Abstract
Accumulation of advanced glycation end‐products (AGEs) is thought to contribute to muscle weakness in a diabetic animal model. Skin autofluorescence is a proposed marker for accumulation of AGEs in the skin. We aimed to investigate the relationship between AGEs accumulation, sarcopenia and muscle function of Japanese patients with type 1 diabetes. A total of 36 patients with type 1 diabetes participated in the present cross‐sectional study. Sarcopenia parameters (skeletal muscle mass index and knee extension strength) were compared with subcutaneous AGEs accumulation using skin autofluorescence. The prevalence of sarcopenia and impaired knee extension strength was 16.6% (men 0.0%, women 22.2%) and 47.2% (men 22.2%, women 55.6%), respectively. Knee extension strength was negatively correlated with skin autofluorescence (r² = 0.14, P < 0.05), but not with skeletal muscle mass index. In conclusion, the AGEs accumulation might be one of the reasons of impaired lower limb muscle function in Japanese patients with type 1 diabetes.
Keywords: Advanced glycation end‐products, Sarcopenia, Type 1 diabetes
Introduction
Sarcopenia is characterized by loss of skeletal muscle mass and its function1, 2, and is associated with aging1. The prevalence rates of sarcopenia in people aged 65–74 years are 2.3% in men and 5.0% in women, whereas those in people aged 75–84 years are 15.3% in men and 11.7% in women in a healthy Japanese population3. Sarcopenia has recently been recognized as one of the complications of type 2 diabetes4, 5. However, there have been no reports on the prevalence of sarcopenia in patients with type 1 diabetes.
Advanced glycation end‐products (AGEs), the products of non‐enzymatic binding of glucose and proteins, accumulate in various tissues in older adults6. Chronic hyperglycemia accelerates the AGEs accumulation, and increases the risk of diabetic vascular complications, such as macroangiopathy and microangiopathy7, 8, 9, 10, 11. A previous study suggested that hyperglycemia was associated with lower muscle function in middle‐aged and older patients12. However, there have been no reports on the relationship between the accumulation of AGEs in patients with type 1 diabetes and a reduction of muscle function.
Skin autofluorescence (AF) has been proposed as a marker of AGEs accumulation in the skin13. Skin AF is strongly correlated with the specific AGEs content in skin biopsies, as shown in multiple validation studies14, 15, 16, 17.
The aim of the present study was to ascertain the status of sarcopenia, and compare it with clinical characteristics in Japanese patients with type 1 diabetes. Furthermore, the relationship between AGEs accumulation and reduced muscle function was cross‐validated.
Materials and Methods
Study design and participants
The present study was approved by the ethical committee of Tokushima University Hospital (approved #2281‐1). The inclusion criteria of this study were outpatients with type 1 diabetes aged over 40 years at Tokushima University Hospital. Written informed consent was obtained from all participants. Patients who were taking steroids and those with myopathy, severe peripheral neuropathy or motor function disorders were excluded. A cross‐sectional analysis was carried out for each set of data obtained from July until October 2015. There were 36 participants, aged 42–75 years (men n = 9, women n = 27; mean age 55.7 ± 10.3 years (mean ± SD); duration of disease 13.9 ± 10.2 years).
Measures
Skeletal muscle mass was measured with a body composition analyzer (In Body bioelectrical impedance analyzer; Bio Space, Seoul, Korea) using multifrequency bioelectrical impedance analysis. Skeletal muscle mass index (SMI) was calculated by dividing extremity skeletal muscle by height squared. A previous validation study showed that lean body mass evaluated by this device was highly correlated with the data measured by dual‐energy X‐ray absorptiometry (intraclass correlation coefficient: men = 0.96 and women = 0.95, respectively)18.
Muscle function measurements included grip strength, knee extension strength and gait speed. Grip strength and knee extension strength were measured using handheld dynamometers (T.K.K5401; Takei Scientific Instruments, Tokyo, Japan; and μ‐tus F‐100; ANIMA, Tokyo, Japan). A previous validation study showed that interrater reliability of isometric knee extension strength measurements was highly correlated with the data measured by the handheld dynamometers (intraclass correlation coefficient: men and women = 0.99, respectively)19. Gait speed was measured using a stopwatch in time units of 0.01 s. Participants were asked to walk straight ahead for 11 m at their usual walking speed. The walking speed was measured at the middle 5 m. Measurements were carried out twice and the faster speed (m/s) was recorded20. Muscle morphology and physical functional parameters were applied to the Asian Working Group for Sarcopenia diagnostic algorithm to identify sarcopenia20. The cut‐off values for sarcopenia were SMI <7.0 and <5.7 kg/m2 for men and women, respectively, grip strength <26 and <18 kg for men and women, respectively, and gait speed <0.8 m/s. Also, the cut‐off value that shows a low value for lower limb muscle strength was set as <0.3 kg/kg of body weight (kg/kgBW) for both men and women20, 21, 22. Glycated hemoglobin (HbA1c) was measured with high‐performance liquid chromatography. HbA1c was expressed as a National Glycohemoglobin Standardization Program equivalent value. Blood pressure was measured in the upper arm, in the supine position. Skin AF was measured with the AGE Reader™ (DiagnOptics Technologies BV, Groningen, the Netherlands), a device that estimates AGEs skin accumulation using AGEs fluorescent properties13. A previous study showed that repeated skin AF measurements on 1 day show an overall Altman error rate of 5.03%14.
Statistical analysis
Spss Statistics 22 (IBM Japan, Tokyo, Japan) was used for statistical processing. All data are presented as the mean ± SD. Relationships among age, diabetes duration, skin AF, HbA1c, SMI and physical function parameters were determined using the Pearson's correlation coefficient. Statistical significance was defined as a P‐value <0.05.
Results
The physical characteristics of the participants are shown in Table 1. The presence of hypertension, SMI and grip strength were significantly lower in women than in men. Of the patients with type 1 diabetes, 16.6% (men 0.0%, women 22.2%) were diagnosed as sarcopenia according to the Asian Working Group for Sarcopenia criteria19 (Table 2). The prevalence of SMI, grip strength, gait speed and lower limb strength below the cut‐off value was 16.6% (men 0.0%, women 22.2%), 25.0% (men 11.1%, women 29.6%) 8.3% (men 0.0%, women 11.1%) and 47.2% (men 22.2%, women 55.6%), respectively.
Table 1.
Characteristics of type 1 diabetes patients
| All | Male | Female | |
|---|---|---|---|
| No. participants | 36 | 9 | 27 |
| Age (years) | 55.7 ± 10.3 | 53.8 ± 7.8 | 56.3 ± 11.0 |
| Duration of diabetes (years) | 13.9 ± 10.2 | 13.9 ± 10.3 | 14.2 ± 10.9 |
| BMI (kg/m2) | 23.0 ± 4.0 | 24.0 ± 4.9 | 22.7 ± 3.7 |
| Systolic blood pressure (mmHg) | 125 ± 15 | 122 ± 10 | 126 ± 17 |
| Diastolic blood pressure (mmHg) | 72 ± 10 | 77 ± 11 | 70 ± 9 |
| HbA1c (%) | 7.6 ± 1.3 | 7.4 ± 1.1 | 7.6 ± 1.4 |
| Skin AF | 2.49 ± 0.41 | 2.43 ± 0.36 | 2.50 ± 0.43 |
| eGFR (mL/min/1.73 m2) | 76 ± 23 | 72 ± 29 | 77 ± 21 |
| ≥90 mL/min/1.73 m2 (%) | 22.2 | 22.2 | 22.2 |
| 60 to <90 mL/min/1.73 m2 (%) | 58.3 | 33.3 | 66.7 |
| 30 to <60 mL/min/1.73 m2 (%) | 16.7 | 33.3 | 11.1 |
| <30 mL/min/1.73 m2 (%) | 2.8 | 11.2 | 0.0 |
| Serum creatinine (mg/dL) | 0.81 ± 0.43 | 1.1 ± 0.6 | 0.71 ± 0.34 |
| Method of insulin delivery MDI/CSII (%) | 11.1 | 11.1 | 11.1 |
| Treatment with statin (%) | 27.8 | 33.3 | 25.9 |
| Treatment with ACE Inhibitor (%) | 2.8 | 11.1 | 0.0 |
| Treatment with ARB (%) | 16.7 | 33.3 | 11.1 |
| Presence of dyslipidemia (%) | 36.1 | 44.4 | 33.3 |
| Presence of hypertension (%) | 36.1 | 66.7 | 25.9* |
| Presence of neuropathy | 47.2 | 66.7 | 40.7 |
| Presence of retinopathy, NDR/SDR/PPDR/PDR (%) | 66.7/19.4/8.3/5.6 | 55.6/22.2/22.2/0.0 | 70.4/18.5/3.7/7.4 |
| Presence of cardiovascular disease (%) | 2.8 | 0.0 | 3.7 |
| SMI (kg/m2) | 6.7 ± 1.1 | 8.0 ± 1.1 | 6.3 ± 0.8** |
| Grip strength (kg) | 26.2 ± 8.5 | 36.9 ± 6.3 | 22.6 ± 5.6*** |
| Knee extension strength/weight (kg/kg BW) | 0.30 ± 1.00 | 0.36 ± 0.10 | 0.28 ± 0.09 |
| Gait speed (m/s) | 1.23 ± 0.23 | 1.24 ± 0.19 | 1.23 ± 0.24 |
Data are shown as mean value ± SD. P‐value for sex difference: *P < 0.05, **P < 0.01, ***P < 0.001. ACE, angiotensin‐converting enzyme; AF, autofluorescence; ARB, angiotensin II receptor blocker; BMI, body mass index; BW, bodyweight; CSII, continuous subcutaneous insulin infusion; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; MDI, multiple daily injections; NDR, Non‐diabetic retinopathy; PDR, proliferative diabetic retinopathy; PPDR, preproliferative diabetic retinopathy; SDR, simple diabetic retinopathy; SMI, skeletal muscle mass index.
Table 2.
Prevalence of sarcopenia and cut‐off value parameters
| All | Male | Female | |
|---|---|---|---|
| Prevalence of sarcopenia (%) | 16.6 | 0.0 | 22.2 |
| SMI ≤ cut‐off value (%) | 16.6 | 0.0 | 22.2 |
| Grip strength ≤ cut‐off value (%) | 25.0 | 11.1 | 29.6 |
| Knee extension strength/weight ≤ cut‐off value (%) | 47.2 | 22.2 | 55.6 |
| Gait speed ≤ cut‐off value (%) | 8.3 | 0.0 | 11.1 |
SMI, skeletal muscle mass index.
There was no significant correlation between knee extension strength and SMI. Knee extension strength was negatively correlated with duration of diabetes (r² = 0.16, P < 0.05) and skin AF (r² = 0.14, P < 0.05), but not with HbA1c (Figure 1). SMI was negatively correlated with age (r² = 0.17, P = 0.01), but not HbA1c, skin AF or duration of diabetes. Skin AF was not correlated with age and duration of diabetes. Multiple regression analyses were carried out using knee extension strength/weight and SMI indices as dependent variables, and age, estimated glomerular filtration rate, dyslipidemia, hypertension, neuropathy, retinopathy, cardiovascular disease and skin AF as independent variables. Skin AF was negatively associated with knee extension strength/weight (β = −0.37, P = 0.03).
Figure 1.
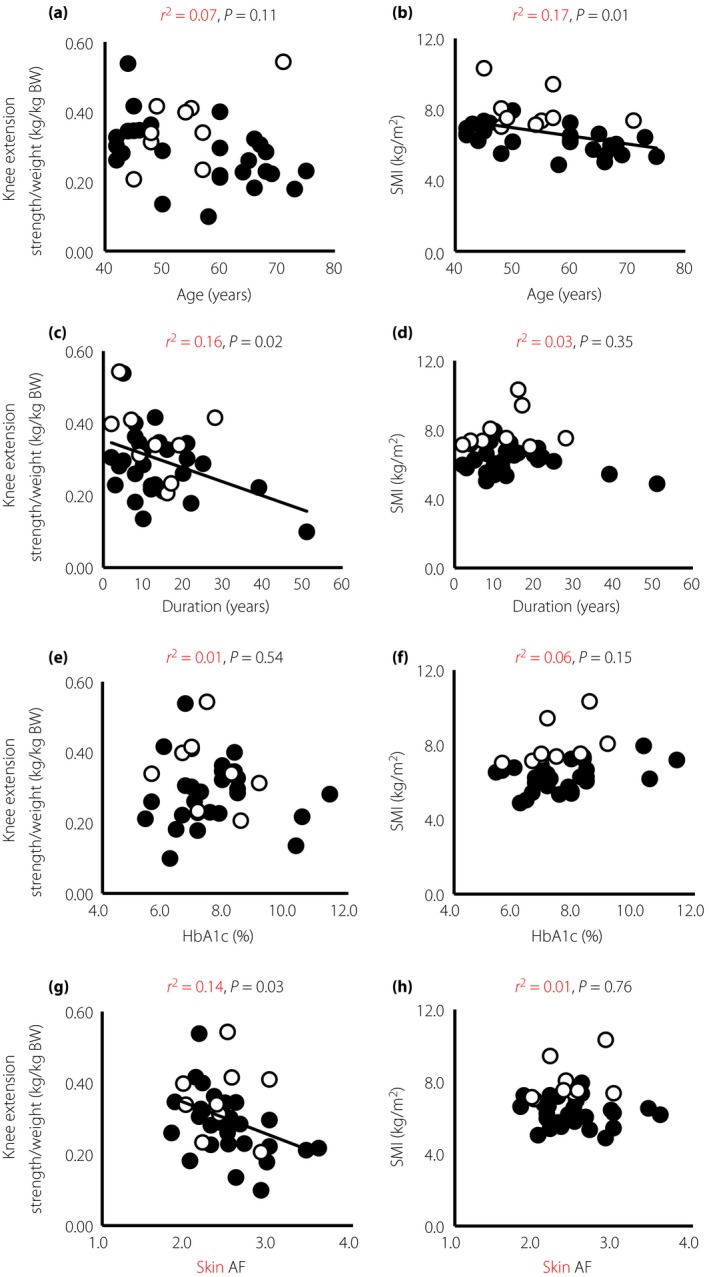
The correlation between knee extension strength/weight, skeletal muscle mass index (SMI) and clinical characteristics. (a,b) Age, (c,d) duration of diabetes, (e,f) glycated hemoglobin (HbA1c) and (g,h) skin autofluorescence (AF) in male (open circle) and female (closed circle) patients with type 1 diabetes. BW, bodyweight.
Discussion
The aim of the present study was to investigate the prevalence rate of sarcopenia in patients with type 1 diabetes, and to validate the relationship between AGEs accumulation and reduced muscle function.
Compared with the previous report by Yuki et al.3, the prevalence of sarcopenia in patients with type 1 diabetes in the present study, where the mean age was 55.7 ± 10.3 years, was unexpectedly higher, and the proportion of women was markedly higher. Furthermore, the percentage of participants whose grip strength values were below the cut‐off values was higher than that found by Yuki et al.3 In addition, the prevalence of participants whose knee extension strength was less than the cut‐off value was very high (47.2%).
Muscle mass has been reported to correlate with muscle function in healthy individuals23, 24. However, there was no significant correlation between knee extension strength and SMI in the present study. This possibility suggests that muscle function is impaired independently from age‐related reduction of muscle mass in patients with type 1 diabetes.
In contrast, skin AF and the duration of disease had a significant negative correlation with knee extension strength. HbA1c levels had an insignificant negative correlation with knee extension strength. Previous reports have shown that the accumulation of AGEs in fast twitch muscle fibers cross‐links muscle collagen, hardens muscle stiffness and reduces the tonic force of muscle contraction25, 26, 27. Several studies suggested that muscle function declined with accumulation of AGEs in patients with type 2 diabetes mellitus28, and healthy middle‐aged29 and older people30. Sugisawa et al.31 reported that skin AF reflects integration of past long‐term glycemic control in patients with type 1 diabetes. Taken together, the results of the present study suggest that sustained hyperglycemia associated with long duration of disease and accumulation of AGEs could be involved as a mechanism in the impaired muscle function together with aging in patients with type 1 diabetes. However, there was no correlation between SMI and skin AF. Because a significant negative correlation between the SMI and age was observed, skin AF was not associated with lower muscle mass in patients with type 1 diabetes. Skin AF and age were independent factors for knee extension strength/weight and SMI, respectively.
The limitations of the present study were that the study design was cross‐sectional without control subjects, so we were not able to achieve detailed verification of sarcopenia incidence, along with muscular dysfunction and the influence of AGEs accumulation in the muscular tissue of type 1 diabetes patients. We were also unable to evaluate the influence of lifestyle, diabetic complications (such as neuropathy) and pharmacotherapy of patients with type 1 diabetes on the decline of muscle strength. These are the topics for future investigations. Finally, another limitation was the small sample size of the analyzed participants in the present study.
In conclusion, the present study shows that the prevalence rate of lower limb muscular dysfunction was higher than sarcopenia in Japanese patients with type 1 diabetes. The AGEs accumulation might be one of the reasons for impaired lower limb muscle function in these patients.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
We acknowledge Ineko Takikawa for excellent secretarial work.
J Diabetes Investig 2017; 8: 377–382
Clinical Trial Registry
University Hospital Medical Information Network
UMIN000020901
References
- 1. Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci 2000; 55: 16–24. [DOI] [PubMed] [Google Scholar]
- 2. Santilli V, Bernetti A, Mangone M, et al Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 2014; 11: 177–180. [PMC free article] [PubMed] [Google Scholar]
- 3. Yuki A, Ando F, Otsuka R, et al Epidemiology of sarcopenia in elderly Japanese. J Phys Fitness Sports Med 2015; 4: 111–115. [Google Scholar]
- 4. Umegaki H. Sarcopenia and diabetes: Hyperglycemia is a risk factor for age‐associated muscle mass and functional reduction. J Diabetes Investig 2015; 6: 623–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim TN, Park MS, Yang SJ, et al Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010; 33: 1497–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldin A, Beckman JA, Schmidt AM, et al Advanced glycation end products. Sparking the development of diabetic vascular injury. Circulation 2006; 114: 597–605. [DOI] [PubMed] [Google Scholar]
- 7. Beisswenger PJ, Makita Z, Curphey TJ, et al Formation of immunochemical advanced glycosylation end products precedes and correlates with early manifestations of renal and retinal disease in diabetes. Diabetes 1995; 44: 824–829. [DOI] [PubMed] [Google Scholar]
- 8. Dyer DG, Dunn JA, Thorpe SR, et al Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest 1993; 91: 2463–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCance DR, Dyer DG, Dunn JA, et al Maillard reaction products and their relation to complications in insulin‐dependent diabetes mellitus. J Clin Invest 1993; 91: 2470–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monnier VM, Vishwanath V, Frank KE, et al Relation between complications of type I diabetes mellitus and collagen‐linked fluorescence. N Engl J Med 1986; 314: 403–408. [DOI] [PubMed] [Google Scholar]
- 11. Genuth S, Sun W, Cleary P, et al Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10‐year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 2005; 54: 3103–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalyani RR, Metter EJ, Egan J, et al Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care 2015; 38: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meerwaldt R, Graaff R, Oomen PH, et al Simple non‐invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004; 47: 1324–1330. [DOI] [PubMed] [Google Scholar]
- 14. Meerwaldt R, Links T, Graaff R, et al Simple noninvasive measurement of skin autofluorescence. Ann N Y Acad Sci 2005; 1043: 290–298. [DOI] [PubMed] [Google Scholar]
- 15. Lutgers HL, Graaff R, Links TP, et al Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care 2006; 29: 2654–2659. [DOI] [PubMed] [Google Scholar]
- 16. Singh R, Barden A, Mori T, et al Advanced glycation end‐products: a review. Diabetologia 2001; 44: 129–146. [DOI] [PubMed] [Google Scholar]
- 17. Gerrits EG, Lutgers HL, Kleefstra N, et al Skin autofluorescence: a tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care 2008; 31: 517–521. [DOI] [PubMed] [Google Scholar]
- 18. Ling CH, de Craen AJ, Slagboom PE, et al Accuracy of direct segmental multi‐frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle‐aged adult population. Clin Nutr 2011; 30: 610–615. [DOI] [PubMed] [Google Scholar]
- 19. Katoh M, Isozaki K. Reliability of Isometric Knee Extension Muscle Strength Measurements of Healthy Elderly Subjects Made with a Hand‐held Dynamometer and a Belt. J Phys Ther Sci 2014; 26: 1855–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen LK, Liu LK, Woo J, et al Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 21. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci 2012; 67: 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manini TM, Visser M, Won‐Park S, et al Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc 2007; 55: 451–457. [DOI] [PubMed] [Google Scholar]
- 23. Maughan RJ, Watson JS, Weir J. Strength and cross‐sectional area of human skeletal muscle. J Physiol 1983; 338: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukunaga T, Miyatani M, Tachi M, et al Muscle volume is a major determinant of joint torque in humans. Acta Physiol Scand 2001; 172: 249–255. [DOI] [PubMed] [Google Scholar]
- 25. Snow LM, Thompson LV. Influence of insulin and muscle fiber type in nepsilon‐ (carboxymethyl) ‐lysine accumulation in soleus muscle of rats with streptozotocin‐induced diabetes mellitus. Pathobiology 2009; 76: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Snow LM, Fugere NA, Thompson LV. Advanced glycation end‐product accumulation and associated protein modification in type II skeletal muscle with aging. J Gerontol A Biol Sci Med Sci 2007; 62: 1204–1210. [DOI] [PubMed] [Google Scholar]
- 27. Haus JM, Carrithers JA, Trappe SW, et al Collagen, cross‐linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 2007; 103: 2068–2076. [DOI] [PubMed] [Google Scholar]
- 28. Shah KM, Clark BR, McGill JB, et al Relationship between skin intrinsic fluorescence–an indicator of advanced glycation end products‐and upper extremity impairments in individuals with diabetes mellitus. Phys Ther 2015; 95: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Momma H, Niu K, Kobayashi Y, et al Skin advanced glycation end product accumulation and muscle strength among adult men. Eur J Appl Physiol 2011; 111: 1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dalal M, Ferrucci L, Sun K, et al Elevated serum advanced glycation end products and poor grip strength in older community‐dwelling women. J Gerontol A Biol Sci Med Sci 2009; 64: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sugisawa E, Miura J, Iwamoto Y, et al Skin autofluorescence reflects integration of past long‐term glycemic control in patients with type 1 diabetes. Diabetes Care 2013; 36: 2339–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]


