. 2012;7:e44768. doi: 10.1371/journal.pone.0044768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zong CS, Chan J, Levy DE, Horvath C, Sadowski HB, Wang LH. Mechanism of STAT3 activation by insulin-like growth factor I receptor. The Journal of biological chemistry. 2000;275:15099–15105. doi: 10.1074/jbc.M000089200. [DOI] [PubMed] [Google Scholar]
- 112.Zou Y, Chiu H, Zinovyeva A, Ambros V, Chuang CF, Chang C. Developmental decline in neuronal regeneration by the progressive change of two intrinsic timers. Science. 2013;340:372–376. doi: 10.1126/science.1231321. [DOI] [PMC free article] [PubMed] [Google Scholar]